Abstract
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase aberrantly expressed in neuroblastoma, a devastating pediatric cancer of the sympathetic nervous system. Germline and somatically acquired ALK aberrations induce increased autophosphorylation, constitutive ALK activation and increased downstream signaling. Thus, ALK is a tractable therapeutic target in neuroblastoma, likely to be susceptible to both small-molecule tyrosine kinase inhibitors and therapeutic antibodies–as has been shown for other receptor tyrosine kinases in malignancies such as breast and lung cancer. Small-molecule inhibitors of ALK are currently being studied in the clinic, but common ALK mutations in neuroblastoma appear to show de novo insensitivity, arguing that complementary therapeutic approaches must be developed. We therefore hypothesized that antibody targeting of ALK may be a relevant strategy for the majority of neuroblastoma patients likely to have ALK-positive tumors. We show here that an antagonistic ALK antibody inhibits cell growth and induces in vitro antibody-dependent cellular cytotoxicity of human neuroblastoma-derived cell lines. Cytotoxicity was induced in cell lines harboring either wild type or mutated forms of ALK. Treatment of neuroblastoma cells with the dual Met/ALK inhibitor crizotinib sensitized cells to antibody-induced growth inhibition by promoting cell surface accumulation of ALK and thus increasing the accessibility of antigen for antibody binding. These data support the concept of ALK-targeted immunotherapy as a highly promising therapeutic strategy for neuroblastomas with mutated or wild-type ALK.
Keywords: neuroblastoma, anaplastic lymphoma kinase, immunotherapy, receptor tyrosine kinase
INTRODUCTION
Anaplastic lymphoma kinase (ALK) was originally identified in an oncogenic fusion protein1 expressed in anaplastic large-cell lymphoma. Other oncogenic ALK fusions have been identified in human cancers, including non-small-cell lung cancer,2,3 squamous cell carcinoma4 and inflammatory myofibroblastic tumors.5 The full-length ALK RTK has also been linked to neuroblastoma,6 a pediatric cancer of the sympathetic nervous system accounting for 10% of childhood cancer mortality.7,8 The ALK gene is amplified in 2 –3% of neuroblastoma cases.9 In addition, activating mutations within the tyrosine kinase domain of ALK were recently identified as the major cause of familial neuroblastoma,10 also arising somatically in up to 10% of sporadic cases. Amplification or mutation of ALK can lead to constitutive autophosphorylation and activation,11–13 and may be associated with a more aggressive clinical course.10,14,15 These findings argue that therapeutic manipulation of intact ALK is a promising strategy for neuroblastoma treatment.
Approaches for therapeutically targeting RTKs include monoclonal antibodies and small-molecule tyrosine kinase inhibitors (TKIs), both of which have led to dramatic increases in survival and time to progression in multiple cancers.16,17 The trastuzumab antibody was approved for treatment of human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer over 10 years ago, and is thought to exert its effects through blockade of aberrant signaling by amplified HER2 and antibody-dependent cellular cytotoxicity (ADCC).18 Similarly, the epidermal growth factor receptor (EGFR) antibody cetuximab inhibits binding of activating ligands and induces ADCC.19 Clinical activity of TKIs that inhibit HER2 and EGFR has been amply demonstrated; moreover, these TKIs have been found to potentiate and enhance the activity of HER2- and EGFR-targeted antibodies in breast and lung cancer, respectively.20–22
Analogous approaches should be effective for targeting intact ALK. Recent studies have shown that crizotinib, a dual Met/ALK TKI, induces remarkable tumor regression in non-small-cell lung cancer patients harboring ALK translocations.23 Crizotinib is also currently in early-phase clinical trial testing in patients with neuroblastoma. However, preclinical studies have shown that cell lines harboring the F1174L mutation, the second most common ALK mutation seen in neuroblastoma tumors, are significantly more resistant to crizotinib than those harboring the most common mutation, R1275Q.24–26 Moreover, acquired resistance to TKIs is largely inevitable,27 and resistance mutations in oncogenic ALK fusions have already emerged in early studies with crizotinib.28,29 These findings underline an important need for developing additional therapeutic options in neuroblastoma, an often-lethal childhood cancer.7,30 One such option is immunotherapy, for which proof of concept was recently demonstrated in a phase 3 trial of high-risk neuroblastoma patients using GD2 antibodies.31 We therefore sought to identify antibody-based strategies for therapeutic targeting of ALK. We show here that ALK antibodies inhibit the growth of neuroblastoma cells, and demonstrate the utility of combining ALK antibodies with TKIs as a potentially important therapeutic strategy. Our findings provide a strong rationale for the immediate development of clinical grade ALK antibodies.
