Abstract
Nanoparticles (NPs) offer a great possibility for biomedical application, not only to deliver pharmaceutics, but also to be used as novel diagnostic and therapeutic approaches. Currently, there are no data available regarding to what extent the degree of the toxicity and the accumulation of gold nanoparticles (GNPs) are present in in vivo administration. This study aimed to address the GNP size and exposure duration effect on the liver and kidney function of rats: in vivo.
Methods
A total of 30 healthy male Wistar-Kyoto rats of the same age (12 weeks old) and weighing 220–240 g of King Saud University colony were used. Animals were randomly divided into groups, two GNP-treated rat groups and one control group (CG). The 50 μl of 10 and 50 nm GNPs was intraperitoneally administered in rats for exposure duration of 3 days. Then, several biochemical parameters such as aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alanine transaminase (ALT), alkaline phosphatase (ALP), urea (UREA) and creatinine (CREA) were evaluated.
Results
In this study, the AST values increased with the administration of 10 and 50 nm GNPs compared with the control. The AST values significantly increased with 10 nm GNPs compared with 50 nm GNPs and control. The GGT and ALT values decreased with the administration of 10 and 50 nm GNPs compared with the control. The GGT and ALT values significantly decreased with 50 nm GNPs compared with 10 nm GNPs and control. The ALP values significantly decreased with the administration of 10 and 50 nm GNPs compared with the control. The decrease in ALP values with 10 nm GNPs was higher than those compared with 50 nm GNPs. In this study, the levels of UREA and CREA values increased in a non significant manner after the administration of 10 and 50 nm GNPs compared with the control.
Conclusions
This study demonstrates that the increase in the enzymes AST and the decrease in ALP are smaller GNPs (10 nm) size-dependent for exposure duration of 3 days; while the decrease in the enzymes GGT and ALT are bigger GNPs (50 nm) size-dependent. The levels of UREA and CREA values indicated no significant changes with the administration of 10 and 50 nm GNPs for exposure duration of 3 days compared with the control. The administration of 10 and 50 nm GNPs for short exposure duration of 3 days induced only significant variations with some liver enzymes while kidney showed no significant variations. This study suggests that synthesis and metabolism of GNPs as well as the protection of the liver will be more important issues for medical applications of gold-based nanomaterials in future.
Keywords: Gold nanoparticle, Sizes, Exposure duration, Rats, Liver and kidney function
1. Introduction
The rats exposed to gold nanoparticles (GNPs) revealed that the GNPs were rapidly taken into the system with the highest accumulation in the lungs and aorta (Lanone and Boczkowski, 2006). Moreover, GNPs are believed to be more biologically reactive than their bulk material due to their small size and larger surface area to volume ratio (Lanone and Boczkowski, 2006; Yu et al., 2007).
Although some scientists consider NPs as nontoxic, other studies reported the toxic effects of NPs (Chithrani and Chan, 2007; Pan et al., 2007; BarathManiKanth et al., 2010). The toxicity of NPs is being addressed by a number of standardized approaches with in vitro, in vivo as well as detailed genomic or biodistribution studies (Schrand et al., 2008). In addition, GNPs are used as carriers for drug delivery and genes (Gibson et al., 2007).
The GNPs imply that they could get close to a biological target of interest. The environmental impact of NPs is evident; however, their nanotoxicity due to the reduction in size to nanoscale is rarely discussed. GNPs may serve as a promising model to address this size-dependent toxicity, since gold is extraordinarily biocompatible. There are differing reports of the extent of the toxic nature of these particles owing to the different modifications of GNPs, surface functional attachments, shape and diameter size of the nanospheres (Takahashi et al., 2006; Pan et al., 2007).
Abdelhalim, 2011a,b and Abdelhalim, 2012a,b; Abdelhalim and Jarrar, 2011a,b,c,d have reported that the bioaccumulation of GNPs in several rat tissues (liver, heart, kidney and lung) is size and exposure duration dependent.
The GNP size and exposure duration effect on the liver and kidney function of rats: in vivo have not been well documented before. In the present study we aimed to address the particle-size and exposure duration effect on the liver and kidney function in an attempt to cover and understand the toxicity and potential therapeutic and diagnostic tool of GNPs.
