Abstract
Biosynthesis of selenium-containing proteins requires insertion of the unusual amino acid selenocysteine by alternative translation of a UGA codon, which ordinarily serves as a stop codon. In eukaryotes, selenoprotein translation depends upon one or more selenocysteine insertion sequence (SECIS) elements located in the 3′-untranslated region of the mRNA, as well as several SECIS-binding proteins. Our laboratory has previously identified nuclease sensitive element binding protein 1 (NSEP1) as another SECIS-binding protein, but evidence has been presented both for and against its role in SECIS binding in vivo and in selenoprotein translation. Our current studies sought to resolve this controversy, first by investigating whether NSEP1 interacts closely with SECIS elements within intact cells. After reversible in vivo cross-linking and ribonucleoprotein immunoprecipitation, mRNAs encoding two glutathione peroxidase family members co-precipitated with NSEP1 in both human and rat cell lines. Co-immunoprecipitation of an epitope-tagged GPX1 construct depended upon an intact SECIS element in its 3′-untranslated region. To test the functional importance of this interaction on selenoprotein translation, we used small inhibitory RNAs to reduce the NSEP1 content of tissue culture cells and then examined the effect of that reduction on the activity of a SECIS-dependent luciferase reporter gene for which expression depends upon readthrough of a UGA codon. Co-transfection of small inhibitory RNAs directed against NSEP1 decreased its expression by approximately 50% and significantly reduced luciferase activity. These studies demonstrate that NSEP1 is an authentic SECIS binding protein that is structurally associated with the selenoprotein translation complex and functionally involved in the translation of selenoproteins in mammalian cells.
Selenoproteins comprise a small but biochemically important set of polypeptides that incorporate the micronutrient selenium within the unusual amino acid selenocysteine (Sunde, 1990; Burk and Hill, 1993; Stadtman, 1996; Köhrle et al., 2000; Driscoll and Copeland, 2003). Selenium closely resembles sulfur, the element immediately above it in the periodic table, so selenocysteine functions similarly to cysteine except greater reactivity with nucleophilic substrates (Hu and Tappel, 1987). Prokaryotic selenoproteins include formate dehydrogenases, selenophosphate synthetases, hydrogenase, glycine reductase, nicotinic acid hydroxylase, and xanthine dehydrogenase (Stadtman, 1990; Wilting et al., 1998; Thanbichler and Bock, 2002). Mammalian selenoproteins include important antioxidants such as the glutathione peroxidase (GPx) family (Chambers et al., 1986; Esworthy et al., 1991; Schuckelt et al., 1991; Chu et al., 1993) and thioredoxin reductase (Gladyshev et al., 1996), the iodothyronine 5′deiodinase family (Berry et al., 1991b; Larsen and Berry, 1995), and members with unknown function such as selenoproteins P and W (Burk and Hill, 1994; Vendeland et al., 1995; Hill and Burk, 1997).
Synthesis of both prokaryotic and eukaryotic selenoproteins requires insertion of the selenocysteine by alternative translation of a UGA codon by a unique selenocysteine-charged tRNA (Lee et al., 1989; Sunde, 1990; Böck et al., 1991; Diamond et al., 1993). Thus, the codon that otherwise serves as a termination signal instead encodes the “21st amino acid.” A critical question in the understanding of the recoding process is how the ribosomal translation assembly can distinguish between the far more common UGA “stop” signals as opposed to those in selenoprotein-encoding transcripts that are read as selenocysteine.
In prokaryotes, translation of the UGA codon depends upon a 40-base stem-loop structure in the mRNA. The selenocysteine insertion sequence (SECIS), located within the open reading frame immediately downstream from the UGA codon (Zinoni et al., 1990; Böck et al., 1991), binds a specialized translation elongation factor, termed SelB, which is required for selenocysteine incorporation in bacteria (Forchhammer et al., 1989; Baron et al., 1993).
In contrast, translation of eukaryotic selenoprotein mRNA depends upon an 80–90 nucleotide SECIS element in the 3′-untranslated region (Berry et al., 1991a, 1993; Shen et al., 1993), located anywhere from 50 to more than 4,000 nucleotides downstream of the UGA codon (Lee et al., 1993; Martin et al., 1996). Eukaryotic SECIS elements have been classified into two structurally-related classes that share a wellconserved secondary structure comprised of a stem-loop with several bulges and incorporating very short highly-conserved sequences in the loop and a quartet of non-Watson-Crick base pairs at the base of the stem (Walczak et al., 1998; Grundner-Culemann et al., 1999). Aside from these conserved motifs, the primary nucleotide sequences are highly variable, and function of the SECIS depends largely on the secondary structure of the entire element (Shen et al., 1995).
In prokaryotic cells, ribosomal translation of the UGA codon as selenocysteine requires the assembly of a quaternary complex including not only the UGA codon, SECIS element, and specific aminoacylated tRNA (tRNASec), but also a specialized elongation factor, SelB, that binds to the bacterial SECIS and tRNASec, tethers them to the ribosome, and catalyzes the GTP hydrolysis necessary for polypeptide chain elongation (Forchhammer et al., 1989; Thanbichler and Böck, 2002). Its mammalian homolog, termed mSelB or eEFSEC, has been identified by sequence homology searches of EST databases (Tujebajeva et al., 2000; Fagegaltier et al., 2000b). This selenocysteine-specific elongation factor recognizes tRNASec, binds GTP in the presence of the aminoacylated tRNA and contributes to selenoprotein synthesis in intact cells, but does not interact directly with the SECIS RNA (Tujebajeva et al., 2000; Fagegaltier et al., 2000b; Driscoll and Copeland, 2003).
Thus, the mammalian selenocysteine translation apparatus appears to be more complex than the prokaryotic, with additional proteins involved in SECIS recognition and binding. The best-established of these constituents is SECIS-binding protein 2 (SBP2), a 120 kDa protein originally isolated and cloned from rat testis (Lesoon et al., 1997; Copeland and Driscoll, 1999; Copeland et al.,2000).The amino acid sequence includes domains homologous to ribosomal RNA-binding regions and a yeast suppressor of translation termination, suggesting that it functions to prevent termination, and hence to favor selenocysteine insertion, at the UGA codon (Copeland and Driscoll, 1999; Copeland et al., 2000; Lescure et al., 2002). It recognizes the SECIS of selenoprotein-encoding mRNAs from multiple mammalian species, with binding sites at the conserved non-Watson-Crick base pairs and the proximal helical region of the stem-loop (Fletcher et al., 2001).
