Abstract
Natural remedies from medicinal plants are considered to be effective and safe alternative treatment for diabetes mellitus. The aim of the present study was to investigate the hypoglycemic activity of the crude tea leaves extract on streptozotocin (STZ)-induced diabetic mice. The average body weight of animals with diabetes and their percentage changes of body weight gain after 15 and 30 days were significantly lower than that of the normal control mice. In diabetic mice, supplementation with tea leaves extract decreased the loss of body weight. After 15 and 30 days, significant increases in the levels of serum glucose, triglycerides, cholesterol, creatinine, urea, uric acid, glutamic pyruvic acid transaminase (GPT) and glutamic oxaloacetic acid transaminase (GOT) were noted in STZ-diabetic mice fed with normal diet. Also, the values of total protein in this group were statistically declined after 15 and 30 days. The levels of serum glucose and GPT were significantly elevated after 15 and 30 days in diabetic mice supplemented with tea leaves extract. Moreover, the level of serum GOT was notably increased after 30 days. Insignificant alterations were observed in the levels of serum triglycerides, cholesterol, total protein, creatinine, urea and uric acid in diabetic mice supplemented with tea leaves extract. Thus, the present results have shown that tea leaves extract has the antihyperglycemic, antihyperlipidemic, and antihyperproteinemic effects and consequently may alleviate liver and kidney damage associated with STZ-induced diabetes in mice.
Keywords: Streptozotocin, Diabetes, Tea leaves, Body weight, Serum chemistry, Mice
1. Introduction
Diabetes mellitus is an increasingly common, potentially devastating, expensive, treatable but incurable life long disease. Globally, the estimated incidence of diabetes mellitus and projection for year 2010, as given by International Diabetes Federation is 239 million (Gandhi, 2001). Diabetes mellitus is a chronic metabolic disorder of multiple aetiologies, characterized by a state of insulin deficiency that leads to a rise in glycemia (Gupta et al., 2005), initially involving changes in carbohydrate metabolism and secondarily of lipids and proteins (Fontes, 2002, Negri, 2005). The most common symptoms observed in type I diabetes patients are polydipsia, polyuria, glycosuria, weakness with no apparent cause, and slow healing of wounds (Fontes, 2002). The levels of glycemia and insulinemia must be controlled in order to avoid later complications of diabetes, such as atherosclerosis, hypertension, hypertriglyceridemia, hypercholesterolemia, myocardial infarction, ischemic attacks, impotence, retinopathy and nephropathy (Stadler et al., 2003). As yet there are no effective therapies to cure diabetes (Maiti et al., 2004). Many hypoglycemiant agents, such as the biguanides and sulfonylureas, are used alone or together with insulin to treat this disease. However, these medications can cause serious side effects (Hwang et al., 2005), motivating a search for safer, more efficacious agents to control diabetes.
In recent years, interest has increased in using natural products for pharmacological purposes, as a form of complementary or replacement therapy. Particularly in the case of diabetes, published reports show that numerous extracts obtained from plants are effective in reducing glycemia, causing fewer side effects and with lower cost than the usual antidiabetic agents (Pushparaj et al., 2000, Gupta et al., 2005, Lee and Sohn, 2009, Sohn et al., 2010). The majority of the plants that are used in popular medicine for treatment of diabetes have been shown to possess biologically active chemical constituents (alkaloids, carbohydrates, cumarins, flavonoids, terpenoids, phenolic substances, and other constituents) that can be used as new hypoglycemiant agents (Marles and Fansworth, 1995, Gupta et al., 2005, Negri, 2005). Tea is one of the most widely consumed beverages in the world, second only to water, and its medicinal properties have been widely explored. The tea plant, Camellia sinensis, is a member of the Theaceae family, and black, oolong, and green tea are produced from its leaves. It is an evergreen shrub or tree and can grow to heights of 30 feet, but is usually pruned to 2–5 feet for cultivation. The leaves are dark green, alternate and oval, with serrated edges, and the blossoms are white, fragrant, and appear in clusters or singly. Unlike black and oolong tea, green tea production does not involve oxidation of young tea leaves. Black tea, however, undergoes fermentation, while green tea does not (Hamilton-miller, 1995). Green tea is produced from steaming fresh leaves at high temperatures, thereby inactivating the oxidizing enzymes and leaving the polyphenol content intact. The polyphenols found in tea are more commonly known as flavonols or catechins, and comprise 30–40 percent of the extractable solids of dried green tea leaves. The main catechins in green tea are epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatechin-3-gallate (EGCG), with the latter being the highest in concentration. Green tea polyphenols have demonstrated significant antioxidant, anticarcinogenic, anti-inflammatory, thermogenic, probiotic, and antimicrobial properties in numerous human, animal, and in vitro studies (Graham, 1992, Alschuler, 1998). Therefore, the present study was undertaken to evaluate the effectiveness of the crude leaves extract of Camellia sinensis as antihyperglycemic agents in streptozotocin (STZ)-induced diabetic mice.
