Abstract
Improved medical therapies have increased survivorship rates for children with posterior fossa tumors; resultantly, morbidities associated with survivorship, such as executive function deficits, have become increasingly important to identify and address. Executive dysfunction can impact academic achievement as well as functional outcomes. We summarize studies describing executive functioning deficits in pediatric posterior fossa tumor survivors who received cranial radiation therapy and intervention studies that have targeted executive functioning deficits. Previous theoretical models describing the etiology of these deficits are reviewed, and a new, more comprehensive model is proposed. Future research should move toward incorporating neuroimaging, longitudinal designs, and multiple informants.
Childhood brain tumors, the second most common type of childhood cancer, account for approximately 20% of the pediatric oncology population (Kaatsch, 2010). The overall incidence of pediatric brain tumor is approximately 4.5 in 100,000, with males comprising about 57% of the population (CBTRUS, 2008). Medulloblastoma, a tumor arising in the posterior fossa (PF), is the most commonly occurring brain tumor in childhood (Crawford, MacDonald & Packer, 2007). Other tumors typically occurring in the PF include ependymoma and astrocytoma. Surgical resection is standard treatment for all types of malignant PF tumors, and cranial radiation therapy (CRT) is considered standard for most medulloblastoma and ependymoma patients over the age of 3 years (Muzamdar & Ventureyra, 2010). By its very nature, a PF tumor can have a profound impact on children’s neurocognitive status given its location within the brain. Evidence of this has been substantiated by studies showing neurocognitive deficits in PF tumor survivors treated with surgical resection only (Levisohn, Cronin-Golomb, & Schmahmann, 2000; Riva & Giorgi, 2000; Steinlin et al., 2003; Ris et al., 2008; Zuzak et al., 2008). Secondary effects may also occur, compounded with those related to surgical resection, when CRT is a part of the treatment regimen.
Improved medical technology including CRT has resulted in increased 5-year survival rates for children diagnosed with PF tumors, and heightened the importance of functional outcomes and quality of life after completion of treatment (American Cancer Society, 2007). Nonetheless, pediatric PF tumor survivors experience significant treatment-related late effects across multiple domains of functioning including neurological, endocrinologic, motor, and neurocognitive difficulties (Anderson, 2005; Aarsen et al., 2009; Gurney et al., 2003). These late effects are generally chronic in nature and tend to worsen over time. The repercussions of these late effects often leave PF tumor survivors unable to manage their complex health problems independently and to function autonomously in society (Ellenberg et al., 2009).
Of particular concern following CRT is the impact of neurocognitive deficits in the domain of executive functions (Riva & Giorgi, 2000; Aarsen, Van Dongen, Paquier, Van Mourik, & Catsman-Berrevoets, 2004; Maddrey et al., 2005). Executive functions are a diverse set of cognitive processes broadly conceptualized according to four primary domains: volition, planning (e.g., working memory, organization), purposive action (e.g., inhibition, set-shifting), and effective performance (e.g., self-monitoring; Lezak, 2004). However, most neurocognitive tests of executive functions focus on the domains of attention, working memory, processing speed, and “general executive functions,” a term encompassing planning, metacognition, and problem-solving abilities. Developmentally these processes have been studied in infants and toddlers (e.g. Marcovitch & Zelazo, 2009), and are widely considered to continue developing into young adulthood (Blakemore & Choudhury, 2006; Luna et al., 2001).
Neuroanatomically, the prefrontal cortex has been implicated in the principal component processes of executive function, such as working memory, attention, and response inhibition (Goldman-Rakic, 1987; Fuster, 1989; Diamond, 1988). In fact, in one investigation children ages 9 to 11 years exhibited similar patterns of prefrontal neural activation as an adult group on a working memory task (Casey et al., 1995). Comparable activation patterns in the prefrontal cortex on a response inhibition task have also been observed in children between the ages of 7–12 years and adults, although children had more widely distributed activation overall (Casey et al., 1997). Such distributed patterns of activation in children may be related to the maturation of executive function processes. Development of these processes corresponds with neuroanatomical changes seen in late adolescence, such as the proliferation and subsequent pruning of synaptic connections during puberty (McGivern, Andersen, Byrd, Mutter, & Reilly, 2002).
Histological studies of monkey prefrontal cortices have shown synapse proliferation in the subgranular layers of the prefrontal cortex just before puberty. A plateau phase is postulated to occur during puberty with subsequent synaptic pruning and reorganization (Woo, Pucak, Kye, Matus, & Lewis, 1997). Structural imaging comparing children with adolescents has found larger volume of grey matter in the frontal and parietal cortices during childhood, which declines during adolescence and is replaced by greater volume of white matter in these regions (Sowell et al., 1999). White matter volume has been shown to increase linearly over time during late childhood and adolescence (Barnea-Goraly et al., 2005; Giedd 2008). These findings align with a study showing that the behavioral performances of 11–17-year-olds on tasks of selective attention, working memory, and problem-solving improved linearly across the age span with older participants performing better (Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001). Additional studies have substantiated improvements in executive functions during the course of adolescence in tasks of inhibition (Luna and Sweeney 2004), working memory (Luciana, Conklin, Cooper, & Yarger, 2005), and processing speed (Luna and Sweeney 2004). Thus, the development of executive functions appears to co-occur with increasing myelination (i.e., white matter), particularly in the frontal lobes.
A growing body of literature has examined neurocognitive sequelae status post completion of CRT for childhood PF tumor survivors. Whole brain CRT results in equal amounts of radiation to all parts of the brain; however, the frontal lobe is more susceptible to radiation-induced injury. One study examined white matter fractional anisotropy (WMFA), a marker of white matter integrity, in pediatric medulloblastoma survivors and found that frontal lobe WMFA was more severely reduced compared with parietal lobe WMFA, although all parts of the brain had received the same dose of radiation (Qiu, Kwong, Chan, Leung, & Khong, 2007). In addition, decreased normal appearing white matter volumes in the bilateral prefrontal cortices and right frontal cortex has been shown to correlate with multiple indices of sustained attention in survivors of malignant brain tumor treated with CRT (Mulhern et al., 2004a). A longitudinal structural imaging study, comparing pediatric medulloblastoma survivors ages 6–12 years who had received whole-brain CRT plus a boost to the posterior fossa region with healthy control subjects (Reddick et al., 2003), indicated that the survivors had reduced white matter volume. In fact, they showed relative white matter decreases of −1.1% per year, while the control group showed a normal rate of white matter growth at 5.4% per year. Interestingly, the cohort of medulloblastoma survivors who were 12 years and older at the time of radiation showed white matter volume and growth trajectories comparable to the age-matched healthy control group. This suggests that white matter development is particularly susceptible to radiation during childhood, and may not be as vulnerable when exposed after age 12 years.
The susceptibility of white matter to CRT-induced injury may be related to reduced vasculature density in white matter compared to grey matter (Reinhold, Calvo, Hopewell, & Van Den Breg, 1990). In normal adults, positron emission tomography (PET) studies have noted cerebral blood flow to be lower in the frontal cortices compared with temporal, parietal, and occipital cortices (Ito, Kanno, Takahashi, Ibaraki, & Miura, 2003). As a result, the frontal lobe may be especially prone to insufficient perfusion after CRT-related white matter damage (Qiu et al., 2007). Because the myelination process continues throughout adolescence and young adulthood, particularly in the frontal lobes (Ullen, 2009), the neuroanatomical substrates of executive functions may have increased vulnerability to injury and delayed development after irradiation.
In addition to the effects of CRT, injury of the PF including a tumor and its resection may be associated with cerebellar cognitive affective syndrome (CCAS) which can involve language disturbances, blunting of affect, disinhibited behavior, spatial cognition deficits, and particularly executive function deficits (Schmahmann & Sherman, 1998). Several studies have found marked executive function deficits in survivors of PF tumor treated with surgical resection only (e.g. Levisohn et al., 2000). The pathophysiology of these executive deficits remains unclear; however, a recent neuroimaging investigation found decreased cerebral perfusion in frontal areas of the brain following PF tumor resection (De Smet et al., 2009). Thus, it is plausible that the resection of a PF tumor and subsequent CRT may additively exacerbate hypoperfusion in the frontal lobes. The exact etiology of CCAS, however, has yet to be defined.
