Abstract
An efficient somatic embryogenesis system has been established in six date palm (Phoenix dactylifera L.) cultivars (Barhee, Zardai, Khalasah, Muzati, Shishi and Zart). Somatic embryogenesis (SE) was growth regulators and cultivars dependent. Friable embryogenic callus was induced from excised shoot tips on MS medium supplemented with various auxins particularly 2,4-dichlorophenoxyacetic acid (2,4-D, 1.5 mg 1−l). Suspension culture increased embryogenesis potentiality. Only a-naphthaleneacetic acid (NAA, 0.5 mg 1−1) produced somatic embryos in culture. Somatic embryos germinated and converted into plantlets in N6-benzyladenine (BAP, 0.75 mg 1−l) added medium following a treatment with thidiazuron (TDZ, 1.0 mg 1−l) for maturation. Scanning electron microscopy showed early stages of somatic embryo particularly, globular types, and was in masses. Different developing stages of embryogenesis (heart, torpedo and cotyledonary) were observed under histological preparation of embryogenic callus. Biochemical screening at various stages of somatic embryogenesis (embryogenic callus, somatic embryos, matured, germinated embryos and converted plantlets) of date palm cultivars has been conducted and discussed in detail. The result discussed in this paper indicates that somatic embryos were produced in numbers and converted plantlets can be used as a good source of alternative propagation. Genetic modification to the embryo precursor cell may improve the fruit quality and yield further.
Abbreviations: ANOVA, analysis of variance; BA, N6-benzyladenine; 2,4-D, 2,4-dichlorophenoxyacetic acid; 2,4,5-T, 2,4,5-trichlorophenoxyacetic acid; CPA, chlorophenoxyacetic acid; TDZ, thidiazuron; IAA, indole-3-acetic acid; NAA, a-naphthaleneacetic acid; MS, Murashige and Skoog’s (1962) medium; SE, somatic embryogenesis
Keywords: Amino acid, Date palm cultivars, Somatic embryogenesis, Histological analysis, Scanning electron microscopy, Protein, Sugar, Amino acids
1. Introduction
Somatic embryogenesis is the potency of somatic cells to produce somatic embryos (SEs) and has been reported in a number of plant systems (Mohanty and Ghosh, 1988; Bajaj, 1995; Brown et al., 1995; Mujib and Sama, 2006; Junaid et al., 2007a,b; Moon et al., 2008; Nasim et al., 2009; Ghanti et al., 2010). The induction of SEs is a unique mode of in vitro propagation. It offers numerous advantages which includes production of synthetically coated seeds, unlimited production of clones with elite traits. Initial cell population can be used as a single cellular system and their genetic manipulation appears to be easy (Redenbaugh, 1993; Gray et al., 1995) and it also provides a source of regenerable protoplasts (Chang and Wong, 1994; Jimenez, 1996; Mujib and Sama, 2006).
The process of somatic embryogenesis is directly regulated by a number of factors that are used to induce SEs. Plant growth regulators (PGRs) in particular play a vital role (Koh and Loh, 2000; Nuutila et al., 2002; Van Winkle et al., 2003; Cheong and Pooler, 2004; Pullman et al., 2005; Junaid et al., 2006, 2008; Feng et al., 2009; Nasim et al., 2010), and the right balance or the ratio of these PGRs is often the primary empirical basis for the optimization of in vitro SEs development (Ochatt et al., 2000; Moon et al., 2008; Ghanti et al., 2010).
Date palm (Phoenix dactylifera L.) is an important cash crop belonging to the family Arecaceae. It is a monocotyledonous and dioecious species cultivated through arid regions of the Middle East and North Africa (AlKharyi and AlMaarri, 1997; AlKhayri, 2001); almost 95% of the total world production is reported from Middle East. The propagation is of both types (sexual and vegetative). Sexual propagation is through seeds; and vegetative propagation by offshoot (Bonga, 1982). Plants propagated vegetatively accumulate numerous diseases (bacterial, fungal, viral and mycoplasmal) which decrease productivity (Anonymous, 1969). The generation of the offshoots is limited because their number produced by each palm tree is very low (Popenoe, 1973). Second sources of the propagation are seeds, but it has many limitations like low rate of germination and progeny variations (Venkataramaiah et al., 1980; Chand and Singh, 2004). To overcome the propagation problems and to maintain the germplasm, the in vitro micropropagation (somatic embryogenesis/organogenesis) is the successful technique (Mujib et al., 2004; Bhattacharjee, 2006) which provides a rapid production of genetically uniform and disease free plantlets. A number of organogenesis and somatic embryogenesis studies have been carried out previously in date palm (Rhiss et al., 1979; Tisserat, 1979; Beauchesne, 1983; Sharma et al., 1984; Daquin and Letouze, 1988; Junaid and Khan, 2009). In the present investigation we have studied somatic embryogenesis in six date palm cultivars (Barhee, Zardai, Khalasah, Muzati, Shishi, Zart) growing throughout United Arab Emirates (U.A.E.), and observed the effect of growth regulators on SEs. Biochemical variations at different stages of somatic embryogenesis, histological and scanning electron microscopic (SEM) studies have also been conducted. Fig. 1 summarized the pathway of somatic embryogenesis, scanning electron microscopy, histology and biochemical analysis at different developing stages of embryogenesis. To our knowledge, it is the first ever report compiling a detailed documentation on somatic embryogenesis and plant regeneration in six important date palm’s cultivars. The optimized protocol would be highly valuable to germplasm conservation and plantlets production at commercial level as it takes many years conventionally.
Figure 1.
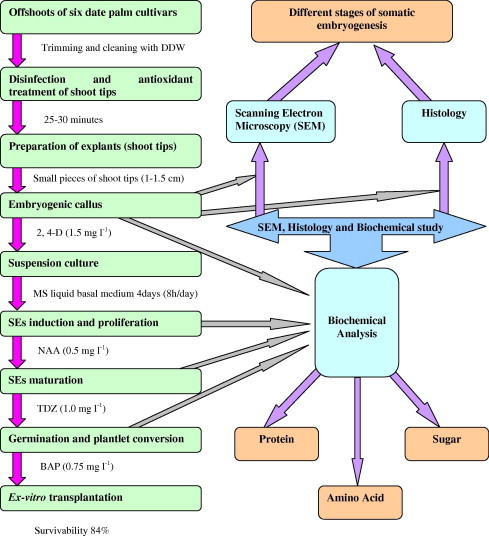
Sketch diagram representing the pathway of somatic embryogenesis, scanning electron microscopy, histology and biochemical analysis at different developing stages of embryogenesis in date palm.