Our laboratory has previously identified nuclease sensitive element binding protein 1 (NSEP1) as another SECIS-binding protein (Shen et al., 1998). This protein was originally isolated and named DNA binding protein B (DBPB) in accordance with its DNA-binding properties (Didier et al., 1988; Sakura et al., 1988; Horwitz et al., 1994) and has also been termed Y box binding protein 1 (YB-1) in recognition of its role as a transcription factor (Didier et al., 1988; Kohno et al., 2003). However, it also contains four arginine-rich motifs characteristic of a group of RNA-binding proteins (Burd and Dreyfuss, 1994) and specifically binds to the SECIS element of human cellular glutathione peroxidase mRNA. NSEP1 lacks properties, such as tRNASec binding and GTPase activity, associated with mSelB; so, like SBP2, it probably functions as part of a large selenocysteine translation complex. However, Krol’s group independently identified NSEP1 as a possible SECIS-binding protein, but raised doubts about its importance in selenoprotein biosynthesis (Fagegaltier et al., 2000a), so its functional role has remained controversial.
In the present study, we have further investigated the function of NSEP1 in tissue culture cells by ribonucleoprotein immunoprecipitation (RIP) (Niranjanakumari et al., 2002) to determine whether it associates in vivo with selenoprotein-encoding mRNA and by small interfering RNA (siRNA) silencing (Zamore et al., 2000;Mello and Conte, 2004; Novina and Sharp, 2004) of NSEP1 expression to determine its functional importance in selenoprotein translation.
MATERIALS AND METHODS
Constructs, formaldehyde crosslinking and cell lysis
RIP was performed by modification of a previously-described technique (Niranjanakumari et al., 2002). Formaldehyde solution (AR grade, Mallinckrodt, St. Louis) was added to the final concentration of 1%, to McArdle 7777 or HeLa cells actively growing as a monolayer in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) plus 10% fetal bovine serum (Invitrogen), sodium selenite 5 × 10−9 g/ml, and penicillin–streptomycin (Invitrogen). For experiments examining RIP of influenza hemagglutinin (HA) epitope-tagged rather than endogenous GPx, the procedures were performed on Saos-2 osteosarcoma cells (Rodan et al., 1987) previously stably transfected with the tTA tetracycline regulator plasmid (Shockett and Schatz, 1996) (kindly provided by Dr. Janet Stein, University of Massachusetts Medical School) and then stably transfected with the Tet-off plasmid pTet-Bsd-IRES-EGFP (Rabiet et al., 2002) (kindly provided by Prof. Franc¸ois Boulay, Commissariat à l’Energie Atomique, Grenoble) containing cDNA encoding epitope-tagged human GPx1 with either the native 3′UTR or a partial or complete SECIS deletion mutant as described and diagrammed in the “Results” section, below.
Epitope-tagged GPX1 cDNA was constructed by inserting a DNA fragment encoding the HA epitope into the 5′ end of the GPX1 sequence just upstream to the TAG stop codon, using a standard overlap extension technique. PCR was performed on a wild type GPX1 cDNA in pBluscriptKS-GPxR construct (Shen et al., 1993) using a standard reverse T7 primer for the flanking pBluscript KS sequence and the following forward primers: GPX1 -HA fusion: GCTGTCTCAAGGGCTCAGCTGTGCCTACCCATAC-GACGTCCCAGACTACGCTTAGGGCGCCCCTCCTACCCC; flanking GPX1 sequence: ATATATTCTAGAATGTGTGCT-GCTCGGCTAGCGGCG; After two round of PCR, the final amplicon was digested with restriction endonucleases Xba1 and Spe1, then ligated into pTet-Bsd-EGFP. The resultant clones from E. coli transformants were verified by DNA sequencing. Subsequently, using the this GPX1 -HA construct in pTet-Bsd-EGFP as a template, the overlap extension was used to make SECIS deletions with the following primers: Forward primer for deletion of entire SECIS element: CTGCTTGGCAGTTGCAGTGCTGCTGTGGGTGCTGGTCC-TGTTGATCCCAG; Forward primer for deletion of the 5′ basal stem: CTGCTTGGCAGTTGCAGTGCTGCTGATGAGGGTG-TTTCCTCTAAACCTACGAGGG; Forward primer for deletion of the distal stem and terminal loop: TGTCTCGGG-GGGGTTTTCATCTAGAAAATACCACCTCGAGATGGGTGC; Forward flanking primer in pTet-Bsd-EGFP: GACCTCCATA-GAAGACACCGGGACC; Reverse flanking primer in pTet-Bsd-EGFP: GCGGCTTCGGCCAGTAACGTTAGGG; The final PCR products were subcloned back into pTet-Bsd-EGFP as described above.
The cross-linking mixture was incubated with gentle shaking at room temperature for 10 min, then 2 M glycine (pH 7.0) solution was added to a final concentration of 0.25 M and the mixture incubated at room temperature for 5 min. After decanting the solution, cells were washed twice with 10 ml of ice-cold phosphate-buffered saline, then suspended with a plastic scraper into1.5 ml of cold phosphate-buffered saline for each 10 cm plate. The cells were centrifuged at 1,000g for 10 min at 4°C and the cell pellet frozen at −80°C.
The cell pellets representing each 10 cm plate were resuspended in 2 ml of ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.5, 1% NP-40, 0.5% sodium deoxycholate, 0.05% SDS, 1 mM EDTA, 150 mM NaCl) containing complete, EDTA-free protease inhibitor cocktail tablet (Roche Molecular Biochemicals, Indianapolis, IN), sonicated three to five times at 4°C for 15 s at an amplitude setting 5 to 10 on a Microson XL 2000 Ultrasonic Liquid Processor (Misonix, Inc., Farmingdale, NY).
The cell lysate was cleared by centrifugation at 14,000g at 4°C for 10 min.