In addition to treatment variables, neurocognitive function post completion of treatment is also impacted by other child-specific risk factors. For example, female gender and younger age at treatment have been associated with poorer outcomes (Mulhern et al., 2004a, 2001; Maddrey et al., 2005; Ellenberg et al., 2009). Few studies have had the statistical power to adequately investigate the impact of various child-specific factors on executive function deficits, however. Clearly, this is an area that warrents further study.
Several studies have designed interventions to ameliorate executive deficits in pediatric brain tumor survivors, only one specific to PF tumor survivors, but with limited success. Extant literature describing both deficits and interventions will be delineated in the present review. Following the literature review, we describe two published theoretical models of executive function deficits in pediatric brain tumor survivors who received CRT. The limitations of these models are addressed through a new proposed model, incorporating the findings of the present review, that provides a more comprehensive explanation of the neuroanatomical, neurocognitive, and contextual factors affecting the executive functions of pediatric PF tumor survivors who have received CRT.
METHODS
Literature searches were first conducted within PubMed, PsychInfo, and the Cochrane Controlled Trials databases using all possible combinations of the following search terms: “pediatric” or “childhood” + “brain tumor” or “posterior fossa tumor” + “executive function” or “neurocognitive” or “cognitive” or “attention.” Inclusion criteria for studies were: (1) Examined some aspect of executive functions in pediatric brain tumor survivors or intervention to improve executive functions, with “executive functions” being defined as attention, working memory, processing speed, or general executive functions (e.g., planning, problem-solving); (2) Inclusion of only PF tumor survivors who had received CRT, or analyzed a cohort of PF survivors who all received CRT separately from other diagnoses and treatment types (except in intervention articles due to limited number of studies); (3) Study included original data; and (4) Inclusion of only participants that were off-treatment.
Initial searches using the aforementioned combinations of search terms yielded 260 peer-reviewed scientific articles. Of these, 14 met all inclusion and no exclusion criteria. The bibliographies of these 14 articles were subsequently perused and potential articles of interest not found in the initial search stage were examined. Resultantly, three additional articles were found to meet inclusion criteria. In total, 17 articles are analyzed in the present review (see Tables 1–5). Articles will be summarized by the domain of executive functions examined. Limitations inherent to each study may be found in the corresponding tables. When we list “small sample size,” we refer to a study’s overall number of participants as being below the typical rule for adequate statistical power given the analyses utilized. When we list “heterogeneity in terms of treatment/age at diagnosis/tumor type/etc.” it means that the cohort differed amongst themselves on this variable, and thus it is a potential confounding variable in assessments. Other limitations, such as “no control group,” “unequal group sizes,” or “missing data” are unambiguous.
TABLE 1.
Studies Examining Attention
| Reference | N, Population, Design | Time Since Treatment | Treatment Regimen | Outcome | Limitations |
|---|---|---|---|---|---|
| Mabbott et al. (2008) | 60 survivors of PF tumor; 10 non-CNS tumor control subjects; Cross-sectional | Mean since diagnosis: PF group = 5.4 y; non-CNS tumor group = 5.9 y | PF group: 32/60 received CRT; 28/60 received surgery only; Non-CNS group received chemo + surgery | No differences in sustained attention were found between PF groups and/or healthy control group. | Heterogeneity in type of treatment received; Unequal group sizes. |
| Mabbott et al. (2009) | 22 survivors of MDB; 17 survivors of low-grade PF glioma; 15 survivors of non-CNS tumor; 10 healthy controls; Cross-sectional | MDB group: Mean = 4.9y (SD = 0.55 y) | MDB group received CRT + surgery, 10/22 also received chemo. | Overall, both PF groups performed worse on selective attention tasks than the non-CNS and control groups. MDB group who received CRT had more errors and slower reaction times compared to non-CNS and control groups. | Potentially confounded by MDB group having 18% with residual complications of tumor (0% in all other groups); Unequal group sizes. |
| Maddrey et al. (2005) | 16 long-term survivors of MDB; Cross-sectional | Mean = 14.6 y (SD = 3.5 y) | All received surgery + CRT | The majority were impaired on measures of attention. | Small sample size; No control group. |
| Mulhern et al. (2001) | 42 MDB survivors; Cross-sectional | Mean = 4.9 y; Range (1.8–11.0 y) | All received surgery + CRT; 29/42 also received chemo | Cohort showed deficits in selective attention; these were linked to younger age at time of CRT. | No control group; Heterogeneity of treatment. |
| Reeves et al. (2006) | 38 MDB survivors; Cross-sectional | Time since diagnosis: Mean = 1.9 y; Range (0.1–4.7 y) | Surgery and CRT: 25/38; Surgery, CRT, and chemo: 13/38 | 8/11 attention variables were significantly below sample mean; omission errors associated with lower reading and math. | No control group; Wide range of time since treatment. |
| Ronning et al. (2005) | 23 survivors of MDB (n=11) or astrocytoma (n=12); Cross-sectional | Mean = 15.9 y, Range (10.2–20.8 y) | MDB: Surgery, CRT + chemo; Astroctyoma: Surgery | Both groups performed below the mean; MDB group performed worse than astrocytoma group in attention. | Small sample size; No control group. |
Note. MDB = medulloblastoma; PF = posterior fossa; Y = years; SD = standard deviation; CRT = cranial radiation therapy; chemo = chemotherapy; CNS = Central Nervous System.
TABLE 5.
Intervention Studies
| Reference | Study Design | Population, N | Intervention | Domain(s) Assessed | Outcome | Limitations |
|---|---|---|---|---|---|---|
| Butler et al. (2008) | Randomized (2:1); Tested before, after, and 6 mo. post; Wait-list control group | 161 childhood cancer survivors that had CNS treatment | 20 2-hour sessions over 4–5 mo focused on cognitive training | 5 indices: Academics, brief attention, WM, memory, vigilance | Improvements found in academics & parent report attention after intervention compared to control group. | Moderate compliance; No long-term follow-up; Wide variation in cancer diagnoses and treatment types. |
| Conklin et al. (2007) | Double-blind, crossover in clinic testing sessions; Subjects served as own controls | 122 survivors of BT or ALL with attention problems | Received either MPH (60 mg) or placebo before testing | NP battery, focused on attention, and processing speed | Improvements in selective attention, impulsivity, cognitive flexibility, and processing speed, but not sustained attention. | Combined ALL and BT groups; Heterogeneous treatment types. |
| Conklin et al. (2010) | 12-month, open-label MPH trial. If participants declined medication or were not classified as responders, they were in control group | 122 survivors of BT or ALL (control group = 58) with attention problems. | After 3-week trial, those who responded well to MPH continued taking it for 12 mo. Pre- and post-NP testing occurred. | IQ, achievement, attention, and behavior indices | Improvements in attention through objective, parent-report, teacher-report, and self-report measures, as well as parent-report social skills and behavior problems. | Groups not randomized; MPH group biased towards those who responded to MPH initially; Heterogenous treatment types; Combined BT and ALL participants for analyses. |
| Hardy et al. (2011) | Tested at baseline, post-intervention, and three months post intervention | Nine survivors of childhood cancer, including six PF tumor survivors | Computerized, home-based, 12-week cognitive training intervention | Attention, working memory | Working memory and parent-reported attention scores improved from baseline to post-assessment | Combined ALL and BT groups; Small sample size; Variable compliance. |
| Jain et al. (2008) | Cross-sectional data comparing standard CRT with IMRT; Standard treatment control group | 25 survivors of medulloblastoma | Treated with either standard CRT or IMRT, and were evaluated afterwards | IQ, visuomotor, and processing speed indices. | No differences between IMRT and standard CRT group on any NP variables; both groups performed below norms on processing speed. | Small sample size; Not randomized into groups; No long-term follow-up. |
| Mulhern et al. (2004b) | Randomized, 3-week, double-blind, crossover study; Subjects were own controls | 83 long-term survivors of childhood ALL (n = 40) or BT (n = 43) | Received low-dose MPH, moderate-dose MPH, and placebo for one week each | Social skills and attention (both teacher & parent report) | Significant improvement with MPH compared to placebo; no effect for high-dose over low-dose MPH. | Combined ALL and BT groups; No long-term follow-up; Wide range of ages at treatment. |
| Patel et al. (2009) | Pre/post testing, intervention in between; No control group | 12 survivors of childhood cancer w/CNS involvement (9-CNS tumors) | 15 sessions focused on learning strategies and problem solving | IQ, attention, memory, academics, social skills, and behavior indices | Gains were seen in all attention scores, though none statistically significant | Small sample size; No control group; Wide range of ages and diagnoses; Moderate compliance. |
| Thompson et al. (2001) | Baseline testing, then repeat next day after receiving MPH or placebo; Used placebo control group | 32 survivors of childhood ALL (n = 7) or BT (n = 25) | Baseline NP testing; then randomly assigned to MPH or placebo, did NP tests again. | Baseline: IQ, achievement, attention, and memory. MPH/Placebo: Attention, and memory | Improvements for MPH over placebo noted in overall attention and omission indices. | Small sample size; Combined ALL and BT groups; No long-term follow-up. |
Note. Mo = months; CNS = central nervous system; BT = brain tumor; ALL = acute lymphoblastic leukemia; MPH = methylphenidate; NP = neuropsychological; IMRT = intensity-modulated radiation therapy; and CRT = cranial radiation therapy.
