Abstract
In this study, antibiofilm activity of coconut husk extract (CHE) was tested by various assays in the laboratory. The effects of CHE on extracellular polymeric substance (EPS) production, hydrophobicity and adhesion ability of Pseudomonas sp., Alteromonas sp. and Gallionella sp. and the antimicrobial activity of the extract against these bacteria were assessed. CHE was found to possess antibacterial activity against all the bacterial strains and affected the EPS production. The CHE affected the growth of the biofilm-forming bacteria in a culture medium. The hydrophobicity of the bacterial cells was also changed due to the CHE treatment. The active compound of the CHE was characterised by thin-layer chromatography (TLC), high performance liquid chromatography (HPLC) and fourier transform infrared (FT-IR) analysis. HPLC spectrum showed a single peak and the FT-IR spectrum indicated the presence of an OH-group-containing compound in the extract. In conclusion the CHE could be used as a source for the isolation of antifouling compounds.
Keywords: Biofouling, Biofilm, Polyphenol, Extracellular polymeric substance, Adhesion
1. Introduction
Natural products and their analogues exhibiting inhibitory or repellent activity against microbes, algae and larval forms of invertebrates are preferred as potential agents to control biofouling. The search for natural-product antifoulants has been greatly encouraged by the fact that these compounds are less or non-toxic to the environment. The majority of natural-product antifoulants identified so far are terpenoids, steroids, carotenoids, phenolics, furanones, alkaloids, peptides and lactones (Fusetani, 2004). They have been isolated from a wide range of organisms such as sponges, corals, seaweeds and microbes (Thompson et al., 1985; Goto et al., 1992). As biofouling causes huge loss to the marine domain, intensive studies towards the development of eco-friendly antifouling agents are progressing worldwide (Clare, 1998; Yebra et al., 2004; Sipkema et al., 2005; Raveendran et al., 2008). Most of the studies on natural-product antifoulants are concentrated mainly on marine invertebrates, seaweeds, tunicates and microbes. There is a lack of information on antifouling agents from terrestrial sources.
Cocos nucifera L. (family Arecaceae), commonly known as ‘coconut’, is considered as an important cash crop in tropical countries. Coconuts are unique in terms of their fruit (a drupe) morphology. Coir fibres are found between the husk and the fibrous outer shell (mesocarp) of a coconut. The individual fibre cells are narrow and hollow, with thick walls made of cellulose. The coarse fibre is used as a raw material for the preparation of various coir products. Various polyphenols are present in the husk (Sueli and Gustavo, 2007) and the aqueous extract of the coconut husk has antimicrobial activity (Esquenazi et al., 2002). In the present study, an attempt has been made to screen the antifouling activity of the coconut husk extract (CHE). The major objectives of the present study were: (1) to assess the inhibitory activity of the CHE against bacterial adhesion onto surfaces; (2) to assess the effect of CHE on extracellular polymeric substance synthesis in biofilm forming bacteria; and (3) to characterise the bioactive principle present in the CHE. A study of this kind will expand our knowledge on the inhibitory activity of natural-products from terrestrial plants against fouling organisms. This study will also provide leads to the formulation of natural- product antifoulants from coconut husk, a by-product of agriculture.
2. Materials and methods
2.1. Collection and concentration of coconut husk extract (CHE)
The coconut husk extract was obtained from coir retting effluent collected from a coir-retting field near to the centre for Marine Science and Technology (Rajakkamangalam, Kanyakumari Dist, Tamil Nadu, India). The term ‘retting’ refers to the decomposition of tissues surrounding the fibres of the coconut husk. Normally, the retting process was carried out by soaking the coconut husks in water (small pools near to backwaters or rivers). The effluent from the coir retting field (brown in colour due to the release of organics from the husk) was collected and filtered (0.47 μm, Whatman) to remove the debris. The filtrate was dried and used for the antifouling assays against target bacteria. The filtrate is hereafter referred to as ‘coconut husk extract’ (CHE). About 5 L of the coir retting effluent was filtered to obtain 100 mg of coconut husk extract. Similarly, the coconut husk was kept in small plastic tanks (5 L) in fresh water for 15 days and the resulting brown coloured effluent was collected. The effluent was filtered (0.47 μm, Whatman) to remove the debris and dried. This laboratory prepared coconut husk extract was also used for anti-biofilm assays.