2. Material and methods
2.1. Plant material
The offshoots of the six date palm cultivars (Barhee, Zardai, Khalasah, Muzati, Shishi, and Zart) were collected from (Fig. 2) the residential premises of the Chairman, Dubai Pharmacy College. The selected offshoots were 3–4 years old, each weighting approximately 30–40 kg.
Figure 2.

Mother date palm cultivars: (A) date palm at the fruiting stage and (B) date palm with offshoots.
2.2. Cleaning of explant
Cleaning of the explants was done according to Junaid and Khan (2009). In short, the offshoots were washed with the tap water to remove the attached soil and other debris. The outer large leaves and fibres were carefully removed with the sharp knife until the shoot tip zone was exposed. Shoot tips were then trimmed to approximately 60 cm in length and 40 cm in width.
2.3. Disinfection and antioxidant treatment
The disinfection and antioxidant treatment were also carried out according to the Junaid and Khan (2009). In brief, the excised shoot tips of the cultivars were washed 3–4 times with double distilled water. Thereafter, the cleaned shoot tips were subjected to two steps of disinfection: (a) the washed shoot tips were dipped for 20 min in a fungicide (Benlate, 5 g l−l) solution; (b) later dipped in 33% commercial clorox solution for 25–30 min. The explants were then rinsed three times with autoclaved distilled water inside the laminar hood and soaked in an antioxidant solution to minimize oxidation of phenolic compounds (responsible for the browning of tissues), and to protect them from desiccation. The antioxidant solution consisted of 2 g 1−l polyvinylpyrolydon (PVP, Mw = 40,000), 200 mg−l anhydrous caffeine and 100 mg−l sodium diethyldithiocarbonate. The shoot tips were kept in this solution for 20 min and finally washed with double distilled water.
2.4. Embryogenic callus induction and maintenance
For induction of embryogenic callus, small pieces of shoot tips (1–1.5 cm) were cultured on Murashige and Skoog (1962) medium supplemented with different auxins (2,4-D, CPA, 2,4,5-T) and concentrations (0.0–2.0 mg l−l). The cultures were maintained with periodic subculturing at an interval of four weeks.
2.5. Suspension culture
For establishing suspension culture, embryogenic calluses were dissected from small pieces of shoot tips and cultured in liquid MS medium supplemented with 2,4-D (1.5 mg l−l). Cultures were placed on a rotary shaker at 120 rpm at 25 ± 2 °C. After four days (8 h per day), the suspension was filtered in a laminar hood using sterile Whatman filter paper No. 2.
2.6. Scanning electron microscopy
For scanning electron microscopy (SEM), embryogenic calli of different date palm cultivars were fixed in 2% glutaraldehyde adjusted to pH 6.8 in 0.1 M phosphate buffer for 24 h at 4 °C. The tissue was washed in the buffer, postfixed for 2 h in similarly buffered 1% osmium tetroxide, dehydrated in a graded ethanol series and finally coated with gold palladium. The prepared samples were examined and photographed in a LEO 435 VP (Zeiss, Oberkochen, Germany) scanning electron microscope operating at 15–25 kV.
2.7. Histological analysis
For light microscopy, nodular embryogenic calluses of different cultivars produced on induction medium were fixed in a 2.5% glutaraldehyde solution, dehydrated through a graded series of ethanol and embedded in paraffin wax. Longitudinal sections (10 μm) were cut and stained for general observations according to Pintos et al. (2002).
2.8. Somatic embryo initiation
The suspended cells of the embryogenic calluses (20–30 mg) were implanted on MS medium supplemented with a range of NAA (0.0–2.5 mg 1−l) concentrations, where undifferentiated heterogeneous masses of somatic embryos were produced. Data were scored in terms of somatic embryogenesis percentage and morphogenetic callus morphology was noticed.
2.9. Somatic embryo maturation
Advanced globular embryos were separated out from the callus masses and placed on MS medium fortified with different concentrations of TDZ (0.0–2.0 mg 1−l), Data was scored after 4th of SEs maturation in terms of somatic embryo maturation percentage and length of SEs in each cultivars.
2.9.1. Somatic embryo germination and plantlet conversion
To achieve the germination and subsequent plantlet formation, matured somatic embryos (20 SEs per conical flask) were cultured on MS medium supplemented with various concentrations of BAP and KIN (0.0–2.5 mg 1−l). Five replicates were tested for each concentration. The data were scored in terms of SEs germination percentage, plantlet conversion percentage, only shoot length and complete plant length (mm).
2.9.2. Preparation and establishment of plantlets for outdoor transfer
Somatic embryo regenerated plants in all the cultivars with well developed shoots and roots were cultured on 1/2 MS medium supplemented with BAP (1.0 mg 1−l) for further development of new shoots. Within 2–3 weeks, the plantlets developed multiple shoots and roots which grew well on liquid medium. After additional 10 weeks they could be transferred outdoor.
2.9.3. Culture conditions
The pH of all the cultures was adjusted to 5.6–5.8 before autoclaving. The media were sterilized in an autoclave for 15 min at 121 °C. Cultures were incubated at 25 ± 2 °C under 16 h photoperiod with cool white fluorescent light (100 μmol m−2 s−1 PFD).
2.9.4. Estimation of protein
Protein estimation was carried out according to Bradford (1976). 0.5 g tissue was ground in a mortar and pestle with 1.0 ml (0.1 M) phosphate buffer (pH 7.0), placed on ice and centrifuged at 5000 rpm for 10 min. With 0.5 ml TCA, the sample was again centrifuged at 5000 rpm for 10 min. The supernatant was discarded, and the pellet was dissolved in 1.0 ml of 0.1 N NaOH after washing with double distilled water. After adding 5.0 ml of Bradford reagent the optical density was measured at 595 nm as described above.
2.9.5. Estimation of free amino acid
Amino acids were estimated by the method of Lee and Takahashi (1966). In short, 0.1 g tissue was incubated overnight in 70% ethanol followed by washing with double distilled water. Then 1.5 ml of 55% glycerol and 0.5 ml ninhydrin solution were added, boiled at 100 °C for 20 min and cooled down. The final volume was made up to 6 ml with double distilled water, and the optical density was measured at 570 nm as described above.
2.9.6. Estimation of total sugar
Total sugars in developing somatic embryos and different parts of somatic embryo-derived plantlets were estimated according to Dey (1990). All samples (0.5 g) were extracted twice with 90% ethanol, and the extracts were pooled. The final volume of the pooled extract was made up to 25 ml with double distilled water. To an aliquot of the extract, 1.0 ml of 5% phenol and 5.0 ml concentrated analytical-grade sulphuric acid were added, and the final volume was made up to 10 ml with double distilled water. The optical density was measured at 485 nm as described above. A solution containing 1.5 ml of 55% glycerol, 0.5 ml ninhydrin and 4.0 ml double distilled water was used as a calibration standard.