LITERATURE REVIEW
Attention
Attention is a cognitive task involving allocating processing resources in order to selectively focus on one aspect of the environment while ignoring others (Anderson, 2004). Various models have been proposed to describe this multidimensional construct. Mirsky’s original model (Mirsky, Anthony, Duncan, Ahearn, & Kellam, 1991), as one example, included such abilities as focus/execute, sustain, encode, and shift. Attention is key for performing most tasks in daily life, and is particularly relevant to children because it is crucial for learning. Since subsequent learning-related processes such as permanent encoding and recall cannot take place without basic attentional processes, children with attention problems may miss important information in the classroom and consequently do poorly in school. This may affect not only their immediate academic progress but also their friendships, self-esteem, and future educational achievement. Attention deficits have been well documented in pediatric PF tumor survivors who received CRT, with the majority of studies documenting significant attention deficits (Table 1).
In one such study, Ronning, Sundet, Tonnessen, Lundar, and Helseth (2005) compared the attentional abilities of 11 childhood medulloblastoma survivors treated with surgery, chemotherapy, and CRT (whole brain = 27.0–35.0 Gy; with posterior fossa boost = 45.0–47.0 Gy) with 12 childhood astrocytoma survivors who had received surgical resection only. While both groups performed well below the normative means, the medulloblastoma group performed more poorly on all three measures. Given the small sample size, and corresponding low statistical power, this finding is particularly noteworthy and suggests greater impairment in attentional processes for PF tumor survivors to be associated with CRT.
In another study, Mabbott, Snyder, Penkman, and Witol (2009) specifically examined selective attention, or the ability to attend to particular stimuli while filtering out irrelevant information (Pashler, 1988). A cohort of PF tumor survivors who received CRT (whole brain = 0–3,600 cGY, and 5,110–5,580 cGY to the PF) was compared with a group of PF tumor survivors who were treated with surgical resection only, with a non-CNS tumor group, and with a healthy control group. Overall, both PF tumor groups performed worse than the non-CNS tumor and healthy control groups on tasks of selective attention. Further analysis showed that both the PF tumor groups as well as the non-CNS tumor group had slower reaction times compared to the healthy control groups. Additionally, the PF tumor group treated with CRT showed increased error rates when a target was present compared to the non-CNS tumor and healthy control groups, but did not differ from the PF tumor group treated with surgery only. As most studies in this area have investigated sustained attention in this population, Mabbott et al.’s study of selective attention adds to the body of literature supporting global attention impairments to frequently present in pediatric PF tumor survivors, particularly those who have received CRT.
A few studies have examined the neuroanatomical and neurocognitive effects of CRT on attention in childhood brain tumor survivors. Mulhern and colleagues (2001) explored the relationships between normal appearing white matter (NAWM), age at CRT, and sustained attention performance in 42 childhood medulloblastoma survivors who had received whole brain CRT (23.4–36.0 Gy) with a boost to the PF (total dose of 49–54 Gy). Younger age at CRT was associated with both lower performance on a measure of sustained attention, and decreased NAWM. This study contributed to converging evidence that younger age at CRT is likely a moderating factor of worse cognitive outcomes, an important finding which has prompted investigations of the efficacy of lower doses of CRT (e.g. Thomas et al., 2000).
While neurocognitive studies often divide cognition into discrete and separable domains of functioning, it is likely that a deficit in one domain may have a significant impact on other domains, and vice versa. For example, if a child has an attentional deficit, her visuomotor skills may also be impaired because of decreased thoughtful planning and organization while drawing or taking notes. In one study seeking to investigate the interconnectedness between deficits in various cognitive domains, Reeves and colleagues (2006) examined the relationship between attention and academic achievement among 38 childhood medulloblastoma survivors, who had received whole brain CRT and a boost to the PF (total dose = 55.8–59.4 Gy). When compared to normative scores, medulloblastoma survivors scored more poorly on eight of 11 attention variables, and inattention predicted worse performance in reading and mathematical reasoning. By examining the relationships between attention and academic achievement, Reeves and colleagues emphasized the importance of interconnections between different domains of cognition, suggesting the need for addressing cognitive sequelae in pediatric cancer survivors at a global level since performance in one domain may likely affect performance in others.
Because longitudinal studies are often costly and labor intensive, long-term follow-up studies of brain tumor survivors also shed light on late sequelae of diagnosis and treatment. In one such study, Maddrey and colleagues (2005) investigated executive functioning in 10-year survivors of pediatric medulloblastoma, all of whom had received CRT (37.9 Gy to the whole brain and a 15.5 Gy boost to the PF) and found the majority of participants demonstrated marked deficits in attention. More specifically, 78% of participants were classified as impaired (≥1.5 SD below the mean) on a computerized sustained attention task, and 90% were considered to be impaired on a motor-based attention task. The high prevalence of attentional deficits in this group of long-term survivors is concerning, particularly because the radiation dosages are not significantly greater than many of the other, more recent studies described here. Thus, the deficits observed in these long-term survivors may foreshadow what is to come for cohorts of more recently treated survivors.
In a single study finding no impairments in attentional abilities, Mabbott, Penkman, Witol, Strother, and Bouffet (2008) examined attention in 60 survivors of posterior fossa tumor, 32 of whom had received CRT (0–36.0 Gy to the whole brain and a total of 51.1–55.8 Gy to the PF). Subjects were compared with 10 non-CNS tumor controls. No differences were found on indices of attention between groups, and all groups performed within the normal range. It is possible that since approximately one-third of the CRT cohort received radiation only to the PF tumor bed, this may have conserved some attentional functioning. Mabbot and colleagues’ findings are encouraging for the preservation of attentional functioning in some PF tumor survivors; future research will need to tease apart the various protective factors that may influence the manifestation of attentional deficits in this population.
In sum, in PF tumor survivors who received CRT, attentional deficits have been widely noted. These are possibly related to the CCAS, decreased white matter, and decreased perfusion of frontal lobe areas. Further research in this area should continue to utilize neuroimaging methods to investigate mechanisms of deficits, and move towards using longitudinal or prospective designs to characterize changes in white matter over time and how those changes relate to attentional abilities.
Working Memory
Working memory is typically conceptualized as a temporary mental workspace, lasting 30 seconds or less, in which information is either maintained or manipulated (Mesulam, 2000). Because the manipulation component of working memory is more difficult than simply maintaining information in mind for a period of time, studies have utilized tasks that demand the participant to comprehend information, perform some mental operation(s) on it, and then formulate a correct response. Working memory is crucial for many aspects of academic learning, for example, mental mathematical operations, reading comprehension, and remembering the last few words said by the teacher until they can be written down. A couple of studies have investigated working memory deficits in survivors of pediatric PF tumor (Table 2).