2.2. Antimicrobial assay
Coconut husk extract was screened for antimicrobial activity against Pseudomonas sp., Gallionella sp. and Alteromonas sp. These bacteria had been isolated from the marine biofilm developed on acrylic panels submerged in Kudankulam coastal waters (8° 9′ 5″ N and 77° 39′ 59″ E). Bacterial strains were identified at genus level using morphological and physiological characters and maintained in our laboratory (centre for Marine Science and Technology, Rajakkamangalam, India). Antimicrobial activity was assessed by the disc diffusion method. 100 mg of dried CHE was dissolved in 500 μL of distiled water. From this extract, 50 μL was loaded onto a sterile disc (6 mm, HIMEDIA, India) and allowed to saturate. The disc was aseptically transferred to agar plates (Zobell Marine Agar 2216, HIMEDIA, India) seeded with target bacteria (Pseudomonas sp., Gallionella sp. and Alteromonas sp.). Sterile discs loaded with distiled water were used as control. The plates were incubated at room temperature for a period of 24 h. After the incubation, inhibition zones if any around the discs were measured.
2.3. Influence of coconut husk extract on the EPS production of biofilm-forming bacteria
This experiment was carried out to assess the influence of CHE on the extracellular polymeric substance (EPS) production of biofilm-forming bacteria. The culture broth (overnight grown) of biofilm-forming bacteria (maintained in Zobell marine broth) was centrifuged at 5000g for 15 min. The cell pellets obtained after centrifugation were washed with phosphate-buffered saline (10 mM PBS) and re-suspended in the same buffer to obtain a cell number of 106 cells ml−1(number of cells were quantified by preparing a standard graph based on the optical density of the medium). This cell suspension was used for further assay.
Three millilitres of biofilm-forming bacterial culture was taken in a test tube containing Zobell Marine broth (3 ml) and 500 μl of CHE (100 mg of dried coconut husk extract powder dissolved in 500 μl of distiled water) was added to the tube. Bacterial culture without the addition of CHE was kept as the control. The tubes were incubated at 37°C for 24 h. After incubation, the culture broth was centrifuged at 5000g for 5 min at 4°C. The pellets were discarded and the supernatant was filtered through a membrane filter (0.47 μm, Whatman). The filtrate was mixed with an equal volume of cold absolute ethanol to precipitate the EPS. The volume of the precipitated EPS was measured and diluted to a known volume with distiled water and stored at 4°C. The EPS was estimated by quantifying the carbohydrate and protein concentration and expressed as micrograms in a litre of total EPS collected.
Carbohydrate concentration of the EPS was estimated by the modified phenol sulphuric acid method using glucose as the standard (Dubois et al., 1956). The total protein content of EPS was estimated by the modified Lowry et al. (1951) method using bovine serum albumin as the standard.
2.4. Effect of CHE on biofilm-forming bacterial growth
For the growth inhibition assay, the bacterial cells (Pseudomonas sp., Gallionella sp. and Alteromonas sp.) were grown overnight at room temperature (28 ± 2 °C) and the next day transferred into 3 ml of nutrient broth (Zobell Marine broth) in a test tube. Five hundred microlitres of CHE was added to the test tubes and incubated at 37°C. The test tubes without CHE were kept as the control. The absorbance of the bacterial cultures was read at 600 nm in a spectrophotometer after every 3 h interval. This experiment was conducted for a period of 24 h. The student’s t-test was used to find out the variation between the control and experimental samples.
