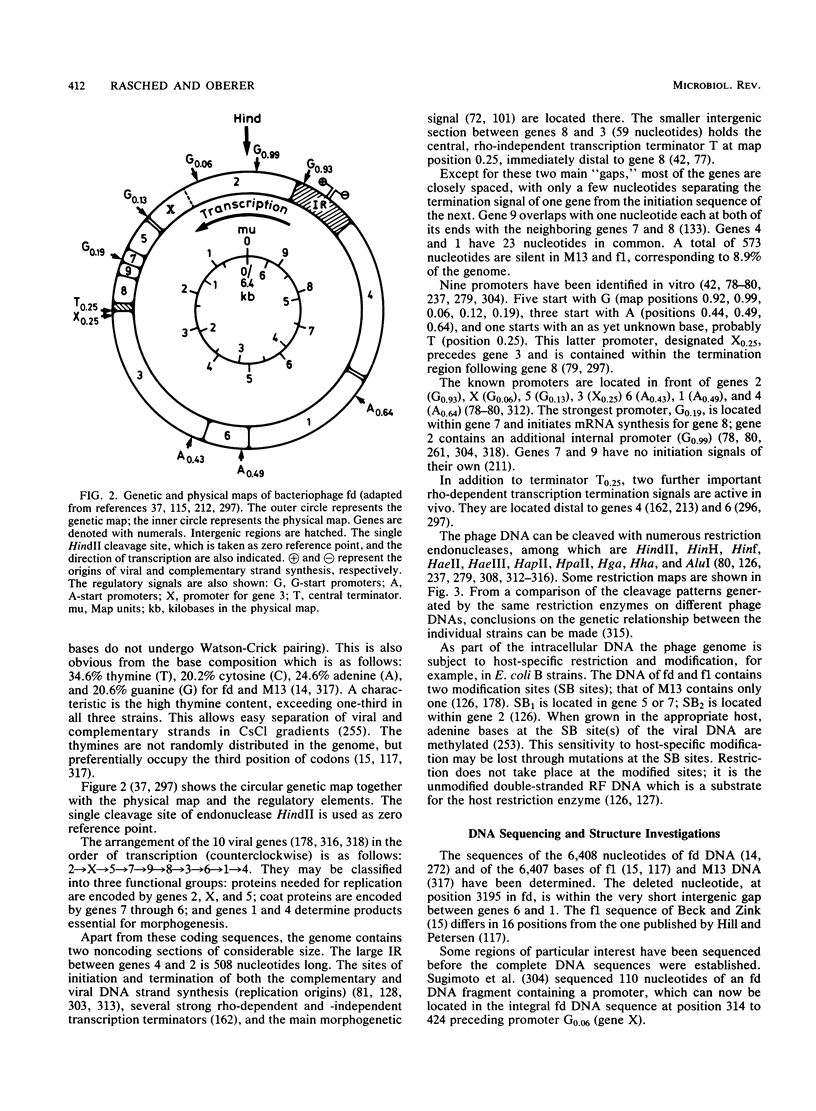
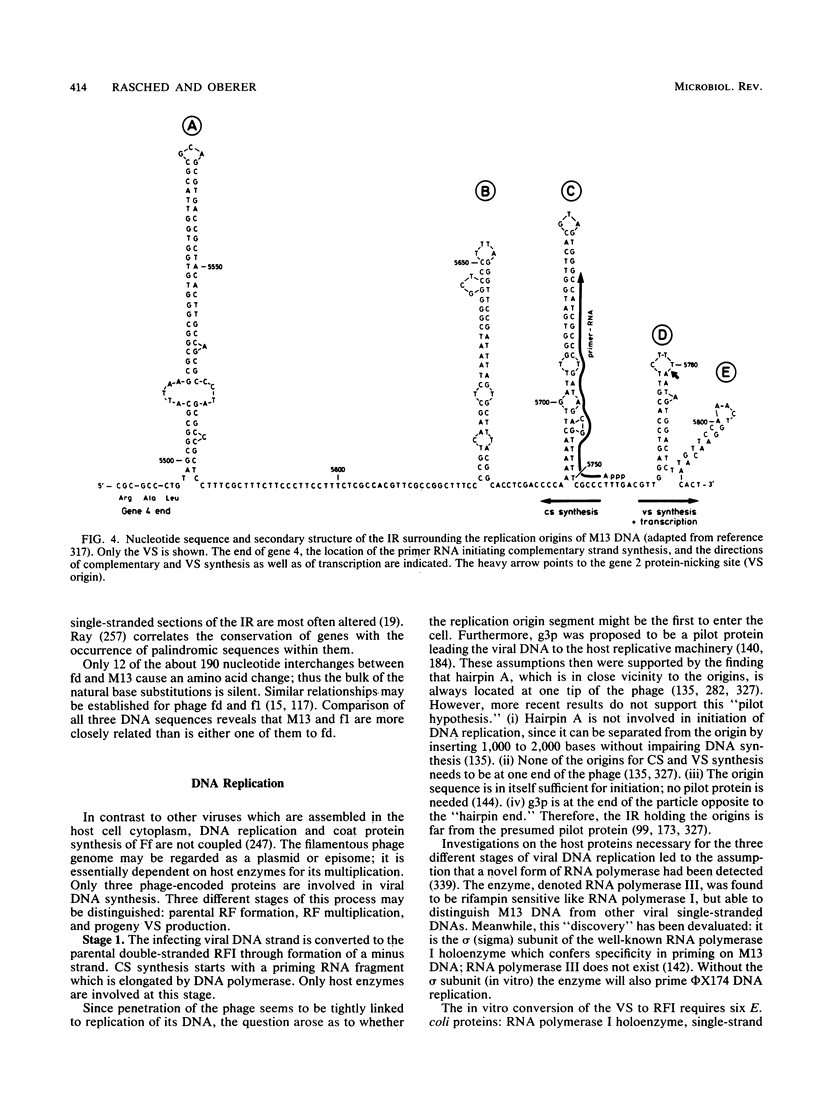
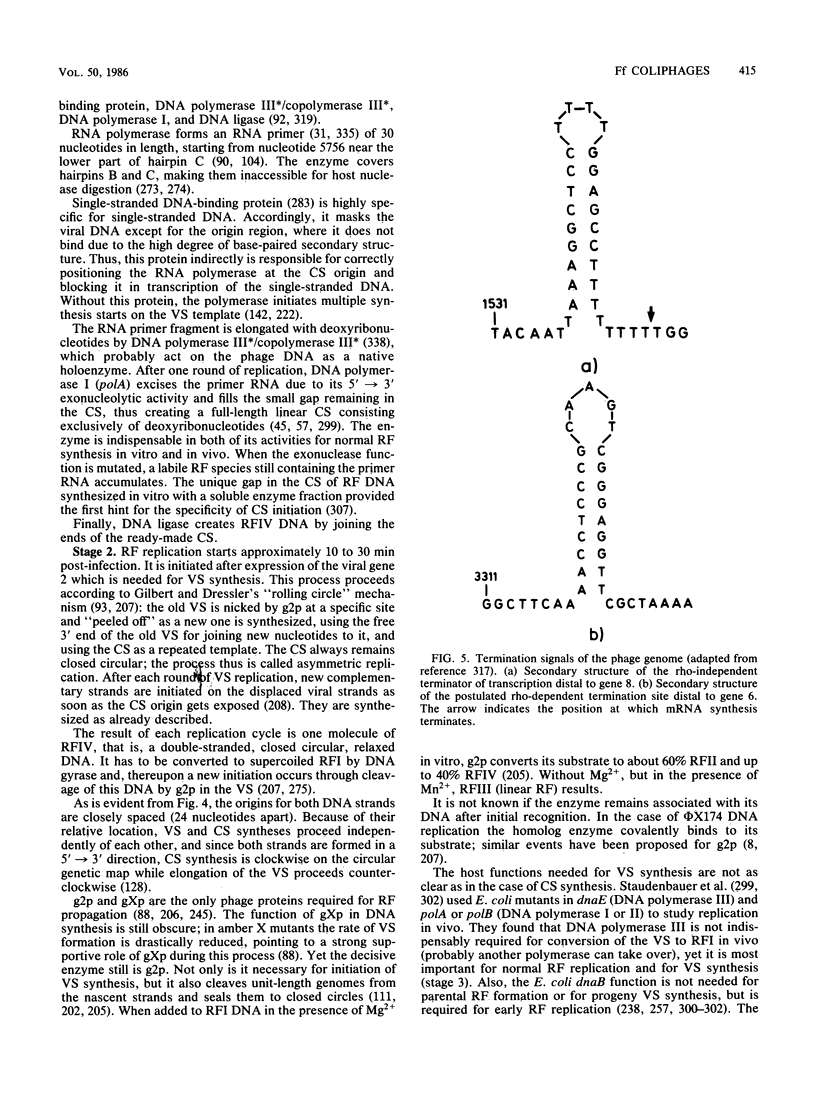
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- Alma N. C., Harmsen B. J., van Boom J. H., van der Marel G., Hilbers C. W. A 500-MHz proton nuclear magnetic resonance study of the structure and structural alterations of gene-5 protein-oligo(deoxyadenylic acid) complexes. Biochemistry. 1983 Apr 26;22(9):2104–2115. doi: 10.1021/bi00278a010. [DOI] [PubMed] [Google Scholar]
- Anderson E., Nakashima Y., Konigsberg W. Photo-incuced cross-linkage of gene-5 protein and bacteriophage fd DNA+. Nucleic Acids Res. 1975 Mar;2(3):361–371. doi: 10.1093/nar/2.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. A., Nakashima Y., Coleman J. E. Chemical modifications of functional residues of fd gene 5 DNA-binding protein. Biochemistry. 1975 Mar 11;14(5):907–917. doi: 10.1021/bi00676a006. [DOI] [PubMed] [Google Scholar]
- Armstrong J., Hewitt J. A., Perham R. N. Chemical modification of the coat protein in bacteriophage fd and orientation of the virion during assembly and disassembly. EMBO J. 1983;2(10):1641–1646. doi: 10.1002/j.1460-2075.1983.tb01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J., Perham R. N., Walker J. E. Domain structure of bacteriophage fd adsorption protein. FEBS Lett. 1981 Nov 30;135(1):167–172. doi: 10.1016/0014-5793(81)80969-6. [DOI] [PubMed] [Google Scholar]
- Asbeck F., Beyreuther K., Köhler H., von Wettstein G., Braunitzer G. Virusproteine, IV. Die Konstitution des Hüllproteins des Phagen fd. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1047–1066. [PubMed] [Google Scholar]
- Baas P. D. DNA replication of single-stranded Escherichia coli DNA phages. Biochim Biophys Acta. 1985 Jun 24;825(2):111–139. doi: 10.1016/0167-4781(85)90096-x. [DOI] [PubMed] [Google Scholar]
- Banner D. W., Nave C., Marvin D. A. Structure of the protein and DNA in fd filamentous bacterial virus. Nature. 1981 Feb 26;289(5800):814–816. doi: 10.1038/289814a0. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. Construction of an M13 histidine-transducing phage: a single-stranded cloning vehicle with one EcoRI site. Gene. 1979 Feb;5(2):127–139. doi: 10.1016/0378-1119(79)90098-2. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H. Effects of bacteriophage fd infection on Escherichia coli HB11 envelope: a morphological and biochemical study. J Virol. 1986 Jan;57(1):258–266. doi: 10.1128/jvi.57.1.258-266.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne S., Rasched I. The lysine residues implicated in the gene 5 protein association sites. Biosci Rep. 1983 May;3(5):469–474. doi: 10.1007/BF01121958. [DOI] [PubMed] [Google Scholar]
- Beaudoin J., Henry T. J., Pratt D. Purification of single- and double-length M13 virions by polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):470–477. doi: 10.1128/jvi.13.2.470-477.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Zink B. Nucleotide sequence and genome organisation of filamentous bacteriophages fl and fd. Gene. 1981 Dec;16(1-3):35–58. doi: 10.1016/0378-1119(81)90059-7. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. A., Day L. A. Mass, length, composition and structure of the filamentous bacterial virus fd. J Mol Biol. 1976 Apr 15;102(3):531–547. doi: 10.1016/0022-2836(76)90332-6. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. A., Day L. A. Turbidity measurements in an analytical ultracentrifuge. Determinations of mass per length for filamentous viruses fd, Xf, and Pf3. Biochemistry. 1980 Jun 10;19(12):2696–2702. doi: 10.1021/bi00553a025. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Model P. A prokaryotic membrane anchor sequence: carboxyl terminus of bacteriophage f1 gene III protein retains it in the membrane. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5200–5204. doi: 10.1073/pnas.79.17.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Model P. Molecular basis of the am8H1 lesion in bacteriophage M13. Virology. 1979 Jul 15;96(1):299–301. doi: 10.1016/0042-6822(79)90198-3. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Model P., Zinder N. D. Effects of bacteriophage f1 gene III protein on the host cell membrane. Mol Gen Genet. 1982;186(2):185–192. doi: 10.1007/BF00331849. [DOI] [PubMed] [Google Scholar]
- Boeke J. D. One and two codon insertion mutants of bacteriophage f1. Mol Gen Genet. 1981;181(3):288–291. doi: 10.1007/BF00425599. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Russel M., Model P. Processing of filamentous phage pre-coat protein. Effect of sequence variations near the signal peptidase cleavage site. J Mol Biol. 1980 Dec 5;144(2):103–116. doi: 10.1016/0022-2836(80)90027-3. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Vovis G. F., Zinder N. D. Insertion mutant of bacteriophage f1 sensitive to EcoRI. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2699–2702. doi: 10.1073/pnas.76.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogusky M. J., Tsang P., Opella S. J. One- and two- dimensional 15N/1H NMR of filamentous phage coat proteins in solution. Biochem Biophys Res Commun. 1985 Mar 15;127(2):540–545. doi: 10.1016/s0006-291x(85)80193-5. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Dewar C. A. Intracellular changes in cells of Escherichia coli infected with a filamentous bacteriophage. J Gen Virol. 1967 Apr;1(2):179–188. doi: 10.1099/0022-1317-1-2-179. [DOI] [PubMed] [Google Scholar]
- Brayer G. D., McPherson A. A model for intracellular complexation between gene-5 protein and bacteriophage fd DNA. Eur J Biochem. 1985 Jul 15;150(2):287–296. doi: 10.1111/j.1432-1033.1985.tb09019.x. [DOI] [PubMed] [Google Scholar]
- Brayer G. D., McPherson A. Mechanism of DNA binding to the gene 5 protein of bacteriophage fd. Biochemistry. 1984 Jan 17;23(2):340–349. doi: 10.1021/bi00297a025. [DOI] [PubMed] [Google Scholar]
- Brayer G. D., McPherson A. Refined structure of the gene 5 DNA binding protein from bacteriophage fd. J Mol Biol. 1983 Sep 15;169(2):565–596. doi: 10.1016/s0022-2836(83)80065-5. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Day L. A. DNA packing in the filamentous viruses fd, Xf, Pf1 and Pf3. Nucleic Acids Res. 1982 Apr 10;10(7):2467–2481. doi: 10.1093/nar/10.7.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Day L. A. Silver and mercury probing of deoxyribonucleic acid structures in the filamentous viruses fd, If1, IKe, Xf, Pf1, and Pf3. Biochemistry. 1983 Sep 27;22(20):4831–4842. doi: 10.1021/bi00289a033. [DOI] [PubMed] [Google Scholar]
- Cashman J. S., Webster R. E. Bacteriophage f1 infection of Escherichia coli: identification and possible processing of f1-specific mRNAs in vivo. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1169–1173. doi: 10.1073/pnas.76.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman J. S., Webster R. E. Effect of cessation of phospholipid synthesis on the synthesis of a specific membrane-associated bacteriophage protein in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1245–1249. doi: 10.1128/jb.129.3.1245-1249.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman J. S., Webster R. E., Steege D. A. Transcription of bacteriophage fl. The major in vivo RNAs. J Biol Chem. 1980 Mar 25;255(6):2554–2562. [PubMed] [Google Scholar]
- Cavalieri S. J., Goldthwait D. A., Neet K. E. The isolation of a dimer of gene 8 protein of bacteriophage fd. J Mol Biol. 1976 Apr 25;102(4):713–722. doi: 10.1016/0022-2836(76)90287-4. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Neet K. E., Goldthwait D. A. Gene 5 protein of bacteriophage fd: a dimer which interacts co-operatively with DNA. J Mol Biol. 1976 Apr 25;102(4):697–711. doi: 10.1016/0022-2836(76)90286-2. [DOI] [PubMed] [Google Scholar]
- Chamberlain B. K., Nozaki Y., Tanford C., Webster R. E. Association of the major coat protein of fd bacteriophage with phospholipid vesicles. Biochim Biophys Acta. 1978 Jun 16;510(1):18–37. doi: 10.1016/0005-2736(78)90127-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain B. K., Webster R. E. Lipid-protein interactions in Escherichia coli. Membrane-associated f1 bacteriophage coat protein and phospholipid metabolism. J Biol Chem. 1976 Dec 25;251(24):7739–7745. [PubMed] [Google Scholar]