TABLE 2.
Studies Examining Working Memory
| Reference | Population, N, Design | Time Since Treatment | Treatment Regimen | Outcome | Limitations |
|---|---|---|---|---|---|
| Mabbott et al. (2008) | 62 survivors of PF tumor; 10 non-CNS tumor control subjects; Cross-sectional | Mean since diagnosis: PF groups = 5.4 y; non-CNS group = 5.9 y | PF group: 31/62 received CRT; 31/62 received surgery only; Non-CNS group received chemo + surgery | No differences were found between PF CRT, PF Surgery, and non-CNS tumor groups on working memory. | Heterogeneity in type of treatment received; Unequal group sizes. |
| Spiegler et al. (2004) | 34 survivors of pediatric posterior fossa tumor; Longitudinal. | Range (0–10 y) | Surgery + CRT: 10/34; Surgery, CRT, + chemo: 24/34 | Working memory declined by 1.86 standard points per year; with worse declines just after treatment. | Heterogeneity in treatment received and complications; No control group; Missing data. |
Note. PF = posterior fossa; CNS = central nervous system; Y = years; CRT = cranial radiation therapy; chemo = chemotherapy.
As with attention deficits, the long-term nature of working memory deficits in childhood brain tumor survivors is still unclear. Spiegler, Bouffet, Greenberg, Rutka, and Mabbott (2004) utilized growth curve analyses to predict change in working memory abilities over time after treatment for posterior fossa tumor including CRT of 23.4–36.0 Gy to the whole brain and up to 45.0–55.8 Gy to the PF. Working memory declined by 1.86 standard score points per year after diagnosis. Interestingly, the greatest decline was seen immediately following treatment, followed by a gradual decline over time. This was a relatively young sample (M = 6.08 years, SD = 2.73), and thus it may not be representative of cognitive trajectories in childhood cancer survivors diagnosed as adolescents. As the neural substrates of working memory are known to involve areas of the frontal cortex, such as the dorsolateral prefrontal cortex (DLPFC; Casey et al., 1995), and the myelin of the frontal lobes is particularly sensitive to the harmful effects of CRT as it is still forming throughout childhood and adolescence, progressive failure to develop normal myelination over time may help explain the worsening deficits in working memory observed in this study. Rather than a loss of already acquired skills, these deficits are likely to reflect instead a failure to develop cognitive abilities at expected rates (Palmer, 2008). Spiegler et al.’s study suggests that cognitive interventions for working memory might be most effective when implemented during treatment or immediately post completion of medical therapy.
One study of working memory in pediatric PF tumor survivors who received CRT did not find deficits. Mabbott and colleagues (2008) found that both cohorts who received CRT (doses described earlier) and who received surgery only scored within the normal range on three measures of working memory, and did not differ significantly from non-CNS tumor control subjects. As previously mentioned, the lack of deficits noted may be related to one-third of Mabbott and colleagues’ CRT cohort not receiving whole-brain irradiation; alternatively, protective factors yet to be directly examined may have been present in this cohort.
In conclusion, one major study of childhood PF tumor survivors receiving CRT has noted deficits in working memory. Moreover, these deficits were shown to worsen over time and to predict poorer educational and social outcomes in adulthood. Studies examining survivors of other types of pediatric brain tumor who received CRT have also noted deficits in working memory (Briere, Scott, McNall-Knapp, & Adams, 2008; Scott et al., 2001; Waber et al., 2006). Future research should focus on substantiating the presence of working memory deficits in PF tumor survivors and elucidating their mechanisms and moderators, particularly whether they are related to changes in NAWM.
Processing Speed
Processing speed is typically conceptualized as the rapidity with which one can perform relatively automatic mental tasks. Processing speed interacts with other cognitive functions, for example faster processing speed places less demand on maintaining information in working memory. Slow processing speed can affect comprehension of verbally presented information at school, as children may be struggling to keep up with what the teacher is explaining. Outside the classroom, children may have difficulty keeping up with conversations or may spend hours longer on homework than their classmates to do the same amount of work. Several studies have examined processing speed deficits in pediatric PF tumor survivors (see Table 3).
TABLE 3.
Studies Examining Processing Speed
| Reference | Population, N | Time Since Treatment | Treatment Regimen | Outcome | Limitations |
|---|---|---|---|---|---|
| Aukema et al. (2009) | 6 medulloblastoma survivors; Cross-sectional | Median = 3.5 y since diagnosis | All had surgery, chemo + CRT | Medulloblastoma group scored worse on processing speed, had lower WMFA | Small sample size; No control group |
| Mabbott et al. (2008) | 62 survivors of PF tumor; 10 non-CNS tumor control subjects; Cross-sectional | Mean since diagnosis: PF group = 5.4 y; non-CNS tumor group = 5.9 y | PF group: 31/62 received CRT; 31/62 received surgery only; Non-CNS group received chemo + surgery | PF-CRT group had lowest processing speed scores, followed by PF-surgery group. Non-CNS tumor group had best scores. | Heterogeneity in type of treatment received; Unequal group sizes. |
| Ribi et al. (2005) | 18 medulloblastoma survivors; Cross-sectional | Mean age at diagnosis: 6.8 y; Mean age at assessment: 18.9 y | Surgery + CRT: 4/18; Surgery + Chemo: 12/18; Chemo only: 2/18 | 11/14 showed deficits in attention/processing speed factor | Heterogeneity in treatment; No control group; Small sample size. |
| Spiegler et al. (2004) | 34 posterior fossa tumor survivors; Longitudinal | Range (0–10 y) | Surgery + CRT: 10/34; Surgery, CRT, + chemo: 24/34 | Processing speed declined steadily after treatment. | Heterogeneity in treatment; No control group; Missing data. |
Note. Y = years; chemo = chemotherapy; CRT = cranial radiation therapy; WMFA = white matter fractional anisotropy; PF = posterior fossa; CNS = central nervous system.
Ribi and colleagues (2005) investigated processing speed and attention (combined into one factor score) in 16 pediatric medulloblastoma survivors, all of whom had received 23.4–36.0 Gy of CRT and a total of 48.0–55.0 Gy to the PF. Eleven subjects showed deficits on this composite factor; however, interpretation of specific deficits is difficult due to the lack of reporting separate scores for attention and processing speed.
Mabbott and colleagues (2008) investigated processing speed in 62 survivors of posterior fossa tumor, half of whom had received CRT (doses described above). Subjects were compared with a non-CNS tumor control group. A between-group effect was found such that the group that had received CRT had the lowest scores in processing speed, followed by the surgery-only posterior fossa group, and the non-CNS tumor control group had the highest scores. Additionally, processing speed was found to predict IQ after accounting for treatment type in a multiple regression model. The authors proposed that reduced processing speed may be a critical mechanism for intellectual deficits in pediatric brain tumor survivors.
Few studies have examined the longitudinal course of processing speed deficits in childhood posterior fossa tumor survivors. Spiegler et al. (2004) found that processing speed declined by about two standard score points per year for participants who received CRT (doses reported above), the steepest decline across all factor scores. When only subjects observed from baseline were considered, the estimated decline was even steeper at about three standard score points per year. These reported trends warrant additional long-term studies to validate or correct these estimates.
Speed of cognitive processing relies on connections between various areas of the brain, particularly white matter tracts (e.g. Turken et al., 2008). Thus, imaging techniques such as diffusion tensor imaging (DTI) that examine the structural integrity of white matter in the brain have been utilized in an effort to establish whether reduced processing speed in childhood brain tumor survivors is directly linked to reduced white matter integrity. Aukema et al. (2009) compared childhood medulloblastoma survivors, who had received 25.2–34.5 Gy whole brain CRT and a total of 53.3–55.4 Gy to the PF, with childhood acute lymphocytic leukemia (ALL) survivors who had received high-dose or low-dose intrathecal chemotherapy, and age-matched healthy controls. Medulloblastoma survivors had slower cognitive processing speed than both the high-dose and low dose ALL groups, as well as the healthy controls. Furthermore, the medulloblastoma group had lower WMFA when compared to the high-dose ALL group and age-matched controls. Reduced WMFA in the splenium was specifically related to slower processing speed. As intrathe-cal chemotherapy crosses the blood-brain barrier, it has been shown to affect cognition (Mennes et al., 2005). However, the results of this study suggest that intrathecal chemotherapy did not affect white matter as strongly as did the CRT. Additional factors to consider include the effects of a solid tumor such as medulloblastoma in the cerebellar region, which may have also impacted processing speed.