2.5. Influence of coconut husk extract on the hydrophobicity of biofilm-forming bacteria
The hydrophobicity of the biofilm-forming bacterial cell suspension was evaluated using BATH (bacterial adherence to hydrocarbon) assay described by Rosenberg et al. (1980). For this experiment, 3 ml of biofilm-forming bacterial suspension was taken in a test tube and 500 μl of CHO (100 mg dissolved in 500 μl distiled water) added to it. The bacterial suspension without the addition of CHE was taken as the control. The tubes were incubated for 24 h. After incubation, 100 μl of hydrocarbon (n-hexane) was added to the bacterial suspension and vortexed for 1 min to ensure mixing and then left undisturbed for 15 min to allow the separation of the two phases. The absorbance of the aqueous phase was taken at 400 nm using a spectrophotometer. If the cells are hydrophobic, then they adhere to the hydrocarbon phase and form an upper ‘cream’ like layer. The hydrophilic cells remain in the polar aqueous phase. The measure of hydrophobicity of the bacterial cell wall is the proportion of bacterial cells bound to the hydrocarbon determined by recording the decrease in light absorbance in the aqueous phase. The percentage of cells bound to hydrocarbon (n-hexane) was calculated using the formula.
Where A0 is the optical density (OD) (400 nm) of the aqueous cell suspension before adding n-hexane and A is the OD (400 nm) after adding n-hexane.
2.6. Adhesion assay using coconut husk extract
Bacterial adhesion assay was carried out in 500-ml glass beakers using microscopic slides (7.5 × 2.5 cm) as substratum (coupons). The glass beakers were filled with 300 ml of sterile seawater. One hundred milligrams of coconut husk extract was added to the seawater and the coupons were (n = 5) placed inside the beaker in a slanting position. The whole setup was kept undisturbed for 24 h for the formation of a conditioning film from the surrounding medium. Coupons immersed in sterile seawater (without CHE) were used as the control. After 24 h, the coupons were retrieved from the beaker and placed in another beaker containing 300 ml of sterile seawater along with a 3 ml biofilm bacterial culture. Three millilitres of Zobell marine broth was also added to the medium in order to provide the essential nutrients. The coupons were incubated for 5 h. The coupons were removed after 1, 2, 3, 4 and 5 h for the enumeration of bacterial cells. The coupons were rinsed in filter-sterilized seawater to remove the unattached cells, dried, heat-fixed and stained with crystal violet. The number of bacteria that adhered onto the coupons was counted under the microscope. The experiment was repeated (n = 5) and the mean (5 × 2 = 10 coupons) ± standard deviation values were taken. Student’s t-test was used to find out the variation in the adhesion of bacteria onto control and treated coupons.
2.7. Partial purification of coconut husk extract by thin layer chromatography
The CHE was analysed by thin layer chromatography. The crude extract was placed on silica gel plates and the solvent system used was benzene, acetic acid and methanol in the ratio of 1:1:3. The distinct spot observed on the thin-layer chromatogram was scraped and kept in a vial. The active compound was recovered by adding a known volume of water in the vial and centrifuged at 5000 rpm for 15 min. The supernatant was collected and stored at 4°C. The antimicrobial activity of the partially purified compound was also assessed using the disc assay method.
2.8. Test for phenolic compounds
The ferric chloride method was used for the identification of the active compound present in the coconut husk extract. One millilitre of coconut husk extract was taken in a test tube and 1 ml of ethyl alcohol and 1 ml of 1 N HCl were added. One or two drops of 3% FeCl3 solution were added to the mixture, and the presence of phenolic compounds in the extract was confirmed by the formation of deep red colour.