To remove non-specific RNA-binding proteins, an aliquot of cell lysate supernatant, typically 250–500 µl, was incubated with rotation at 4°C for 1 h with 20 µl of Protein A-Sepharose beads (Amersham, Piscataway, NJ) plus E. coli tRNA (100 µg/ ml). The supernatant was recovered by brief centrifugation at 3,000g for 45 s and then used for immunoprecipitation.
Ribonucleoprotein immunoprecipitation and RNA detection
Protein A-Sepharose beads (20 µl) were coated with specific polyclonal antibody of interest at 4°C for 2 h, then washed four times with 1 ml of RIPA buffer containing protease inhibitors, as described above. Subsequently, the beads were incubated at 4°C for 10 min with 0.5 µl of RNasin (Promega, San Luis Obispo, CA) 40 U/µl. The coated beads were incubated at room temperature for 60–90 min with 250–500 µl of precleared cell lysate diluted with equal volume of RIPA buffer. The beads were then centrifuged and washed five times by rotation for 10 min at room temperature with 1 ml of high-stringency RIPA buffer (1 M urea, 50 mM Tris-HCl, pH 7.5, 1% NP-40. 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, and 0.2 mM phenylmethylsulfonyl fluoride). The beads containing the immunoprecipitated ribonucleoprotein complex were then resuspended in 100 µl of 50 mM Tris-HCl, pH 7.0, 5 mM EDTA, 10 mM dithiothreitol, and 1% SDS.
To reverse the crosslinking, the beads were incubated at 70°C for 45 min, then 300 µl of Trizol (Invitrogen) and 80 µl of chloroform were added and mixed thoroughly; the beads were then incubated tumbling at room temperature for 10 min. The aqueous phase was collected after centrifugation at 10,000g for 10 min, then a 5/6 volume of isopropanol and 1 µl of glycoblue (Ambion, Inc., Austin, TX) were added. Precipitate was collected by centrifugation at 14,000g for 10 min at 4°C, washed with 70% ethanol, air-dried at room temperature, and re-suspended in RNase free water. Finally, the sample was subjected to DNase I treatment following the manufacturer’s protocol (DNA-free™, Ambion, Inc.). Complete DNA removal was verified by using the material as template for PCR, using the same primers as for RT-PCR but with no RT reaction.
One tenth of each purified RNA sample served as the template for reverse transcription, using either GPx-specific or random hexamers as reverse primers, using SuperScript III (Invitrogen, Inc.)for reverse transcription (RT) and following manufacturer’s protocol. The following oligonucleotide primer pairs, targeting human and rat mRNA open reading frames for glutathione peroxidase1 (GPx1), phospholipid hydroperoxide glutathione peroxidase (GPx4) and β-actin were used for polymerase chain reaction (PCR) amplification: human GPx1 forward: GTGGCGTCCCTCTGAGGCACCACGGTCCGG;reverse:AAG-CAGCCGGGGTAGGAGGGGCGCCCTAGG;humanGPx1with influenza hemagglutinin (HA) epitope tag forward: TTCCC-TCAAGTACGTCCGGCCTGG; reverse: GTAGTCTGGGACGT-CGTATGGGTA (within the HA sequence) human GPx4 forward: CATGTGCGCGTCCCGGGACGACTGGCGC; reverse: CAGGGGCTCGGGCGGGGCCACACACTTG human β-actin forward: ATGGTGGGCATGGGTCAGAAGGATTC; reverse: TCTTGATCTTCATTGTGCTGGGTGCC; rat GPx1 forward: CTGGGCTCCCTGCGGGGCAAGGTGCTGC; reverse:TCCCC-CCGGAGGGCAGCCAGCCATCACC; rat GPx4 forward: GGCTCTGGCTGTGCCTGGCCTGGCTGGC; reverse: CAGG-GGGGCTCGGTGCAGGGGCCAACAC; rat β-actin forward: GTGTGATGGTGGGTATGGGTCAGAAG; reverse: TCTTGA-TCTTCATGGTGCTAGGAGCC.
PCR was performed using AccuPrime Taq DNA polymerase (Invitrogen) according to the manufacturer’s instructions for 35 cycles.
75Se labeling and selenoprotein detection
Saos-2 cells transfected with pTet-Bsd-IRES-EGFP containing epitope-tagged GPX1 cDNA with either the native 3′UTR or with SECIS deletion mutations (as described above) were cultured with 10 µCi of 75Se as selenous acid (obtained at an original specific activity of 750–1,000 Ci/g from the University of Missouri Research Reactor Facility) for 2 h at37°C. The cells were lysed and immunoprecipitated with antibody to human GPx1 (Shen et al., 1993) or to the influenza HA (Roche), then the proteins were analyzed by SDS–PAGE and autoradiography, all as previously described (Shen et al., 1993).
Transfection of siRNA duplexes and reporter constructs
Four NSEP1 siRNA duplexes were designed and synthesized by Dharmacon, Inc. (Lafayette, CO). Each pair of 21-base RNA oligonucleotides formed a 19 base-pair duplex core, with two-base symmetrical 3′-overhangs and 5 ′ phosphorylated antisense strands in order to optimize the silencing effect. Three of the duplexes proved to be effective for NSEP1 silencing in pilot experiments. The 19-base duplex core sequences of the effective siRNAs were: 1: GAACGGA-TATGGTTTCATC; 2: GCGGAGGCAGCAAATGTTA; 3: GCA-GACCGTAACCATTATA.
The ineffective duplex sequence was: GAGAGACTGTG-GAGTTTGA. A negative control siRNA duplex of similar design but directed against jellyfish green fluorescent protein (GFP) was obtained from Dr. Zhoshang Xu (University of Massachusetts Medical School).
Twenty-four hours before transfection, approximately 12,000 HEK 293 cells (American Type Culture Collection) were split into each well of 96-well tissue culture plates, with each well containing 100 µl DMEM (high glucose; Invitrogen) with 10% fetal bovine serum and 5 × 10−9 g/ml sodium selenite. After incubation at 37°C overnight, the cells were transfected, in accordance with the Invitrogen protocol, by the addition of 50 µl of Opti-MEM1 reduced serum medium (Invitrogen) containing 0.5 µl Lipofectamine 2000 (Invitrogen), 1.5 pmol siRNA duplex in Tris-EDTA buffer, and a reporter construct (200 ng pBPH-sec or pH9A– see descriptions in “Results”) and a transfection efficiency control (20 ng pRL-TK; Promega). After 48 h incubation at 378C, cells were lysed with single detergent lysis buffer (50 mM HEPES pH 7.8, 10 mM EDTA, 1% Triton X-100, 5% glycerol) for Western blot analysis (see below) or with passive lysis buffer (Promega) for measurement of luciferase activity in the Dual-Glo™ (Promega) luminescence assay system in accordance with the manufacturer’s instructions.