- Chan T. S., Model P., Zinder N. D. In vitro protein synthesis directed by separated transcripts of bacteriophage f1 DNA. J Mol Biol. 1975 Dec 15;99(3):369–382. doi: 10.1016/s0022-2836(75)80132-x. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Model P., Blobel G. Membrane biogenesis: cotranslational integration of the bacteriophage f1 coat protein into an Escherichia coli membrane fraction. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1251–1255. doi: 10.1073/pnas.76.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. C., Ray D. S. Replication of bacteriophage M13. X. M13 replication in a mutant of Escherichia coli defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. J Mol Biol. 1976 Sep 25;106(3):589–604. doi: 10.1016/0022-2836(76)90253-9. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Ray D. S. Replication of bacteriophage M13. XIII. Structure and replication of cloned M13 miniphage. J Mol Biol. 1978 Oct 25;125(2):107–121. doi: 10.1016/0022-2836(78)90340-6. [DOI] [PubMed] [Google Scholar]
- Cleary J. M., Ray D. S. Deletion analysis of the cloned replication origin region from bacteriophage M13. J Virol. 1981 Oct;40(1):197–203. doi: 10.1128/jvi.40.1.197-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary J. M., Ray D. S. Replication of the plasmid pBR322 under the control of a cloned replication origin from the single-stranded DNA phage M13. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4638–4642. doi: 10.1073/pnas.77.8.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. E., Anderson R. A., Ratcliffe R. G., Armitage I. M. Structure of gene 5 protein-oligodeoxynucleotide complexes as determined by 1H, 19F, and 31P nuclear magnetic resonance. Biochemistry. 1976 Dec 14;15(25):5419–5430. doi: 10.1021/bi00670a001. [DOI] [PubMed] [Google Scholar]
- Coleman J. E., Oakley J. L. Physical chemical studies of the structure and function of DNA binding (helix-destabilizing) proteins. CRC Crit Rev Biochem. 1980 Jan;7(3):247–289. doi: 10.3109/10409238009105463. [DOI] [PubMed] [Google Scholar]
- Crissman J. W., Smith G. P. Gene-III protein of filamentous phages: evidence for a carboxyl-terminal domain with a role in morphogenesis. Virology. 1984 Jan 30;132(2):445–455. doi: 10.1016/0042-6822(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Cross T. A., Opella S. J. Protein structure by solid state nuclear magnetic resonance. Residues 40 to 45 of bacteriophage fd coat protein. J Mol Biol. 1985 Apr 5;182(3):367–381. doi: 10.1016/0022-2836(85)90197-4. [DOI] [PubMed] [Google Scholar]
- Cross T. A., Opella S. J. Structural properties of fd coat protein in sodium dodecyl sulfate micelles. Biochem Biophys Res Commun. 1980 Jan 29;92(2):478–484. doi: 10.1016/0006-291x(80)90358-7. [DOI] [PubMed] [Google Scholar]
- Cuypers T., van der Ouderaa F. J., de Jong W. W. The amino acid sequence of gene 5 protein of bacteriophage M 13. Biochem Biophys Res Commun. 1974 Jul 24;59(2):557–563. doi: 10.1016/s0006-291x(74)80016-1. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Transitory recombination between plasmid pHV33 and phage M13. EMBO J. 1983;2(12):2117–2122. doi: 10.1002/j.1460-2075.1983.tb01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J Biol Chem. 1985 Dec 15;260(29):15925–15931. [PubMed] [Google Scholar]
- Dasgupta S., Mitra S. Structure of nascent replicative form DNA of coliphage M13. Proc Natl Acad Sci U S A. 1978 Jan;75(1):153–157. doi: 10.1073/pnas.75.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Inuzuka M., Tomoeda M. Purification and characterization of F pili from Escherichia coli. Biochemistry. 1977 Dec 13;16(25):5579–5585. doi: 10.1021/bi00644a030. [DOI] [PubMed] [Google Scholar]
- Date T., Wickner W. T. Procoat, the precursor of M13 coat protein, inserts post-translationally into the membrane of cells infected by wild-type virus. J Virol. 1981 Mar;37(3):1087–1089. doi: 10.1128/jvi.37.3.1087-1089.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Zwizinski C., Ludmerer S., Wickner W. Mechanisms of membrane assembly: effects of energy poisons on the conversion of soluble M13 coliphage procoat to membrane-bound coat protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):827–831. doi: 10.1073/pnas.77.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. G., Boeke J. D., Model P. Fine structure of a membrane anchor domain. J Mol Biol. 1985 Jan 5;181(1):111–121. doi: 10.1016/0022-2836(85)90329-8. [DOI] [PubMed] [Google Scholar]
- Day L. A., Berkowitz S. A. The number of nucleotides and the density and refractive index increments of fd virus DNA. J Mol Biol. 1977 Nov 5;116(3):603–606. doi: 10.1016/0022-2836(77)90087-0. [DOI] [PubMed] [Google Scholar]
- Day L. A. Circular dichroism and ultraviolet absorption of a deoxyribonucleic acid binding protein of filamentous bacteriophage. Biochemistry. 1973 Dec 18;12(26):5329–5339. doi: 10.1021/bi00750a017. [DOI] [PubMed] [Google Scholar]
- Day L. A., Wiseman R. L., Marzec C. J. Structure models for DNA in filamentous viruses with phosphates near the center. Nucleic Acids Res. 1979 Nov 24;7(6):1393–1403. doi: 10.1093/nar/7.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Enea V., Zinder N. D. Functional analysis of bacteriophage f1 intergenic region. Virology. 1981 Oct 30;114(2):463–473. doi: 10.1016/0042-6822(81)90226-9. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Enea V., Zinder N. D. Gene II of phage f1: its functions and its products. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5421–5424. doi: 10.1073/pnas.78.9.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Horiuchi K., Jakes K. S., Zinder N. D. Replication origin of bacteriophage f1. Two signals required for its function. J Mol Biol. 1982 Dec 5;162(2):335–343. doi: 10.1016/0022-2836(82)90530-7. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Horiuchi K. Replication of a plasmid containing two origins of bacteriophage. J Mol Biol. 1981 Nov 25;153(1):169–176. doi: 10.1016/0022-2836(81)90532-5. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Horiuchi K., Zinder N. D. Initiation and termination of phage f1 plus-strand synthesis. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7122–7126. doi: 10.1073/pnas.79.23.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Horiuchi K., Zinder N. D. The functional origin of bacteriophage f1 DNA replication. Its signals and domains. J Mol Biol. 1984 Feb 5;172(4):507–521. doi: 10.1016/s0022-2836(84)80020-0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Zinder N. D. Increased intracellular concentration of an initiator protein markedly reduces the minimal sequence required for initiation of DNA synthesis. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1336–1340. doi: 10.1073/pnas.81.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., Zinder N. D. Reduction of the minimal sequence for initiation of DNA synthesis by qualitative or quantitative changes of an initiator protein. Nature. 1984 Sep 20;311(5983):279–280. doi: 10.1038/311279a0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Zinder N. D. The morphogenetic signal of bacteriophage f1. Virology. 1983 Oct 15;130(1):252–256. doi: 10.1016/0042-6822(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Edens L., Konings R. N., Schoenmakers J. G. A cascade mechanism of transcription in bacteriophage M13 DNA. Virology. 1978 May 15;86(2):354–367. doi: 10.1016/0042-6822(78)90076-4. [DOI] [PubMed] [Google Scholar]
- Edens L., Konings R. N., Schoenmakers J. G. Physical mapping of the central terminator for transcription on the bacteriophage M13 genome. Nucleic Acids Res. 1975 Oct;2(10):1811–1820. doi: 10.1093/nar/2.10.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens L., Konings R. N., Schoenmakers J. G. Transcription of bacteriophage M13 DNA: existence of promoters directly preceding genes III, VI, and I. J Virol. 1978 Dec;28(3):835–842. doi: 10.1128/jvi.28.3.835-842.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens L., van Wezenbeek P., Konings N. H., Schoenmakers J. G. Mapping of promoter sites on the genome of bacteriophage M13. Eur J Biochem. 1976 Nov 15;70(2):577–587. doi: 10.1111/j.1432-1033.1976.tb11049.x. [DOI] [PubMed] [Google Scholar]
- Enea V., Horiuchi K., Turgeon B. G., Zinder N. D. Physical map of defective interfering particles of bacteriophage f1. J Mol Biol. 1977 Apr 25;111(4):395–414. doi: 10.1016/s0022-2836(77)80061-2. [DOI] [PubMed] [Google Scholar]