2.9. Analysis of coconut husk extract by high performance liquid chromatography (HPLC)
The active fraction of the coconut husk extract was analysed by HPLC (Cyberlab, USA) using acetonitrile and water (1:1) as the mobile phase. The reagents for the mobile phase were of HPLC grade and the mobile phase was filtered and degassed on a Millipore filtration system with 0.45 μm pore size. The fraction eluted from the TLC was mixed with the HPLC mobile phase and injected into HPLC system consisting of reverse phase C16 column and UV detector. The flow rate was fixed as 1 ml min−1.
2.10. Fourier transform analysis (FT-IR)
The (IR) Infrared spectrum was recorded in a SHIMADZU FT-IR system. A small quantity of TLC purified fraction was placed on the face of a highly polished KBr salt plate and another KBr plate was positioned on the top to spread the compound as a thin layer. The FT-IR spectra were recorded in a wave number from 400 to 4000 cm−1.
3. Results
3.1. Antimicrobial activity of coconut husk extract (CHE)
The results of the present study revealed that the coconut husk extract was capable of inhibiting the growth of biofilm-forming bacteria such as Pseudomonas sp., Alteromonas sp. and Gallionella sp. The zone of inhibition of CHE obtained from the coir retting field against Pseudomonas sp. and Alteromonas sp. was 8 mm and that against Gallionella sp. was 7 mm. Similarly, the CHE obtained from the retting process in the laboratory also showed inhibitory activity against Pseudomonas sp., Alteromonas sp. and Gallionella sp. The zone of inhibition against Alteromonas sp. was 10 mm and that against Pseudomonas sp. and Gallionella sp. were 11 and 8 mm respectively. For further experiments, the CHE obtained from the natural coir retting pond was used.
3.2. Influence of coconut husk extract on EPS production of biofilm-forming bacteria
The EPS produced by Pseudomonas sp. culture (without coconut husk extract) showed a carbohydrate concentration of 1.469 mg l−1 after 24 h. The culture treated with coconut husk extract exhibited a carbohydrate concentration of 1.205 mg l−1 after 24 h (Table 1). The EPS produced by Alteromonas sp. culture (without coconut husk extract) showed a carbohydrate concentration of 1.21 mg l−1 after 24 h. The Alteromonas sp. culture treated with coconut husk extract showed a carbohydrate concentration of 0.952 mg l−1 after 24 h of incubation. Similarly, the EPS produced by Gallionella sp. culture (without coconut husk extract) showed a carbohydrate concentration of 1.189 mg l−1 after 24 h. The Gallionella sp. culture treated with coconut husk extract showed a carbohydrate concentration of 1.139 mg l−1 after 24 h of incubation.
Table 1.
Effect of coconut husk extract on extracellular polymeric substance production (EPS) in biofilm-forming bacteria. The carbohydrate and protein concentrations were used to measure EPS production.
| Bacteria | Carbohydrate (mg l−1) | Protein (mg l−1) | ||
|---|---|---|---|---|
| Control | CHE treated | Control | CHE treated | |
| Pseudomonas sp. | 1.469 | 1.205 | 0.501 | 0.364 |
| Alteromonas sp. | 1.21 | 0.952 | 0.539 | 0.292 |
| Gallionella sp. | 1.189 | 1.139 | 0.547 | 0.392 |
The EPS produced by Pseudomonas sp. control culture showed a protein concentration of 0.501 mg 1−1 after 24 h, and that produced by Pseudomonas sp. treated with CHE was 0.364 mg 1−1 (Table 1). The EPS produced by Alteromonas sp. control culture had a protein concentration of 0.539 mg 1−1 after 24 h, whereas that produced by the same species treated with CHE was 0.292 mg 1−1 after 24 h. The EPS produced by Gallionella sp. control culture showed a protein concentration of 0.547 mg 1−1. The protein concentration of EPS produced by Gallionella sp. treated with coconut husk extract was found to be 0.392 mg 1−1.