2.9.7. Statistical analysis
The data on the effects of growth regulators on different stages of somatic embryogenesis and other parameters were analysed by one-way analysis of variance (ANOVAs). Values are means of six replicates from two experiments, and the presented mean values were separated using Duncan’s Multiple Range Test (DMRT) at at P < 0.05.
3. Results
3.1. Embryogenic callus induction and maintenance
The excised shoot (Fig. 3A) tips of six different date palm cultivars responded well in culture on which three tested auxins had profound influence to produce callus. Amongst the tested auxins, only 2,4-D (1.5 mg 1−l) produced embryogenic calluses (Fig. 3B), however, callus induced at the higher concentrations characterized by their compact and nodular appearance, are grown relatively slow. The maximum callus induction was noticed in ‘Khalasah’ followed by ‘Zadai’ and ‘Muzati’ (Fig. 4A) on MS medium supplemented with 2,4-D (1.5 mg 1−l). The effective concentration, however, varied generally lay within 0.5–1.5 mg 1−l range; higher concentration inhibited callus induction and growth.
Figure 3.

Somatic embryogenesis in date palm’s cultivars: (A) trimmed date palm’s offshoot, (B) embryogenic callus on MS medium supplemented with 2,4-D (1.5 mg l−1), (C) SEs proliferation on MS medium added with NAA (0.5 mg l−1), (D) scanning electron microscopy of embryogenic calluse showing globular type of embryos, (E) SEs maturation on MS medium fortified with TDZ (1.0 mg l−1), (F) Es germination and plantlet conversion on MS medium supplemented with BAP (0.75 mg l−1) and (G) date palm cultivars growing in natural conditions.
Figure 4.

Somatic embryogenesis in Date Palm’ cultivars: (A) Embryogenic callus induction on MS medium supplemented with 2,4-D (1.5 mg l−1), (B) somatic embryogenesis induction percentage on MS medium supplemented with NAA (0.5 mg l−1), (C) SEs maturation percentage on MS medium fortified with TDZ (1.0 mg l−1), (D and E) SEs germination and plantlet conversion percentage on MS medium added with BAP (0.75 mg l−1) supplemented medium.
Fresh and dry callus masses of cultivars were weighted up to 9th weeks (Table 1). The maximum fresh and dry weight was observed from ‘Barhee’ followed by ‘Muzati’ cultivar. A visible morphological variation was noticed in callusing appearance of different cultivars. Table 2 showing morphological variations in embryogenic calluses of different date palm cultivars. Calli were routinely maintained on media supplemented with 2,4-D (1.5 mg 1−l). High embryogenic callus induction was achieved by continuous subculturing on fresh nutrient medium. The calluses were friable light yellow greenish (Barhee), creamy (Zardai), yellow (Khalasah), light green (Muzati), creamy with greenish appearances (Shishi) and light green (Zart) respectively.
Table 1.
Callus biomass (fresh and dry weight) growth in optimized auxin concentrations (2,4-D 1.5 mg 1−l). Data were scored up to 9 weeks of culture.
| Date palm cultivar | After 5 weeks |
After 7 weeks |
After 9 weeks |
|||
|---|---|---|---|---|---|---|
| F.W.A (g) | D.W. (g) | F.W. (g) | D.W. (g) | F.W. (g) | D.W. (g) | |
| Barhee | 0.81aB | 0.27ab | 1.36a | 0.39ab | 2.84abc | 0.78ab |
| Zardai | 0.70cd | 0.19de | 1.18de | 0.22de | 2.31de | 0.52e |
| Khalasah | 0.75bc | 0.20cd | 1.20cde | 0.28d | 2.50dc | 0.62cde |
| Muzati | 0.78ab | 0.23bc | 1.31cd | 0.31cde | 2.61abc | 0.69bc |
| Shishi | 0.68abc | 0.16fg | 1.10fg | 0.20ef | 2.05ef | 0.45efg |
| Zart | 0.79ab | 0.21cd | 1.34bc | 0.34bc | 2.12e | 0.55de |
F.W. = Fresh weight; D.W. = Dry weight.
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
Table 2.
Morphological behaviour of embryogenic callus of six date palm cultivars cultivated on MS medium contained 2,4-D (1.5 mg 1−l). Data were scored after 6 weeks of culture.
| Date palm’s cultivars | Morphogenetic appearance |
|---|---|
| Barhee | Light yellow greenish |
| Zardai | Creamy |
| Khalasah | Yellow |
| Muzati | Light green |
| Shishi | Creamy with greenish appearances |
| Zart | Light greenish |
3.2. Scanning electron microscopy
Scanning electron microscopic study of embryogenic callus showed undifferentiated forms of somatic embryos (Fig 3D). A remarkable variation in somatic embryo origin was observed in different cultivars. In ‘Barhee’ and ‘Muzati’, globular and torpedo types were maximum in numbers; in ‘Zardai’, ‘Khalasah’, ‘Shishi’ and ‘Zart’, heart and cotyledonary were highest in numbers. They were developed on the surface of callus. Globular embryo, were high in number and produced regularly. Embryos were clustered together in a common mass, but they could not be easily detached from the parental tissue. The other forms of the embryos (torpedo, heart and cotyledonary) were in less numbers compared to the globular type. Beside the normal forms and appearance, SEM studies also show various morphological deviations in embryos structure.
3.3. Histological analysis
A histological study of the embryogenic callus was performed after 4 weeks of somatic embryos induction. It revealed that the somatic embryos appear simultaneously at different development stages. The somatic embryos started their development at the stage of meristematic centres through globular to cotyledonary shape. Most of the embryos formed a typical single layer of protoderm on their surface very soon. Somatic embryos are mainly composed of small compact meristematic cells, characterized by the dense cytoplasm and dense nucleus (photographs not shown due to the space).
3.4. Somatic embryo initiation and proliferation
Embryogenic calli was induced on MS medium supplemented with 2,4-D (1.5 mg 1−l). Rapid development of somatic embryos (Fig. 3C) was observed on medium containing NAA (1.5 mg 1−l) following a suspension culture. Maximum somatic embryogenesis potency was noticed in ‘Khalasah’ followed by ‘Muzati’ cultivar. Comparative account of somatic embryogenesis induction percentage in six date palm cultivars is shown in Fig. 4B. Induced somatic embryos were in mixed population and could not be separated easily.