2. Materials and methods
2.1. Collection of tea leaves and preparation of the extract
Fresh tea leaves were directly collected from the tea plantation farms in Cameron Highlands, Malaysia. The leaves were air-dried at room temperature and stored in dry plastic container until use for extract preparation. The leaves extract was prepared every three days. The dried leaves (5 g) were powdered in an electric blender, put in one liter boiling water for 5 min and the mixture then left to cool to room temperature. The mixture was filtered and the resulting extract was kept in refrigerator until use in the experiment.
2.2. Animals and experimental protocol
Male albino mice of MF1 strain, weighing 26.4–32.2 g were used in this study. The experimental animals were obtained from the Experimental Animal Unit of King Fahd Medical Research Center, King Abdul Aziz University, Jeddah, Saudi Arabia. The experimental animals were housed in standard plastic cages and maintained under controlled laboratory conditions of humidity (65%), temperature (20 ± 1 °C) and 12:12 h light: dark cycle. Mice were fed ad libitum on normal commercial chow and had free access to water. For the experimental induction of diabetes, STZ (Sigma–Aldrich Corp, St. Louis, MO, USA) was freshly prepared by dissolving in 0.9% NaCl before administration. A single dose of 60 mg/kg STZ was injected intraperitoneally to mice. Five days after STZ injection, only mice with blood glucose concentration higher than 310 mg/dL (at fasting state) were considered as diabetic and included in the present study. A total of 60 mice were categorized into 4 groups, each group consisting of 15 mice. Group 1 was normal healthy control, intraperitoneally received saline solution. Group 2 was diabetic control. Group 3 was diabetic mice, administered orally with tea leaves extract at a dose of 0.5 mL/day. Group 4 was non diabetic mice, intraperitoneally received saline solution and treated with tea leaves extract at the same dose given to group 3. The body weights of mice were measured at the start of the experimental period, after 15 and 30 days, using a digital balance. These weights were determined at the same time during the morning. After 15 and 30 days of treatment, mice were anaesthetized with diethyl ether, the blood samples were collected from orbital venous plexus after overnight fasting and it was used for separating the serum for analyzing the biochemical parameters. Serum glucose, triglycerides, cholesterol, creatinine, urea, uric acid, glutamic pyruvic acid transaminase (GPT) and glutamic oxaloacetic acid transaminase (GOT) were measured using an automatic analyzer (Reflotron® Plus System, Roche, Germany). Serum total protein was estimated using Automated Clinical Chemistry Analysis System, Dimension® type RXL Max (Dade Behring Delaware, DE 19714, USA).
2.3. Statistical analysis
Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Tests (DMRT). All values were expressed as mean with their standard deviation (SD). P values <0.05 were considered as significant.
3. Results
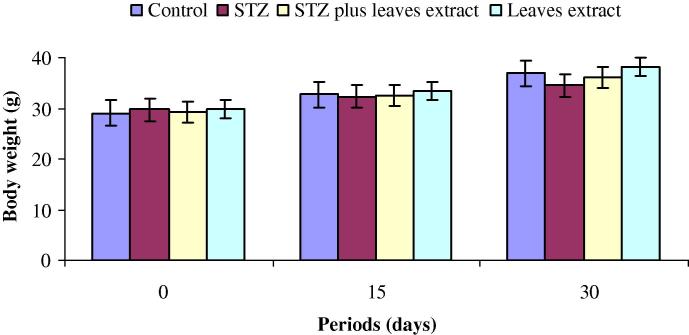
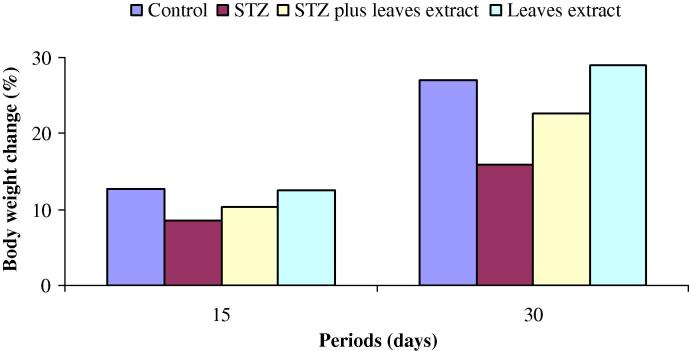
The body weights in all experimental groups are represented in Fig. 1. The percentage changes of body weight gain in control mice were 12.7% after 15 days and 27.0% after 30 days (Fig. 2). Likewise, the changes of body weight gain were 12.5% and 28.9% in fourth group after 15 and 30 days respectively. The average of body weight of animals with diabetes (group 2) and their body weight gain (8.7% and 16.1%) after 15 and 30 days were significantly lower than that of the normal control mice (Figure 1, Figure 2). In diabetic mice, supplementation of tea leaves extract decreased the body weight loss. The changes of body weight gain were 10.4% and 22.7% after 15 and 30 days respectively in third group (Fig. 2).