3.3. Effect of CHE on biofilm-forming bacterial growth
The CHE showed inhibitory activity on the growth of all the bacterial strains tested. The absorbance of the culture medium treated with CHE exhibited lower values than the control (Fig. 1A–C). Significant variations in the absorbance were also observed between control and experimental mediums of Pseudomonas sp. (t stat: 6.25; d.f: 8), Alteromonas sp. (t stat: 4.01; d.f: 8) and Gallionella sp. (t stat: 6.97; d.f: 8).
Figure 1.
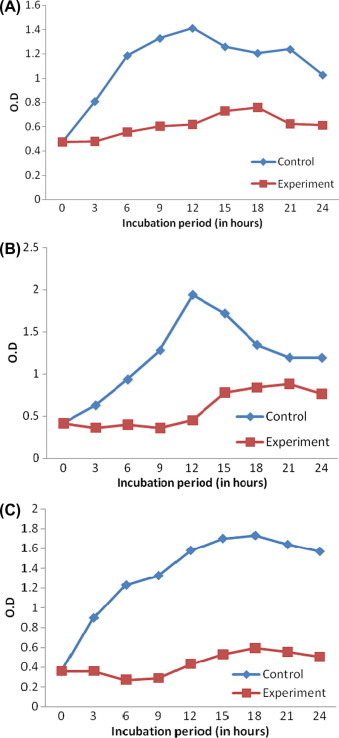
Growth inhibitory activity of the coconut husk extract against Pseudomonas sp. Alteromonas sp. and Gallionella sp. The absorbance of the bacterial culture medium was measured in spectrophotometer at 600 nm; (A). Pseudomonas sp.; (B). Alteromonas sp.; (C). Gallionella sp.
3.4. Measurement of hydrophobicity
The hydrophobicity of Alteromonas sp. control culture was 52.45. It increased to 66.40 after CHE treatment. Similarly, the hydrophobicity of Pseudomonas sp. control culture was 66.23 and it increased to 70.06 when treated with CHE. The hydrophobicity of Gallionella sp. culture was 76.56, and this value decreased to 63.00 after the CHE treatment (Table 2).
Table 2.
Changes in the hydrophobicity of bacterial cells treated with coconut husk extract.
| Bacteria | Control | CHE treated |
|---|---|---|
| Pseudomonas sp. | 66.23 | 70.06 |
| Alteromonas sp. | 52.45 | 66.40 |
| Gallionella sp. | 76.56 | 63.00 |
3.5. Bacterial adhesion assay
The adhesion of bacterial cells to glass slides submerged in CHE medium was assessed using Pseudomonas sp., as target organism. This assay showed that the number of Pseudomonas sp. cells that adhered to the glass surface was reduced significantly. The coupons were submerged for a period of one hour showing an abundance of 4784 ± 918 cells cm−2 on the coupons coated with the CHE and 5772 ± 857 cells cm−2 on the control coupons (Fig. 2). After 2 h, 5620 ± 340 cells cm−2 were observed on the coupons coated with the extract and 6864 ± 554 cells cm−2 on the control coupons. After 3 h, 5500 ± 720 cells cm−2 were observed on the coupons coated with the CHE and 7696 ± 1061 cells cm−2 cells on the control coupons. The coupons observed after 4 h showed the abundance of 5112 ± 865 cells cm−2 on CHE coated coupons and 9256 ± 934 cells cm−2 on the control coupons. After 5 h, 4516 ± 276 cells cm−2 were observed on the coupons coated with CHE and 10608 ± 1812 cells cm−2 on the control coupon. Student’s t test showed a significant variation on the adhesion of bacteria between the control and CHE coated coupons (t stat: 3.039; d.f. 4).
Figure 2.
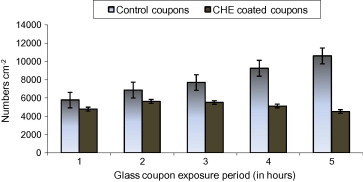
Adhesion of Alteromonas sp. on control and coconut husk extract treated glass coupons.