RESULTS
ALK is widely expressed in neuroblastoma tumors and cell lines
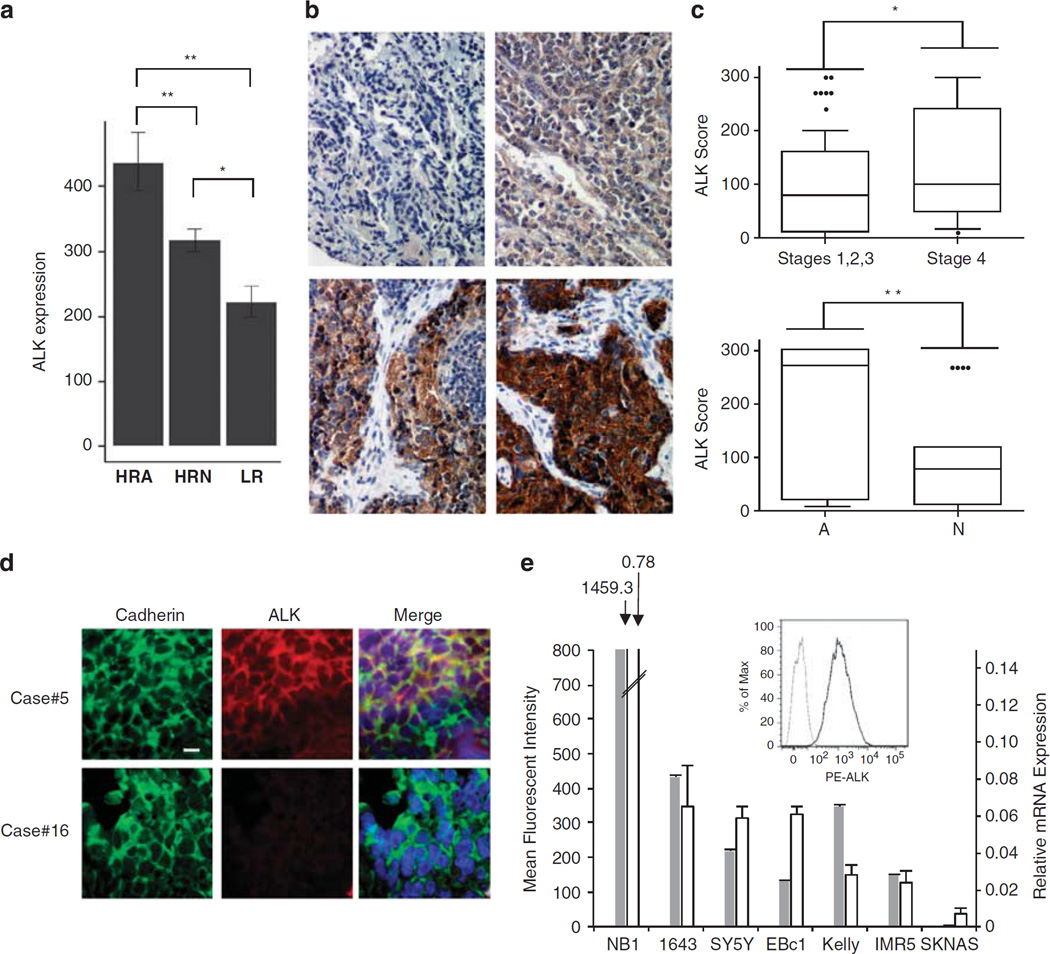
Successful immunotherapy requires the targeted antigen to be expressed selectively (or at much greater levels–for ubiquitously expressed receptors) in tumors, but not in normal tissue. The targeted antigen must be expressed on the majority of tumors for immunotherapy to be relevant to a large proportion of patients, and expression levels should correlate with disease severity. Intact ALK is normally found only in the developing embryonic and neonatal brain,32 a finding confirmed by the lack of consistent ALK staining of a normal tissue microarray (TMA; Supplementary Table 1), which suggests that ALK is a valuable target for immunotherapy. To assess ALK expression in primary patient tumors, we analyzed our own collection33 as well as data from the TARGET initiative (Therapeutically Applicable Research to Generate Effective Treatments: http://target.cancer.gov/). ALK mRNA expression is seen in tumors from patients with clinically aggressive disease, especially in those with high-risk metastatic disease and/or MYCN amplification (Figure 1a; P=5.06E-05 for high-risk MYCN-amplified neuroblastoma (HRA) versus low risk (LR), P=0.0022 for HRA versus high-risk MYCN non-amplified neuroblastoma (HRN), P=0.0211 for HRN versus LR). We next analyzed a TMA of diagnostic neuroblastomas and ganglioneuroblastomas. We stained samples from 126 patients for native ALK expression and graded staining intensity on a scale of 0–3 (representative samples shown in Figure 1b) and percent positivity. Among the samples analyzed, 109 (86.5%) were ALK positive, with 75 samples (59.5%) having either moderate or strong staining. As shown in Figure 1c, ALK expression was significantly stronger in patients with INSS stage 4 (P¼0.0108; top panel) or amplified MYCN (P¼ 0.0065; ‘A’ in bottom panel). These data argue that ALK is expressed in the majority of neuroblastoma tumors, and that expression levels are higher in those patients with the worst prognoses.
Figure 1.
ALK expression in neuroblastoma. (a) ALK expression in 229 neuroblastoma patient tumors analyzed by Affymetrix Human Exon Array and normalized using quantile normalization (high-risk MYCN amplified (HRA), n=64; high-risk MYCN non-amplified (HRN), n=141; low-risk (LR), n= 24). (b) Representative images for immunohistochemical staining of ALK in neuroblastoma patient tumors. ALK staining was positive overall in 109 of 126 (86.5%) samples analyzed. In all, 17 samples showed no positive staining (upper left panel); 35 showed weak staining (upper right panel); 55 showed moderate staining (lower left panel); and 19 showed strong staining (lower right panel). (c) Box plots showing 10th percentile, 90th percentile and mean ALK score by immunohistochemistry for INSS stage (top panel) and MYCN status (bottom panel; A, MYCN amplified; N, MYCN non-amplified). **P<0.01; *P<0.05. ****indicate data-points outside of percentiles. (d) Representative confocal micrographs of formalin-fixed paraffin-embedded primary neuroblastoma patient tumor slides showing staining for ALK and the cell surface antigen cadherin. All samples showed strong plasma membrane cadherin staining, and of 17 samples stained, 1 exhibited strong ALK staining (ALK score=3; top row), 10 showed intermediate ALK staining, 2 had weak staining and 3 had no ALK staining (ALK score=0, bottom row). Bar represents 10µm. (e) Comparison of flow cytometry results with ALK mRNA expression levels for several neuroblastoma cell lines. Gray bars show flow cytometry results for ALK cell surface staining. White bars represent an ALK mRNA expression ‘index’ (relative expression) measured as ALK levels relative to HPRT1. Inset shows mean fluorescence intensity of NB1 cells for ALK staining (black line) and isotype control (gray line).
We next used confocal microscopy to assess cell surface ALK expression on primary tumors. ALK was readily detected on 13 of 16 tumors analyzed, and co-localization of ALK with the cell surface protein cadherin was confirmed by the generation of Pearson’s correlation coefficient values (Supplementary Table 2 and Figure 1d). Although microscopy using the nuclear stain 4’,6-diamidino-2-phenylindole (DAPI) and anti-cadherin antibody generated Pearson’s correlation coefficient values <0.5, reflecting a known lack of co-localization, ALK and cadherin Pearson’s correlation coefficient values ranged from 0.6 to >0.8 for 11 of the 13 ALK-positive tumors. Abundant cell surface ALK expression was also confirmed by flow cytometry (Figure 1e) for cell lines expressing wild-type ALK (NB1, EBc1 and IMR5), R1275Q-mutated ALK (1643) or F1174L-mutated ALK (SY5Y, Kelly), but not the control SKNAS cell line shown. Plasma membrane ALK expression corresponded closely with relative ALK mRNA expression (Figure 1e), and was also observed in immunofluorescence microscopy studies (Supplementary Figure 1). These data suggest widespread expression and cell surface localization of full-length ALK in neuroblastoma primary tumors and cell lines.