For Western blot analysis of NSEP1 suppression, transfected HEK 293 cell lysates were subjected to 10% SDS-polyacrylamide gel electrophoresis, followed by blotting of proteins from the gels to PVDF membranes (Bio-Rad, Hercules, CA). The membranes were sequentially incubated with blocking buffer (LI-COR Biosciences, Lincoln, NE), primary antibody, and horseradish peroxidase-conjugated secondary antibody, with extensive washes between each incubation, according to the protocol provided by LI-COR Biosciences. The protein bands were visualized by chemilumi-nescence, using the ECL Western blotting system (Amersham Biosciences, Uppsala) and Hyperfilm (Amersham). The primary antibodies were affinity-purified rabbit IgG specific for human NSEP1 (Shen et al., 1998), and goat IgG specific for human beta-actin (Santa Cruz Scientific, Santa Cruz, CA). Secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Scientific, Santa Cruz, CA) and rabbit anti-goat IgG (Santa Cruz Scientific).
Reporter genes and luciferase assay
Plasmids pBPH-sec (also known as pBPHGP3′UTR) and pH9A express a fusion gene construct containing a 5′ LacZ cDNA, a 3′ firefly luciferase cDNA, a short intervening sequence containing a TGA codon, and a porcine GPx4 SECIS element in the 3′-untranslated region (Kollmus et al., 1996; Nasim et al., 2000). In pBPH-sec, the TGA is derived from nucleotides 165–255 of the porcine phospholipid hydroperoxide glutathione peroxidase (GPx4) gene; pH9A contains cDNA encoding amino acids 300–318 of human selenoprotein P (Burk and Hill, 1994), including two TGA sequences corresponding to the selenocysteines at positions 300 and 318. The respective control plasmids pBLUGA and pH8A lack the SECIS elements in their 3′-untranslated regions. The reporter and control plasmids (Kollmus et al., 1996; Nasim et al., 2000) were kindly provided by Prof. Regina Brigelius-Flohé (German Institute of Human Nutrition, Bergholz-Rehbruecke). The plasmid pRL-TK (Promega), containing a cDNA (Rluc) encoding a modified form of Renilla (sea pansy) luciferase, served as an internal control reporter for transfection efficiency.
Firefly and Renilla luciferase activity were measured with a Mediators PhL™ luminometer (ImmTech, New Windsor, MD), using procedures and reagents of the Dual-Glo Luciferase Assay System (Promega). The relative inhibition of luciferase expression by NSEP1 siRNA was calculated from the ratio of firefly to Renilla luciferase activity in lysates of HEK 293 cells transfected with each NSEP1 siRNA, compared to the firefly:Renilla luciferase activity in lysates of negative control cells transfected with two plasmids alone. Statistical comparisons were performed using ANOVA and the Mann Whitney test (Bhattacharyya and Johnson, 1977).
RESULTS
Our previous studies of interactions of NSEP1 with the SECIS element in selenoprotein gene transcripts examined binding only by means of in vitro measures such as library screening and electrophoretic mobility shift assays (Shen et al., 1998). In order to determine whether NSEP1 functions as a SECIS-binding protein in intact cells, we first tested their in vivo association by ribonucleoprotein immunoprecipitation (RIP), using formaldehyde as a reversible crosslinker to fix RNA molecules with their specific binding proteins (Niranjanakumari et al., 2002). The crosslinking between the two species can be reversed so that the RNA can be released from its binding proteins and identified by RT-PCR.
Antibodies used for the immunoprecipitation included rabbit pre-immune serum as negative control; a previously-described, purified rabbit IgG against human NSEP1 (Shen et al., 1998); and rabbit antiserum against human poly (A) binding protein (Penalva et al., 2004) (a kind gift from Dr. Jack D. Keene, Duke University), as a positive control. Transcripts encoding the human and rat glutathione peroxidase selenoenzymes GPx1 and GPx4 served as targets for PCR amplification from the RIP products, along with human and rat β-actin as a negative control.
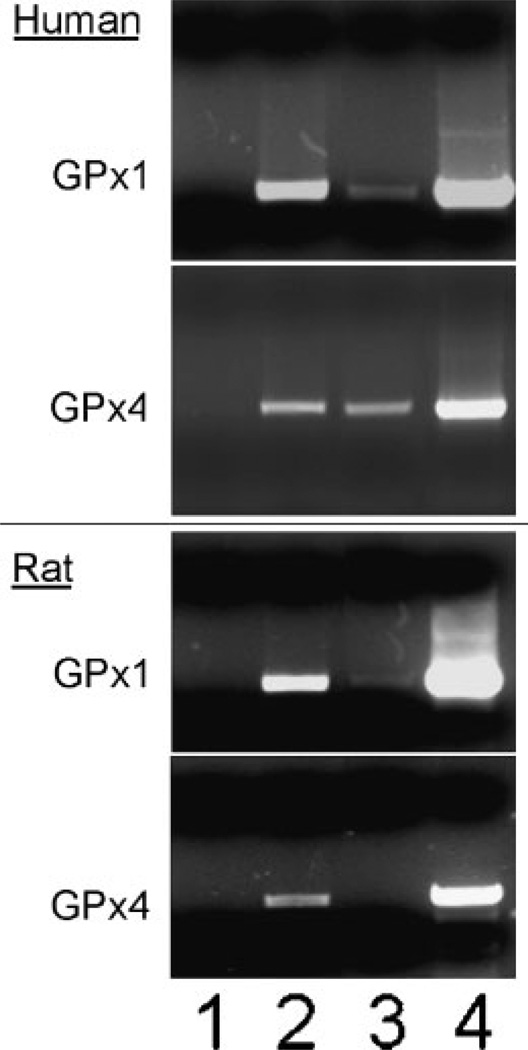
As shown in the two upper parts of Figure 1, the RIP assay detected human GPx1 mRNA as a 500 base-pair RT-PCR product only in ribonucleoproteins immuno-precipitated from HeLa cells by purified anti-NSEP1 IgG (Lane 2) or antiserum against human poly (A) binding protein (Lane 3); RT-PCR of the 550 base-pair product from GPx4 mRNA produced similar results. As expected, the positive control RT-PCR products for total RNA extracts were very strong for both transcripts (Lane 4), whereas immunoprecipitation with preimmune serum resulted in no signal (lane 1). Additional negative control immunoprecipitates amplified without RT produced no PCR signal (data not shown). More RT-PCR product was observed in RIP using IgG against NSEP1 than using antiserum against poly (A) binding protein, probably reflecting the greater efficiency of GPx transcript capture by purified IgG directed against a specific SECIS-binding protein, relative to whole antiserum that recognizes a protein bound to all polyadenylated mRNAs. In order to rule out non-specific crosslinking of NSEP1 to all mRNA species, we also determined that the RIP assay with anti-NSEP1 did not detect transcripts for β-actin (data not shown).