Figure 1.
Body weights (mean ± SD) at 0, 15 and 30 days in groups 1 (control), 2 (STZ), 3 (STZ plus tea leaves extract) and 4 (tea leaves extract).
Figure 2.
Percentage changes of body weight at 15 and 30 days in groups 1 (control), 2 (STZ), 3 (STZ plus tea leaves extract) and 4 (tea leaves extract).
After 15 and 30 days, significant increases in the levels of serum glucose (336.3% and 453.5%), triglycerides (38.6% and 45.4%), cholesterol (33.4% and 55.4%), creatinine (55.0% and 81.6%), urea (23.2% and 46.5%), uric acid (16.8% and 58.6%), GPT (29.6% and 58.6%) and GOT (38.7% and 82.6%) were noted in STZ-diabetic mice fed with normal diet (Table 1, Table 2). Also, the values of total protein in this group were statistically declined after 15 (8.4%) and 30 days (18.6%). In third group, the levels of serum glucose (249.5% and 282.6%) and GPT (19.5% and 23.8%) were significantly elevated after 15 and 30 days. The level of serum GOT was notably increased (15.3%) after 30 days in diabetic mice supplemented with tea leaves extract. Insignificant alterations were observed in the levels of serum triglycerides, cholesterol, total protein, creatinine, urea and uric acid in diabetic mice supplemented with tea leaves extract. The values of all serum biochemical parameters were remarkably unchanged in normal mice supplemented with tea leaves extract.
Table 1.
Hematobiochemical values (mean ± SD) of control, STZ, STZ plus tea leaves extract and tea leaves extract treated mice after 15 and 30 days.
| Parameters | Periods (days) | Treatments |
|||
|---|---|---|---|---|---|
| Control | STZ | STZ + leaves extract | Leaves extract | ||
| Glucose (mg/dL) | 15 | 77.86 ± 3.89 | 339.71 ± 34.30a, b | 272.14 ± 32.42a, c | 75.29 ± 4.61 |
| 30 | 76.14 ± 4.85 | 421.43 ± 15.13a, b | 291.29 ± 14.85a, c | 76.28 ± 2.75 | |
| Triglycerides (mg/dL) | 15 | 122.57 ± 8.62 | 169.86 ± 13.31a, b | 128.86 ± 5.31 | 126.71 ± 6.42 |
| 30 | 129.71 ± 4.89 | 188.57 ± 13.39a, b | 134.29 ± 12.66 | 132.29 ± 3.55 | |
| Cholesterol (mg/dL) | 15 | 83.43 ± 5.03 | 111.29 ± 9.12a, b | 85.86 ± 9.37 | 81.43 ± 4.79 |
| 30 | 81.06 ± 1.03 | 125.94 ± 5.45a, b | 80.27 ± 2.46 | 79.87 ± 2.24 | |
| Total protein (g/dL) | 15 | 6.69 ± 0.50 | 6.13 ± 0.21a, b | 6.51 ± 0.31 | 6.60 ± 0.27 |
| 30 | 7.17 ± 0.26 | 5.84 ± 0.14a, b | 7.20 ± 0.256 | 7.06 ± 0.31 | |
| Creatinine (mg/dL) | 15 | 0.40 ± 0.04 | 0.62 ± 0.05a, b | 0.38 ± 0.06 | 0.41 ± 0.03 |
| 30 | 0.38 ± 0.02 | 0.69 ± 0.03a, b | 0.38 ± 0.03 | 0.36 ± 0.05 | |
| Urea (mg/dL) | 15 | 18.24 ± 1.05 | 22.47 ± 1.61a,b | 18.06 ± 1.05 | 17.50 ± 1.51 |
| 30 | 17.09 ± 0.94 | 25.04 ± 5.33a | 18.27 ± 1.58 | 17.20 ± 0.95 | |
| Uric acid (mg/dL) | 15 | 3.46 ± 0.34 | 4.04 ± 0.37a, b | 3.36 ± 0.34 | 3.45 ± 0.20 |
| 30 | 3.89 ± 0.37 | 4.59 ± 0.47a, b | 3.86 ± 0.39 | 3.74 ± 0.40 | |
| GPT (U/L) | 15 | 22.71 ± 2.29 | 29.43 ± 3.82a, b | 27.14 ± 2.6a, c | 23.14 ± 2.19 |
| 30 | 20.50 ± 0.91 | 32.51 ± 1.47a, b | 25.37 ± 0.93a, c | 21.19 ± 2.07 | |
| GOT (U/L) | 15 | 43.57 ± 3.21 | 60.43 ± 5.00a, b | 41.43 ± 2.57 | 40.29 ± 2.81 |
| 30 | 45.26 ± 2.60 | 82.63 ± 4.18a, b | 52.19 ± 6.11a, c | 44.14 ± 2.38 | |
Indicates a significant difference between control and treated groups.