An ALK antibody induces growth inhibition and cytotoxicity
We next asked whether an antagonistic ALK monoclonal antibody34 can inhibit growth of a neuroblastoma cell line driven by expression of activated ALK. We first treated F1174L-expressing SY5Y cells with a 3-log dose range of a mixture of two antagonistic ALK antibodies (mAb30 and mAb49) and measured growth. Antibody treatment resulted in significant dose-dependent growth inhibition as compared with cells treated with control immunoglobulin–whether we used the mAb30/mAb49 mixture (Figure 2a) or the individual antibodies (not shown). We next treated a panel of well-characterized neuroblastoma cell lines harboring wild-type (IMR5), mutated (1643 and SY5Y), amplified (NB1) or low/undetectable ALK (SKNAS) with a fixed concentration of mAb30/mAb49 ALK antibody mixture, and saw a direct correlation between ALK expression level and antibody-induced cytotoxicity (Figure 2b), with the ALK amplified line NB1 showing the greatest sensitivity to antibody treatment, and SKNAS showing no growth inhibition. As an additional negative control, we treated retinal-pigmented epithelial-1 cells–a non-neuroblastoma, ALK-negative, neural crest-derived cell line–with ALK antibody or murine immunoglobulin, and saw no growth inhibition (Supplementary Figure S2).
Figure 2.
ALK antibody-induced growth inhibition and ADCC of neuroblastoma cells. To measure growth inhibition upon antibody exposure, cell lines were plated in 96-well plates and treated with anti-ALK (mAb30 plus mAb49) or a negative control murine IgG1. (a) Growth inhibition of SY5Y cells treated with indicated amounts of anti-ALK as compared with control Ig. (b) The indicated cell lines were treated with 10 µg/ml ALK antibody, and growth inhibition was measured after 144h. (c) ADCC was measured using an in vitro assay as described in Materials and methods, in which IL-2-activated peripheral blood lymphocytes were co-incubated with neuroblastoma cells in the presence (black line) or absence (gray line) of 1 µg/ml ALK antibody. Shown are percent (%) cytotoxicities at the indicated effector:target ratios when NB1 cells (left panel), SY5Y cells (middle panel) or cell surface ALK-negative SKNAS cells (right panel) were used as targets.
Immune cell-mediated ADCC has been shown to be important for the mechanism of action of the GD2 antibody in neuroblastoma, and this effect is substantially enhanced in the presence of interleukin-2 (IL-2).35 To explore whether an ALK antibody could also induce an immune-mediated anti-tumor response in neuroblastoma, we conducted in vitro ADCC assays using normal donor peripheral blood lymphocytes as effectors and neuroblastoma cell lines as targets. Treatment with ALK antibody greatly enhanced cytotoxicity in NB1 cells induced by lymphocytes preincubated with IL-2 (Figure 2c, left panel). SY5Y cells also showed antibody-enhanced cytotoxicity in this assay (Figure 2c, middle panel), although less than seen for NB1 cells, possibly because of the lower cell surface ALK levels seen by flow cytometry (Figure 1e). No ADCC was detected when SKNAS cells that lack cell surface ALK expression were used as targets (Figure 2c, right panel). Thus, antibody targeting of ALK can both inhibit cell growth and induce cytotoxicity of neuroblastoma cell lines harboring either wild-type or mutated forms of activated ALK.
Crizotinib treatment induces cell surface accumulation of ALK
Treatment with a combination of TKIs and therapeutic antibodies targeting the same tumor antigen, such as EGFR20 or HER221 has been shown to enhance the tumor growth inhibition and cytotoxicity elicited by either agent alone. One possible mechanism for this is cell surface accumulation of the targeted RTK resulting from RTK stabilization upon TKI binding.36,37 Certain activating RTK mutations, including several in c-Kit, may destabilize the RTK and cause intracellular accumulation that can be reversed by TKI treatment–leading to increased cell surface expression.38,39
To explore the effects of TKIs on surface ALK levels in neuroblastoma, we treated SY5Y cells with crizotinib and then used surface biotin labeling of membrane proteins to quantify changes in cell surface ALK. As shown in Figure 3a, crizotinib treatment increased the levels of ALK protein detected at the cell surface by 2.15 times over that found on vehicle-treated cells. Flow cytometry studies also showed a substantial crizotinib-induced increase in cell surface ALK levels for SY5Y cells (Figure 3a), with a 51.4% increase in mean fluorescence intensity for cells treated with crizotinib (mean fluorescence intensity=636) compared with vehicle (mean fluorescence intensity=420). This phenomenon was dose dependent (Figure 3c), and increased over time (Figure 3d). To control for the possibility that crizotinib binding induces conformational changes in ALK that stabilize or enhance exposure of the epitope to which our staining antibody (mAb14) binds (increasing fluorescence signal), we repeated this experiment using an anti-ALK antibody (mAb46) that binds to an epitope distant from that of mAb14.34 Crizotinib-induced enhancement of cell surface ALK levels was also observed in this experiment (Supplementary Figure S3), arguing that crizotinib functions similarly to other TKIs in promoting cell surface accumulation of its target RTK.
Figure 3.
Effect of crizotinib on cell surface ALK expression. (a) SY5Y cells were incubated for 24h with vehicle or 1000 nm crizotinib, then biotin labeled, precipitated with NeutrAvidin beads and immunoblotted for ALK (upper left panel) or cadherin (lower left panel). The results of densitometric analysis of ALK protein, normalized to cadherin, are shown in the bar chart (right panel), and indicate an increase in total ALK from 1.39 to 2.91 in arbitrary units. (b) Representative flow cytometry results showing mean fluorescence intensity (MFI) for SY5Y cells incubated for 72 h with either vehicle (medium gray line; MFI = 420) or 1000 nm crizotinib (black line; MFI= 636), resulting in a 51.4% increase in MFI for crizotinib- versus vehicle-treated cells. The single-peaked light gray line represents the isotype control. (c) Concentration dependence of the percent change in cell surface ALK, as measured by MFI, for cells incubated for 72 h with varying concentrations of crizotinib as compared with vehicle. (d) Time course of percentage change in cell surface ALK levels (over that seen for vehicle treatment) when cells were incubated with 1000 nm crizotinib.