Fig. 1.
Co-immunoprecipitation of selenoprotein mRNAs bound to NSEP1 in human and rat cell lines. Upper parts: RT-PCR detection of mRNA encoding human cytosolic glutathione peroxidase (GPx1) or phospholipid hydroperoxide glutathione peroxidase (GPx4), as indicated in the left margin, from human HeLa cells. Lower parts: RT-PCR detection of mRNA encoding rat GPx1 or GPx4, as indicated in the left margin, from rat McArdle 7777 cells. Intact cells were treated with formaldehyde to crosslink mRNA to associated binding proteins, then lysed and the lysates immunoprecipitated with the antibodies listed below and protein A-sepharose beads; the crosslinking was reversed and the released RNA species identified by RT-PCR. RT-PCR products were detected by ethidium bromide staining of agarose gels. Lane 1, normal rabbit serum; Lane 2, purified rabbit IgG against human NSEP1; Lane 3, rabbit antiserum against human poly (A) binding protein. Lane 4, RT-PCR using total cellular RNA prior to protein A-sepharose bead extractions.
The lower two parts of Figure 1 show RIP assay results for detection of rat GPx1 and GPx4 mRNA in rat McArdle 7777 cells. Again, immunoprecipitation using IgG against human NSEP1 yielded a positive RT-PCR product from rat GPx1 mRNA, shown as a 580 base-pair band in the agarose gel (Fig. 1, lower part, lane 2) or from rat GPx 4 mRNA as a 570 base-pair band (lane 2). However, antiserum against human poly (A) binding protein did not cross-react sufficiently with the rat homolog to immunoprecipitated sufficient mRNA for consistent detection of GPx transcripts: in lane 3, only the GPx1 PCR product is detectable at a low level. As in HeLa cells, RIP with anti-NSEP1 did not co-precipitate any detectable β-actin mRNA (data not shown). Together, the RIP results indicate that NSEP1 binds selenoprotein mRNAs in living HeLa and McArdle 7777 cells, presumably through the SECIS elements in their 3′ UTR regions.
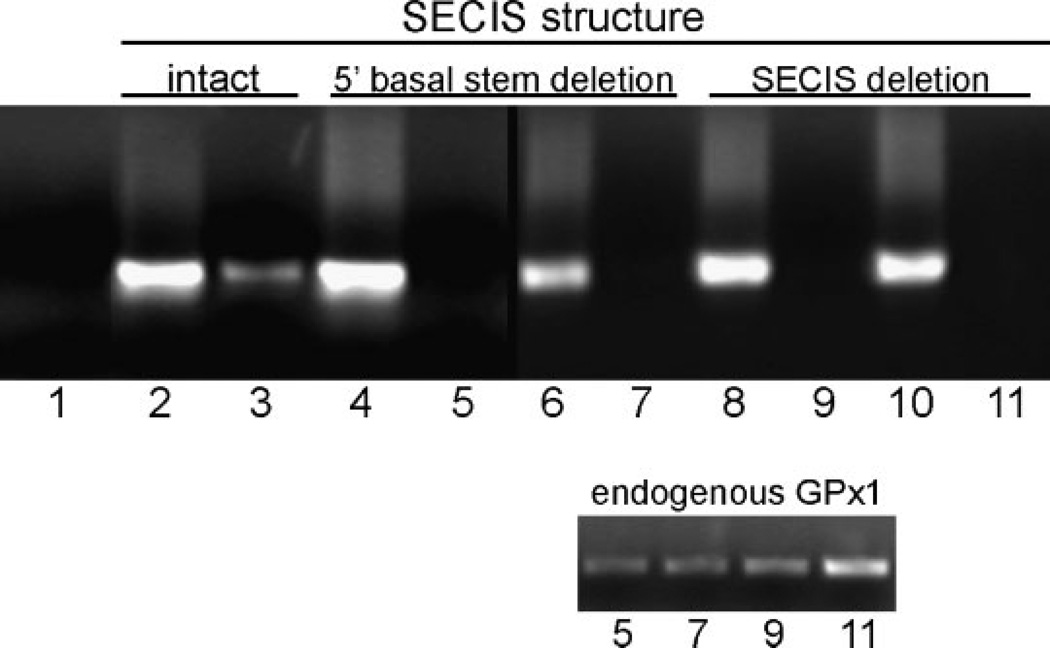
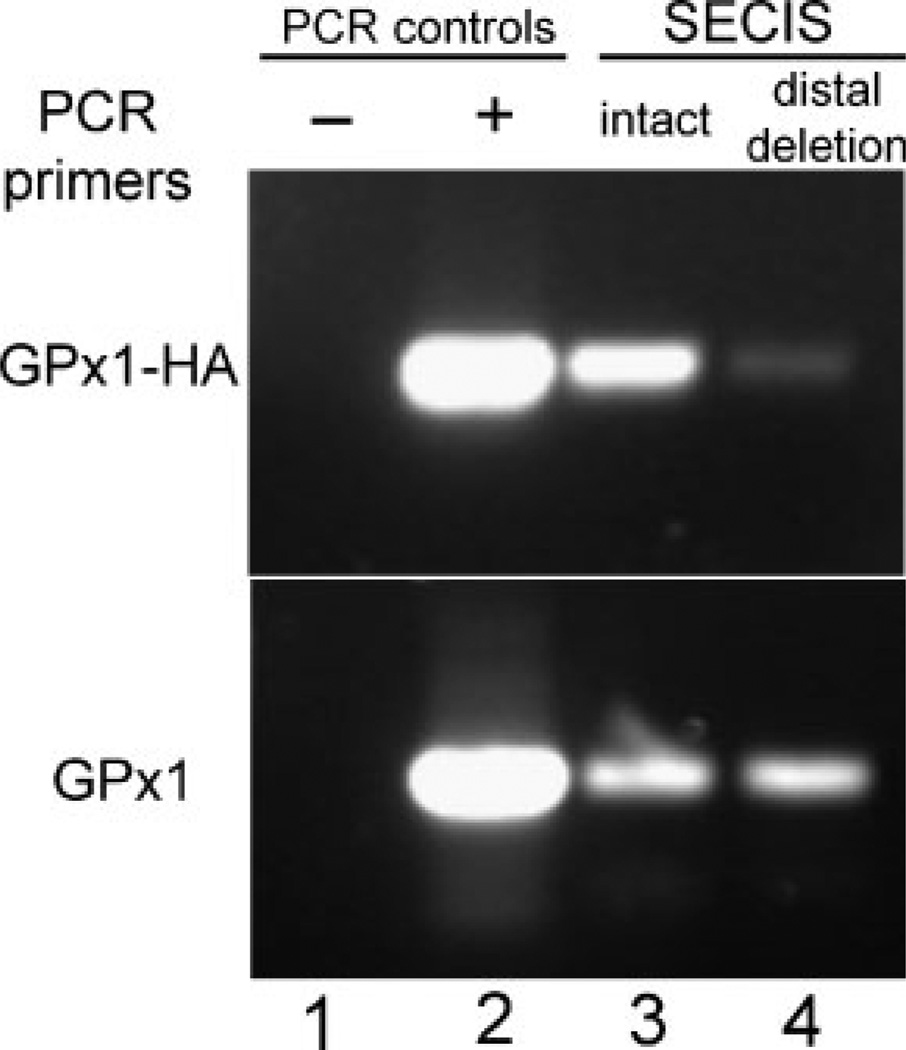
In addition, we expressed epitope-tagged human GPX1 mRNA from a stably-transfected pTet-Bsd-IRES-EGFP “Tet-off” plasmid in a Saos-2-derived cell line that expresses the tTA tetracycline regulator (Shockett and Schatz, 1996). The GPX1 constructs included one with a native 3′UTR and several SECIS deletion constructs, including one with a deletion of the entire 87 nucleotide SECIS element (illustrated schematically in Fig. 2) and another with deletions of 20 nucleotides on the 5′ side of the basal stem segment plus four nucleotides in a region of conserved sequence in a mid-stem bulge, indicated by the darker grey shaded areas in Figure 2. As shown in Figure 3, RT-PCR amplification of total cell RNA (lanes 2, 4, 6, 8, 10) detected roughly equivalent levels of expression of