Indicates a significant difference between mice exposed to STZ and STZ plus tea leaves extract or tea leaves extract.
Indicates a significant difference between mice exposed to STZ plus tea leaves extract and tea leaves extract.
Table 2.
Percentage changes of hematobiochemical values of STZ, STZ plus tea leaves extract and tea leaves extract treated mice after 15 and 30 days compared with control values.
| Parameters | Periods (days) | Treatments |
||
|---|---|---|---|---|
| STZ | STZ + leaves extract | Leaves extract | ||
| Glucose | 15 | +336.3 | +249.5 | −3.3 |
| 30 | +453.5 | +282.6 | +0.2 | |
| Triglycerides | 15 | +38.6 | +5.13 | +3.4 |
| 30 | +45.4 | +3.5 | +2.0 | |
| Cholesterol | 15 | +33.4 | +2.9 | −2.4 |
| 30 | +55.4 | −1.0 | −1.5 | |
| Total protein | 15 | −8.4 | −2.7 | −1.4 |
| 30 | −18.6 | +0.4 | −1.5 | |
| Creatinine | 15 | +55.0 | −5.0 | +2.5 |
| 30 | +81.6 | 0.0 | −5.3 | |
| Urea | 5 | +23.2 | −1.0 | −4.1 |
| 30 | +46.5 | +6.9 | +0.6 | |
| Uric acid | 15 | +16.8 | −0.8 | −0.3 |
| 30 | +58.6 | −0.8 | −3.9 | |
| GPT | 15 | +29.6 | +19.5 | +1.9 |
| 30 | +58.6 | +23.8 | +3.4 | |
| GOT | 15 | +38.7 | −4.9 | −7.5 |
| 30 | +82.6 | +15.3 | −2.5 | |
4. Discussion
Diabetes mellitus is associated with several complications such as atherosclerosis, myocardial infarction, nephropathy, etc. (Pushparaj et al., 2007). These complications are usually related to chronically elevated blood glucose level. Strepotozotocin has been used as a diabetogenic agent in experimental animals. The present investigation demonstrated that STZ induced significant decreases in body weight gain of mice after 15 and 30 days. Similar observations were detected in many experimental studies (Satav and Katyare, 2004, Kavalali et al., 2007, Sridevi et al., 2007, Zari and Al-Attar, 2007, Subash-Babu et al., 2008, Sellamuthu et al., 2009, Salahuddin et al., 2010). One of the parameters to consider the amelioration of the diabetic state is to ascertain the effect of treatment on the body weight. An increase in body weight implies that anabolic effects have overridden the catabolic ones. No variation means protection against weight loss. Decrease in body weight would mean that catabolism has persisted. Effects of certain plant/plant products in body weight gain in diabetic state have been reported by Stanley et al. (2000). In the present study, mice group treated with tea leaves extract was effective in exerting protection against body weight loss. Insulin is a major anabolic hormone in the body. Its deficiency not only affects glucose metabolism but also protein and fat metabolism. Unopposed actions of the counter-regulatory hormones also play an important role in metabolic derangements. With deficiency of insulin the scale swings from insulin promoted anabolism to catabolism of proteins and fats. Proteolysis follows and gluconeogenic amino acids are removed by liver and used as building blocks for glucose. The catabolism of proteins and fat tends to induce negative nitrogen balance. This results in increased appetite (polyphagia). The combination of polyphagia coupled with weight loss is paradoxical and always raises the suspicion of diabetes (Crowford and Cotran, 2000). Disturbance in the metabolisms results in wasting of muscles and early fatigability. Either a protection against weight loss alone or an increase of body weight has their own distinctive role to play. The normalization of carbohydrate, protein and fat metabolisms would alleviate the diabetic symptoms like loss of weight and fatigability. This would certainly improve the quality of life of the individual. From the present study it can be concluded that tea leaves extract is obviously effective not only in preventing body weight loss but also in helping to gain weight. STZ induced highly significant increases in the levels of serum glucose, triglycerides, cholesterol, creatinine, urea, uric acid, GPT and GOT. These findings are generally in agreement with previous experimental diabetes studies (Ene et al., 2006, Al-Attar and Zari, 2007, Jasmine and Daisy, 2007, Subash-Babu et al., 2008, Andallu et al., 2009, Farswan et al., 2009, Sellamuthu et al., 2009, Salahuddin et al., 2010). Kasetti et al. (2010) reported that STZ induced significant increases in the levels of serum glucose, triglycerides, cholesterol, low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C), creatinine, urea, GPT, GOT and alkaline phosphatase (ALP) in male rats. Also, they demonstrated that the levels of serum insulin and high density lipoprotein cholesterol (HDL-C) were statistically decreased in diabetic rats. The present decline of serum total protein in diabetic rats is clearly associated with reduction of body weight. The decrease in body weight in diabetic rats clearly shows a loss or degradation of structural proteins due to diabetes. The structural proteins are known to contribute for the body weight (Rajkumar and Govindarajulu, 1991). Protein synthesis may be decreased in all tissues due to absolute or relative deficiency of insulin in STZ-induced diabetic rats. Additionally, several studies showed that the levels of serum total protein were declined in diabetic animals (Surana et al., 2008, Yokozawa et al., 2008, Gupta and Gupta, 2009).