Crizotinib sensitizes cells to ALK antibody-mediated growth inhibition
To test the hypothesis that crizotinib-induced accumulation of cell surface ALK sensitizes cells to ALK antibody treatment, we next compared the ability of the ALK antibody to inhibit growth of SY5Y cells alone or together with crizotinib. Treatment with crizotinib alone (at a sub-IC50 dose of 333 nm) or ALK antibody alone led to measurable (but limited) growth inhibition (Figure 4a). However, combined TKI and antibody treatment had a significantly larger inhibitory effect as compared with TKI (P<0.0001) or antibody alone (P<0.001), leading to almost complete growth inhibition of SY5Y cells. Increases in total ALK levels were also seen by western blotting (Figure 4b) in cells treated with crizotinib (alone or in combination with ALK antibody), consistent with the results shown in Figure 3. On the other hand, phosphorylated ALK levels were substantially diminished by crizotinib treatment, alone or together with antibody (Figure 4b), suggesting that the TKI stabilizes ALK while simultaneously blocking its activation. We also considered whether crizotinib-induced upregulation of cell surface ALK might promote antibody-mediated ADCC. To address this, we repeated the in vitro ADCC assays using SY5Y cells preincubated with crizotinib or vehicle as targets. As shown in Figure 4c, crizotinib preincubation significantly increased ADCC at effector to target ratios of 50:1 (crizotinib-treated mean = 55.7±9.3%; vehicle-treated mean=31.1±14.7%; P=0.0331) and 25:1 (crizotinib-treated mean =33.7±2.5%; vehicle-treated mean = 17.3±7.9%; P=0.0262), providing further evidence for the ability of crizotinib to sensitize neuroblastoma cells to ALK antibody treatment.
Figure 4.
Dual antibody/TKI targeting of ALK. SY5Y cells were treated with either 333 nm crizotinib or 10 µg/ml anti-ALK antibody (mAb30+mAb49) or both. As negative control, cells were treated with equal volumes of dimethyl sulfoxide and 10µg/ml murine IgG1. (a) Cell growth was monitored by Real-Time Cell Electronic Sensing. (b) Immunoblot analysis of native ALK protein levels (upper panel) and phospho-ALK (middle panel). β-Actin levels are shown as a loading control (lower panel). (c) Effect of crizotinib pretreatment on anti-ALK antibody-mediated ADCC was measured. SY5Y cells were preincubated in the presence of crizotinib or vehicle for 48h, harvested and then used as target cells in the in vitro ADCC assay. **P<0.01 and *P<0.05.
ALK antibody improves sensitivity to a broad range of crizotinib doses
We next explored whether ALK antibody treatment could improve sensitivity of SY5Y cells to growth inhibition by crizotinib across a range of doses. We treated SY5Y cells with a 4-log range of crizotinib in the presence or absence of ALK antibody. Figure 5a shows that the addition of ALK antibody significantly enhanced growth inhibition at all crizotinib doses, except for the highest dose of 10 000 nm, a supralethal dose at which the majority of TKI effect is likely to be off-target. These data also reveal that addition of the ALK antibody shifts the crizotinib dose-response curve. Although 333 nm crizotinib is required to achieve 25% growth inhibition when added alone (Figure 5a), the same effect (mean=33.7±2.8%) can be achieved with just 10 nm crizotinib when antibody is included. As suggested by this finding, antibody treatment reduced the IC50 for crizotinib treatment of SY5Y cells from 3018 nm (crizotinib alone) to 1745 nm for dual treatment (Figure 5b). This is a particularly important result for SY5Y cells and other neuroblastoma cell lines that harbor an F1174L ALK mutation, as we have shown in other studies that this mutation causes resistance to crizotinib inhibition at typical inhibitor levels.24 The data in Figure 5a reveal that simultaneous application of antibody treatment can restore crizotinib sensitivity in these cells.
Figure 5.
Effects of an antagonist ALK antibody on crizotinib dose - response curve. SY5Y cells were treated with crizotinib at the indicated doses, either alone or in combination with 10µg/ml (total) ALK antibody mAb30 and mAb49. Cell growth was measured at day 7 using Real-Time Cell Electronic Sensing. (a) Comparison of growth inhibition for monotherapy with dual ALK targeting: white bars represent crizotinib alone and gray bars represent crizotinib treatment in the presence of 10µg/ml anti-ALK. **P<0.01 and *P<0.05. (b) IC50 was calculated over a range of 10 doses of crizotinib alone (circles) or crizotinib plus 10 µg/ml anti-ALK (squares), yielding IC50 values of 3018 nm and 1745 nm, respectively.