2. Materials and methods
A total of 30 healthy male Wistar-Kyoto rats were obtained from the Laboratory Animal Center (College of Pharmacy, King Saud University, Saudi Arabia). The rats nearly of the same age (12 weeks old) and weighing 220–240 g of King Saud University colony were used. Animals were randomly divided into groups, two GNP-treated rats groups and one control group (CG). The 10 and 50 nm GNPs were intraperitoneally administered in rats at the rate of 3 days as follows: Group 1 (G1): received infusion of 50 μl GNPs of size 10 nm for 3 days (n = 10); Group 2 (G2): received infusion of 50 μl GNPs of size 50 nm for 3 days (n = 10); The rats were maintained on standard laboratory rodent diet pellets and housed in humidity and temperature-controlled ventilated cages on a 12 h day/night cycle. All experiments were conducted in accordance with the guidelines approved by King Saud University Local Animal Care and Use Committee.
3. Serum biochemical analysis
Whole blood was centrifuged twice at 3000 rpm for 10 min in order to separate serum. Using a biochemical autoanalyzer (Type 7170, Hitachi), serum biochemical analysis was carried out. To evaluate the liver function, the levels of aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alanine transaminase (ALT) and alkaline phosphatase (ALP) were measured; while to evaluate the kidney function, the levels of urea (UREA) and creatinine (CREA) were measured.
4. Results and discussions
For exposure duration of 3 days, the effects of intraperitoneal administration of 10 and 50 nm of GNPs on the blood serum of rats were evaluated through the measurement of different biochemical parameters such as AST, GGT, ALT, ALP, UREA and CREA.
In this study, the AST values increased with the administration of 10 (228.18 ± 43.53; Mean ± SE); and 50 (200.96 ± 21.02) nm GNPs compared with the control (180.08 ± 23.26) (Fig. 1). The AST values significantly increased with 10 nm GNPs compared with 50 nm GNPs and control (Wim et al., 2008). This study suggests that the liver might be slightly damaged with the administration of GNPs, and the GNPs have a direct effect on the liver function.
Figure 1.
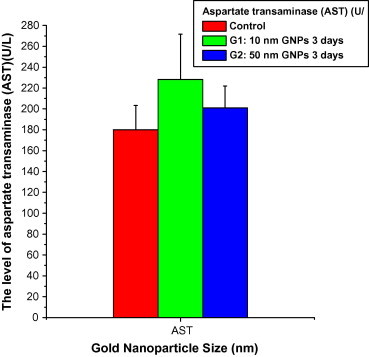
The levels of aspartate transaminase (AST) versus the gold nanoparticle size in rats’ blood serum.
Serum AST and ALT levels are common markers for hepatic toxicity: levels of these proteins are rapidly increased when the liver is damaged by any cause, including hepatitis or hepatic cirrhosis (Sheth et al., 1998).
The hepatocyte swelling might be exhibited as a result of disturbances of membrane function that lead to a massive influx of water and Na+ due to GNP effects accompanied by leakage of lysosomal hydrolytic enzymes that lead to cytoplasmic degeneration and macromolecular crowding (Abdelhalim, 2012a,b; Abdelhalim and Jarrar, 2011a,b,c,d).
The vacuolated swelling of the cytoplasm of the hepatocytes of the GNP treated rats might indicate an acute and subacute liver injury induced by the GNPs. These alterations were size-dependent with smaller ones inducing most effects and related to time exposure of GNPs (Abdelhalim, 2012a,b; Abdelhalim and Jarrar, 2011a,b,c,d).
For 13 nm sized colloidal gold beads, the highest amount of gold was observed in liver and spleen after intraperitoneal administration (Hillyer and Albrecht, 1999). In another study, intravenously injected gold nanorods (65 ± 5 nm) revealed that within minutes these particles accumulated predominantly in the liver (Niidome et al., 2006).
At exposure duration of 3 days, the GGT values decreased with the administration of 10 (14.8 ± 1.36; Mean ± SE) and 50 (10.2 ± 1.36) nm GNPs compared with the control (15.8 ± 1.74). The GGT values significantly decreased with 50 nm GNPs compared with 10 nm GNPs and control (Fig. 2). The ALT values decreased with the administration of 10 (65.86 ± 6.01; Mean ± SE) and 50 (60.38 ± 7.42) nm GNPs for exposure duration of 3 days compared with the control (70.52 ± 10.09). The ALT values significantly decreased with 50 nm GNPs compared with 10 nm GNPs and control (Fig. 3).
Figure 2.

The levels of gamma glutamyl transferase (GGT) versus the gold nanoparticle size in rats’ blood serum.
Figure 3.
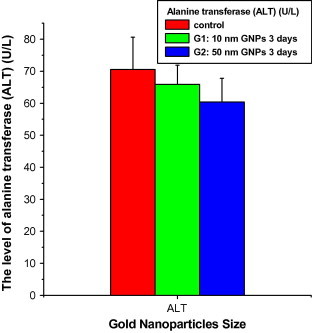
The levels of alanine transferase (ALT) versus the gold nanoparticle size in rats’ blood serum.