In sum, pediatric PF tumor survivors have shown deficits in processing speed when compared to survivors of other types of cancer as well as healthy controls. The longitudinal course of these deficits appears bleak, with progressive decline shown in one study. White matter integrity has been examined as a candidate mechanism for processing speed deficits in childhood brain tumor survivors, and reduced WMFA was associated with slower processing speed in one cohort. Further study of the mechanisms and moderating factors of processing speed deficits should be undertaken in the future, utilizing neuroimaging methods. Additionally, further longitudinal studies are necessary to validate or modify the current information regarding worsening of deficits over time.
General Executive Functions
Although attention, working memory, and processing speed are often considered parts or necessary companions of global executive functions in a conceptual framework, assessment tools may vary in the specific domains they assess. Consequently, “general executive functions” is a catch-all term encompassing other aspects of executive functions not included in the more commonly assessed domains already discussed. This category includes planning, metacognition, organization, reasoning, and problem solving; all components of executive functions that are closely related to daily living, coping, interpersonal, and academic skills (Baron, 2004). Several studies have examined these abilities in survivors of pediatric PF tumor (Table 4).
TABLE 4.
Studies Examining General Executive Functions
| Reference | Population, N | Time Since Treatment | Treatment Regimen | Outcome | Limitations |
|---|---|---|---|---|---|
| Maddrey et al. (2005) | 16 MDB survivors; Cross-sectional | Mean age at diagnosis: 7.2y; Mean age at assessment: 22.2y | Surgery + CRT: 16/16; Surgery, CRT + chemo: 9/16 | 79% and 85% found to be impaired on measures of EF | Small sample size; No control group; Heterogeneity of treatment. |
| Ronning et al. (2005) | 23 survivors of MDB (n = 11) or astrocytoma (n = 12); Cross-sectional | Mean = 15.9 y, Range (10.2–20.8 y) | MDB: All had surgery, chemo, + CRT; Astroctyoma: All had surgery only | Both groups performed below the mean on measures of EF | Small sample size; No control group. |
| Spiegler et al. (2004) | 34 brain tumor survivors; Longitudinal | Range (0–10 y) | Surgery + CRT: 10/34; Surgery, CRT, + chemo: 24/34 | EF was within average range at first evaluation, but declined over time | Heterogeneity in treatment received and complications; No control group; Missing data. |
Note. MDB = medulloblastoma; y = years; CRT = cranial radiation therapy; chemo = chemotherapy; EF = executive function.
Ronning and colleagues (2005) compared a cohort of young adults treated for childhood astrocytoma with surgery only, and young adult survivors of medulloblastoma treated with surgery, chemotherapy, and CRT (27.0–35.0 Gy to the whole brain, and 45.0–57.0 Gy to the PF). While both groups performed below the mean on a measure of executive functions, the groups’ scores did not significantly differ. This study reinforces the deleterious outcomes that can be associated with surgical resection even in the absence of subsequent CRT.
Characterizing the long-term course of executive function deficits following treatment for PF brain tumor in childhood is an important step toward addressing these deficits comprehensively. Maddrey and colleagues (2005) investigated executive functioning in 10-year survivors of pediatric medulloblastoma, all of whom had received CRT (doses reported above). In this sample, 79–85% showed deficits on tests of planning and cognitive set-shifting. Performance on these tests of executive abilities correlated with age at diagnosis, with younger age being linked to worse performance. This finding is supported by the literature documenting the importance of waiting as long as possible to administer CRT (e.g., Mulhern et al., 2001).
Spiegler et al. (2004) examined problem solving, fluency, and cognitive flexibility over time in survivors of childhood PF tumor, all of whom received CRT (doses reported above). Significant negative slopes were noted for all three domains, indicating worsening performance over time when compared to same-age peers. For measures of problem solving, a decline of approximately one standard deviation every five years was observed.
In conclusion, deficits in general executive functions have been found in PF tumor survivors who received CRT, and worse scores were related to younger age at treatment. Longitudinal data indicates that these general executive deficits worsen over time. Future research should investigate neuroanatomical mechanisms of these general executive deficits using neuroimaging methods and standardize which aspects of general executive functions (e.g., planning) are most important to assess in this population.
Interventions and Treatment-Related Studies
There is a well-established body of literature delineating the multifaceted deficits in executive functions experienced by pediatric PF tumor survivors. Not surprisingly therefore, the focus has recently shifted to developing interventions to ameliorate the documented deficits. A few focal avenues for treatment have been studied including modification of medical treatment protocols, pharmaceutical interventions, and cognitive training programs (see Table 5).
Modifying treatment protocols
Given the well-documented negative effects of radiation therapy on cognitive functions, various techniques to modulate these repercussions while retaining treatment efficacy have been examined in recent years. One such technique is intensity-modulated radiation therapy (IMRT), which is able to precisely deliver radiation to the target tissue with relative sparing of the surrounding tissue. Unfortunately, the only study using cognitive outcome measures after treatment with IMRT found equivocal results. Jain, Krull, Prouwers, Chintagumpala, and Woo (2008) found no differences on two measures of processing speed in children treated for medulloblastoma with IMRT compared with traditional CRT. Although the mean scores for the IMRT cohort were generally higher than the CRT cohort, no conclusions can be drawn for cognitive sparing of IMRT given the lack of statistically significant differences in this study. Further studies should compare IMRT with traditional CRT using larger sample sizes and examining additional domains of executive functions. Using proton beam radiotherapy instead of conventional radiation is another up-and-coming avenue for sparing maximal healthy tissue and potentially preserving cognitive functioning; however, cognitive outcomes after proton beam radiotherapy have yet to be published.
Cognitive intervention
Most cognitive interventions currently available in childhood cancer focus on treating deficits in long-term survivors. In a seminal multi-center randomized clinical trial, Butler and colleagues (2008) evaluated the efficacy of the “Cognitive Remediation Program,” a collection of cognitive training modules based on the principles of pediatric traumatic brain injury, cognitive rehabilitation, educational psychology, and child clinical psychology, with childhood cancer survivors who received CNS treatment. Findings from this labor-intensive intervention revealed improvements in learning strategies, parents’ perceptions of cognitive problems and attention, and in all academic domains except reading comprehension.
The efficacy of the “Cognitive Remediation Program” for survivors of childhood cancer was also tested by Patel, Katz, Richardson, Rimmer, and Kilian (2009). While significant improvements were only noted in tests of social skills and writing achievement, the small sample size of this pilot study (N = 12) likely contributed to difficulty finding statistical significance. Improvements in scores approaching statistical significance included measures of focused and sustained attention, memory, and externalizing behavior. The lack of a control group limits findings, particularly since Butler and colleagues’ (2008) study showed significant improvements over time in the wait-list control group on some measures of attention.
More recently, Hardy, Willard, and Bonner (2011) tested the efficacy of a computerized, home-based, 12-week intervention. Nine survivors of childhood cancer including six PF tumor survivors completed the program, entitled “Captain’s Log.” Overall working memory scores showed a trend (p = .07) toward improvement from baseline to 3 months post-intervention; this finding may have been statistically significant with a larger cohort. Changes in working memory abilities were associated with greater time spent training. Additionally, parent-reported attention problems decreased from baseline to post-assessment. As the first published intervention to examine change in working memory in childhood cancer survivors over time, Hardy and colleagues set an important precedent to include executive function outcome measures other than those pertaining to attention, as working memory, processing speed, and general executive functions may benefit as well from these intervention programs.