Dual ALK targeting enhances programmed cell death
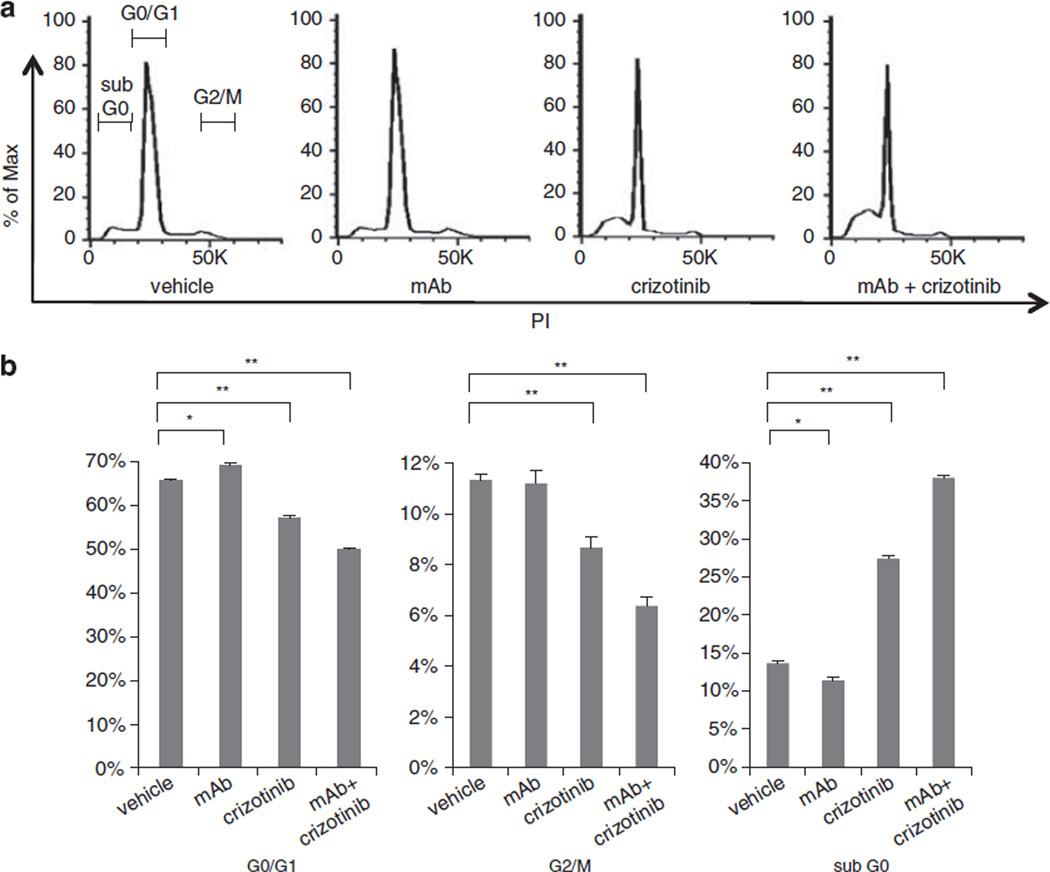
To determine the effect of ALK targeting on cell cycle progression, we next analyzed the impact of TKI and/or antibody exposure on SY5Y cellular DNA content by flow cytometry. As shown in Figure 6a, and quantified in Figure 6b, treatment with antibody alone led to a small, but significant, increase in the G0/G1 fraction (antibody-treated mean=69.2±0.5%; vehicle-treated mean =65.7±0.3%) and a small but significant decrease in the sub G0 fraction (antibody-treated mean=11.4±0.3%; vehicle-treated mean=13.7±0.2%), suggesting that the main mechanism of antibody action may be G1 arrest. On the other hand, dual antibody and TKI ALK targeting led to large and significant increases in the sub-G0 fraction (mean=38.0±0.4%), suggesting induction of programmed cell death as the dominant mechanism of dual ALK targeting. Treatment with crizotinib alone also increased the sub-G0 fraction, but to a smaller extent than seen with dual treatment, consistent with the findings above that anti-ALK potentiates crizotinib effects.
Figure 6.
Cell cycle analysis of inhibitor-treated cells. SY5Y cells were treated with 1000 nm crizotinib, 10 µg/ml antibody, both, or vehicle/IgG1, and were then harvested, fixed, stained with propidium iodide and analyzed by flow cytometry. (a) Representative histograms showing proportion of cells in sub-G0/apoptosis, G0/G1 and G2/mitosis. (b) Quantification of flow cytometry results. **P<0.01 and *P<0.05.
DISCUSSION
Cure rates among children with high-risk neuroblastoma have shown only modest improvement, despite dramatic escalations in the intensity of therapy provided, and survivors of modern high-risk neuroblastoma therapy are at risk for major morbidities.40,41 New approaches are required for treating these patients, and advances in our understanding of its molecular basis have identified tractable therapeutic targets that may respond to novel agents. One promising approach is to target the RTK ALK, which can be inhibited by TKIs and may also be a valuable target for immunotherapy. A recent phase 3 clinical trial using an antibody against the disialoganglioside GD2 has shown the promise of targeted immunotherapy in neuroblastoma.42 We show here that ALK is also expressed in the vast majority of neuroblastoma cases, suggesting that it may represent another tractable immunotherapy target. Moreover, similar to GD2, ALK expression is largely limited to tumor tissue,32 making it an ideal immunotherapy target, while minimizing the risk of cytotoxicity in nonmalignant tissue and simplifying patient selection.
Similar to the GD2 antibody, and several clinically successful antibodies such as trastuzumab and cetuximab, we find that an ALK antibody can mediate ADCC of ALK-positive neuroblastoma cells. In our in vitro ADCC system, neuroblastoma cells were killed when treated with 1µg/ml anti-ALK antibody, a lower dose than the 10 µg/ml clinically achieved in trastuzumab-treated breast cancer patients,43 and well below the trough plasma antibody concentrations achieved in two phase I studies of lung cancer patients treated with cetuximab.44,45 Our experiments were not designed to determine the optimal ADCC dose, but nonetheless argue that antibody targeting of ALK is likely to be an important therapeutic avenue based on its ADCC effects alone. Additional studies will be required to determine the minimum ALK antibody dose at which ADCC can be induced, the range of effective concentrations and dependence of ADCC sensitivity on ALK expression level and genotype.
Unlike GD2 antibodies, ALK antibodies bind to and inhibit an oncogenic receptor–permitting an additional immune cell-independent component of its inhibitory mechanism. ALK overexpression or mutation leads to its hyperactivation, autophosphorylation and elevated downstream signaling,13 as also seen for HER2 and EGFR in breast and lung cancer.46 The oncogenic role of full-length ALK in neuroblastoma was originally described when activating mutations were discovered in the germline of patients with familial neuroblastoma, and were subsequently found to be somatically acquired.9,10,47,48 More recently, it was shown that high levels of ALK expression are associated with decreased survival.49 Thus, ALK signaling–enhanced by mutation or overexpression–has an important role in initiation and/or maintenance of neuroblastoma. ALK inhibition with crizotinib or other TKIs is likely to be an important tool in treating ALK-dependent neuroblastoma, but it is already clear that certain ALK-activating mutations reduce sensitivity to this drug.24 Antagonistic ALK antibodies may offer a parallel approach to ALK inhibition, just as has been reported for antibodies that target HER2 or EGFR. We show here that an antagonist ALK antibody inhibits growth of neuroblastoma cells over a range of doses, all of which are below (or equal to) those reported to be clinically achievable in patients with trastuzumab or cetuximab. The ALK antibody inhibits growth of neuroblastoma cell lines that harbor either amplified/overexpressed ALK (NB1) or ALK with the most common constitutively activating mutations (R1275Q in 1643 cells and F1174L in SY5Y cells). Notably, SY5Y cells are relatively resistant to TKI targeting of ALK, but respond to antibody inhibition. ALK antibody therapy may therefore be relevant for patient tumors exhibiting a broad range of ALK expression levels and aberrations.