The ALP values significantly decreased with the administration of 10 (196.60 ± 33.95; Mean ± SE) and 50 (251.60 ± 38.52) nm GNPs compared with the control (347 ± 52.52). The decrease in ALP values with 10 nm GNPs was higher than those with 50 nm GNPs (Fig. 4).
Figure 4.

The levels of alkaline phosphatase (ALP) versus the gold nanoparticle size in rats’ blood serum.
In this study, the levels of UREA values increased in a non significant manner after the administration of 10 (43.56 ± 1.68; Mean ± SE) and 50 (44.18 ± 2.59) nm GNPs compared with the control (38.36 ± 3.36) (Fig. 5). In this study, the levels of CREA values increased in a non significant manner after the administration of 10 (0.284 ± 0.02; Mean ± SE) and 50 (0.294 ± 0.05) nm GNPs compared with the control (0.274 ± 0.06) (Fig. 6). The non significant changes observed in the levels of UREA and CREA might be attributed to the highest clearance of GNPs through the kidney and/or less exposure duration of GNPs.
Figure 5.

The levels of urea (UREA) versus the gold nanoparticle size in rats’ blood serum.
Fig. 6.
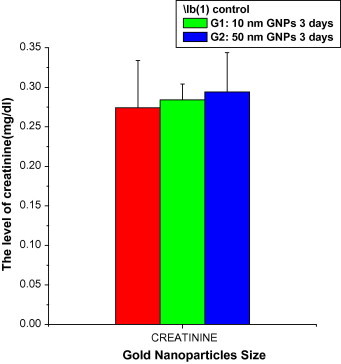
The levels of creatinine (CREA) versus the gold nanoparticlesize in rats’ blood serum.
In kidney, there was a significant increase in the amount of gold after repeated injection of GNPs. Interestingly the% gold accumulated decreases when the GNP dose increases, suggesting an efficient clearance of GNPs from the body (Lasagna-Reeves et al., 2010).
The levels of UREA, CREA, total bilirubin and ALP in rats’ blood serum were tested as a measure of kidney, hepatic and biliary functionality. A detailed analysis of all these metabolites in serum of animals treated with different doses of GNPs as compared to controls showed no statistically significant differences in any of the parameters tested (Lasagna-Reeves et al., 2010).
After absorption of GNP following, e.g. inhalation, oral or dermal exposure, further distribution in the body then occurs via the blood circulation. The distribution of GNPs proved to depend on the size and exposure duration of the injected particles. The smallest GNPs revealed the most widespread tissue distribution at 3 days after injection (Abdelhalim, 2011a,b; Abdelhalim, 2012a,b; Abdelhalim and Jarrar, 2011a,b,c,d; Wim et al., 2008).
Exposure to GNP doses produced cloudy swelling, vacuolar degeneration, hyaline droplets and casts, karyorrhexis and karyolysis. The glomeruli showed moderate congestion with no hypercellularity or basement membrane thickening. These changes were mainly seen in the cortex and the proximal renal convoluted tubules than the distal ones. Moreover, cloudy swelling, renal tubular necrosis and intertubular blood capillary dilatation and hemorrhage, and inflammatory cell infiltrations were observed (Abdelhalim and Jarrar, 2011a,b,c,d).
This study suggests that synthesis and metabolism of different GNP sizes as well as protection of the liver will be more important issues for medical applications of gold-based nanomaterials in future. Therefore, further kinetic and toxicokinetic studies are required to extend the existing knowledge on particle behavior in vivo.
5. Conclusions
This study aimed to address the GNP size and exposure duration effects of GNPs on the liver and kidney function of rats: in vivo.
A total of 30 healthy male Wistar-Kyoto rats of the same age (12 weeks old) and weighing 220–240 g of King Saud University colony were used in this study. Several biochemical parameters such as AST, GGT, ALT, ALP, UREA and CREA were evaluated.
This study demonstrates that for exposure duration of 3 days, the increase in the enzymes AST and the decrease in ALP are smaller GNPs (10 nm) size-dependent; while the decrease in the enzymes GGT and ALT are bigger GNPs (50 nm) size-dependent. The administration of 10 and 50 nm GNPs for short exposure duration of 3 days induced only significant variations with some liver enzymes; while the levels of UREA and CREA values indicated no significant changes with the administration of GNPs for exposure duration of 3 days compared with the control.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohamed Anwar K. Abdelhalim, Email: abdelhalimmak@yahoo.com, mabdulhleem@ksu.edu.sa.