Pharmacological intervention
In addition to cognitive remediation programs, a few studies have endeavored to test the effects of methylphenidate (MPH), an effective psychostimulant in the traditional treatment of attention deficit disorders (Committee on Children with Disabilities and Committee on Drugs: Medication for children with attention disorders, 1996), for attentional difficulties in childhood cancer survivors. The first study to investigate this hypothesis found significant improvements for MPH over placebo in omission and overall attention indices (Thompson et al., 2001). While the sample size in this study was relatively modest for a clinical trial (N = 32), the results nonetheless provided encouraging support for the effectiveness of MPH for attention problems and established rationale for future, larger studies exploring the efficacy of MPH in this population.
In a more recent, larger investigation, Conklin and colleagues (2007) found significant improvements associated with taking MPH on one index of executive functioning. No measures of attention were shown to improve with MPH over placebo. It is unclear why MPH did not improve attention in this study; perhaps it was dose-related, or perhaps MPH had positive effect for some subjects, but not others, and so the overall group effect was washed out. Regardless, in a recently published follow-up to this original study, Conklin et al. (2010) found more promising effects, noting improvements in sustained attention, parent-report, teacher-report, and self-report attention, and parent ratings of social skills and behavioral problems. The control group only improved on parent ratings of social skills and attention.
In another study investigating the efficacy of MPH in childhood cancer survivors, Mulhern and colleagues (2004b) found that teachers (but not parents) reported improvements in overall social skills and academic competence in both moderate-dose and low-dose MPH groups, and improvements in problem behaviors for the moderate-dose MPH group. No relative benefit was found for moderate doses of MPH compared with low doses. Although the efficacy of MPH for attentional difficulties observed by teacher report is encouraging, the short-term nature of this study makes it difficult to extrapolate findings regarding long-term clinical effectiveness. The authors mention future plans to follow up with the participants who chose to continue using MPH after the study ended. This will be enlightening, as Conklin and colleagues (2010) is currently the only long-term study of the effects of MPH on cognition, social skills, and achievement.
Not specifically discussed in this section were ecological interventions, for example special education services in schools and classroom accommodations. These interventions are certainly vital to the academic success of pediatric brain tumor survivors; however, as they do not aim to directly address primary cognitive deficits, their specifics are outside the scope of the present review. Overall, while interventions directed at modifying treatment protocols have not been definitively shown to be efficacious, cognitive training programs and pharmacological intervention techniques have shown efficacy in addressing attentional deficits in some pediatric brain tumor survivors. However, a theoretical model describing the relationships between contextual, neurocognitive, and neuroanatomical factors is the next step towards designing more comprehensive interventions to effectively address executive function deficits in this population.
EXISTING CONCEPTUAL MODELS
Seminal models of executive function deficits in pediatric brain tumor survivors include those of Reddick and colleagues (2003) and Palmer (2008). While Reddick et al.’s model focuses on neural substrates to understand only attention deficits, Palmer’s model employs a broader conceptualization of executive function and includes both child and treatment variables.
Reddick and colleagues’ (2003) model incorporated data from structural neuroimaging, positing that reductions in NAWM affect attention negatively and these deficits in turn affect IQ and academic achievement. Direct links between deficits in attention and academic achievement were also proposed in the final model. A main contribution of Reddick and colleagues’ model is how it related the neurocognitive deficits back to underlying neuroanatomical substrates. However, as the model did not include executive functions other than attention, nor child risk factors or treatment-related variables, it may not be a comprehensive description of executive function deficits in pediatric brain tumor survivors.
Palmer’s (2008) model suggested that both the brain tumor diagnosis and its associated treatment affect processing speed, attention, and working memory, potentially leading to lower IQ and academic achievement. These effects are moderated by idiopathic factors, such as the age of the patient. This model took into account multiple contextual and neurocognitive factors in explaining executive functions in pediatric brain tumor survivors. Furthermore, it indicated areas for future study, particularly in the domain of interventions. Limitations of this model, however, include no mention of general executive functions such as planning and metacognition, and not including the neuroimaging literature that has linked deficits in executive functions with underlying white matter deficiencies.
Each model makes a viable contribution but has limitations. What is needed is a more comprehensive approach taking into account contextual (e.g., patient gender, age, brain tumor, and treatment type), neurocognitive (e.g., executive functions), and neuroanatomical (e.g., white matter) factors. Such a model will not only sum up the existing body of literature, but also delineate future directions for research. In particular, a comprehensive model will give inspiration and focus for new targets of intervention strategies.
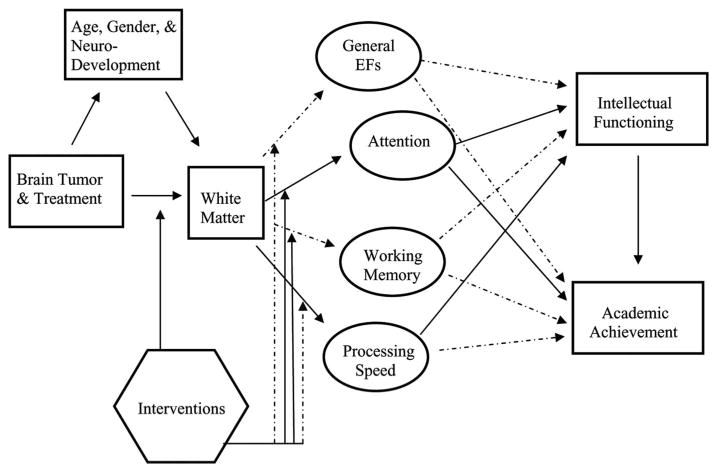
A NEW MODEL
The proposed model (Figure 1), derived from the literature review, aligns with and expands on aspects of previously published models from Palmer (2008) and Reddick and colleagues (2003). Unique contributions of the new model include incorporating executive functions as an important neurocognitive subdomain and revising the targets of published interventions. As in previous models, the tumor and its treatment negatively impact white matter (Reddick et al., 2003) and this effect may be moderated by the child’s age, gender, and/or stage of neurodevelopment (Palmer, 2008). Decrements in white matter negatively affect executive functions; this has been documented in attention (Mulhern et al., 2004a) and processing speed (Aukema et al., 2009), and it is likely that working memory and general executive functions are also affected similarly. Future research should examine this hypothesis. Deficits in core executive functions over time lead to decreased intellectual functioning and academic achievement. Recent modifications in delivery of CRT (e.g., intensity-modulated radiation therapy) may preemptively impact the effects of the brain tumor and treatment on NAWM (Jain et al., 2008). In contrast to this treatment-related approach most other interventions have focused on ameliorating the deficits in core executive functions for survivors, mostly in attention, through pharmacological aids or cognitive training (Conklin et al., 2007; Thompson et al., 2001; Butler et al., 2008; Patel et al., 2009; Hardy et al., 2011).
FIGURE 1.
A theoretical model of the effects of pediatric brain tumor and its treatment on intellectual functioning and academic achievement. Solid lines represent findings from studies with pediatric brain tumor survivors. Dotted lines represent relationships that are theoretically plausible but have yet to be investigated in the literature.
By expanding previous models, our model outlines the current state of knowledge regarding the relationships between factors from contextual, neurocognitive, and neuroanatomical levels which interact to produce executive function deficits in pediatric brain tumor survivors. While the majority of the extant literature focuses on characterizing deficits in executive functions, fewer studies have examined the relationships between these deficits and other variables that certainly impact the presence and severity of executive dysfunction. Importantly, comprehensive intervention strategies should be designed to address multiple levels of factors contributing to deficits. For example, delaying CRT in very young children addresses a contextual variable; modified treatment protocols that ameliorate white matter damage focus on neuroanatomical factors; and cognitive training programs soon after completion of therapy address neurocognitive variables. Clearer understanding of the multifaceted contributing factors to executive dysfunction, and the ways in which they are interrelated in pediatric brain tumor survivors, will produce more creative, comprehensive and effective intervention strategies.
CONCLUSIONS AND FUTURE DIRECTIONS
There is a substantial body of literature documenting the executive function deficits in pediatric PF tumor survivors who received CRT. Executive abilities including attention, working memory, processing speed, and general executive functions (e.g., planning and metacognition) are often negatively impacted by both the brain tumor diagnosis and its associated treatment. Additional documented risk factors include younger age at treatment, gender (female), and longer time post completion of treatment. Recent neuroimaging research has begun to link these neurocognitive deficits to reduced white matter integrity in the brains of survivors. Knowledge of these factors is important for researchers as they strive to decrease neurocognitive toxicity while maintaining treatment effectiveness.