3.5. Somatic embryo maturation
Advanced globular embryos were separated out from the callus mass and placed on MS medium fortified with different concentrations of TDZ (0.0–2.0 mg l−l). Amongst the various TDZ concentrations highest SEs maturation was noticed in ‘Muzati’ (Fig. 3D) on MS medium fortified with TDZ (1.0mg 1−l) (Fig. 4C). After 2–3 weeks in maturation medium embryos started to become greenish morphologically, but significant difference in SE maturation was observed amongst the cultivars. In ‘Barhee’ and ‘Muzati’, maturation started after the 2 weeks of implantation; in ‘Zardai’, ‘Khalasah’, ‘Shishi’ and ‘Zart’ it was after 3 weeks. Maximum somatic embryogenesis was noticed in ‘Muzati’ cultivars followed by ‘Shishi’ cultivars. The embryos of ‘Khalash’, ‘Zardai’ and ‘Zart’ were dark green in colour, whereas they were light green with brown spots in ‘Barhee’, ‘Muzati’, ‘Khalasah’. The somatic embryos increased in size in maturation medium. Table 3 shows differences in size of matured somatic embryos of six date palm’s cultivars.
Table 3.
Length of matured SEs on MS medium supplemented optimization concentration of TDZ (1.0 mg 1−l). Data were scored after 6 weeks of inoculation.
| Date palm’s cultivars | Initial length of somatic embryos (mm) | Length after 9th week (mm) |
|---|---|---|
| Barhee | 5.0cdeA | 10.0def |
| Zardai | 4.5defg | 9.5cde |
| Khalasah | 6.0abc | 12.5ab |
| Muzati | 5.5bc | 10.5cde |
| Shishi | 5.5bc | 11.0abc |
| Zart | 4.0efgh | 8.0fgh |
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
3.6. Germination and plantlet conversion
Highly green somatic embryos were isolated from the maturation medium and placed on MS medium supplemented with different concentrations of BAP and KN. In this study, two different types of responses were mainly observed: (1) only shoot (without root); (2) plants with shoot and root (complete plantlets). Of the two cytokinins tested, BAP was found to be more active as compared to KN in SEs germination and plantlet conversion. BAP (0.75 mg 1−l) proved to be highly effective compared to the other concentrations (Fig. 3E). The highest SE germination was in ‘Shishi’ (Fig. 4D), however, plantlet conversion was noticed in ‘Muzati’ (Fig. 4E). A comparative account of only shoot and complete plantlet length raised through somatic embryos is shown in Tables 4 and 5.
Table 4.
Only shoot length (mm) of germinated somatic embryo on MS media supplemented with BAP and KN. Data were scored after 7 weeks of culture.
| PGR |
Date palm’s cultivars |
||||||
|---|---|---|---|---|---|---|---|
| BAP | KN | Barhee | Zardai | Khalasah | Muzati | Shishi | Zart |
| 0.0 | 3.4ghA | 0.0gh | 3.0def | 3.5ijk | 0.0hi | 0.0ij | |
| 0.25 | 9.0ef | 3.9efg | 6.1efg | 9.5ghi | 4.4fjk | 5.8 h | |
| 0.50 | 15.8abc | 9.8cd | 9.5abc | 16.5ab | 11.9a | 16.8ab | |
| 0.75 | 18.8bcd | 7.6bcd | 12.7cd | 20.4cdef | 12.6bc | 18.2bc | |
| 1.00 | 12.3def | 10.3ab | 8.7def | 12.5efj | 11.3b | 13.4de | |
| 0.0 | 2.9gh | 0.0hi | 2.1cde | 2.5jkl | 0.0hij | 0.0ij | |
| 0.25 | 7.3fgh | 2.7efg | 5.5abc | 7.3hij | 3.6gh | 6.8gh | |
| 0.50 | 14.0bc | 5.4ef | 9.0def | 17.4bcd | 10.2cde | 14.6cd | |
| 0.75 | 14.4cd | 7.6cd | 10.3bc | 13.9df | 9.6cd | 12.6ef | |
| 1.00 | 12.0def | 8.3cd | 7.3bcd | 10.4fjk | 8.4efj | 10.8fjh | |
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
Table 5.
Length of complete plantlet (mm) derived through germinated somatic embryo on MS media supplemented with constant NAA (0.5 mg 1−l) with various concentrations of BAP. Data were scored after 7 weeks of culture.
| PGR |
Length of complete plantlet of Date palm’s cultivars |
|||||
|---|---|---|---|---|---|---|
| BAP | Barhee | Zardai | Khalasah | Muzati | Shishi | Zart |
| 0.0 | 5.9eA | 0.0de | 6.0de | 6.5ef | 0.0de | 0.0ef |
| 0.25 | 11.2de | 6.9d | 7.1d | 12.5de | 7.4bc | 9.8de |
| 0.50 | 23.8ab | 10.8bc | 14.5ab | 24.5a | 15.9a | 21.8ab |
| 0.75 | 17.8b | 12.8b | 13.7b | 19.4b | 14.6ab | 20.2b |
| 1.00 | 15.7cd | 13.7ab | 12.7bc | 16.5bc | 14.3b | 15.4bc |
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
3.7. Acclimatization
A large number of somatic embryos derived plantlets were successfully acclimatized to ex vitro conditions (Fig. 3F). The plantlets were placed for hardening at incubation room temperature up to 45 days. Maximum survivability was noticed in ‘Khalasah’ followed by ‘Muzati’ and ‘Zart’ cultivars (Table 6). After 45 days plantlets were exposed at natural environmental conditions. It was observed that after four months plantlets showed a remarkable growth performance. Table 7 shows survivability percentage of plantlets after four months at natural environmental conditions. Morphologically, there were no differences with respect to growth and development, size and type of leaf between the established groups of plants. Five months after the beginning of the acclimatization process these plants were 15 cm in length.
Table 6.
Survivability percentage of various date palm cultivars at 25 ± 2 °C temperature.
| Date palm cultivars | 15 days | 30 days | 45 days |
|---|---|---|---|
| Barhee | 100aA | 90.5abc | 80.6cdef |
| Zardai | 100a | 84.5cdef | 70.2defg |
| Khalasah | 100a | 95.6abcd | 86.4abc |
| Muzati | 100a | 90.0bcde | 83.7bcd |
| Shishi | 100a | 70.5defg | 68.2efg |
| Zart | 100a | 90.2abcde | 83.5bcde |
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
Table 7.