Sherif A. Abdelmottaleb Moussa, Email: sherifmoussa96@yahoo.com.
References
- Abdelhalim M.A.K., Jarrar B.M. Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia. Lipids in Health and Disease. 2011;10:133. doi: 10.1186/1476-511X-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim M.A.K., Jarrar B.M. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids in Health and Disease. 2011;10:166. doi: 10.1186/1476-511X-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim M.A.K., Jarrar B.M. Renal tissue alterations were size-dependent with smaller ones induced more effects and related with time exposure of gold nanoparticles. Lipids in Health and Disease. 2011;10:163. doi: 10.1186/1476-511X-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim M.A.K., Jarrar B.M. The appearance of renal cells cytoplasmic degeneration and nuclear destruction might be an indication of GNPs toxicity. Lipids in Health and Disease. 2011;10:147. doi: 10.1186/1476-511X-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim M.A.K. Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure. Lipids in Health and Disease. 2011;10:205. doi: 10.1186/1476-511X-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim M.A.K. Exposure to gold nanoparticles produces pneumonia, fibrosis, chronic inflammatory cell infiltrates, congested and dilated blood vessels, and hemosiderin granule and emphysema foci. Journal of Cancer Science & Therapy. 2012;4(3):046–050. [Google Scholar]
- Abdelhalim M.A.K. Gold nanoparticles administration induces disarray of heart muscle, hemorrhagic, chronic inflammatory cells infiltrated by small lymphocytes, cytoplasmic vacuolization and congested and dilated blood vessels. Lipids in Health and Disease. 2011;10:233. doi: 10.1186/1476-511X-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim M.A.K. Optimizing a novel method for synthesizing gold nanoparticles: biophysical studies. Journal of Cancer Science & Therapy (Letter of Acceptance May 2 2012).
- BarathManiKanth S., Kalishwaralal K., Sriram M., Pandian S.R.K., Youn H., Eom S., Gurunathan S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. Journal of Nanobiotechnology. 2010;8:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chithrani B.D., Chan W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Letters. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- Gibson J.D., Khanal B.P., Zubarev E.R. Paclitaxel-functionalized gold nanoparticles. Journal of American Chemical Society. 2007;129:11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- Hillyer J.F., Albrecht R.M. Correlative instrumental neutron activation analysis, light microscopy, transmission electron microscopy, and X-ray microanalysis for qualitative and quantitative detection of colloidal gold spheres in biological specimens. Microscopy and Microanalysis. 1999;4:481–490. doi: 10.1017/s143192769898045x. [DOI] [PubMed] [Google Scholar]
- Lanone S., Boczkowski J. Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Current Molecular Medicine. 2006;6:651–663. doi: 10.2174/156652406778195026. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves C., Gonzalez-Romero D., Barria M.A., Olmedo I., Clos A., Ramanujam V.M.S.A., Urayama A., Vergara L., Kogan M.J., Soto C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochemical and Biophysical Research Communications. 2010;393:649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Niidome T., Yamagata M., Okamoto Y., Akiyama Y., Takahishi H., Kawano T. PEG-modified gold nanorods with a stealth character for in vivo application. Journal of Control Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Pan Y., Neuss S., Leifert A., Fischler M., Wen F., Simon U., Schmid G., Brandau W., Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- Schrand A.M., Bradich-Stolle L.K., Schlager J.J., Dai L., Hussain S.M. Can silver nanoparticles be useful as potential biological labels? Nanotechnology. 2008;9:1–13. doi: 10.1088/0957-4484/19/23/235104. [DOI] [PubMed] [Google Scholar]
- Sheth S.G., Flamm S.L., Gordon F.D., Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. The American Journal of Gastroenterology. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Niidome Y., Niidome T., Kaneko K., Kawasaki H., Yamada S. Modification of gold nanorods using phosphatidylcholineto reduce cytotoxicity. Langmuir. 2006;22(1):2–5. doi: 10.1021/la0520029. [DOI] [PubMed] [Google Scholar]
- De Jong Wim H., Hagens Werner I., Krystek Petra, Burger Marina C., Slips Adrie¨nne J.A.M., Geertsma Robert E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Yu L.E., Yung L.-Y.L., Ong C.-N., Tan Y.-L., Balasubramaniam K.S., Hartono D. Et al. Translocation and effects of gold nanoparticles after inhalation exposure in rats. Nanotoxicology. 2007;1(3):235–242. [Google Scholar]