- Enea V., Vovis G. F., Zinder N. D. Genetic studies with heteroduplex DNA of bacteriophage fl. Asymmetric segregation, base correction and implications for the mechanism of genetic recombination. J Mol Biol. 1975 Aug 15;96(3):495–509. doi: 10.1016/0022-2836(75)90175-8. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. A delection mutant of bacteriophage f1 containing no intact cistrons. Virology. 1975 Nov;68(1):105–114. doi: 10.1016/0042-6822(75)90152-x. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Heteroduplex DNA: a recombinational intermediate in bacteriophage f1. J Mol Biol. 1976 Feb 15;101(1):25–38. doi: 10.1016/0022-2836(76)90064-4. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Interference resistant mutants of phage f1. Virology. 1982 Oct 15;122(1):222–226. doi: 10.1016/0042-6822(82)90395-6. [DOI] [PubMed] [Google Scholar]
- Folkhard W., Leonard K. R., Malsey S., Marvin D. A., Dubochet J., Engel A., Achtman M., Helmuth R. X-ray diffraction and electron microscope studies on the structure of bacterial F pili. J Mol Biol. 1979 May 15;130(2):145–160. doi: 10.1016/0022-2836(79)90423-6. [DOI] [PubMed] [Google Scholar]
- Fulford W., Model P. Gene X of bacteriophage f1 is required for phage DNA synthesis. Mutagenesis of in-frame overlapping genes. J Mol Biol. 1984 Sep 15;178(2):137–153. doi: 10.1016/0022-2836(84)90136-0. [DOI] [PubMed] [Google Scholar]
- Fulford W., Model P. Specificity of translational regulation by two DNA-binding proteins. J Mol Biol. 1984 Feb 25;173(2):211–226. doi: 10.1016/0022-2836(84)90190-6. [DOI] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Beck E., Schaller H. An RNA transcribed from DNA at the origin of phage fd single strand to replicative form conversion. Proc Natl Acad Sci U S A. 1978 Feb;75(2):645–649. doi: 10.1073/pnas.75.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Hohmeyer C., Haas R., Meyer T. F. A plasmid cloning system utilizing replication and packaging functions of the filamentous bacteriophage fd. Gene. 1985;33(3):341–349. doi: 10.1016/0378-1119(85)90242-2. [DOI] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. E., Konigsberg W. H. Adsorption protein of the bacteriophage fd: isolation, molecular properties, and location in the virus. Biochemistry. 1977 Jun 14;16(12):2686–2694. doi: 10.1021/bi00631a016. [DOI] [PubMed] [Google Scholar]
- Goodman J. M., Watts C., Wickner W. Membrane assembly: posttranslational insertion of M13 procoat protein into E. coli membranes and its proteolytic conversion to coat protein in vitro. Cell. 1981 May;24(2):437–441. doi: 10.1016/0092-8674(81)90334-2. [DOI] [PubMed] [Google Scholar]
- Grant R. A., Lin T. C., Konigsberg W., Webster R. E. Structure of the filamentous bacteriophage fl. Location of the A, C, and D minor coat proteins. J Biol Chem. 1981 Jan 10;256(1):539–546. [PubMed] [Google Scholar]
- Grant R. A., Webster R. E. Minor protein content of the gene V protein/phage single-stranded DNA complex of the filamentous bacteriophage f1. Virology. 1984 Mar;133(2):315–328. doi: 10.1016/0042-6822(84)90398-2. [DOI] [PubMed] [Google Scholar]
- Grant R. A., Webster R. E. The bacteriophage f1 morphogenetic signal and the gene V protein/phage single-stranded DNA complex. Virology. 1984 Mar;133(2):329–340. doi: 10.1016/0042-6822(84)90399-4. [DOI] [PubMed] [Google Scholar]
- Gray C. P., Sommer R., Polke C., Beck E., Schaller H. Structure of the orgin of DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1978 Jan;75(1):50–53. doi: 10.1073/pnas.75.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. W., Brown R. S., Marvin D. A. Adsorption complex of filamentous fd virus. J Mol Biol. 1981 Mar 15;146(4):621–627. doi: 10.1016/0022-2836(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Gray C. W., Page G. A., Gray D. M. Complex of fd gene 5 protein and double-stranded RNA. J Mol Biol. 1984 Jun 5;175(4):553–559. doi: 10.1016/0022-2836(84)90184-0. [DOI] [PubMed] [Google Scholar]
- Griffith J., Kornberg A. Mini M13 bacteriophage: circular fragments of M13 DNA are replicated and packaged during normal infections. Virology. 1974 May;59(1):139–152. doi: 10.1016/0042-6822(74)90211-6. [DOI] [PubMed] [Google Scholar]
- Griffith J., Manning M., Dunn K. Filamentous bacteriophage contract into hollow spherical particles upon exposure to a chloroform-water interface. Cell. 1981 Mar;23(3):747–753. doi: 10.1016/0092-8674(81)90438-4. [DOI] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Hagen D. S., Weiner J. H., Sykes B. D. Fluorotyrosine M13 coat protein: fluorine-19 nuclear magnetic resonance study of the motional properties of an integral membrane protein in phospholipid vesicles. Biochemistry. 1978 Sep 5;17(18):3860–3866. doi: 10.1021/bi00611a028. [DOI] [PubMed] [Google Scholar]
- Hagen D. S., Weiner J. H., Sykes B. D. Investigation of solvent accessibility of the fluorotyrosyl residues of M13 coat protein in deoxycholate micelles and phospholipid vesicles. Biochemistry. 1979 May 15;18(10):2007–2012. doi: 10.1021/bi00577a026. [DOI] [PubMed] [Google Scholar]
- Harth G., Bäumel I., Meyer T. F., Geider K. Bacteriophage fd gene-2 protein. Processing of phage fd viral strands replicated by phage T7 enzymes. Eur J Biochem. 1981 Oct;119(3):663–668. doi: 10.1111/j.1432-1033.1981.tb05659.x. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Achtman M. Cell-cell interactions in conjugating Escherichia coli: purification of F pili with biological activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1237–1241. doi: 10.1073/pnas.75.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. J., Pratt D. The proteins of bacteriophage M13. Proc Natl Acad Sci U S A. 1969 Mar;62(3):800–807. doi: 10.1073/pnas.62.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R., Neugebauer K., Pirkl E., Zentgraf H., Schaller H. Conversion of bacteriophage fd into an efficient single-stranded DNA vector system. Mol Gen Genet. 1980 Jan;177(2):231–242. doi: 10.1007/BF00267434. [DOI] [PubMed] [Google Scholar]
- Herrmann R., Neugebauer K., Zentgraf H., Schaller H. Transposition of a DNA sequence determining kanamycin resistance into the single-stranded genome of bacteriophage fd. Mol Gen Genet. 1978 Feb 16;159(2):171–178. doi: 10.1007/BF00270890. [DOI] [PubMed] [Google Scholar]
- Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage f1 DNA. J Virol. 1982 Oct;44(1):32–46. doi: 10.1128/jvi.44.1.32-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J. C., Ray D. S. Construction and characterization of new coliphage M13 cloning vectors. Gene. 1980 Nov;11(3-4):207–218. doi: 10.1016/0378-1119(80)90061-x. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Greulich K. O., Ludwig H., Marvin D. A. Calorimetric, density and circular dichroism studies of the reversible structural transition in Pf1 filamentous bacterial virus. J Mol Biol. 1980 Dec 15;144(3):281–289. doi: 10.1016/0022-2836(80)90091-1. [DOI] [PubMed] [Google Scholar]
- Hohn B., von Schütz H., Marvin D. A. Filamentous bacterial viruses. II. Killing of bacteria by abortive infection with fd. J Mol Biol. 1971 Feb 28;56(1):155–165. doi: 10.1016/0022-2836(71)90091-x. [DOI] [PubMed] [Google Scholar]
- Hondel C. A., Konings R. N., Schoenmakers J. G. Regulation of gene activity in bacteriophage M13 DNA: Coupled transcription and translation of purified genes and gene-fragments. Virology. 1975 Oct;67(2):487–497. doi: 10.1016/0042-6822(75)90449-3. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Webster R. E. Morphogenesis of f1 filamentous bacteriophage. Increased expression of gene I inhibits bacterial growth. J Mol Biol. 1986 Apr 5;188(3):403–413. doi: 10.1016/0022-2836(86)90164-6. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Co-evolution of a filamentous bacteriophage and its defective interfering particles. J Mol Biol. 1983 Sep 15;169(2):389–407. doi: 10.1016/s0022-2836(83)80057-6. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Origin of DNA replication of bacteriophage f1 as the signal for termination. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5226–5229. doi: 10.1073/pnas.77.9.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Vovis G. F., Enea V., Zinder N. D. Cleavage map of bacteriophage f1: location of the Escherichia coli B-specific modification sites. J Mol Biol. 1975 Jun 25;95(2):147–165. doi: 10.1016/0022-2836(75)90388-5. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Cleavage of bacteriophage fl DNA by the restriction enzyme of Escherichia coli B. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3220–3224. doi: 10.1073/pnas.69.11.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Origin and direction of synthesis of bacteriophage fl DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2341–2345. doi: 10.1073/pnas.73.7.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Hu V. W., Wisnieski B. J. Photoreactive labeling of M13 coat protein in model membranes by use of a glycolipid probe. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5460–5464. doi: 10.1073/pnas.76.11.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E. Fine mapping of secondary structures of fd phage DNA in the region of the replication origin. Nucleic Acids Res. 1981 Nov 11;9(21):5587–5599. doi: 10.1093/nar/9.21.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E. Pauses at positions of secondary structure during in vitro replication of single-stranded fd bacteriophage DNA by T4 DNA polymerase. Anal Biochem. 1980 Mar 15;103(1):127–139. doi: 10.1016/0003-2697(80)90246-8. [DOI] [PubMed] [Google Scholar]