Survivability percentage of in vitro raised plantlets at natural environmental conditions (48 ± 2 °C).
| Date palm cultivars | Month |
|||
|---|---|---|---|---|
| One | Two | Three | Four | |
| Barhee | 90.6ab A | 85.5abc | 84.5abc | 84.5abc |
| Zardai | 84.7cde | 81.6bcde | 80.5bcd | 80.0bcd |
| Khalasah | 87.8bcd | 80.1abcd | 79.0abc | 79.0abc |
| Muzati | 83.2cde | 80.0bcde | 80.0bcd | 80.0bcd |
| Shishi | 76.5abcde | 72.3defg | 70.1def | 70.0def |
| Zart | 81.6defg | 78.4cdef | 77.5cde | 77.0cdef |
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
3.8. Biochemical analysis
As all the six date palm’s cultivars were different in morphology and embryogenic response, they were also characterized for their biochemical changes as well. A significant variation was noticed in protein sugar and amino acids contents at different stages of somatic embryogenesis. Maximum protein was noticed in ‘Muzati’ followed by the ‘Zart’, ‘Shishi’ and ‘Barhee’, however, a linear increased in protein content was noticed from embryogenic callus to complete plantlet production (Table 8). There was a decline in sugar content with increasing complexities in embryogenic process and it was high in ‘Khalasah’ cultivar (Table 9). Amino acid content was high in ‘Shishi’ cultivar followed by ‘Zardai’. Similar pattern of amino acid was noticed as in case of protein (Table 10). In six date palm’s cultivars we observed significant quantitative changes in protein, sugar and amino acid contents at different developing stages somatic embryogenesis.
Table 8.
Protein content characterization of six date palm cultivars at different developing stages of somatic embryogenesis.
| Date palm cultivars | ECA | SEs | MEs | GEs | CPs |
|---|---|---|---|---|---|
| Barhee | 2.3bB | 3.5bc | 3.8cde | 4.0def | 5.2cde |
| Zardai | 1.9def | 3.0efg | 3.4defg | 4.6cde | 6.1bce |
| Khalasah | 2.1bcd | 2.9fgh | 3.1edef | 3.9efgh | 4.8fgh |
| Muzati | 2.5abc | 3.7abc | 4.3abcd | 4.9bcde | 6.7abc |
| Shishi | 2.3bcd | 3.4cdef | 3.8cdf | 4.9bcd | 5.1efg |
| Zart | 2.4abc | 3.3defg | 4.0bc | 5.0abcd | 6.0abcd |
ECs = Embryogenic callus; SEs = Somatic embryos; MEs, matured embryos, GEs = germinated embryos; CPs = Converted plantlet.
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
Table 9.
Sugar content characterization of six date palm cultivars at different stages of somatic embryogenesis.
| Date palm cultivars | ECsA | SEs | MEs | GEs | CPs |
|---|---|---|---|---|---|
| Barhee | 20.5bcB | 18.5ab | 17.0bc | 16.9abc | 11.7bcd |
| Zardai | 16.3fgh | 14.7def | 13.2def | 12.4defg | 9.9cde |
| Khalasah | 23.4abc | 20.1bc | 18.4ab | 16.8bcd | 12.2abc |
| Muzati | 19.8def | 17.3cde | 16.8def | 13.7abc | 10.4cdfg |
| Shishi | 18.6ef | 16.8def | 13.4efg | 11.9efg | 9.8def |
| Zart | 22.5bcd | 19.8abc | 18.0abc | 17.4abc | 12.4abcd |
ECs = Embryogenic callus, SEs, Somatic embryos, MEs, matured embryos, GEs, germinated embryos, CPs, Converted plantlet.
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
Table 10.
Amino acids contents of six date palm cultivars at different developing stages of somatic embryogenesis.
| Date palm cultivars | ECssA | SEs | MEs | GEs | CPs |
|---|---|---|---|---|---|
| Barhee | 1.0deB | 1.3bc | 1.4bc | 1.5bc | 1.7de |
| Zardai | 1.1bcd | 1.5ab | 1.6bc | 1.7ab | 2.1ab |
| Khalasah | 0.9efg | 1.1dc | 1.3de | 1.4def | 1.6efg |
| Muzati | 1.3bcd | 1.5bcd | 1.6ab | 1.7abc | 1.9bcd |
| Shishi | 1.5ab | 1.8ab | 1.8abc | 2.0ab | 2.2ab |
| Zart | 0.9bcde | 1.1cd | 1.2efg | 1.3efg | 1.5fg |
ECs = Embryogenic callus, SEs, Somatic embryos, MEs, Matured embryos, GEs, germinated embryos, CPs, Converted plantlet.
Means with common letters within a column are not significantly different at P < 0.05, according to Duncan’s Multiple Range Test (DMRT).
4. Discussion
Two different types of embryogenesis have been observed; direct embryogenesis when embryos developed directly on the surface of explants without any intermediate callus formation and indirect embryogenesis where embryos arose on meristematic callus masses. In date palm cultivars (‘Barhee’, ‘Zardai’, ‘Khalasah’, ‘Muzati’, ‘Shishi’ and ‘Zart’) indirect type of somatic embryogenesis has been reported. There has been no previous report on the screening of somatic embryogenesis in date palm cultivars nor has any comparison been ever made. In the present study, embryogenic callus was observed from off shoot apical meristematic tissues but cultivars showed a significant variation at callus induction and embryogenesis level. These variable responses may be due to the different levels of endogeneous plant growth regulators and other physiological gradients which are present in off shoots meristematic tissues collected from different cultivars. The influence of plant growth regulators at different developing stages of somatic embryogenesis has been reported earlier for some other plant species (Etienne et al., 1993; Mujib et al., 1996; Pintos et al., 2002; Tokuji and Kuriyama, 2003; Murthy et al., 2006; Junaid et al., 2007a,b; Shen et al., 2008). A remarkable variation in calluses morphology was observed in the studied date palm cultivars. The difference in callus morphology and subsequent embryogenic competence was also reported in some other plants (Wernicke and Milkovits, 1986; Mujib et al., 1996; Afreen et al., 2002; Junaid et al., 2007a,b).
In the present investigation, different forms of somatic embryos have been seen by histological and Scanning Electron Microscopic (SEM) studies using embryogenic callus. Embryogenic calluses have been used for histological (Mohanty and Ghosh, 1988; Faure, 1990; Chengalrayan et al., 2001; Feng et al., 2004; Li et al., 2008; You et al., 2008; Feng et al., 2009, 2010) and scanning electron microscopic studies in a number of plant systems (Jason et al., 2009; Wang et al., 2010; Ulisses et al., 2010). We noticed that in date palm cultivars 2,4-D only induced embryogenic callus, but was less effective compared to NAA at the time of embryo induction and proliferation. These results agreed with the previous reports carried out in other plant species, whereas 2,4-D influences embryo induction and participation at initial stages of development (Mujib and Sama, 2006; Junaid et al., 2007a,b; Liu et al., 2008; Nasim et al., 2009; Yang et al., 2010). SEs induction, maturation and germination showed varied response in the studied date palm cultivars. Here, a short application of TDZ was found to be very effective in maturation before conversion. The embryo maturation and subsequent conversion represent a complex process. TDZ and various others such as sugars, polyethylene glycol and sugar-alcohol GA3 in the medium substantially improved maturation (Corredoira et al., 2003; Robichaud et al., 2004; Junaid et al., 2006; Joshi et al., 2008; Sahal et al., 2010).