Our results strongly argue for dual antibody and TKI targeting of ALK–the utility of which has been demonstrated for several other oncogenic RTKs.21,22,50–52 For non-small-cell lung cancer expressing erlotinib-resistant EGFRT790M, such dual targeting was the only therapeutic approach among several studied that induced tumor regression.53 The parallels between T790M-mutated EGFR and (crizotinib resistant) F1174L-mutated ALK are robust,24 suggesting that dual antibody/TKI therapy may be particularly important in this case. Binding of crizotinib promotes cell surface accumulation of ALK, as has been observed for several other therapeutically targeted TKIs–a likely key mechanism for the efficacy of dual TKI/antibody targeting.21 Combined antibody/TKI treatment in F1174L-expressing cells led to almost complete growth inhibition, induced a significantly higher level of apoptosis than either treatment alone and reduced the IC50 for crizotinib treatment by half. This implies that dual ALK targeting may be a relevant therapeutic strategy for decreasing the dose-dependent toxicities associated with TKI therapy and may even provide potential for delaying or preventing TKI resistance.
Our results point to the urgent need for development of clinically relevant inhibitory ALK antibodies and their evaluation with in vivo preclinical models and clinical trials. Ongoing work to develop a clinically relevant anti-ALK antibody includes humanization to reduce immunogenicity,54 defucosylation to maximize ADCC,55 and possible conjugation to immunostimulatory cytokines, such as IL-2 or cytotoxic agents.56 Preclinical optimization of anti-ALK antibody design will be crucial for maximizing success in the clinic.
MATERIALS AND METHODS
Cell culture and reagents
Cell lines were maintained in a 5% CO2 incubator at 37°C in complete RPMI (Invitrogen, Carlsbad, CA, USA). Mouse monoclonal immunoglobulin IgG1 antibodies 14, 30, 46 and 49 were generated to the extracellular domain of human ALK as previously described57 and provided by Dr Marc Vigny. Murine IgG1 (Sigma-Aldrich, St Louis, MO, USA) and dimethyl sulfoxide were used as negative controls.
Quantitative mRNA expression
RNA from primary diagnostic tumor specimens of 229 neuroblastomas was analyzed using Affymetrix (Santa Clara, CA, USA) Human Exon Arrays (HuEx), normalized by quantile normalization and summarized using robust multichip average (Affymetrix Power Tools software package version 1.12). ALK expression levels were obtained by averaging the core unique probe sets for the ALK transcript (transcript ID: 2546409). Significance was determined among patients diagnosed with LR (stage 1 and 2, n = 24), HRN (>18 months of age at diagnosis, n = 141) and HRA (n = 64). The P-value was calculated using likelihood ratio χ2-tests in the context of a general linear model. Differences between subgroups were assessed using the Wald test. Analyses were performed using the R statistical package (R version 12.1, Auckland, New Zealand).
Immunohistochemistry
Cases were selected from a review of tumors accessioned to the Pathology Department of the Children’s Hospital of Philadelphia (CHOP) from 1987 to 2004. Specimen selection and construction of the TMA followed approval by the CHOP Institutional Review Board. Tumors were reviewed by a pediatric pathologist for adequacy, and classified using International Neuroblastoma Pathology Classification criteria. The TMA comprised two paraffin blocks and contained tumor cores from 126 patients (117 neuroblastomas and 9 nodular ganglioneuroblastomas). MYCN amplification status and stage were obtained by tumor registry review. Staining was conducted with pre-diluted anti-ALK-1 (Ventana Medical Systems, Oro Valley, AZ, USA) using pressure cooker antigen retrieval, overnight incubation and avidin-biotin complex conjugation. An ALK staining score was calculated as the product of staining intensity grade (1–3; weak, moderate and strong) and percentage of neuroblasts stained. The Food and Drug Administration normal human organ TMA (US Biomax, Rockville, MD, USA) was similarly analyzed.
Microscopy
Formalin-fixed paraffin-embedded tissue slides of 16 neuroblastoma patient samples were rinsed in xylene, rehydrated with ethanol and treated in a pressure cooker with High pH Retrieval Solution (Dako, Carpinteria, CA, USA). Slides were incubated with primary ALK-1 antibody (Ventana Medical Systems), followed by rabbit pan-cadherin antibody (Abcam, Cambridge, MA, USA). Slides were then incubated with secondary antibodies Alexa-488 anti-Rabbit (Invitrogen) and Alexa-594 anti-Mouse (Invitrogen) and counterstained in DAPI hydrochloride (Sigma, Ronkonkoma, NY, USA). Tissue sections were visualized using Olympus IX-81 DSU spinning disk confocal microscope (Olympus, Center Valley, PA, USA) through a 60 × 1.49 NA oil immersion objective. Images were analyzed and Pearson’s correlation coefficient values generated using Volocity software (Improvision, Waltham, MA, USA). To grade staining, settings were determined at the beginning of the experiment, so that unstained controls were negative and single-stained controls were not emitting signal detectable using filter sets intended for imaging other fluorophores.
Immunofluorescence
Cell lines were plated overnight on Lab Tek II Chamber Slides (Nunc Thermo Scientific, Roskilde, Denmark), then transferred to ice and washed with ice-cold phosphate-buffered saline before blocking. Cells were incubated for 60 min on a rocker table with ALK antibody mAb14, washed and incubated for 45 min with rhodamine-conjugated goat anti-mouse secondary antibody (Jackson, West Grove, PA, USA), washed and cover-slipped with DAPI-containing mounting media (Santa Cruz Biotechnology, Santa Cruz, CA) for evaluation with a fluorescence microscope.