3.6. Analysis of coconut husk extract by TLC, HPLC and FT-IR
The coconut husk fibre extract treated with ferric chloride showed a deep red colour indicating the presence of phenolic compounds. The coconut husk extract showed a single spot in the thin layer chromatogram. The active component was eluted from the silica gel and used for HPLC analysis. The HPLC spectrum of the TLC-purified extract showed one peak. The peak was observed at the retention time of 2.193 min and the peak height was 99 mAU (Fig. 3). The FT-IR spectrum of the TLC-resolved coconut husk extract showed a broad band in the region of 3600–3400, and a stretch at 1600–1200 and 800–600 cm−1 area (Fig. 4).
Figure 3.
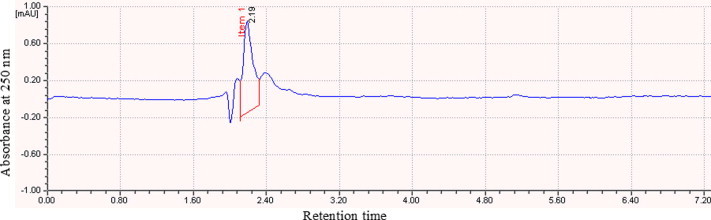
HPLC spectrum of coconut husk extract. The fraction eluted from the thin-layer chromatogram is used for the analysis.
Figure 4.
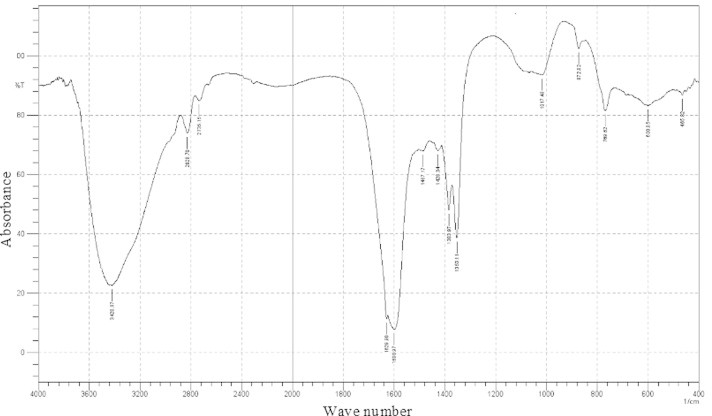
FT-IR spectrum of the coconut husk extract. The fraction eluted from the thin-layer chromatogram was used for the FT-IR analysis.
4. Discussion
Various methods are applied to control biofilm and biofouling growth on artificial substrata submerged in aquatic environments (Yebra et al., 2004). The antifouling compounds inhibit the attachment of bacteria and other higher organisms by certain mechanisms of action. For bacteria, the most important mechanisms of actions are the inactivation of enzymes (Cross et al., 2003), oxidation of the organic materials in the cells (Chapman, 2003), reduction of cellular metabolism and growth (Haque et al., 2005) and change in cell surface properties like hydrophobicity and cell surface charge (Jain et al., 2007). Hence in the present study, in addition to the adhesion assay, the effect of CHE on EPS production, hydrophobicity and growth of biofilm bacteria was analysed.
Plant phenols are an important group of natural antioxidants and some of them are potent antimicrobial compounds. Coconut husk is similar to hard wood in chemical composition, composed mainly of lignin and cellulose. Traditionally, coir fibre is extracted from the coconut husk by a process known as retting (soaking of husks in water). Coconut husk is a source of chemical compounds, mainly phenolic compounds. During the retting process, organic substances such as pectin, tannins and phenols may be liberated into the water. Phenolic compounds exhibit a wide range of physiological properties, such as anti-allergenic, anti-artherogenic, anti-inflammatory, antimicrobial, anti-oxidant, anti-thrombotic, cardioprotective and vasodilatory effects (Samman et al., 1998; Benavente et al., 2000; Middleton et al., 2000; Puupponen-pimia et al., 2001; Manach et al., 2005). Results of this study showed that CHE possess antimicrobial activity against the bacteria involved in biofilm formation. Since, the extract obtained from coir retting pond and laboratory showed antimicrobial activities, we have selected the coir retting effluent CHE for adhesion and growth inhibition assays. This is due to the easy availability of coir retting effluent from the retting ponds.