- Hulsebos T., Schoenmakers J. G. Nucleotide sequence of gene VII and of a hypothetical gene (IX) in bacteriophage M13. Nucleic Acids Res. 1978 Dec;5(12):4677–4698. doi: 10.1093/nar/5.12.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara K., Utiyama H., Kurata M. Studies on the structure of filamentous bacteriophage fd. II. All-or-none disassembly in guanidine-HCl and sodium dodecyl sulfate. Virology. 1975 Jul;66(1):306–315. doi: 10.1016/0042-6822(75)90200-7. [DOI] [PubMed] [Google Scholar]
- Ikoku A. S., Hearst J. E. Identification of a structural hairpin in the filamentous chimeric phage M13Gori1. J Mol Biol. 1981 Sep 15;151(2):245–259. doi: 10.1016/0022-2836(81)90514-3. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Ito K., Mandel G., Wickner W. Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1199–1203. doi: 10.1073/pnas.76.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. Role of F pili in the penetration of bacteriophage fl. J Virol. 1972 Oct;10(4):835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Marco R., Kornberg A. A coat protein of the bacteriophage M13 virion participates in membrane-oriented synthesis of DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):205–209. doi: 10.1073/pnas.70.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S., Lee J. H., Ray D. S. High-level expression of M13 gene II protein from an inducible polycistronic messenger RNA. Gene. 1985;34(2-3):137–145. doi: 10.1016/0378-1119(85)90121-0. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. The rho subunit of RNA polymerase holoenzyme confers specificity in priming M13 viral DNA replication. J Biol Chem. 1982 May 25;257(10):5437–5443. [PubMed] [Google Scholar]
- Kaguni J., LaVerne L. S., Ray D. S. Cloning and expression of the Escherichia coli replication origin in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6250–6254. doi: 10.1073/pnas.76.12.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J., Ray D. S. Cloning of a functional replication origin of phage G4 into the genome of phage M13. J Mol Biol. 1979 Dec 25;135(4):863–878. doi: 10.1016/0022-2836(79)90516-3. [DOI] [PubMed] [Google Scholar]
- Kim M. H., Hines J. C., Ray D. S. Viable deletions of the M13 complementary strand origin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6784–6788. doi: 10.1073/pnas.78.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Ray D. S. Mutational mechanisms by which an inactive replication origin of bacteriophage M13 is turned on are similar to mechanisms of activation of ras proto-oncogenes. J Virol. 1985 Mar;53(3):871–878. doi: 10.1128/jvi.53.3.871-878.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Tecoma E. S., Wolber P. K., Hudson B. S., Wickner W. T., Simoni R. D. Protein-lipid interactions. Studies of the M13 coat protein in dimyristoylphosphatidylcholine vesicles using parinaric acid. Biochemistry. 1979 Dec 25;18(26):5874–5880. doi: 10.1021/bi00593a018. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. II. Interaction of the protein with DNA in vitro. J Mol Biol. 1966 Nov 14;21(2):293–304. doi: 10.1016/0022-2836(66)90100-8. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings R. N., Hulsebos T., Van den Hondel C. A. Identification and characterization of the in vitro synthesized gene products of bacteriophage M13. J Virol. 1975 Mar;15(3):570–584. doi: 10.1128/jvi.15.3.570-584.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings R. N., Jansen J., Cuypers T., Schoenmakers J. G. Synthesis of bacteriophage M13-specific proteins in a DNA-dependent cell-free system. II. In vitro synthesis of biologically active gene 5 protein. J Virol. 1973 Dec;12(6):1466–1472. doi: 10.1128/jvi.12.6.1466-1472.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings R. N., Schoenmakers J. G. Bacteriophage M13 DNA-directed in vitro synthesis of gene 5 protein. Mol Biol Rep. 1974 Feb;1(5):251–256. doi: 10.1007/BF00417579. [DOI] [PubMed] [Google Scholar]
- Konings R. N. Synthesis of phage M13 specific proteins in a DNA-dependent cell-free system. FEBS Lett. 1973 Sep 1;35(1):155–160. doi: 10.1016/0014-5793(73)80600-3. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Wickner W. Conserved residues of the leader peptide are essential for cleavage by leader peptidase. J Biol Chem. 1985 Dec 15;260(29):15914–15918. [PubMed] [Google Scholar]
- Kuhn A., Wickner W. Isolation of mutants in M13 coat protein that affect its synthesis, processing, and assembly into phage. J Biol Chem. 1985 Dec 15;260(29):15907–15913. [PubMed] [Google Scholar]
- Kumamoto C. A., Oliver D. B., Beckwith J. Signal sequence mutations disrupt feedback between secretion of an exported protein and its synthesis in E. coli. 1984 Apr 26-May 2Nature. 308(5962):863–864. doi: 10.1038/308863a0. [DOI] [PubMed] [Google Scholar]
- LOEB T. Isolation of a bacteriophage specific for the F plus and Hfr mating types of Escherichia coli K-12. Science. 1960 Mar 25;131(3404):932–933. doi: 10.1126/science.131.3404.932. [DOI] [PubMed] [Google Scholar]
- La Farina M., Model P. Transcription in bacteriophage F1-infected Escherichia coli. I. Translation of the RNA in vitro. Virology. 1978 May 15;86(2):368–375. doi: 10.1016/0042-6822(78)90077-6. [DOI] [PubMed] [Google Scholar]
- La Farina M., Model P. Transcription in bacteriophage f1-infected Escherichia coli. Messenger populations in the infected cell. J Mol Biol. 1983 Mar 5;164(3):377–393. doi: 10.1016/0022-2836(83)90057-8. [DOI] [PubMed] [Google Scholar]
- La Farina M. Transcription in bacteriophage f1-infected Escherichia coli: very large RNA species are synthesized on the phage DNA. Mol Gen Genet. 1983;191(1):22–25. doi: 10.1007/BF00330884. [DOI] [PubMed] [Google Scholar]
- La Farina M., Vitale M., Enea V. Transcription in bacteriophage f1-infected Escherichia coli: RNA synthesized on DNA of deletion mutant PII shows the existence of a two-site terminator. Mol Gen Genet. 1984;195(3):411–417. doi: 10.1007/BF00341441. [DOI] [PubMed] [Google Scholar]
- La Farina M., Vitale M. Rho-dependence of the terminator active at the end of the I region of transcription of bacteriophage f1. Mol Gen Genet. 1984;195(1-2):5–9. doi: 10.1007/BF00332715. [DOI] [PubMed] [Google Scholar]
- Lerner T. J., Model P. The "steady state" of coliphage f1: DNA synthesis late in infection. Virology. 1981 Dec;115(2):282–294. doi: 10.1016/0042-6822(81)90111-2. [DOI] [PubMed] [Google Scholar]
- Lica L., Ray D. S. Replication of bacteriophage M13. XII. In vivo cross-linking of a phage-specific DNA binding protein to the single-stranded DNA of bacteriophage M13 by ultraviolet irradiation. J Mol Biol. 1977 Sep;115(1):45–59. doi: 10.1016/0022-2836(77)90245-5. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Haller B., Fuchs J. A. Thioredoxin is the bacterial protein encoded by fip that is required for filamentous bacteriophage f1 assembly. J Bacteriol. 1985 Feb;161(2):799–802. doi: 10.1128/jb.161.2.799-802.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N. S., Pratt D. Bacteriophage M 13 gene 2 protein: increasing its yield in infected cells, and identification and localization. Virology. 1974 Oct;61(2):334–342. doi: 10.1016/0042-6822(74)90271-2. [DOI] [PubMed] [Google Scholar]
- Lin N. S., Pratt D. Role of bacteriophage M13 gene 2 in viral DNA replication. J Mol Biol. 1972 Dec 14;72(1):37–49. doi: 10.1016/0022-2836(72)90066-6. [DOI] [PubMed] [Google Scholar]
- Lin T. C., Bendet I. J. The A protein of bacteriophage fd: its interference with viral infection. Biochem Biophys Res Commun. 1976 Sep 7;72(1):369–372. doi: 10.1016/0006-291x(76)91003-2. [DOI] [PubMed] [Google Scholar]