these constructs, but RIP with anti-NSEP1 antibody brought down only GPx1 mRNA with an intact SECIS element (lane 3).NoRT-PCR signal was detected in repeated RIP assays of cells transfected with two independently-generated constructs with either the SECIS total deletion (lanes 5, 7) or 5′ stem deletion (lanes 9, 11). RT-PCR amplification of the same immunoprecipitates detected the Saos-2 cells′ endogenous GPX1 transcripts in all of these samples (lower part). Another construct with deletion of the distal stem and terminal loop, leaving the basal stem structure intact (Fig. 2, light gray shading), produced a RIP product for the epitope-tagged GPx1 that was consistently detectable, but at a level much below that of the intact SECIS construct (Fig. 4).
Fig. 2.
Schematic diagram of the SECIS element in the 3′UTR of mRNA encoding human GPx1. Regions of short conserved sequences are indicated by bold face type. Shading indicates regions of deletions, as described in the text. Light shading indicates the extent of the distal stem and terminal loop deletion; dark shading indicates the extent of the 5′ stem and conserved sequence deletion The oval area of dark shading indicates a conserved sequence region deleted in both constructs. The total SECIS deletion construct removed the entire sequence.
Fig. 3.
Co-immunoprecipitation of epitope-tagged human GPX1 transcripts bound to NSEP1. mRNA from Saos-2 cells was analyzed as described in the legend to Figure 1. Upper part: RT-PCR products were detected by ethidium bromide staining of agarose gels. Lane 1: no-template control; Lanes 2, 4, 6, 8, 10: RT-PCR using total cellular RNA prior to protein A-sepharose bead extractions from cells transfected with the constructs indicated in the upper margin; Lanes 3, 5, 7, 9, 11: co-immunoprecipitated epitope-tagged GPX1 mRNA from cells transfected with the constructs indicated in the upper margin. Lower part: co-immunoprecipitated endogenous GPX1 mRNA from the same preparations as the corresponding lanes from the main part of the figure.
Fig. 4.
Co-immunoprecipitation of epitope-tagged and endogenous human GPX1 transcripts bound to NSEP1. mRNA from Saos-2 cells was analyzed as described in the legend to Figure 1. RT-PCR products were detected by ethidium bromide staining of agarose gels. The upper part shows the products amplified by PCR primers directed to the epitope-tagged transcripts (“GPx1-HA”); the lower part, to endogenous transcripts (“GPx1”). Lane 1: no-RT control; Lane 2: total cellular RNA; Lane 3: co-immunoprecipitated RNA from cells transfected with a construct containing the SECIS element; Lane 4: co-immunopreci-pitated RNA from cells transfected with a construct with the distal stem and loop of the SECIS element deleted (as indicated by light shading in Fig. 2).