We used a range of concentration of BA and KN to study somatic embryo germination and plantlet conversion and found that BA was highly effective for high germination and plantlet conversion. The same cytokinin alone or in combinations with auxin was earlier reported to be very effective in somatic embryogenesis in a number of plants (Junaid et al., 2007a,b; Bhattacharya et al., 2008; Moon et al., 2008; Nasim et al., 2009; Ghanti et al., 2010). Different stages of somatic embryogenesis in six date palm cultivars varied at biochemical level, their differential responses to somatic embryogenesis (process) might be due to variation in the level of endogenous plant growth regulators. The morphology of somatic embryos and simultaneous accumulation of storage reserves have been shown to be a good indicator of their maturity and development (Merkle et al., 1995). It has also been demonstrated that this process is positively influenced by various compounds like carbohydrates, sugar alcohols, PEG, etc. (Lipavska and Konra´dova, 2004; Tang and Newton, 2005; Junaid et al., 2006, 2008; Nasim et al., 2010).
Plantlets with healthy roots were transplanted for acclimatization purpose, showed a good survivability percentage, and normal morphological appearance. Successful transplantation has been achieved in several other plants earlier (Zhang and Davis, 1986; Debergh and Read, 1991; Preece and Sutter, 1991; Shibli and Smith, 1996; Kim et al., 1997; Dhar and Joshi, 2005; Junaid et al., 2008; Ghanti et al., 2010).
In date palm cultivars, we have been able to demonstrate an efficient somatic embryogenesis system originating from off shoot’s meristematic tissues. Somatic embryos and plantlets were obtained in large numbers in all the studied cultivars. The embryo precursor cell could be used in date palm genetic manipulation studies to improve fruit quality. Somatic embryogenesis based plant regeneration reported in the present communication could be very useful for continuous regeneration and production of somatic embryos/plantlets for ex vitro transplantation.
Acknowledgement
The first author (Dr. Junaid Aslam), gratefully acknowledges the Chairman, Board of Trustees of Dubai Pharmacy College for providing all facilities to carry out the present research work.
References
- Afreen F., Zobayed S.M.A., Kozai T. Photoautotrophic culture of arabusta somatic embryos Development of a bioreactor for large-scale plantlet conversion from cotyledonary embryos. Ann. Bot. 2002;90:21–29. doi: 10.1093/aob/mcf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlKharyi J.M., AlMaarri K.W. Effect of seasonal variation on the regeneration capacity of date palm. In Vitro. 1997;33:22–26. [Google Scholar]
- AlKhayri J.M. Optimization of biotin and thiamine requirements for somatic embryogenesis of date palm (Phoenix dactylifera L.) In Vitro Cell Dev. Biol. Plant. 2001;37:453–456. [Google Scholar]
- Anonymous, 1969. The wealth of India: a dictionary of Indian raw materials and industrial products, vol. VIII, New Delhi CSIR, pp. 303–305.
- Bajaj Y.P.S. Somatic embryogenesis and its applications for crop improvement. In: Bajaj Y.P.S., editor. Springer; Berlin: 1995. pp. 105–125. (Biotechnology in Agriculture and Forestry, Somatic Embryogenesis and Synthetic Seed). [Google Scholar]
- Beauchesne, G., 1983. Vegetative propagation of date palm (Phoenix dactylifera L.) by in vitro culture. In: Proc of first symposium on date palm, King Faisal University, Hofuf, Saudi Arabia, pp. 698–699.
- Bhattacharjee S.K. Advances in Ornamental Horticulture. Pointer Jaipur. 2006;6(2065) ISBN 8171324320. [Google Scholar]
- Bhattacharya S., Dey T., Bandopadhyay T.K., Ghosh P.D. Genetic polymorphism analysis of somatic embryo-derived plantlets of Cymbopogon flexuosus through RAPD assay. Plant Biotechnol. Rep. 2008;2(4):245–252. [Google Scholar]
- Bonga J.M. Clonal propagation of mature trees: problems and possible solutions. In: Bonga JM., editor. Tissue Culture in Forestry. Martinus Nijhoff Publ; Dordrecht: 1982. pp. 249–271. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–541. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown D.C.W., Finstad K.I., Watson E.M. Somatic embryogenesis in herbaceous dicots. In: Thorpe TA., editor. In Vitro Embryogenesis in Plants. Kluwer; Dordrecht: 1995. pp. 345–415. [Google Scholar]
- Chand S., Singh A.J. In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree, Pterocarpus marsupium roxb. In Vitro Cell Dev. Biol. Plant. 2004;40:167–170. [Google Scholar]
- Chang Y.F., Wong J.R. Regeneration of plants from protoplasts of Tritium aestivum L. (Wheat) In: Bajaj YPS., editor. Plant Protoplasts and Genetic Engineering V (Biotechnology in Agriculture and Forestry), vol. 29. Springer; Berlin: 1994. pp. 161–171. [Google Scholar]
- Chengalrayan K., Hazra S., Gallo-Meagher M. Histological analysis of somatic embryogenesis and organogenesis induced from mature zygotic embryo-derived leaflets of peanut (Arachis hypogaea L.) Plant Sci. 2001;161:415–421. [Google Scholar]
- Cheong E.J., Pooler M.R. Factors affecting somatic embryogenesis in Prunus incisa cv. February Pink. Plant Cell Rep. 2004;22:810–815. doi: 10.1007/s00299-004-0771-5. [DOI] [PubMed] [Google Scholar]
- Corredoira E., Ballester A., Vieitez A.M. Proliferation, maturation and germination of Castanea sativa Mill. somatic embryos originated from leaf explants. Ann Bot. 2003;92:129–136. doi: 10.1093/aob/mcg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquin F., Letouze R. Regeneration of date palm (Phoenix dactylifera L.) by somatic embryogenesis, improved effectiveness by dipping in a stirred liquid medium. Fruits. 1988;43:191–194. [Google Scholar]
- Debergh P.C., Read P.E. Micropropagation. In: Debergh PC., Zimmerman RH., editors. Micropropagation: Technology and Application. Kluwer Acad Publishers; Dordrecht: 1991. pp. 1–3. [Google Scholar]
- Dey, P.M., 1990. Methods in plant biochemistry. Carbohydrates, vol. 2. Acad. Press, London.