As we move forward in this field, studies from multiple disciplines that provide convergent evidence of deficits and their contributing mechanisms will become increasingly important. Emphasis is shifting towards neuroimaging research, such as functional and structural magnetic resonance imaging, and psychophysiological tools, such as electroencephalography. These objective and non-invasive measures add another important dimension to our appreciation of the nature of executive function deficits, and flesh out our understanding of why they occur. In spite of the potential artifacts in neuroimaging or vascular abnormalities due to the tumor itself, functional and structural neuroimaging afford opportunities to compare the neural substrates of cognitive abilities in childhood brain tumor survivors to those of typically developing children, and both the similarities and differences found will be meaningful for developing new targets for cognitive intervention.
Because neurocognitive deficits from CRT may not be evident until years after treatment, longitudinal studies of childhood brain tumor survivors are important to fully characterize enduring morbidities; including a longitudinal component should be seriously considered in all future studies in this area. When longitudinal studies are not feasible due to financial or time constraints, long-term follow-up cross-sectional studies should be considered as a snapshot of how survivors are functioning long after the completion of treatment. Also, while objective neurocognitive tests may provide an empirical account of abilities, self-report and proxy-report questionnaires reveal an ecologically valid perspective on how the patient is functioning in real life, and should be included more often.
As this review has documented, there have been several smaller-scale studies that described executive function deficits in pediatric brain tumor survivors and the various factors that can affect these deficits. A potential next step for research in this area will be to collaborate in larger-scale studies that will provide sufficient statistical power for analyzing the relative contributions of both idiopathic and treatment-related variables, as well as accurately characterizing the mechanisms underlying these deficits. The Childhood Cancer Survivors Study (CCSS; Ellenburg et al., 2009) is an excellent example of the potential afforded by larger-scale studies to draw meaningful conclusions regarding a large number of variables. A limitation of the CCSS, however, was the lack of objective neurocognitive test scores. Large-scale studies with objective neuropsychological data will be best accomplished by standardization of neuropsychological testing protocols across treating medical centers. This has already been established for some Children’s Oncology Group protocols, but is often not done for children who are being medically treated off-study. Regardless of whether a child is treated on-study or off-study, the neuropsychological time points and test batteries should be uniform. If centers can collaborate with this standardized data, it would afford an incredible opportunity for large-scale research in this specialized population. A major limitation of studies in this area is the heterogeneity of neurocognitive tests utilized to assess these domains of executive functions, as it impedes the interpretation of inter-study comparisons. Therefore, a first step in this direction would be to standardize testing protocols, including the exact measures to be utilized, across childhood cancer treating centers. Computerized screening batteries may be particularly well-suited for this endeavor, as they tend to be more economical than one-on-one testing and ensure a high level of consistency across administrations.
On the vanguard of research in this area are interventions designed to address these executive function deficits in pediatric brain tumor survivors. Three avenues of intervention that have shown promise are: (1) modifying treatment protocols to reduce or become more precise with radiation dosages, (2) cognitive interventions similar to those used with brain-injured populations, and (3) stimulant medication trials. However, these approaches to intervention are still nascent, with accompanying limitations. For instance, the only study examining cognitive outcomes after IMRT did not show benefits over and above traditional CRT. Proton beam radiotherapy shows promise for a new technique that may spare maximal healthy tissue; however, cognitive outcomes after its use have yet to be established. Cognitive training interventions are both time and labor-intensive, requiring many sessions and often one-on-one training, which may not be feasible for widespread dissemination. Finally, although pharmacological trials using MPH have shown encouraging results, particularly over longer periods of time, the side effects of this medication may be prohibitive for some pediatric PF survivors.
While the current literature on intervention is promising, further research is necessary to explore for whom these interventions are effective and by what mechanisms. Future studies should move toward incorporating neuroimaging and psychophysiological measurement tools in their outcome measures to provide additional insight into the mechanisms behind observed changes. For example, do cognitive training programs play a significant role in white matter connectivity, or in functional activity in relevant brain areas? In addition, while current interventions such as cognitive training seem to affect endpoints such as academic achievement, the evidence for their effect on core deficits (i.e., attention, working memory, processing speed, and general executive functions) is less clear. Future interventions should focus on targeting those core impairments.
The importance of pursuing effective interventions for executive function deficits in pediatric brain tumor survivors cannot be overstated, as these deficits have been shown to affect other cognitive abilities, academic achievement, and social functioning. As larger-scale studies using multiple measurement modalities are able to parse out the effects of idiopathic and treatment-related variables on executive function deficits in pediatric brain tumor survivors, directions for effective intervention strategies will become increasingly clearer, and the interventions themselves, progressively more effective.
Acknowledgments
Ms. Wolfe is supported by a training grant from the National Cancer Institute (NCI # 5R25CA047888-22).
Footnotes
Drs. Kana and Madan-Swain have no financial relationships to disclose.
Contributor Information
Kelly R. Wolfe, Department of Psychology, University of Alabama at Birmingham, Birmingham, Alabama
Avi Madan-Swain, Department of Pediatrics, University of Alabama at Birmingham, Birmingham, Alabama.
Rajesh K. Kana, Department of Psychology, University of Alabama at Birmingham, Birmingham, Alabama
References
- Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, Lequin M, Catsman-Berrevoets CE. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. Journal of Clinical Oncology. 2009;27:3526–3532. doi: 10.1200/JCO.2008.19.6303. [DOI] [PubMed] [Google Scholar]
- Aarsen FK, Van Dongen HR, Paquier PF, Van Mourik M, Catsman-Berrevoets CE. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology. 2004;62:1311–1316. doi: 10.1212/01.wnl.0000120549.77188.36. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. Retrieved from http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf. [Google Scholar]
- Anderson JR. Cognitive psychology and its implications. New York, NY: Worth Publishers; 2004. [Google Scholar]
- Anderson NE. Late complications in childhood central nervous system tumour survivors. Current Opinion in Neurology. 2005;16:677–683. doi: 10.1097/01.wco.0000102623.38669.e5. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20(1):385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Aukema EJ, Caan MWA, Oudhuis N, Majoiem CBLM, Vos FM, Reneman L, Schouten-VanMeeteren MD. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. International Journal of Radiation Oncology Biology Physics. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Baron IS. Neuropsychological evaluation of the child. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Blakemore S, Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Briere ME, Scott JG, McNall-Knapp RY, Adams RL. Cognitive outcome in pediatric brain tumor survivors: Delayed attention deficit at long-term follow-up. Pediatric Blood and Cancer. 2008;50:337–340. doi: 10.1002/pbc.21223. [DOI] [PubMed] [Google Scholar]
- Butler RW, Copeland DR, Fairclough DL, Mulhern RK, Katz ER, Kazak AE, Sahler OJZ. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. Journal of Consulting and Clinical Psychology. 2008;76:367–378. doi: 10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. 2008 Source: Central Brain Tumor Registry of the United States, Hinsdale, IL. website: www.cbtrus.org.