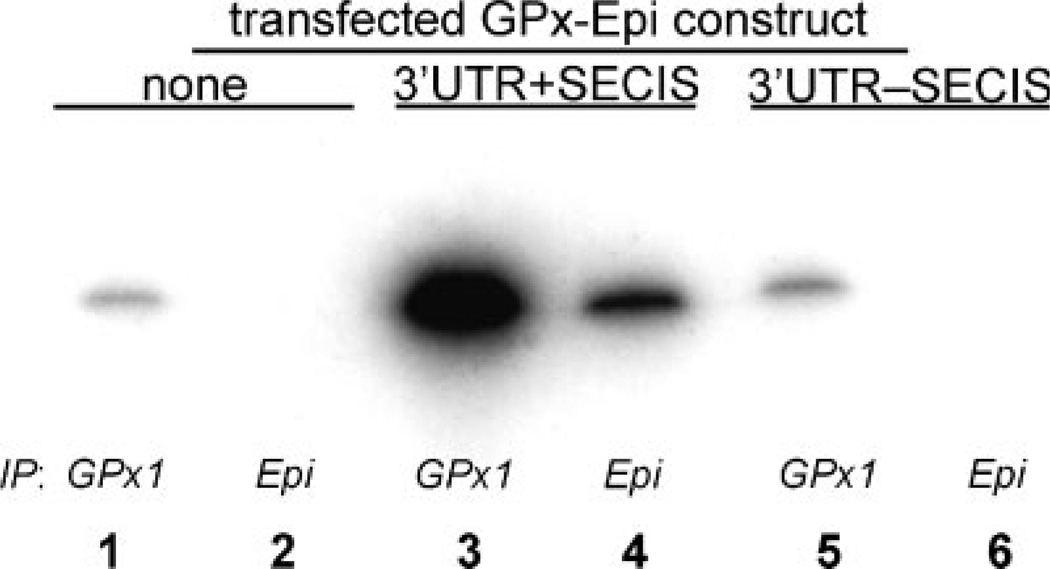
Selenoprotein translation from the epitope-tagged GPX1 constructs was assessed by biosynthetic labeling with 75Se and autoradiography of SDS–PAGE gels of immunoprecipitated GPx1. As shown in Figure 5, cells transfected with a GPX1 construct with an intact SECIS element in the 3′ UTR incorporated 75Se into newly-synthesized epitope-tagged GPx1 protein, indicated by the appearance of a band in lane 4, immunoprecipitated by anti-HA antibody directed against the epitope tag. However, no epitope-tagged protein was synthesized by cells transfected by the construct with the entire SECIS element deleted (lane 6), as in the untransfected cells (lane 2). Selenium incorporation into protein immuno-precipitated by anti-GPx1 antibody, which brought down both endogenous and epitope-tagged GPx1, showed an increase in the cells transfected by the intact construct (lane 3) but those transfected by the deletion mutant (lane 5) expressed only the same baseline level as untransfected cells (lane 1).
Fig. 5.
Biosynthetic incorporation of 75Se into GPx1 selenoprotein. Saos-2 cells were transfected with epitope-tagged GPx1 constructs as indicated in the top margin, incubated with 75Se, lysed and immunoprecipitated with antibody to human GPx1 or to the epitope tag as indicated in the lower margin, then analyzed by SDS–PAGE and autoradiography. The autoradiogram shows Lanes 1, 2: immunoprecipitated selenoprotein from non-transfected cells; Lanes 3, 4: from cells transfected with an epitope-tagged GPx1 construct with an intact SECIS element; Lanes 5, 6: from cells transfected with an epitope-tagged GPx1 construct with the SECIS element deleted. Lanes 1, 3, 5: immunoprecipitated with anti-GPx1 antibody; Lanes 2, 4, 6: immunoprecipitated with antibody to the epitope tag (“Epi”).
In order to determine the functional importance of this interaction for selenocysteine translation, we next applied the technique of small interfering RNA (siRNA) silencing to test whether NSEP1 is necessary for in vivo selenoprotein synthesis. This method, based on the important newly-discovered principle of RNA inhibition or silencing, targets a specific mRNA for degradation by the introduction into growing cells of short doublestranded RNA molecules with sequence homology to the transcript of interest (Zamore et al., 2000; Mello and Conte, 2004; Novina and Sharp, 2004). Our approach used siRNA to test the hypothesis that, if NSEP1 is actively involved in selenoprotein synthesis, suppression of NSEP1 expression would impair the expression of a SECIS-dependent selenoprotein reporter construct.
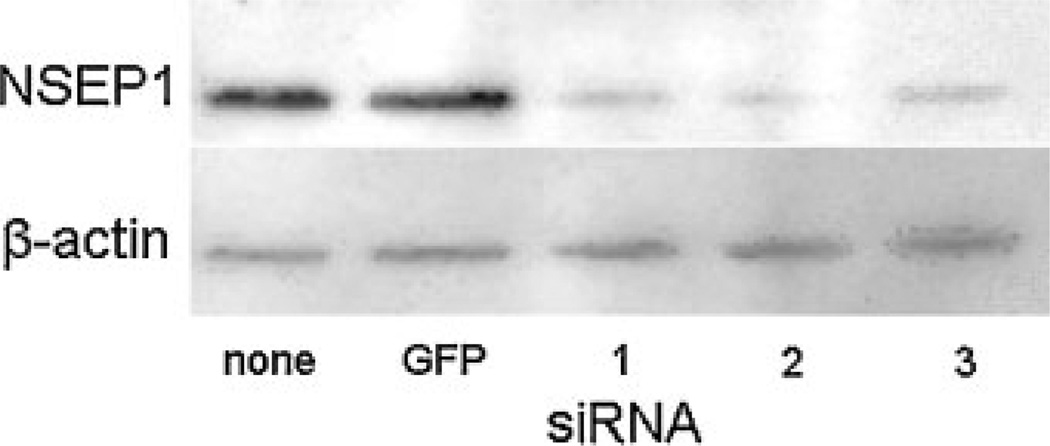
Experimentally, we used three double-stranded siRNA duplexes specifically designed for silencing NSEP1 expression. Each consisted of two 21-nucleotide RNA oligonucleotides forming a 19 base pair duplex core with two-base symmetrical 3′-overhangs and a 5′-phosphorylated antisense strand. We first tested whether the introduction of each siRNA duplex could suppress NSEP1 expression. Figure 6 shows Western blot assays of protein contents, demonstrating lower levels of NSEP1 in lysates of cells transfected with each siRNA (lanes labeled “1–3”) compared to mock-transfected cells (“none”) or cells transfected with an siRNA that silences GFP expression (“GFP”). The lower part indicates equal amounts of β-actin in the same protein extracts. Thus, all three NSEP1 siRNA duplexes are capable of inhibiting NSEP1 expression in HEK293 cells.