The process of biofouling involves a sequence of events, with numerous interactions taking place between fouling organisms. The initial colonisers of newly submerged surfaces in marine waters are bacteria and they are found to affect the subsequent recruitment of macrofouling organisms. Hence, antifouling compounds should have a broad spectrum of activity against micro-and macrofouling organisms. The findings of this study indicate that CHE inhibits the settlement of bacteria onto surfaces. Though, the adhesion assay carried out in this study has limitations due to manual counting under a microscope, we incorporated sufficient replicates to show the variations. The CHE treatment affected the EPS production of biofilm-forming bacterial strains. This was evidenced from the low carbohydrate and protein concentration in the EPS produced by the bacterial strains treated with CHE. Generally, EPS produced by microbes is considered to play an important role during biofilm formation. Azeredo and Oliverira (2000) reported that exopolymers are essential for cell-to-cell adhesion and biofilm formation. EPS bridge microbial cells within the substratum and permit negatively charged bacteria from adhering to both negatively and positively charged surfaces. Hence, the reduction in EPS production by bacteria due to CHE treatment attains significance from the view point of antifouling. The bacterial growth in the culture medium is also affected due to CHE treatment. This was evidenced from the observed significant variation between the control and CHE treated mediums.
Coconut husk extract also affected the hydrophobicity of the biofilm-forming bacterial strains. The measurement of bacterial hydrophobicity is of importance in many research areas, particularly biofouling and industrial microbiology (Davey and O‘Toole, 2000). This is due to the fact that the active attachment of bacterial cells is facilitated by cell surface properties such as adhesion proteins, capsules surface charge, flagella and pili (Kumar and Anand, 1998). In the present study, the cell surface hydrophobicity of Alteromonas sp. and Pseudomonas sp. was found to have increased after CHE treatment, whereas it decreased in Gallionella sp. This indicates that CHE treatment affects the hydrophobicity of the bacterial cell surface. The physico–chemical theory for bacterial adhesion (Bos et al., 1999) implies that a decrease in bacterial cell surface hydrophobicity may result in increased adhesion rate to hydrophobic surfaces. Since, glass surfaces were used as test coupons in the present study, further experiments using hydrophobic and hydrophilic surfaces may provide more details on the influence of bacterial cell hydrophobicity on adhesion.
Thin layer chromatography analysis revealed that the CHE has one active component. The HPLC spectrum of the TLC-resolved fraction also showed a single peak, indicating the purity of the compound. The FT-IR spectrum shows bands at 3600–3400 cm−1 indicating the presence of an alcoholic group. In conclusion, this study revealed that the active principle present in the CHE could be used as an inhibitory compound to control biofilm development on surfaces submerged in aquatic environments. Further studies on the effect of CHE on macrofouling organisms and the identification of the polyphenolic compounds present in the CHE may provide details on the applicability as an eco-friendly antifouling agent for marine applications.
Acknowledgement
We thank the anonymous reviewer for his valuable comments.
Footnotes
Peer review under responsibility of King Saud University.