- Committee on Children with Disabilities and Committee on Drugs: Mediation for children with attention disorders. Pediatrics. 1996;98:301–304. [PubMed] [Google Scholar]
- Conklin HM, Khan RB, Reddick WE, Helton S, Brown R, Howard SC, Mulhern RK. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: A randomized, double-blind, cross-over trial. Journal of Pediatric Psychology. 2007;32:1127–1139. doi: 10.1093/jpepsy/jsm045. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Reddick WE, Ashford J, Ogg S, Howard SC, Morris EB, Khan RB. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. Journal of Clinical Oncology. 2010;28:4465–4472. doi: 10.1200/JCO.2010.28.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: New biological advances. Lancet Neurology. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- De Smet HJ, Baillieux H, Wackenier P, De Praeter M, Engelborghs S, Phillipe F, Marien P. Long-term cognitive deficits following posterior fossa tumor resection: A neuropsychological and functional neuroimaging follow-up study. Neuropsychology. 2009;23:694–704. doi: 10.1037/a0016106. [DOI] [PubMed] [Google Scholar]
- Diamond A. Abilities and neural mechanisms underlying AB performance. Child Development. 1988;59:523–527. [PubMed] [Google Scholar]
- Ellenberg L, Liu Q, Gioia G, Yasui Y, Packer RJ, Mertens A, Zeltzer LK. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. New York, NY: Raven Press; 1989. [Google Scholar]
- Giedd JN. The teen brain: Insights from neuroimaging. Journal of Adolescent Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational memory. In: Mountcastle V, editor. Handbook of physiology: The nervous system. Vol. 5. Bethesda, MD: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, Robison LL. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors. Cancer. 2003;97:663–673. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- Hardy KK, Willard VW, Bonner MJ. Computerized cognitive training in survivors of childhood cancer: A pilot study. Journal of Pediatric Oncology Nursing. 2011;28:27–33. doi: 10.1177/1043454210377178. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanno I, Takahashi K, Ibaraki M, Miura S. Regional distribution of human cerebral vascular mean transit time measured by positron emission tomography. NeuroImage. 2003;19:1163–1169. doi: 10.1016/s1053-8119(03)00156-3. [DOI] [PubMed] [Google Scholar]
- Jain N, Krull KR, Prouwers P, Chintagumpala MM, Woo SY. Neuropsychological outcome following intensity-modulated radiation therapy for pediatric medulloblastoma. Pediatric Blood Cancer. 2008;51:275–279. doi: 10.1002/pbc.21580. [DOI] [PubMed] [Google Scholar]
- Kaatsch P. Epidemiology of childhood cancer. Cancer Treatment Reviews. 2010;36:277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Luciana M, Conklin HM, Cooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam E, Garver KE, Minshew NJ, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Annals of New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159–168. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Snyder JJ, Penkman L, Witol A. The effects of treatment for posterior fossa brain tumors on selective attention. Journal of the International Neuropsychological Society. 2009;15:205–216. doi: 10.1017/S1355617709090249. [DOI] [PubMed] [Google Scholar]
- Maddrey AM, Bergeron JA, Lombardo ER, McDomald NK, Mulne AF, Barenberg PD, Bowers DC. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. Journal of Neurooncology. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Zelazo PD. A hierarchical competing systems model of the emergence and early development of executive function. Developmental Science. 2009;12:1–18. doi: 10.1111/j.1467-7687.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern RF, Andersen J, Byrd D, Mutter KL, Reilly J. Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain and Cognition. 2002;50:73–89. doi: 10.1016/s0278-2626(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Mennes M, Stiers P, Vandenbussche E, Vercruysse G, Uyttebroeck A, De Meyer G, Van Gool SW. Attention and information processing in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Pediatric Blood Cancer. 2005;44:478–486. doi: 10.1002/pbc.20147. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of behavioral and cognitive neurology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: A neuropsychological approach. Neuropsychology Review. 1991;2:109–144. doi: 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Khan RB, Kaplan S, Helton S, Christensen R, Bonner M, Reddick WE. Short-term efficacy of methylphenidate: A randomized, double-blind, placebo-controlled trial among survivors of childhood cancer. Journal of Clinical Oncology. 2004b;22:4795–4803. doi: 10.1200/JCO.2004.04.128. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL, Reddick WE, Glass JO, Kun LE, Taylor J, Gajjar A. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. Journal of Clinical Oncology. 2001;19:472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, White HA, Glass JO, Kun LE, Leigh L, Thompson SJ, Reddick WE. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. Journal of the International Neuropsychological Society. 2004a;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- Muzamdar D, Ventureyra ECG. Treatment of posterior fossa tumors in children. Expert Review of Neurotherapeutics. 2010;10:525–546. doi: 10.1586/ern.10.28. [DOI] [PubMed] [Google Scholar]
- Palmer SL. Neurodevelopmental impacts on children treated for medulloblastoma: A review and proposed conceptual model. Developmental Disabilities Research Reviews. 2008;14:201–210. doi: 10.1002/ddrr.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. The psychology of attention. Cambridge, MA: MIT Press; 1988. [Google Scholar]
- Patel SK, Katz ER, Richardson R, Rimmer M, Kilian S. Cognitive and problem solving training in children with cancer: A pilot project. Journal of Pediatric Hematology Oncology. 2009;31:670–677. doi: 10.1097/MPH.0b013e3181b25a1d. [DOI] [PubMed] [Google Scholar]
- Qiu D, Kwong DLW, Chan GCF, Leung LHT, Khong PL. Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: Reflection of regional white matter radiosensitivity? International Journal of Radiation Oncology Biology Physics. 2007;69:846–851. doi: 10.1016/j.ijrobp.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, Mulhern RK. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- Reeves CB, Palmer SL, Reddick WE, Merchant TE, Buchanan GM, Gajjar A, Mulhern RK. Attention and memory functioning among pediatric patients with medulloblastoma. Journal of Pediatric Psychology. 2006;31:272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- Reinhold HS, Calvo W, Hopewell JW, Van Den Breg AP. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. International Journal of Radiation Oncology Biology Physics. 1990;18:37–42. doi: 10.1016/0360-3016(90)90264-k. [DOI] [PubMed] [Google Scholar]
- Ribi K, Relly C, Landolt MA, Alber FD, Boltshauser E, Grotzer MA. Outcome of medulloblastoma in children: Long-term complications and quality of life. Neuropediatrics. 2005;36:357–365. doi: 10.1055/s-2005-872880. [DOI] [PubMed] [Google Scholar]
- Ris MD, Beebe DW, Armstrong FD, Fontanesi J, Holmes E, Sanford RA, Wisoff JH. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: A report from the Children’s Oncology Group. Journal of Clinical Oncology. 2008;26:4765–4770. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development. Brain. 2000;123:1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Ronning C, Sundet K, Due-Tonnessen B, Lundar T, Helseth E. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumor in childhood. Pediatric Neurosurgery. 2005;41:15–21. doi: 10.1159/000084860. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Scott RB, Stoodley CJ, Anslow P, Paul C, Stein JF, Sugden EM, Mitchell CD. Lateralized cognitive deficits in children following cerebellar lesions. Developmental Medicine & Child Neurology. 2001;43:685–691. doi: 10.1017/s0012162201001232. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999;6:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. Journal of Clinical Oncology. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- Steinlin M, Imfeld S, Zulauf P, Boltshauser E, Lovblad KO, Luthy AR, Kaufmann F. Neuropsychological long-term sequelae after posterior fossa tumour resection during childhood. Brain. 2003;126:1998–2008. doi: 10.1093/brain/awg195. [DOI] [PubMed] [Google Scholar]
- Thomas PRM, Deutsch M, Kepner JL, Boyett JM, Krischer J, Aronin P, Kun LE. Low-stage medulloblastoma: Final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. Journal of Clinical Oncology. 2000;18:3004–3011. doi: 10.1200/JCO.2000.18.16.3004. [DOI] [PubMed] [Google Scholar]
- Thompson SJ, Leigh L, Christensen R, Xiong X, Kun LE, Heideman RL, Mulhern RK. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. Journal of Clinical Oncology. 2001;19:1802–1808. doi: 10.1200/JCO.2001.19.6.1802. [DOI] [PubMed] [Google Scholar]
- Turken AU, Whitfield-Gabrieli S, Baummer R, Baldo J, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage. 2008;2:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullen F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biology. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- Waber DP, Pomeroy SL, Chiverton AM, Kieran MW, Scott RM, Goumnerova LC, Rivkin MJ. Everyday cognitive function after craniopharyngioma in childhood. Pediatric Neurology. 2006;34:13–19. doi: 10.1016/j.pediatrneurol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Zuzak TJ, Poretti A, Drexel B, Zehnder D, Boltshauser E, Grotzer MA. Outcome of children with low-grade cerebellar astrocytoma: Long-term complications and quality of life. Child’s Nervous System. 2008;24:1447–1455. doi: 10.1007/s00381-008-0692-7. [DOI] [PubMed] [Google Scholar]