Flow cytometry
Cells were kept ice cold during staining to minimize receptor endocytosis, harvested and washed, and then ALK antibody mAb14 was added for a 10 µg/ml final concentration. Donkey anti-mouse IgG PE (eBioscience, San Diego, CA, USA) was then added (2.5 µg/ml). In some experiments, cells were treated with crizotinib (or dimethyl sulfoxide as a negative control) and harvested at subsequent time points for flow cytometry. In other experiments, staining was conducted with mAb46, which binds to a distinct epitope from mAb14. Cells were analyzed on an LSR II flow cytometer (BD Biosciences, San Jose, CA, USA). All results shown are representative of at least three independent experiments.
Growth inhibition
We used the Real-Time Cell Electronic Sensing system (ACEA Biosciences, San Diego, CA, USA) to measure the in vitro effect of ALK antibodies mAb30 and mAb49 alone or in combination with crizotinib. Cells were plated in triplicate, and antibody and/or drug added 24 h later. Antibody treatment was continued for 4 days. Growth inhibition was calculated as: 100 ×(1– (cell index treatment/cell index control)). All cell lines were mycoplasma tested and genotyped using the AmpFLSTR Identifiler kit (Applied Biosystems, Foster City, CA, USA). All experiments were conducted a minimum of three times, and results quoted as mean ± s.d. Linear mixed effect models were fitted for statistical analysis of time and treatment effects. To account for nonlinearity, time by treatment and time squared by treatment interaction terms were included in the models. F tests were used to examine the difference of the progression of cell index over time between each individual and the combination treatment. For IC50 calculation, cells were plated in a 5-log dose range of crizotinib alone or in combination with 10µg/ml ALK mAb30 and mAb49. GraphPad Prism Version 5.0 (GraphPad Prism, La Jolla, CA, USA) was used to calculate the IC50 from the Real-Time Cell Electronic Sensing-generated cell index data using the log (inhibitor) versus response–variable slope equation.
Surface biotinylation and western blots
Cells were grown in 10 cm dishes until 70–80% confluency, treated with crizotinib, ALK antibody, the combination, or vehicle at the times and doses indicated. For biotinylation, EZ-LINK Sulfo-Biotin (Pierce, Rockford, IL, USA) was used according to manufacturer instructions. Biotinylation was performed after 24 h treatment with 1000 nm crizotinib (or control). Cells were washed with ice-cold phosphate-buffered saline, lysed and biotin-labeled protein precipitated with NeutrAvidin beads (Thermo Scientific, Rockford, IL, USA). Whole-cell lysates or NeutrAvidin precipitates were harvested and immunoblotted using antibodies against ALK (Cell Signaling, Danvers, MA, USA), phospho-ALK Tyr 1604 (Cell Signaling), actin (Santa Cruz Biotechnology) or cadherin (Abcam). Results shown are representative of at least two independent experiments.
Propidium iodide staining
At 24 h post plating, cells were treated with 1000 nm crizotinib, 10 µg/ml ALK antibody, both agents or negative controls. Antibody treatment was continued for four additional days. Cells were harvested, stained with propidium iodide and analyzed on an LSR II flow cytometer.
Antibody-dependent cell-mediated cytotoxicity assay
The CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA) was used to assess ADCC. Normal donor peripheral blood mononuclear cells were used as effectors and plated for 2 h to allow monocytes to adhere. Non-adherent lymphocytes were replated in complete RPMI containing 1000 IU/ml rhIL-2 (Chiron, Emeryville, CA, USA), collected and washed. Target NB1 or SY5Y cells were harvested and washed. In some experiments, target SY5Y cells were preincubated 48 h in crizotinib. Effector and target cells were plated in quadruplicate at effector: target ratios of 50:1, 25:1 and 10:1, and experimental wells treated with 1 µg/ml ALK mAb30 and mAb49. Control wells were plated according to manufacturer’s specifications. After a 4-h incubation at 37 °C, cell viability was assessed by an enzymatic assay quantifying lactate dehydrogenase release upon cell lysis. Percent (%) cytotoxicity was calculated:
Experiments were conducted comparing untreated wells with treatment with 1µg/ml murine IgG1. No difference was detected between untreated (% cytotoxicity=3.1%+0.3) and IgG1-treated wells (0.64+0.28%; P=0.8822). Results are representative of three independent experiments, quoted as mean±standard deviation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Pfizer for their gift of crizotinib, and Dr Marc Vigny for his gift of the ALK monoclonal antibodies 30, 49, 46 and 14. This work was supported in part by NIH Grants R01-CA140198 (YPM), 2R01 CA60104–16 (RCS), 2R01 CA60104–16S1 (RCS), the Children’s Oncology Group, the Carly Hillman Fund (YPM), NIH Training Grant in Structural Biology T32-GM008275 (SCB), a fellowship grant from the St Baldrick’s Foundation (ACW) and the US Army Peer-Reviewed Medical Research Program (W81XWH-10–1–0212/3 to MAL/YPM).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 2.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4.Jazii FR, Najafi Z, Malekzadeh R, Conrads TP, Ziaee AA, Abnet C, et al. Identification of squamous cell carcinoma associated proteins by proteomics and loss of beta tropomyosin expression in esophageal cancer. World J Gastroenterol. 2006;12:7104–7112. doi: 10.3748/wjg.v12.i44.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 6.Miyake I, Hakomori Y, Shinohara A, Gamou T, Saito M, Iwamatsu A, et al. Activation of anaplastic lymphoma kinase is responsible for hyperphosphorylation of ShcC in neuroblastoma cell lines. Oncogene. 2002;21:5823–5834. doi: 10.1038/sj.onc.1205735. [DOI] [PubMed] [Google Scholar]
- 7.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O’Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George RE, Attiyeh EF, Li S, Moreau LA, Neuberg D, Li C, et al. Genome-wide analysis of neuroblastomas using high-density single nucleotide polymorphism arrays. PLoS One. 2007;2:e255. doi: 10.1371/journal.pone.0000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazot P, Cazes A, Boutterin MC, Figueiredo A, Raynal V, Combaret V, et al. The constitutive activity of the ALK mutated at positions F1174 or R1275 impairs receptor trafficking. Oncogene. 2011;30:2017–2025. doi: 10.1038/onc.2010.595. [DOI] [PubMed] [Google Scholar]