The present investigation indicates the ameliorative of body weight changes, serum hypoglycemic, hypolipidemic, hyperproteinemic, protective of kidney serum markers (creatinine, urea and uric acid) and attenuation of liver serum enzymes (GPT and GOT) effects of crude tea leaves extract in STZ-diabetic mice. Anandh Babu et al. (2006) showed that the administration of green tea extract to diabetic rats resulted in significant recovery in body weight, heart weight: body weight ratio and blood glucose levels. The administration of green tea extract reduced cholesterol, triglyceride, free fatty acid and LDL-C levels, and increased HDL-C levels, in the serum of diabetic rats. In addition, green tea extracts decreased cholesterol, triglyceride, free fatty acids levels and lipoprotein lipase activity in the myocardium of diabetic rats. Also, they reported that these beneficial effects of green tea extract are ascribed to its antihyperglycemic and hypolipidemic activity. According to Juśkiewicz et al. (2008), supplementation of a diet with green tea extract had no influence on elevated food intake, body weight loss, increased glucose concentration, or declined antioxidant capacity of water-soluble substances in plasma in the diabetic rats. In cases of intestinal maltase activity, attenuation of liver and kidney hypertrophy, triacylglycerol concentration, and aspartate aminotransferase activity in the serum, both dietary treatments normalized metabolic disorders caused by STZ injection to a similar extent. Unlike the green tea low dose (0.01%) group, the green tea high dose (0.2%) treatment significantly ameliorated development of diabetes-induced abnormal values for small intestinal saccharase and lactase activities, renal microalbuminuria, thiobarbituric acid-reactive substance content in kidney tissue, as well as total antioxidant status in the serum of rats. The green tea high dose group was also characterized by higher antioxidant capacity of lipid-soluble substances in plasma and superoxide dismutase activity in the serum. Although the higher dose of green tea extract did not completely protect against STZ-induced hyperglycemia and oxidative stress in experimental rats, this study suggests that green tea extract ingested at high amounts may prove to be a useful therapeutic option in the reversal of diabetic dysfunction (Juśkiewicz et al., 2008). Renno et al. (2008) demonstrated that green tea extract provides a beneficial effect on long-term diabetic nephropathy via suppressing hyperglycemia and preventing glycogen accumulation in the proximal tubules. The therapeutic property of green tea seems propitious in improving kidney nephropathy by significantly improving serum and urine parameters. They showed that these findings support the importance of controlling blood glucose levels and may be slowing or even reversing some of the early pathologies of diabetic nephropathy. Additionally, Ramadan et al. (2009) compared the modulatory effects of two different doses of black tea aqueous extract and green tea aqueous extract on experimentally induced hyperglycemia, hyperlipidemia and liver dysfunction by alloxan (which destroys pancreatic β-cells and induces type 1 diabetes) and a cholesterol-rich diet (which induces obesity and type 2 diabetes) in male Wistar albino rats. Both tea extracts significantly alleviated most signs of the metabolic syndrome including hyperglycemia (resulting from type 1 and 2 diabetes), dyslipidemia and impairment of liver functions induced by alloxan or the cholesterol-rich diet in the animals. Also, the tea extracts significantly modulated both the severe decrease and increase in body weight induced by alloxan and the high-cholesterol diet, respectively. Moreover, they showed that these modulatory effects were partial or complete, but significant and dose dependent, and slightly more in green tea in most cases. Also, they reported that the study supports the hypothesis that both black and green teas may have beneficial effects against the risks of the metabolic syndrome and cardiovascular complications as shown in rat models of human obesity and diabetes.