- Dhar U., Joshi M. Efficient plant regeneration protocol through callus for Saussurea obvallata (DC) Edgew. (Asteraceae): effects of explant type, age and plant growth regulators. Plant Cell Rep. 2005;24:195–200. doi: 10.1007/s00299-005-0932-1. [DOI] [PubMed] [Google Scholar]
- Etienne H., Sotta B., Montoro P., Miginiac E., Carron M.P. Relations between exogenous growth regulators and endogenous indole-3-acetic acid and abscisic acid in the expression of somatic embryogenesis in Hevea brasiliensis (Müll Arg.) Plant Sci. 1993;88(1):91–96. [Google Scholar]
- Faure O. Somatic embryos of Vitis rupestris and zygotic embryos of Vitis species: morphology, histology, histochemistry and development. Candian J. Bot. 1990;68(3):2305–2315. [Google Scholar]
- Feng D.L., Li Y., Sun Z.Y. Histology and somatic embryogenesis of Parthenocissus tricuspidata. J Beij For Univer. 2004;26(4):97–99. [Google Scholar]
- Feng D.L., Li W., Li J., Li P.T., Zhao M., Zhao S.G., Shi B.S., Peng W.X. Observation of somatic embryogenesis and histology in Koelreuteria bipinnata Franch var integrifoliola. Chen. Plant Physiol. Commun. 2009;45(9):855–858. [Google Scholar]
- Feng D.L., Meng Q.R., Li W.P., Hu Y.H., Li M., Gu A.X. Morphology of somatic embryogenesis and plantlet formation in tissue cultures of lantern tree (Koelreuteria bipinnata var integrifoliola) For Stud. China. 2010;12(1):31–36. [Google Scholar]
- Ghanti S.K., Sujata K.G., Rao S.M., Kishor P.B.K. Direct somatic embryogenesis and plant regeneration from immature explants of chickpea. Biol. Plant. 2010;54(1):121–125. [Google Scholar]
- Gray D.J., Compton M.E., Harrell R.C., Cantliffe D.J. Somatic embryogenesis and the technology of synthetic seed. In: Bajaj Y.P.S., editor. vol. 30. Springer; Berlin: 1995. pp. 126–151. (Biotechnology in Agriculture and Forestry Somatic Embryogenesis and Seed I). [Google Scholar]
- Jason N., Burris, David G.J., Mann Blake L., Joyce C., Neal Stewart C., Jr. An improved tissue culture system for embryogenic callus production and plant regeneration in switchgrass (Panicum virgatum L.) Bioener. Res. 2009;2:267–274. [Google Scholar]
- Jimenez V.M. El cultivo de protoplastos en citricos y su potencial para el mejoramiento genetico. Agron Costarric. 1996;20:187–204. [Google Scholar]
- Joshi M., Sujatha K., Hazra S. Effect of TDZ and 2,4-D on peanut somatic embryogenesis and in vitro bud development. Plant Cell Tiss. Org. Cult. 2008;94(1):85–90. [Google Scholar]
- Junaid A., Khan S.A. In vitro micropropagation of Khalasah date palm (Phoenix dactylifera L.). An important fruit plant. J. Fruit Orna. Plant Res. 2009;17(1):5–17. [Google Scholar]
- Junaid A., Bhatt M.A., Mujib A., Sharma M.P. Somatic embryo proliferation maturation and germination in Catharanthus roseus. Plant Cell Tiss. Org. Cult. 2006;84:325–332. [Google Scholar]
- Junaid A., Mujib A., Sharma M.P., Tang W. Growth regulators affect primary and secondary somatic embryogenesis in Madagaskar periwinkle (Catharanthus roseus (L) GDon) at morphological and biochemical levels. Plant Growth Regul. 2007;51:271–281. [Google Scholar]
- Junaid A., Mujib A., Bhat M.A., Sharma M.P., Samaj J. Somatic embryogenesis and plant regeneration in Catharanthus roseus. Biol. Plant. 2007;51(4):641–646. [Google Scholar]
- Junaid A., Mujib A., Fatima S., Sharma M.P. Cultural conditions affect somatic embryogenesis in Catharanthus roseus L. (G.) Don. Plant Biotechnol. Rep. 2008;2:179–189. [Google Scholar]
- Kim M.K., Sommer H.E., Bongarten B.C., Merkle S.A. High frequency induction of adventitious shoots from hypocotyl segments of Liquidambar styraciflua L. by thidiazuron. Plant Cell Rep. 1997;16:536–540. doi: 10.1007/BF01142319. [DOI] [PubMed] [Google Scholar]
- Koh W.L., Loh C.S. Direct somatic embryogenesis, plant regeneration and in vitro flowering in rapid-cycling Brassica napus. Plant Cell Rep. 2000;19:1177–1183. doi: 10.1007/s002990000268. [DOI] [PubMed] [Google Scholar]
- Lee Y.P., Takahashi T. Improved calorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966;24:71–77. [Google Scholar]
- Li H., Li F.L., Du C.J., Lu H., He Z.Q. Somatic embryogenesis and histological analysis from zygotic embryos in Vitis vinifera L. ‘Moldova’. For Stud China. 2008;10(4):253–258. [Google Scholar]
- Lipavska ´ H., Konra´dova ´ H. Somatic embryogenesis in conifers: the role of carbohydrate metabolism. In Vitro Cell Dev. Biol. Plant. 2004;40:23–30. [Google Scholar]
- Liu M.Y.J., Lu S., Guo Z., Lin X., Wu H. Somatic embryogenesis and plant regeneration in centipede grass (Eremochloa ophiuroides [Munro] Hack) In Vitro Cell Dev. Biol. Plant. 2008;44(2):100–104. [Google Scholar]
- Merkle S.A., Parrot W.A., Flinn B.S. Morphogenetic aspects of somatic embryogenesis. In: Thorpe TA., editor. In Vitro Embryogenesis in Plants. Kluwer Acad Publ; Dordrecht: 1995. pp. 155–203. [Google Scholar]
- Mohanty B.D., Ghosh P.D. Somatic embryogenesis and plant regeneration from leaf callus of Hordeum vulgare. Ann. Bot. 1988;61:551–555. [Google Scholar]
- Moon H.K., Park S.Y., Kim Y.W., Kim S.H. Embryogenesis and plantlet production using rejuvenated tissues from serial grafting of a mature Kalopanax septemlobus tree. In Vitro Cell Dev. Biol. Plant. 2008;44(2):119–127. [Google Scholar]
- Mujib A., Sama J. Springer; Berlin: 2006. Somatic Embryogenesis. [Google Scholar]
- Mujib A., Bandyopadhyay S., Jana .BK., Ghosh P.D. Growth regulator involvement and somatic embryogenesis in Crinum asiaticum. Indian J. Plant Physiol. 1996;1:84–86. [Google Scholar]
- Mujib, A., Cho, M.J., Predieri, S., Banerjee, S., 2004. In Vitro Application in Crop Improvement. Oxford IBH Publ. Co. Pvt. Ltd, New Delhi.