- 12.George R, Attiyeh E, Li S, Moreau L, Neuberg D, Li C, et al. Genome-wide analysis of neuroblastomas using high-density single nucleotide polymorphism arrays. PLoS One. 2007;2:e255. doi: 10.1371/journal.pone.0000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osajima-Hakomori Y, Miyake I, Ohira M, Nakagawara A, Nakagawa A, Sakai R. Biological role of anaplastic lymphoma kinase in neuroblastoma. Am J Pathol. 2005;167:213–222. doi: 10.1016/S0002-9440(10)62966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout EM, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010;16:4353–4362. doi: 10.1158/1078-0432.CCR-09-2660. [Meta-Analysis Research Support, Non-US Government] [DOI] [PubMed] [Google Scholar]
- 15.Passoni L, Longo L, Collini P, Coluccia AM, Bozzi F, Podda M, et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009;69:7338–7346. doi: 10.1158/0008-5472.CAN-08-4419. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. (Review) [DOI] [PubMed] [Google Scholar]
- 19.Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 20.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–3010. doi: 10.1172/JCI38746. [Research Support, NIH, Extramural Research Support, Non-US Government] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–814. doi: 10.1038/onc.2008.432. [Research Support, Non-US Government] [DOI] [PubMed] [Google Scholar]
- 22.Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213–6221. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 23.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [Clinical Trial, Phase I Multicenter Study Research Support, NIH, Extramural Research Support, Non-US Government] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bresler SC, Wood AC, Haglund EA, Courtright J, Belcastro LT, Plegaria JS, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med. 2011;3:108ra14. doi: 10.1126/scitranslmed.3002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George RE, Sanda T, Hanna M, Frohling S, Luther W, 2nd, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70:10038–10043. doi: 10.1158/0008-5472.CAN-10-2956. [Research Support, NIH, Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18:73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L, et al. The neuroblastoma associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK translocated cancers. Cancer Res. 2010;70:10038–10043. doi: 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haupt R, Garaventa A, Gambini C, Parodi S, Cangemi G, Casale F, et al. Improved survival of children with neuroblastoma between 1979 and 2005: a report of the Italian Neuroblastoma Registry. J Clin Oncol. 2010;28:2331–2338. doi: 10.1200/JCO.2009.24.8351. [DOI] [PubMed] [Google Scholar]
- 31.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [Multicenter Study Randomized Controlled Trial Research Support, NIH, Extramural Research Support, US Government, PHS] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Diskin S, Rappaport E, Attiyeh E, Mosse Y, Shue D, et al. Integrative genomics identifies distinct molecular classes of neuroblastoma and shows that multiple genes are targeted by regional alterations in DNA copy number. Cancer Res. 2006;66:6050–6062. doi: 10.1158/0008-5472.CAN-05-4618. [DOI] [PubMed] [Google Scholar]
- 34.Moog-Lutz C, Degoutin J, Gouzi JY, Frobert Y, Brunet-de Carvalho N, Bureau J, et al. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J Biol Chem. 2005;280:26039–26048. doi: 10.1074/jbc.M501972200. [DOI] [PubMed] [Google Scholar]
- 35.Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50:5234–5239. [Research Support, Non-US Government Research Support, US Government, PHS] [PubMed] [Google Scholar]
- 36.Bougherara H, Subra F, Crepin R, Tauc P, Auclair C, Poul MA. The aberrant localization of oncogenic kit tyrosine kinase receptor mutants is reversed on specific inhibitory treatment. Mol Cancer Res. 2009;7:1525–1533. doi: 10.1158/1541-7786.MCR-09-0138. [DOI] [PubMed] [Google Scholar]
- 37.Tabone-Eglinger S, Subra F, El Sayadi H, Alberti L, Tabone E, Michot JP, et al. KIT mutations induce intracellular retention and activation of an immature form of the KIT protein in gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:2285–2294. doi: 10.1158/1078-0432.CCR-07-4102. [DOI] [PubMed] [Google Scholar]
- 38.Bougherara H, Subra F, Crepin R, Tauc P, Auclair C, Poul MA. The aberrant localization of oncogenic kit tyrosine kinase receptor mutants is reversed on specific inhibitory treatment. Mol Cancer Res. 2009;7:1525–1533. doi: 10.1158/1541-7786.MCR-09-0138. [DOI] [PubMed] [Google Scholar]
- 39.Tabone-Eglinger S, Subra F, El Sayadi H, Alberti L, Tabone E, Michot JP, et al. KIT mutations induce intracellular retention and activation of an immature form of the KIT protein in gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:2285–2294. doi: 10.1158/1078-0432.CCR-07-4102. [DOI] [PubMed] [Google Scholar]
- 40.Hobbie WL, Moshang T, Carlson CA, Goldmuntz E, Sacks N, Goldfarb SB, et al. Late effects in survivors of tandem peripheral blood stem cell transplant for high-risk neuroblastoma. Pediatr Blood Cancer. 2008;51:679–683. doi: 10.1002/pbc.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 42.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 44.Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 45.Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti–epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 46.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 48.Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, Combaret V, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 49.De Brouwer S, De Preter K, Kumps C, Zabrocki P, Porcu M, Westerhout EM, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res. 2010;16:4353–4362. doi: 10.1158/1078-0432.CCR-09-2660. [DOI] [PubMed] [Google Scholar]
- 50.Johns TG, Luwor RB, Murone C, Walker F, Weinstock J, Vitali AA, et al. Antitumor efficacy of cytotoxic drugs and the monoclonal antibody 806 is enhanced by the EGF receptor inhibitor AG1478. Proc Natl Acad Sci USA. 2003;100:15871–15876. doi: 10.1073/pnas.2036503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 52.Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–6501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 53.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- 55.Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, et al. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res. 2005;11:2327–2336. doi: 10.1158/1078-0432.CCR-04-2263. [DOI] [PubMed] [Google Scholar]
- 56.Hughes B. Antibody-drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov. 2010;9:665–667. doi: 10.1038/nrd3270. [DOI] [PubMed] [Google Scholar]
- 57.Moog-Lutz C, Degoutin J, Gouzi JY, Frobert Y, Brunet-de Carvalho N, Bureau J, et al. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J Biol Chem. 2005;280:26039–26048. doi: 10.1074/jbc.M501972200. [Research Support, Non-US Government] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.