Diabetic animal’s models exhibit high oxidative stress due to persistent and chronic hyperglycemia, which depletes the activity of antioxidative defense system and thus promotes free radical generation (Ihara et al., 1999, Coskun et al., 2005). Because the expression levels of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase are known to be very low in the islets of Langerhans compared with other tissues (Tiedge et al., 1997), β-cells may be particularly susceptible to oxidative stress (Kaneto et al., 2001). The hyperglycemic in STZ-treated animals leads to the formation of hydrogen peroxide, which subsequently generates free radicals such as O2− and OH−. These reactive compounds can cause peroxidation of lipids, resulting in the formation of hydroperoxy fatty acids and endoperoxides (Pushparaj et al., 2000). In the present investigation, the mechanism by which supplementation of crude tea leaves extract could be exert a hypoglycemic role may be by antioxidant effect. The traditional tea (Camellia sinensis) infusion is characterized by a high content of flavonoids. Flavonoids are a large group of phenolic products of plant metabolism with a variety of phenolic structures that have unique biological properties and may be responsible for many of the health benefits attributed to tea. Tea is an important source of flavonoids in the diet and the flavonoids found in tea are known to be strong antioxidants (Rietveld and Wiseman, 2003). Cai et al. (2002) studied the antioxidative effects of the principal polyphenolic components extracted from green tea leaves and they showed that the kinetic analysis of the antioxidation process demonstrates that these green tea polyphenols are good antioxidants for liver microsomal peroxidation. An another possible mechanism by which crude tea leaves extract mediates a hypoglycemic effect may be by reduction of glucose absorption in small intestine and the serum insulin effect, either the pancreatic secretion of insulin from β-cells or its release from restrict insulin. Wu et al. (2004) demonstrated that insulin-stimulated glucose uptake of, and insulin binding to, adipocytes were significantly increased in Sprague–Dawley rats supplemented with green tea. Also, they showed that green tea increases insulin sensitivity in rats and that green tea polyphenol is one of the active components.
In summary, in spite of the previous studies focused on the effect of green or black tea leaves extract in diabetic models, this is the first study to investigate the effects of crude extract of tea leaves in STZ-diabetic mice. Also, the present study provided evidence indicating that the crude extract of tea leaves significantly reduces the levels of serum glucose in diabetic mice. In addition, treatment with the extract causes the protective role and recovery of certain altered hematobiochemical parameters and of the body weight of diabetic mice. Additionally, the present results justify the development of additional physiological, pharmacological and biochemical researches in order to clarify the nature of the substances responsible for the effect and the exact mechanisms action of active substances of fresh tea leaves. Finally, further experimental studies are needed to explore the influence of different doses of fresh crude tea leaves extract on diabetic models.
Contributor Information
Atef M. Al-Attar, Email: atef_a_2000@yahoo.com.
Talal A. Zari, Email: talzari@yahoo.com.
References
- Al-Attar A.M., Zari T.A. Modulatory effects of ginger and clove oils on physiological responses in streptozotocin-induced diabetic rats. Int. J. Pharmacol. 2007;3:34–40. [Google Scholar]
- Alschuler L. Green tea: healing tonic. Am. J. Natur. Med. 1998;5:28–31. [Google Scholar]
- Anandh Babu P.V., Sabitha K.E., Shyamaladevi C.S. Green tea extract impedes dyslipidaemia and development of cardiac dysfunction in streptozotocin-diabetic rats. Clin. Exp. Pharmacol. Physiol. 2006;33:1184–1189. doi: 10.1111/j.1440-1681.2006.04509.x. [DOI] [PubMed] [Google Scholar]
- Andallu B., Vinay Kumar A.V., Varadacharyulu N.Ch. Lipid abnormalities in streptozotocin-diabetes: amelioration by Morus indica L. cv Suguna leaves. Int. J. Diabetes Dev. Countries. 2009;29:123–128. doi: 10.4103/0973-3930.54289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.J., Ma L.P., Hou L.F., Zhou B., Yang L., Liu Z.L. Antioxidant effects of green tea polyphenols on free radical initiated peroxidation of rat liver microsomes. Chem. Phys. Lipids. 2002;120:109–117. doi: 10.1016/s0009-3084(02)00110-x. [DOI] [PubMed] [Google Scholar]
- Coskun O., Kanter M., Korkmaz M., Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Crowford, J.M., Cotran, R.S., 2000. Robin’s Pathological Basis of Disease. Chapter 20: Inflammation and healing. Philadelphia, pp. 902–929.