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Murthy B.N.S., Murch S.J., Saxena P.K. Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): Endogenous growth regulator levels and significance of cotyledons. Physiol. Plant. 2006;94(2):268–276. [Google Scholar]
- Nasim S.A., Mujib A., Rashmi K., Fatima S., Junaid A., Mahmooduzzafar Improved Allin yield in somatic embryogenesis of Allium sativum L (CV. YAMUNA SAFED) as analyzed by HPTLC. Acta Biol. Hungarica. 2009;60(4):441–454. doi: 10.1556/ABiol.60.2009.4.10. [DOI] [PubMed] [Google Scholar]
- Nasim S.A., Mujib A., Kapoor R., Fatima S., Junaid A., Mahmooduzzafar Somatic embryogenesis in Allium sativum L. (cv. Yamuna Safed 3): Improving embryo maturation and germination with PGRs and carbohydrates. Anal. Biol. 2010;32:1–9. [Google Scholar]
- Nuutila A.M., Villiger C., Oksman-Caldentey K.M. Embryogenesis and regeneration of green plantlets from oat (Avena sativa L.) leaf-base segments: influence of nitrogen balance, sugar and auxin. Plant Cell Rep. 2002;20:1156–1161. [Google Scholar]
- Ochatt S.J., Pontecaille C., Rancillac M. The growth regulators used for bud regeneration and shoot rooting affect the competence for flowering and seed set in regenerated plants of protein peas. In Vitro Cell Dev. Biol. Plant. 2000;36:188–193. [Google Scholar]
- Pintos B., Martin J.P., Centeno M.L., Villalobos N., Guerra H., Martin L. Endogenous cytokinin levels in embryogenic and non-embryogenic calli of Medicago arborea L. Plant Sci. 2002;163:955–960. [Google Scholar]
- Popenoe P. The date palm. Field Research Projects Coconut Grove Miami. 1973:274. [Google Scholar]
- Preece J.E., Sutter E.G. Acclimatization of micropropagated plants to the greenhouse and field. In: Debergh PC., Zimmerman RH., editors. Micro Propagation: Technology and Application. Kluwer Acad Publ; Dordrecht: 1991. pp. 71–94. [Google Scholar]
- Pullman G.S., Gupta P.K., Timmis R., Carpenter C., Kreitinger M., Welty E. Improved Norway spruce somatic embryo development through the use of abscisic acid combined with activated carbon. Plant Cell Rep. 2005;24:271–279. doi: 10.1007/s00299-005-0933-0. [DOI] [PubMed] [Google Scholar]
- Redenbaugh K. Synseeds. CRC Press; Boca Raton, FL: 1993. (Application of Synthetic Seeds to Crop Improvement). [Google Scholar]
- Rhiss A., Poulain C., Beauchesne G. La culture in vitro appliqué ala multiplication vegetative du palmier dattier (Phoenix dactylifera L.) Fruits. 1979;34:551–554. [Google Scholar]
- Robichaud R.L., Lessard V.C., Merkle S.A. Treatments affecting maturation and germination of American chestnut somatic embryos. J. Plant Physiol. 2004;161:957–969. doi: 10.1016/j.jplph.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sahal A., Shahzad A., Anis M. High frequency plant production via shoot organogenesis and somatic embryogenesis from callus in Tylophora indica, an endangered plant species. Tur. J. Bot. 2010;34:11–20. [Google Scholar]
- Sharma D.R., Sunita D., Chowdhury J.R. Somatic embryogenesis and plant regeneration in date palm (Phoenix dactylifera L.) cv. 437 “Khadravi” through tissue culture. Indian J. Exp. Biol. 1984;22:596–598. [Google Scholar]
- Shen X., Kane M.E., Chen J. Effects of genotype, explant source, and plant growth regulators on indirect shoot organogenesis in Dieffenbachia cultivars. In Vitro Cell Dev. Biol. Plant. 2008;44(4):282–288. [Google Scholar]
- Shibli R.A., Smith M.A.L. Direct shoot regeneration from Vaccinium pahalae (ohelo) and Vaccinium myrtillus (bilberry) leaf explants. Hort Sci. 1996;31:1225–1228. [Google Scholar]
- Tang W., Newton R.J. Plant regeneration from callus cultures derived from mature zygotic embryos in white pine (Pinus strobes L.) Plant Cell Rep. 2005;24:1–9. doi: 10.1007/s00299-005-0914-3. [DOI] [PubMed] [Google Scholar]
- Tisserat B. Propagation of date palm (Phoenix dactylifera L.) in vitro. J. Exp. Bot. 1979;30:1275–1283. [Google Scholar]
- Tokuji Y., Kuriyama K. Involvement of gibberellin and cytokinin in the formation of embryogenic Cell clumps in carrot (Daucus carota) J. Plant Physiol. 2003;160(2):133–141. doi: 10.1078/0176-1617-00892. [DOI] [PubMed] [Google Scholar]
- Ulisses C., Camara T.R., Willadino L., Cavalcanti de Albuquerque C., Zoé de Brito J. Early somatic embryogenesis in Heliconia chartacea Lane ex Barreiros cv Sexy Pink ovary section explants. Brazil Arch. Biol. Technol. 2010;53(1) doi: 10.1590/S1516-89132010000100002. [Google Scholar]
- Van Winkle S.C., Johnson S., Pullman G.S. The impact of gelrite and activated carbon on the elemental composition of two conifer embryogenic tissue initiation media. Plant Cell Rep. 2003;21:1175–1182. doi: 10.1007/s00299-003-0637-2. [DOI] [PubMed] [Google Scholar]
- Venkataramaiah V., Prasad S.V., Rajeswara R.G., Swamy P.M. Levels of phenolic acids in Pterocarpus santalinus L. Indian J. Exp. Biol. 1980;18:887–889. [Google Scholar]
- Wang H.C., Chen J.T., Chang W.C. Morphogenetic routes of long-term embryogenic callus culture of Areca catechu. Biol. Plant. 2010;54(1):1–5. [Google Scholar]
- Wernicke W., Milkovits L. Development gradient in wheat leaves Responses of leaf segments in different genotypes cultured in vitro. J. Plant Physiol. 1986;115:49–58. doi: 10.1016/S0176-1617(84)80050-4. [DOI] [PubMed] [Google Scholar]
- Yang J.L., Seong E.S., Kim M.J., Ghimire B.K., Kang W.H., Yu C.Y., Li C.H. Direct somatic embryogenesis from pericycle cells of broccoli (Brassica oleracea L. var. italica) root explants. Plant Cell Tiss. Org. Cult. 2010;100(1):49–58. [Google Scholar]
- You C.R., Fan L.J., Qu F.N., Bian F.H., Liang L.K., Gong X.Q. Somatic embryogenesis and study on histology and cytology in Cyclamen persicum Mill. J. Sichuan University. 2008;45(6):1477–1484. [Google Scholar]
- Zhang Z.M., Davis F.T. In vitro culture of Crape myrtle. Hort. Sci. 1986;21:1044–1045. [Google Scholar]