- Lin T. C., Webster R. E., Konigsberg W. Isolation and characterization of the C and D proteins coded by gene IX and gene VI in the filamentous bacteriophage fl and fd. J Biol Chem. 1980 Nov 10;255(21):10331–10337. [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Assembly site of bacteriophage f1 corresponds to adhesion zones between the inner and outer membranes of the host cell. J Bacteriol. 1985 Sep;163(3):1270–1274. doi: 10.1128/jb.163.3.1270-1274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Minor coat protein composition and location of the A protein in bacteriophage f1 spheroids and I-forms. J Virol. 1982 Jun;42(3):1099–1107. doi: 10.1128/jvi.42.3.1099-1107.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Morphogenesis of filamentous bacteriophage f1: orientation of extrusion and production of polyphage. Virology. 1983 May;127(1):177–193. doi: 10.1016/0042-6822(83)90382-3. [DOI] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. fipB and fipC: two bacterial loci required for morphogenesis of the filamentous bacteriophage f1. J Bacteriol. 1985 Sep;163(3):900–905. doi: 10.1128/jb.163.3.900-905.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiten R. G., Putterman D. G., Schoenmakers J. G., Konings R. N., Day L. A. Nucleotide sequence of the genome of Pf3, an IncP-1 plasmid-specific filamentous bacteriophage of Pseudomonas aeruginosa. J Virol. 1985 Oct;56(1):268–276. doi: 10.1128/jvi.56.1.268-276.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn C. A., Pigiet V. P. Localization of thioredoxin from Escherichia coli in an osmotically sensitive compartment. J Biol Chem. 1982 Oct 10;257(19):11424–11430. [PubMed] [Google Scholar]
- Lyons L. B., Zinder N. D. The genetic map of the filamentous bacteriophage f1. Virology. 1972 Jul;49(1):45–60. doi: 10.1016/s0042-6822(72)80006-0. [DOI] [PubMed] [Google Scholar]
- MARVIN D. A., HOFFMANN-BERLING H. A FIBROUS DNA PHAGE (FD) AND A SPHERICAL RNA PHAGE (FR) SPECIFIC FOR MALE STRAINS OF E COLI. II. PHYSICAL CHARACTERISTICS. Z Naturforsch B. 1963 Nov;18:884–893. doi: 10.1515/znb-1963-1106. [DOI] [PubMed] [Google Scholar]
- Makino S., Woolford J. L., Jr, Tanford C., Webster R. E. Interaction of deoxycholate and of detergents with the coat protein of bacteriophage f1. J Biol Chem. 1975 Jun 10;250(11):4327–4332. [PubMed] [Google Scholar]
- Mandel G., Wickner W. Translational and post-translational cleavage of M13 procoat protein: extracts of both the cytoplasmic and outer membranes of Escherichia coli contain leader peptidase activity. Proc Natl Acad Sci U S A. 1979 Jan;76(1):236–240. doi: 10.1073/pnas.76.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M., Chrysogelos S., Griffith J. Mechanism of coliphage M13 contraction: intermediate structures trapped at low temperatures. J Virol. 1981 Dec;40(3):912–919. doi: 10.1128/jvi.40.3.912-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M., Moore M., Spremulli L., Griffith J. Coat protein conformation in M13 filaments, I-forms and spheroids. Biochem Biophys Res Commun. 1983 Apr 29;112(2):349–355. doi: 10.1016/0006-291x(83)91469-9. [DOI] [PubMed] [Google Scholar]
- Marco R., Jazwinski S. M., Kornberg A. Binding, eclipse, and penetration of the filamentous bacteriophage M13 in intact and disrupted cells. Virology. 1974 Nov;62(1):209–223. doi: 10.1016/0042-6822(74)90316-x. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Pigram W. J., Wiseman R. L., Wachtel E. J., Marvin F. J. Filamentous bacterial viruses. XIL. Molecular architecture of the class I (fd, If1, IKe) virion. J Mol Biol. 1974 Sep 25;88(3):581–598. doi: 10.1016/0022-2836(74)90409-4. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Wachtel E. J. Structure and assembly of filamentous bacterial viruses. Nature. 1975 Jan 3;253(5486):19–23. doi: 10.1038/253019a0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Wiseman R. L., Wachtel E. J. Filamentous bacterial viruses. XI. Molecular architecture of the class II (Pf1, Xf) virion. J Mol Biol. 1974 Jan 15;82(2):121–138. doi: 10.1016/0022-2836(74)90336-2. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Model P. Regulation of coliphage f1 single-stranded DNA synthesis by a DNA-binding protein. J Mol Biol. 1973 Aug 5;78(2):285–300. doi: 10.1016/0022-2836(73)90117-4. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Zinder N. D. The role of gene V protein in f1 single-strand synthesis. Virology. 1975 Dec;68(2):490–502. doi: 10.1016/0042-6822(75)90289-5. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jurnak F. A., Wang A. H., Molineux I., Rich A. Structure at 2.3 A resolution of the gene 5 product of bacteriophage fd: a DNA unwinding protein. J Mol Biol. 1979 Nov 5;134(3):379–400. doi: 10.1016/0022-2836(79)90359-0. [DOI] [PubMed] [Google Scholar]
- McPherson A., Molineux I., Rich A. Crystallization of a DNA-unwinding protein: preliminary x-ray analysis of fd bacteriophage gene 5 product. J Mol Biol. 1976 Oct 5;106(4):1077–1081. doi: 10.1016/0022-2836(76)90354-5. [DOI] [PubMed] [Google Scholar]
- McPherson A., Wang A. H., Jurnak F. A., Molineux I., Kolpak F., Rich A. X-ray diffraction studies on crystalline complexes of the gene 5 DNA-unwinding protein with deoxyoligonucleotides. J Biol Chem. 1980 Apr 10;255(7):3174–3177. [PubMed] [Google Scholar]
- McPherson A., Wang A., Jurnak F., Molineux I., Rich A. Structure of the gene 5 DNA binding protein from bacteriophage fd and its DNA binding cleft. Mol Biol Biochem Biophys. 1980;32:231–240. doi: 10.1007/978-3-642-81503-4_19. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Beyreuther K., Geider K. Recognition of two initiation codons for the synthesis of phage fd gene 2 protein. Mol Gen Genet. 1980;180(3):489–494. doi: 10.1007/BF00268051. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Bäumel I., Geider K., Bedinger P. Replication of phase fd RF with fd gene 2 protein and phage T4 enzymes. J Biol Chem. 1981 Jun 10;256(11):5810–5813. [PubMed] [Google Scholar]
- Meyer T. F., Geider K. Bacteriophage fd gene II-protein. I. Purification, involvement in RF replication, and the expression of gene II. J Biol Chem. 1979 Dec 25;254(24):12636–12641. [PubMed] [Google Scholar]
- Meyer T. F., Geider K. Bacteriophage fd gene II-protein. II. Specific cleavage and relaxation of supercoiled RF from filamentous phages. J Biol Chem. 1979 Dec 25;254(24):12642–12646. [PubMed] [Google Scholar]
- Meyer T. F., Geider K. Cloning of bacteriophage fd gene 2 and construction of a plasmid dependent on fd gene 2 protein. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5416–5420. doi: 10.1073/pnas.78.9.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Geider K. Enzymatic synthesis of bacteriophage fd viral DNA. Nature. 1982 Apr 29;296(5860):828–832. doi: 10.1038/296828a0. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Geider K., Kurz C., Schaller H. Cleavage site of bacteriophage fd gene II-protein in the origin of viral strand replication. Nature. 1979 Mar 22;278(5702):365–367. doi: 10.1038/278365a0. [DOI] [PubMed] [Google Scholar]
- Model P., McGill C., Mazur B., Fulford W. D. The replication of bacteriophage f1: gene V protein regulates the synthesis of gene II protein. Cell. 1982 Jun;29(2):329–335. doi: 10.1016/0092-8674(82)90149-0. [DOI] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Moses P. B., Boeke J. D., Horiuchi K., Zinder N. D. Restructuring the bacteriophage f1 genome: expression of gene VIII in the intergenic space. Virology. 1980 Jul 30;104(2):267–278. doi: 10.1016/0042-6822(80)90332-3. [DOI] [PubMed] [Google Scholar]
- Moses P. B., Horiuchi K. Effects of transposition and deletion upon coat protein gene expression in bacteriophage f1. Virology. 1982 Jun;119(2):231–244. doi: 10.1016/0042-6822(82)90084-8. [DOI] [PubMed] [Google Scholar]
- Moses P. B., Model P. A rho-dependent transcription termination signal in bacteriophage f1. J Mol Biol. 1984 Jan 5;172(1):1–22. doi: 10.1016/0022-2836(84)90411-x. [DOI] [PubMed] [Google Scholar]
- Müller U. R., Fitch W. M. Evolutionary selection for perfect hairpin structures in viral DNAs. Nature. 1982 Aug 5;298(5874):582–585. doi: 10.1038/298582a0. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Frangione B., Wiseman R. L., Konigsberg W. H. Primary structure of the major coat protein of the filamentous bacterial viruses, If1 and Ike. J Biol Chem. 1981 Jun 10;256(11):5792–5797. [PubMed] [Google Scholar]