- Ene A.C., Bukbuk D.N., Ogunmola O.O. Effect of different doses of black caraway (Carum carvi L.) oil on the levels of serum creatinine in alloxan induced diabetic rats. J. Med. Sci. 2006;6:701–703. [Google Scholar]
- Farswan M., Mazumder P.M., Percha V. Protective effect of Cassia glauca Linn. on the serum glucose and hepatic enzymes level in streptozotocin induced NIDDM in rats. Indian J. Pharmacol. 2009;41:19–22. doi: 10.4103/0253-7613.48887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes C.F.L. Diabetes. In: Poian A.T., Carvalhoalves P.C., editors. Hormôniose metabolismo: Integração e correlações clínicas. São Paulo; Atheneu: 2002. p. 350. [Google Scholar]
- Gandhi H.R. Diabetes and coronary artery disease: importance of risk factors. Cardiol. Today. 2001;1:31–34. [Google Scholar]
- Graham H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- Gupta R., Gupta R.S. Effect of Pterocarpus marsupium in streptozotocin-induced hyperglycemic state in rats: comparison with glibenclamide. Diabetologia Croatica. 2009;38:39–45. [Google Scholar]
- Gupta R.K., Kesari A.N., Murthy P.S., Chandra R., Tandon V., Watal G. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J. Ethnopharmacol. 2005;99:75–81. doi: 10.1016/j.jep.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Hamilton-miller J.H. Antimicrobial properties of tea (Camellia sinenesis L.) Am. Soc. Microbiol. 1995;39:2375–2377. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H.J., Kim S.W., Lim J.M., Joo J.H., Kim H.O., Kim K.M., Yun J.W. Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sci. 2005;76:3069–3080. doi: 10.1016/j.lfs.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Toyokuni S., Uchida K., Odaka H., Tanaka T., Ikeda H., Hiai H., Seino Y., Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats a model of type 2 diabetes. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- Jasmine R., Daisy P. Hypoglycemic and hepatoprotective activity of Eugenia Jambolana in streptozotocin-diabetic rats. Int. J. Biol. Chem. 2007;1:117–121. [Google Scholar]
- Juśkiewicz J., Zduńczyk Z., Jurgoński A., Brzuzan Ł., Godycka-Kłos I., Zary-Sikorska E. Extract of green tea leaves partially attenuates streptozotocin-induced changes in antioxidant status and gastrointestinal functioning in rats. Nutr. Res. 2008;28:343–349. doi: 10.1016/j.nutres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kaneto H., Xu G., Song K.H., Suzuma K., Bonner-Weir S., Sharma A., Weir G.C. Activation of the hexosamine pathway leads to deterioration of pancreatic β-cell function through the induction of oxidative stress. J. Biol. Chem. 2001;276:31099–31104. doi: 10.1074/jbc.M104115200. [DOI] [PubMed] [Google Scholar]
- Kasetti R.B., Rajasekhar M.D., Kondeti V.K., Fatima S.S., Kumar E.G., Swapna S., Ramesh B., Rao C.A. Antihyperglycemic and antihyperlipidemic activities of methanol:water (4:1) fraction isolated from aqueous extract of Syzygium alternifolium seeds in streptozotocin induced diabetic rats. Food Chem. Toxicol. 2010;48:1078–1084. doi: 10.1016/j.fct.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Kavalali G., Tuncel H., Göksel S., Hatemi H.H. Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J. Ethnopharmacol. 2007;84:241–245. doi: 10.1016/s0378-8741(02)00315-x. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Sohn C.B. Anti-diabetic activities of ethanol extracts from persimmon leaves. J. Korean Soc. Appl. Biol. Chem. 2009;52:96–97. [Google Scholar]
- Maiti R., Jana D., Das U.K., Ghosh D. Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2004;92:85–91. doi: 10.1016/j.jep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Marles R.F., Fansworth N.R. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- Negri G. Diabetes melito: plantas e princípios ativos naturais hipoglicemiantes. Rev. Bras. Cienc. Farm. 2005;41:121–142. [Google Scholar]