References
- Azeredo J., Oliveira R. The role of exopolymer in the attachment of Sphingomonas paucimobilis. Biofouling. 2000;1:59–67. [Google Scholar]
- Benavente J., Gracia F.J., Lopez-Aguay F. Empirical model of morphodynamic beachface behaviour for low-energy mesotidal environments. Mar. Geol. 2000;167:375–390. [Google Scholar]
- Bos R., Van der Mei H.C., Busscher H.J. Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol. Rev. 1999;23:179–230. doi: 10.1111/j.1574-6976.1999.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Chapman J.S. Biocide resistance mechanisms. Int. Biodeter. Biodegr. 2003;51:133–138. [Google Scholar]
- Clare A.S. Towards nontoxic antifouling. J. Mar. Biotechnol. 1998;6:3–6. [Google Scholar]
- Cross J.B., Currier R.P., Torraco D.J., Vanderberg L.A., Wagner G.L., laden P.D. Killing of Bacillus spores by aqueous dissolve oxygen, ascorbic acid and copper ions. Appl. Environ. Microbiol. 2003;69:2245–2252. doi: 10.1128/AEM.69.4.2245-2252.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M.E., O’Toole G.A. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol Biol. Rev. 2000;64:847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M., Gills K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Esquenazi D., Wigg M.D., Miranda M.M., Rodrigues H.M., Tostes J.B., Rozental S., Da Silva A.J., Alviano C.S. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res. Microbiol. 2002;153:647–652. doi: 10.1016/s0923-2508(02)01377-3. [DOI] [PubMed] [Google Scholar]
- Fusetani N. Biofouling and antifouling. Nat. Prod. Rep. 2004;21:94–104. doi: 10.1039/b302231p. [DOI] [PubMed] [Google Scholar]
- Goto R., Kado R., Muramoto K., Kamiya H. Fatty acids as antifoulants in a marine sponge. Biofouling. 1992;6:61–68. [Google Scholar]
- Haque H., Cutright T.J., Zhang newby B.M. Effectiveness of sodium benzoate as a freshwater low toxicity antifoulant when dispersed in solution and entrapped in silicone coatings. Biofouling. 2005;21:109–119. doi: 10.1080/08927010500222551. [DOI] [PubMed] [Google Scholar]
- Jain A., Nishad K.K., Bhosle N.B. Effects of DNP on cell surface properties of marine bacteria and its implication for adhesion to surfaces. Biofouling. 2007;23:171–177. doi: 10.1080/08927010701269641. [DOI] [PubMed] [Google Scholar]
- Kumar G.C., Anand S.K. Significance of microbial biofilms in food industry: a review. Int. J. Food Microbiol. 1998;42:9–27. doi: 10.1016/s0168-1605(98)00060-9. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Far A.L., Randall R.J. Protein measurement with the folin-phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manach C., Williamson G., Morand C., Scalbert A., Re´me´sy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Middleton F., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Puupponen-Pimia R., Nohynek L., Meier C., Kahkonen M., Heinonen M., Hopia A., Oksman-Caldentey K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001;90:494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- Raveendran T.V., Limna Mol V.P., Parameswaran P.S. Proceedings of the International Conference on Biofouling and Ballast Water Management. NIO; Goa, India: 2008. Natural product antifoulants – present scenario and future prospects; p. 37. [Google Scholar]
- Rosenberg M., Gutnick D., Rosenberg E. Adherence to bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980;9:29–33. [Google Scholar]
- Samman S., Naghii M.R., Lyons wall P.M., Verus A.P. The nutrional and metabolic effects of boron in humans and animals. Biol. Trace Elem. Res. 1998;66:227–235. doi: 10.1007/BF02783140. [DOI] [PubMed] [Google Scholar]
- Sipkema D., Franssen M.C.R., Osinga R., Tramper T., Wijffels R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005;7:142–162. doi: 10.1007/s10126-004-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueli R., Gustavo A.S.P. Ultrasound extraction of phenolic compounds from coconut (Cocos nucifere) shell powder. J. Food Eng. 2007;80:869–872. [Google Scholar]
- Thompson J.E., Walker R.P., Faulkner D.J. Screening and bioassays for biologically-active substances from forty marine sponge species from San Diego, California, USA. Mar. Biol. 1985;88:11–21. [Google Scholar]
- Yebra D.M., Kiil S., Dam-Johansen K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004;50:75–104. [Google Scholar]