- Nakashima Y., Konigsberg W. Reinvestigation of a region of the fd bacteriophage coat protein sequence. J Mol Biol. 1974 Sep 25;88(3):598–600. doi: 10.1016/0022-2836(74)90410-0. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Wiseman R. L., Konigsberg W., Marvin D. A. Primary structure and sidechain interactions of PFL filamentous bacterial virus coat protein. Nature. 1975 Jan 3;253(5486):68–71. doi: 10.1038/253068a0. [DOI] [PubMed] [Google Scholar]
- Nelson F. K., Friedman S. M., Smith G. P. Filamentous phage DNA cloning vectors: a noninfective mutant with a nonpolar deletion in gene III. Virology. 1981 Jan 30;108(2):338–350. doi: 10.1016/0042-6822(81)90442-6. [DOI] [PubMed] [Google Scholar]
- Newman J., Swinney H. L., Day L. A. Hydrodynamic properties and structure of fd virus. J Mol Biol. 1977 Nov 5;116(3):593–603. doi: 10.1016/0022-2836(77)90086-9. [DOI] [PubMed] [Google Scholar]
- Niyogi S. K., Ratrie H., 3rd, Datta A. K. Effect of Escherichia coli DNA binding protein on the transcription of single-stranded phage M13 DNA by Escherichia coli RNA polymerase. Biochem Biophys Res Commun. 1977 Sep 9;78(1):343–349. doi: 10.1016/0006-291x(77)91260-8. [DOI] [PubMed] [Google Scholar]
- Nomura N., Low R. L., Ray D. S. Identification of ColE1 DNA sequences that direct single strand-to-double strand conversion by a phi X174 type mechanism. Proc Natl Acad Sci U S A. 1982 May;79(10):3153–3157. doi: 10.1073/pnas.79.10.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Low R. L., Ray D. S. Selective cloning of Co1E1 DNA initiation sequences using the cloning vector M13 delta E101. Gene. 1982 Jun;18(3):239–246. doi: 10.1016/0378-1119(82)90161-5. [DOI] [PubMed] [Google Scholar]
- Nomura N., Ray D. S. Expression of a DNA strand initiation sequence of ColE1 plasmid in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6566–6570. doi: 10.1073/pnas.77.11.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Yamagishi H., Oka A. Isolation and characterization of transducing coliphage fd carrying a kanamycin resistance gene. Gene. 1978 Feb;3(1):39–51. doi: 10.1016/0378-1119(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Novotny C. P., Fives-Taylor P. Effects of high temperature on Escherichia coli F pili. J Bacteriol. 1978 Feb;133(2):459–464. doi: 10.1128/jb.133.2.459-464.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C. P., Fives-Taylor P. Retraction of F pili. J Bacteriol. 1974 Mar;117(3):1306–1311. doi: 10.1128/jb.117.3.1306-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C. P., Taylor P. F., Lavin K. Effects of growth inhibitors and ultraviolet irradiation on F pili. J Bacteriol. 1972 Dec;112(3):1083–1089. doi: 10.1128/jb.112.3.1083-1089.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Chamberlain B. K., Webster R. E., Tanford C. Evidence for a major conformational change of coat protein in assembly of fl bacteriophage. Nature. 1976 Jan 29;259(5541):335–337. doi: 10.1038/259335a0. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Reynolds J. A., Tanford C. Conformational states of a hydrophobic protein. The coat protein of fd bacteriophage. Biochemistry. 1978 Apr 4;17(7):1239–1246. doi: 10.1021/bi00600a017. [DOI] [PubMed] [Google Scholar]
- Oey J. L., Knippers R. Properties of the isolated gene 5 protein of bacteriophage fd. J Mol Biol. 1972 Jul 14;68(1):125–138. doi: 10.1016/0022-2836(72)90268-9. [DOI] [PubMed] [Google Scholar]
- Ohkawa I., Webster R. E. The orientation of the major coat protein of bacteriophage f1 in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1981 Oct 10;256(19):9951–9958. [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Wickner W. Reconstitution of rapid and asymmetric assembly of M13 procoat protein into liposomes which have bacterial leader peptidase. J Biol Chem. 1983 Feb 10;258(3):1895–1900. [PubMed] [Google Scholar]
- Okamoto T., Sugimoto K., Sugisaki H., Takanami M. Studies on bacteriophage fd DNA. II. Localization of RNA initiation sites on the cleavage map of the fd genome. J Mol Biol. 1975 Jun 15;95(1):33–44. doi: 10.1016/0022-2836(75)90333-2. [DOI] [PubMed] [Google Scholar]
- Olsen W. L., Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M13: specificity of the Escherichia coli dnaB function for replication of double-stranded M13 DNA. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2570–2573. doi: 10.1073/pnas.69.9.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y. "Phospholipids of virus-induced membranes in cytoplasm of Escherichia coli. J Bacteriol. 1971 Sep;107(3):918–925. doi: 10.1128/jb.107.3.918-925.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opella S. J., Cross T. A., DiVerdi J. A., Sturm C. F. Nuclear magnetic resonance of the filamentous bacteriophage fd. Biophys J. 1980 Oct;32(1):531–548. doi: 10.1016/S0006-3495(80)84988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R., Konigsberg W. Photochemical cross-linking of the gene 5 protein.fd DNA complex from fd-infected cells. J Biol Chem. 1982 Feb 10;257(3):1462–1467. [PubMed] [Google Scholar]
- Paradiso P. R., Nakashima Y., Konigsberg W. Photochemical cross-linking of protein . nucleic acid complexes. The attachment of the fd gene 5 protein to fd DNA. J Biol Chem. 1979 Jun 10;254(11):4739–4744. [PubMed] [Google Scholar]
- Peeters B. P., Konings R. N., Schoenmakers J. G. Characterization of the DNA binding protein encoded by the N-specific filamentous Escherichia coli phage IKe. Binding properties of the protein and nucleotide sequence of the gene. J Mol Biol. 1983 Sep 5;169(1):197–215. doi: 10.1016/s0022-2836(83)80180-6. [DOI] [PubMed] [Google Scholar]
- Peeters B. P., Peters R. M., Schoenmakers J. G., Konings R. N. Nucleotide sequence and genetic organization of the genome of the N-specific filamentous bacteriophage IKe. Comparison with the genome of the F-specific filamentous phages M13, fd and f1. J Mol Biol. 1985 Jan 5;181(1):27–39. doi: 10.1016/0022-2836(85)90322-5. [DOI] [PubMed] [Google Scholar]
- Peeters B. P., Schoenmakers J. G., Konings R. N. Plasmid pKUN9, a versatile vector for the selective packaging of both DNA strands into single-stranded DNA-containing phage-like particles. Gene. 1986;41(1):39–46. doi: 10.1016/0378-1119(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- Pretorius H. T., Klein M., Day L. A. Gene V protein of fd bacteriophage. Dimer formation and the role of tyrosyl groups in DNA binding. J Biol Chem. 1975 Dec 25;250(24):9262–9269. [PubMed] [Google Scholar]
- Putterman D. G., Casadevall A., Boyle P. D., Yang H. L., Frangione B., Day L. A. Major coat protein and single-stranded DNA-binding protein of filamentous virus Pf3. Proc Natl Acad Sci U S A. 1984 Feb;81(3):699–703. doi: 10.1073/pnas.81.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasched I., Pohl F. M. Oligonucleotides and the quaternary structure of gene-5 protein from filamentous bacteriophage. FEBS Lett. 1974 Sep 15;46(1):115–118. doi: 10.1016/0014-5793(74)80347-9. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Horiuchi K., Zinder N. D. Nucleotide sequence of the recognition site for the restriction-modification enzyme of Escherichia coli B. Proc Natl Acad Sci U S A. 1978 May;75(5):2266–2270. doi: 10.1073/pnas.75.5.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Horiuchi K., Zinder N. D. Nucleotide sequences near the origin of replication of bacteriophage f1. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4219–4222. doi: 10.1073/pnas.74.10.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Ohsumi M., Model P., Vovis G. F., Fischhoff D., Zinder N. D. Organization of a hybrid between phage f1 and plasmid pSC101. Proc Natl Acad Sci U S A. 1979 May;76(5):2195–2198. doi: 10.1073/pnas.76.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S., Hines J. C., Kim M. H., Imber R., Nomura N. M13 vectors for selective cloning of sequences specifying initiation of DNA synthesis on single-stranded templates. Gene. 1982 Jun;18(3):231–238. doi: 10.1016/0378-1119(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Kook K. Insertion of the Tn3 transposon into the genome of the single-stranded DNA phage M13. Gene. 1978 Oct;4(2):109–119. doi: 10.1016/0378-1119(78)90024-0. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. IV. Synthesis of M13-specific DNA in the presence of chloramphenicol. J Mol Biol. 1970 Oct 28;53(2):239–250. doi: 10.1016/0022-2836(70)90297-4. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. 3. Identification of the intracellular single-straned DNA. J Mol Biol. 1969 Aug 14;43(3):645–647. doi: 10.1016/0022-2836(69)90365-9. [DOI] [PubMed] [Google Scholar]