- Pushparaj P., Tan C.H., Tan B.K.H. Effects of Averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J. Ethnopharmacol. 2000;72:69–76. doi: 10.1016/s0378-8741(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Pushparaj P.N., Low H.K., Manikandan J., Tan B.K.H., Tan C.H. Antidiabetic effects of Cichorium intybus in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2007;111:430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Rajkumar L., Govindarajulu P. Increased degradation of dermal collagen in diabetic rats. Indian J. Exp. Biol. 1991;29:1081–1083. [PubMed] [Google Scholar]
- Ramadan G., El-Beih N.M., Abd El-Ghffar E.A. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Brit. J. Nutr. 2009;102:1611–1619. doi: 10.1017/S000711450999208X. [DOI] [PubMed] [Google Scholar]
- Renno W.M., Abdeen S., Alkhalaf M., Asfar S. Effect of green tea on kidney tubules of diabetic rats. Brit. J. Nutr. 2008;100:652–659. doi: 10.1017/S0007114508911533. [DOI] [PubMed] [Google Scholar]
- Rietveld A., Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. J. Nutr. 2003;133:3285S–3292S. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- Salahuddin M., Jalalpure S.S., Gadge N.B. Antidiabetic activity of aqueous bark extract of Cassia glauca in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2010;88:153–160. doi: 10.1139/Y09-121. [DOI] [PubMed] [Google Scholar]
- Satav J.G., Katyare S.S. Effect of streptozotocin-induced diabetes on oxidative energy metabolism in rat liver mitochondria – a comparative study of early and late effects. Indian J. Clin. Biochem. 2004;19:23–31. doi: 10.1007/BF02894253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellamuthu P.S., Muniappan B.P., Perumal S.M., Kandasamy M. Antihyperglycemic effect of mangiferin in streptozotocin induced diabetic rats. J. Health Sci. 2009;55:206–214. [Google Scholar]
- Sohn E., Kim J., Kim C.S., Kim Y.S., Jang D.S., Kim J.S. Extract of the aerial parts of Aster koraiensis reduced development of diabetic nephropathy via anti-apoptosis of podocytes in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2010;391:733–738. doi: 10.1016/j.bbrc.2009.11.129. [DOI] [PubMed] [Google Scholar]
- Sridevi M., Chandramohan G., Pugalendi K.V. Protective effect of Solanum surattense leaf-extract on blood glucose, oxidative stress and hepatic marker enzymes in STZ-diabetic rats. Asian J. Biochem. 2007;1:117–121. [Google Scholar]
- Stadler K., Jenei V., Bölcshazy G.V., Somogyi A., Jakus J. Increased nitric oxide levels as an early sign of premature aging in diabetes. Free Rad. Biol. Med. 2003;35:1240–1251. doi: 10.1016/s0891-5849(03)00499-4. [DOI] [PubMed] [Google Scholar]
- Stanley P., Prince M., Menon V.P. Hypoglycemic and other related actions of Tinospora cordifolia roots in alloxan induced diabetic rats. J. Ethnopharmacol. 2000;70:9–15. doi: 10.1016/s0378-8741(99)00136-1. [DOI] [PubMed] [Google Scholar]
- Subash-Babu P., Ignacimuthu S., Prince P.S.M. Restoration of altered carbohydrate and lipid metabolism by hyponidd, a herbal formulation in streptozotocin-induced diabetic rats. Asian J. Biochem. 2008;2:90–98. [Google Scholar]
- Surana S.J., Gokhale S.B., Jadhav R.B., Sawant R.L., Wadekar J.B. Antihyperglycemic activity of various fractions of Cassia auriculata Linn. in alloxan diabetic rats. Indian J. Pharm. Sci. 2008;7:227–229. doi: 10.4103/0250-474X.41461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge M., Lortz J., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Wu L.Y., Juan C.C., Ho L.T., Yung-Pei Hsu Y.P., Hwang L.S. Effect of green tea supplementation on insulin sensitivity in Sprague–Dawley rats. J. Agric. Food Chem. 2004;52:643–648. doi: 10.1021/jf030365d. [DOI] [PubMed] [Google Scholar]
- Yokozawa T., Yamabe N., Kim H.Y., Kang K.S., Hur J.M., Park C.H., Tanaka T. Protective effects of morroniside isolated from Corni fructus against renal damage in streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2008;31:1422–1428. doi: 10.1248/bpb.31.1422. [DOI] [PubMed] [Google Scholar]
- Zari T.A., Al-Attar A.M. Effects of ginger and clove oils on some physiological parameters in streptozotocin-diabetic and non-diabetic rats. J. Med. Sci. 2007;7:267–275. [Google Scholar]