- Rivera M. J., Smits M. A., Quint W., Schoenmakers J. G., Konings R. N. Expression of bacteriophage M13 DNA in vivo. Localization of the transcription initiation and termination signal of the mRNA coding for the major capsid protein. Nucleic Acids Res. 1978 Aug;5(8):2895–2912. doi: 10.1093/nar/5.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando E. F., Bladen H. A. Physical changes associated with heating bacteriophage f1. Virology. 1969 Dec;39(4):921–924. doi: 10.1016/0042-6822(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Rossomando E. F. Studies on the structural polarity of bacteriophage f1. Virology. 1970 Nov;42(3):681–687. doi: 10.1016/0042-6822(70)90313-2. [DOI] [PubMed] [Google Scholar]
- Rossomando E. F., Zinder N. D. Studies on the bacteriophage fl. I. Alkali-induced disassembly of the phage into DNA and protein. J Mol Biol. 1968 Sep 28;36(3):387–399. doi: 10.1016/0022-2836(68)90163-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R., Wu R. Modification of the bacteriophage vector M13mp2: introduction of new restriction sites for cloning. Gene. 1981 Nov;15(2-3):167–176. doi: 10.1016/0378-1119(81)90126-8. [DOI] [PubMed] [Google Scholar]
- Roy A., Mitra S. Increased fragility of Escherichia coli after infection with bacteriophage M13. J Virol. 1970 Sep;6(3):333–339. doi: 10.1128/jvi.6.3.333-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. A bacterial gene, fip, required for filamentous bacteriophage fl assembly. J Bacteriol. 1983 Jun;154(3):1064–1076. doi: 10.1128/jb.154.3.1064-1076.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. A mutation downstream from the signal peptidase cleavage site affects cleavage but not membrane insertion of phage coat protein. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1717–1721. doi: 10.1073/pnas.78.3.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salstrom J. S., Pratt D. Role of coliphage M13 gene 5 in single-stranded DNA production. J Mol Biol. 1971 Nov 14;61(3):489–501. doi: 10.1016/0022-2836(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Uhlmann A., Geider K. A DNA fragment from the origin of single-strand to double-strand DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1976 Jan;73(1):49–53. doi: 10.1073/pnas.73.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck P. K., Staudenbauter W. L., Hofschneider P. H. Replication of bacteriophage M-13. Template specific inhibition of DNA synthesis by nalidixic acid. Eur J Biochem. 1973 Sep 21;38(1):130–136. doi: 10.1111/j.1432-1033.1973.tb03042.x. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Schwartz F. M., Zinder N. D. Morphological changes in Escherichia coli infected with the DNA bacteriophage fl. Virology. 1968 Feb;34(2):352–355. doi: 10.1016/0042-6822(68)90246-8. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Segawa K., Ikehara K., Okada Y. Isolation and chemcial properties of A-protein from filamentous phage Fd. J Biochem. 1975 Jul;78(1):1–7. [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Psoralen-crosslinked secondary structure map of single-stranded virus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2649–2653. doi: 10.1073/pnas.73.8.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Ikoku A., Hearst J. E. A specific DNA orientation in the filamentous bacteriophage fd as probed by psoralen crosslinking and electron microscopy. J Mol Biol. 1979 Jan 15;127(2):163–175. doi: 10.1016/0022-2836(79)90237-7. [DOI] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P., Watts C., Wickner W. Membrane assembly from purified components. I. Isolated M13 procoat does not require ribosomes or soluble proteins for processing by membranes. Cell. 1981 Aug;25(2):341–345. doi: 10.1016/0092-8674(81)90052-0. [DOI] [PubMed] [Google Scholar]
- Silver P., Wickner W. T. Role of M13 gene 1 protein in filamentous virus assembly. J Virol. 1980 Jul;35(1):256–258. doi: 10.1128/jvi.35.1.256-258.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons G. F., Konings R. N., Schoenmakers J. G. Genes VI, VII, and IX of phage M13 code for minor capsid proteins of the virion. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4194–4198. doi: 10.1073/pnas.78.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons G. F., Konings R. N., Schoenmakers J. G. Identification of two new capsid proteins in bacteriophage M13. FEBS Lett. 1979 Oct 1;106(1):8–12. doi: 10.1016/0014-5793(79)80683-3. [DOI] [PubMed] [Google Scholar]
- Simons G. F., Veeneman G. H., Konings R. N., van Boom J. H., Schoemakers J. G. Oligonucleotide-directed mutagenesis of gene IX of bacteriophage M13. Nucleic Acids Res. 1982 Feb 11;10(3):821–832. doi: 10.1093/nar/10.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H. Bacteriophage f1 infection and colicin tolerance. J Virol. 1974 Jan;13(1):100–106. doi: 10.1128/jvi.13.1.100-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H. Bacteriophage f1 infection: fate of the parental major coat protein. J Virol. 1974 Jan;13(1):94–99. doi: 10.1128/jvi.13.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985 Jun 14;228(4705):1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Smith B. A., McConnell H. M. Lateral diffusion of M-13 coat protein in model membranes. Biochemistry. 1979 May 29;18(11):2256–2259. doi: 10.1021/bi00578a019. [DOI] [PubMed] [Google Scholar]
- Smits M. A., Jansen J., Konings R. N., Schoenmakers J. G. Initiation and termination signals for transcription in bacteriophage M13. Nucleic Acids Res. 1984 May 25;12(10):4071–4081. doi: 10.1093/nar/12.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M. A., Schoenmakers J. G., Konings R. N. Expression of bacteriophage M13 DNA in vivo. Isolation, identification and characterization of phage-specific mRNA species. Eur J Biochem. 1980 Nov;112(2):309–321. doi: 10.1111/j.1432-1033.1980.tb07206.x. [DOI] [PubMed] [Google Scholar]
- Smits M. A., Simons G., Konings R. N., Schoenmakers J. G. Expression of bacteriophage M13 dna in vivo. I. Synthesis of phage-specific RNA and protein in minicells. Biochim Biophys Acta. 1978 Nov 21;521(1):27–44. doi: 10.1016/0005-2787(78)90246-0. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M 13. Mechanism of single-strand DNA synthesis in an escherichia coli mutant thermosensitive in chromosomal DNA replication. Eur J Biochem. 1972 Nov 7;30(3):403–412. doi: 10.1111/j.1432-1033.1972.tb02111.x. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M-13. Positive role of gene-5 protein in single-strand-DNA synthesis. Eur J Biochem. 1973 May 2;34(3):569–576. doi: 10.1111/j.1432-1033.1973.tb02797.x. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Involvement of DNA polymerases I and III in the replication of bacteriophage M-13. Eur J Biochem. 1974 Nov 1;49(1):249–256. doi: 10.1111/j.1432-1033.1974.tb03829.x. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Olsen W. L., Hofschneider P. H. Analysis of bacteriophage-M 13-DNA replication in an Escherichia coli mutant thermosensitive in DNA polymerase 3. Eur J Biochem. 1973 Jan 15;32(2):247–253. doi: 10.1111/j.1432-1033.1973.tb02604.x. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Ray D. S. Replication of bacteriophage M13. XI. Localization of the origin for M13 single-strand synthesis. J Mol Biol. 1977 Feb 15;110(1):147–163. doi: 10.1016/s0022-2836(77)80103-4. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. III. Nucleotide sequence preceding the RNA start-site on a promoter-containing fragment. Nucleic Acids Res. 1975 Nov;2(11):2091–2100. doi: 10.1093/nar/2.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. P., Webster R. E. fii, a bacterial locus required for filamentous phage infection and its relation to colicin-tolerant tolA and tolB. J Bacteriol. 1986 Jan;165(1):107–115. doi: 10.1128/jb.165.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Takanami M. Specific cleavage of coliphage fd DNA by five different restriction endonucleases from Haemophilus genus. FEBS Lett. 1973 Aug 15;34(2):318–322. doi: 10.1016/0014-5793(73)80821-x. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Day L. A. Structure similarity, difference and variability in the filamentous viruses fd, If1, IKe, Pf1 and Xf. Investigation by laser Raman spectroscopy. J Mol Biol. 1983 Apr 5;165(2):321–356. doi: 10.1016/s0022-2836(83)80260-5. [DOI] [PubMed] [Google Scholar]
- Torbet J., Gray D. M., Gray C. W., Marvin D. A., Siegrist H. Structure of the fd DNA--gene 5 protein complex in solution. A neutron small-angle scattering study. J Mol Biol. 1981 Mar 5;146(3):305–320. doi: 10.1016/0022-2836(81)90390-9. [DOI] [PubMed] [Google Scholar]
- Trenkner E., Bonhoeffer F., Gierer A. The fate of the protein component of bacteriophage fd during infection. Biochem Biophys Res Commun. 1967 Sep 27;28(6):932–939. doi: 10.1016/0006-291x(67)90069-1. [DOI] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Pennings L., Schoenmakers J. G. Restriction-enzyme-cleavage maps of bacteriophage M13. Existence of an intergenic region on the M13 genome. Eur J Biochem. 1976 Sep;68(1):55–70. doi: 10.1111/j.1432-1033.1976.tb10764.x. [DOI] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Schoenmakers J. G. Cleavage maps of the filamentous bacteriophages M13, fd, fl, and ZJ/2. J Virol. 1976 Jun;18(3):1024–1039. doi: 10.1128/jvi.18.3.1024-1039.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Schoenmakers J. G. Studies on bacteriophage M13 DNA. 1. A cleavage map of the M13 genome. Eur J Biochem. 1975 May 6;53(2):547–558. doi: 10.1111/j.1432-1033.1975.tb04098.x. [DOI] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Weijers A., Konings R. N., Schoenmakers J. G. Studies on bacteriophage M13 DNA. 2. The gene order of the M13 genome. Eur J Biochem. 1975 May 6;53(2):559–567. doi: 10.1111/j.1432-1033.1975.tb04099.x. [DOI] [PubMed] [Google Scholar]
- Vicuna R., Hurwitz J., Wallace S., Girard M. Selective inhibition of in vitro DNA synthesis dependent on phiX174 compared with fd DNA. I. Protein requirements for selective inhibition. J Biol Chem. 1977 Apr 25;252(8):2524–2533. [PubMed] [Google Scholar]
- Vovis G. F., Horiuchi K., Zinder N. D. Endonuclease R-EcoRII restriction of bacteriophage f1 DNA in vitro: ordering of genes V and VII, location of an RNA promotor for gene VIII. J Virol. 1975 Sep;16(3):674–684. doi: 10.1128/jvi.16.3.674-684.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel E. J., Wiseman R. L., Pigram W. J., Marvin D. A. Filamentous bacterial viruses. XIII. Molecular structure of the virion in projection. J Mol Biol. 1974 Sep 25;88(3):601–618. doi: 10.1016/0022-2836(74)90411-2. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., Silver P., Wickner W. Membrane assembly from purified components. II. Assembly of M13 procoat into liposomes reconstituted with purified leader peptidase. Cell. 1981 Aug;25(2):347–353. doi: 10.1016/0092-8674(81)90053-2. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Cashman J. S. Abortive infection of Escherichia coli with the bacteriophage f1: cytoplasmic membrane proteins and the f1 DNA-gene 5 protein complex. Virology. 1973 Sep;55(1):20–38. doi: 10.1016/s0042-6822(73)81005-0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Grant R. A., Hamilton L. A. Orientation of the DNA in the filamentous bacteriophage f1. J Mol Biol. 1981 Oct 25;152(2):357–374. doi: 10.1016/0022-2836(81)90247-3. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Rementer M. Replication of bacteriophage f1: a complex containing gene II protein in gene V mutant-infected bacteria. J Mol Biol. 1980 May 25;139(3):393–405. doi: 10.1016/0022-2836(80)90137-0. [DOI] [PubMed] [Google Scholar]
- Wickner W. T. Role of hydrophobic forces in membrane protein asymmetry. Biochemistry. 1977 Jan 25;16(2):254–258. doi: 10.1021/bi00621a015. [DOI] [PubMed] [Google Scholar]
- Wickner W. Assembly of proteins into membranes. Science. 1980 Nov 21;210(4472):861–868. doi: 10.1126/science.7001628. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4749–4753. doi: 10.1073/pnas.72.12.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Ito K., Mandel G., Bates M., Nokelainen M., Zwizinski C. The three lives of M13 coat protein: a virion capsid, an integral membrane protein, and a soluble cytoplasmic proprotein. Ann N Y Acad Sci. 1980;343:384–390. doi: 10.1111/j.1749-6632.1980.tb47267.x. [DOI] [PubMed] [Google Scholar]
- Wickner W., Killick T. Membrane-associated assembly of M13 phage in extracts of virus-infected Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):505–509. doi: 10.1073/pnas.74.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Kornberg A. A holoenzyme form of deoxyribonucleic acid polymerase III. Isolation and properties. J Biol Chem. 1974 Oct 10;249(19):6244–6249. [PubMed] [Google Scholar]
- Wickner W., Kornberg A. A novel form of RNA polymerase from Escherichia coli. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4425–4428. doi: 10.1073/pnas.71.11.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Mandel G., Zwizinski C., Bates M., Killick T. Synthesis of phage M13 coat protein and its assembly into membranes in vitro. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1754–1758. doi: 10.1073/pnas.75.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Dunker A. K. Circular dichroism studies of fd coat protein in membrane vesicles. J Biol Chem. 1977 Sep 25;252(18):6253–6255. [PubMed] [Google Scholar]
- Wiseman R. L., Berkowitz S. A., Day L. A. Different arrangements of protein subunits and single-stranded circular DNA in the filamentous bacterial viruses fd and Pf1. J Mol Biol. 1976 Apr 15;102(3):549–561. doi: 10.1016/0022-2836(76)90333-8. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Silver P., Wickner W. The isolation of homogeneous leader peptidase from a strain of Escherichia coli which overproduces the enzyme. J Biol Chem. 1982 Jul 10;257(13):7898–7902. [PubMed] [Google Scholar]
- Woolford J. L., Jr, Cashman J. S., Webster R. E. F1 Coat protein synthesis and altered phospholipid metabolism in f1 infected Escherichia coli. Virology. 1974 Apr;58(2):544–560. doi: 10.1016/0042-6822(74)90088-9. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Steinman H. M., Webster R. E. Adsorption protein of bacteriophage fl: solubilization in deoxycholate and localization in the fl virion. Biochemistry. 1977 Jun 14;16(12):2694–2700. doi: 10.1021/bi00631a017. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Webster R. E. Proteolytic digestion of the micellar complex of f1 coat protein and deoxycholate. J Biol Chem. 1975 Jun 10;250(11):4333–4339. [PubMed] [Google Scholar]
- Yamamoto M., Kanegasaki S., Yoshikawa M. Role of membrane potential and ATP in complex formation between Escherichia coli male cells and filamentous phage fd. J Gen Microbiol. 1981 Apr;123(2):343–349. doi: 10.1099/00221287-123-2-343. [DOI] [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Bacteriophage f1 gene II and X proteins. Isolation and characterization of the products of two overlapping genes. J Biol Chem. 1981 Nov 10;256(21):11259–11265. [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Translational control of bacteriophage f1 gene II and gene X proteins by gene V protein. Cell. 1982 Jun;29(2):337–345. doi: 10.1016/0092-8674(82)90150-7. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D., VALENTINE R. C., ROGER M., STOECKENIUS W. F1, A ROD-SHAPED MALE-SPECIFIC BACTERIOPHAGE THAT CONTAINS DNA. Virology. 1963 Aug;20:638–640. doi: 10.1016/0042-6822(63)90290-3. [DOI] [PubMed] [Google Scholar]
- Zacher A. N., 3rd, Stock C. A., Golden J. W., 2nd, Smith G. P. A new filamentous phage cloning vector: fd-tet. Gene. 1980 Apr;9(1-2):127–140. doi: 10.1016/0378-1119(80)90171-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Watts C., Wickner W. The biosynthesis of membrane-bound M13 coat protein. Energetics and assembly intermediates. J Biol Chem. 1982 Jun 10;257(11):6529–6536. [PubMed] [Google Scholar]
- Zimmermann R., Wickner W. Energetics and intermediates of the assembly of Protein OmpA into the outer membrane of Escherichia coli. J Biol Chem. 1983 Mar 25;258(6):3920–3925. [PubMed] [Google Scholar]
- Zinder N. D., Boeke J. D. The filamentous phage (Ff) as vectors for recombinant DNA--a review. Gene. 1982 Jul-Aug;19(1):1–10. doi: 10.1016/0378-1119(82)90183-4. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Horiuchi K. Multiregulatory element of filamentous bacteriophages. Microbiol Rev. 1985 Jun;49(2):101–106. doi: 10.1128/mr.49.2.101-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder N. D. Resistance to colicins E3 and K induced by infection with bacteriophage f1. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3160–3164. doi: 10.1073/pnas.70.11.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Date T., Wickner W. Leader peptidase is found in both the inner and outer membranes of Escherichia coli. J Biol Chem. 1981 Apr 10;256(7):3593–3597. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Studies of asymmetric membrane assembly. Biochim Biophys Acta. 1977 Dec 1;471(2):169–176. doi: 10.1016/0005-2736(77)90247-4. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]