Fig. 6.
Inhibition of NSEP1 protein expression by siRNAs. Western blot assay for detection of NSEP1 (top part) and β-actin (bottom part) from lysates of HEK 293 cells transfected with siRNA duplexes as indicated in the bottom margin: none, no siRNA; GFP, GFP-silencing siRNA duplex; 1, NSEP1 siRNA duplex 1; 2, NSEP1 siRNA duplex 2; 3, NSEP1 siRNA duplex 3. HEK 293 cells were transfected with the indicated siRNA constructs, incubated 48 h, then lysed and analyzed by Western blotting for the proteins indicated in the left margin.
Next, we investigated whether such inhibition in turn affects selenoprotein translation, measured as luciferase activity in HEK 293 cells transfected with SECIS-dependent luciferase reporter gene constructs developed in the McCarthy laboratory (Kollmus et al., 1996; Nasim et al., 2000). The plasmids pBPH-sec (also known as pBPHGP3′UTR) and pH9A carry a gene fusion construct consisting of an upstream LacZ cDNA and downstream firefly luciferase cDNA, a short intervening sequence containing a TGA codon (transcribed as UGA in the resultant mRNA), and a porcine GPx4 SECIS element in the 3′-untranslated region. In cells transfected with these constructs, expression of luciferase activity provides a measurement of SECIS-dependent suppression of termination at the UGA codon (Kollmus et al., 1996; Nasim et al., 2000), and thus of the SECIS recognition and selenocysteine incorporation functions of the selenoprotein translation complex. The respective negative control plasmids pBLUGA and pH8A lack a SECIS element in the 3′-untranslated region, so that any residual luciferase activity represents background SECIS-independent read-through at the UGA codon. Each NSEP1 siRNA duplex was cotransfected into HEK 293 cells with each of the luciferase reporter constructs described above.
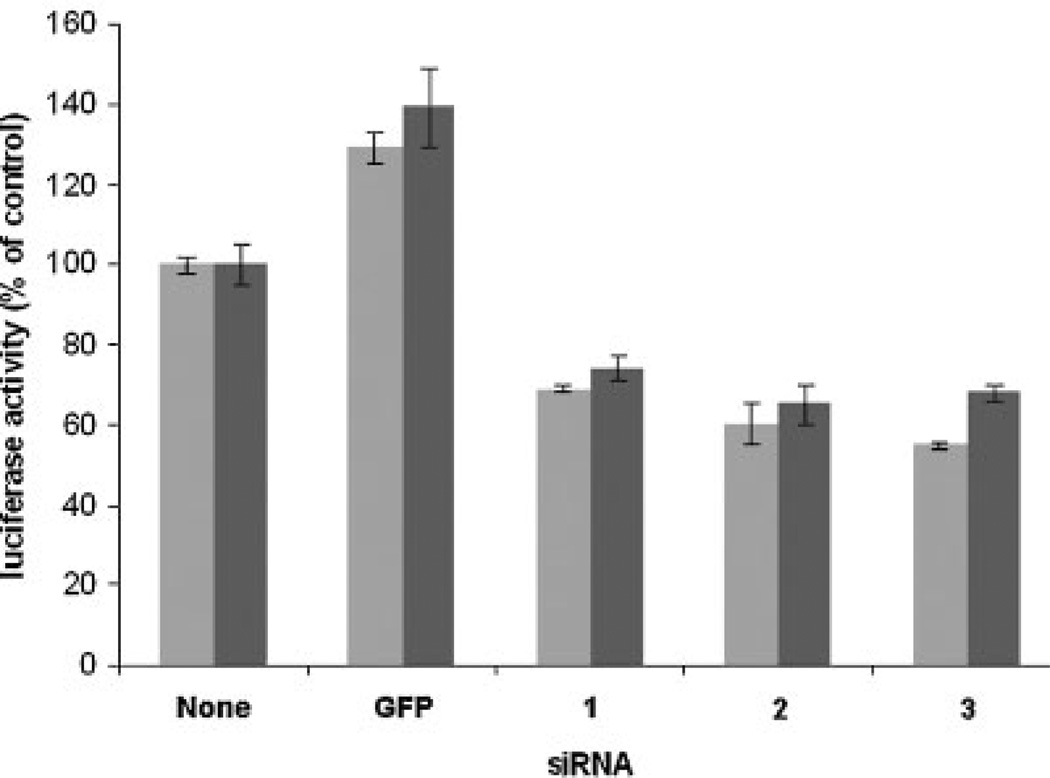
As shown in Figure 7, the siRNAs consistently diminished luciferase activity in transfected cells, compared to negative controls transfected with GFP-silencing siRNA or no RNA duplex. Luciferase activity decreased 50%–70% at 48 h after delivery of the NSEP1 siRNA duplexes into cells cotransfected with either pBPH-sec or pH9A. Compared to the control without siRNA, suppression by each of the siRNAs was significant, as demonstrated by ANOVA (P<0.001, 95% confidence intervals), and confirmed by the Mann– Whitney test (P<0.03). Introduction of the GFP siRNAd uplex actually increased luciferase activity by approximately 30%, a phenomenon observed in siRNA silencing experiments in other laboratories (personal communication, Dr. Zhoshang Xu).
Fig. 7.
Effect of NSEP1 siRNA duplexes on SECIS-dependent luciferase activity in HEK 293 cells. Cells were cotransfected with reporter plasmid pBPHsec (light bars) or pH9A (dark bars) plus siRNA duplexes as indicated in the bottom margin: None, no siRNA; GFP, GFP-silencing siRNA duplex; 1, NSEP1 siRNA duplex 1; 2, NSEP1 siRNA duplex 2; 3, NSEP1 siRNA duplex 3. The ordinate indicates luciferase activity as a percent of no-siRNA controls; bar heights and error bars indicate the mean and standard deviations of triplicate measurements, normalized for transfection efficiency by Renilla luciferase activity derived by co-transfection of plasmid pRL-TK.
DISCUSSION
NSEP1 is a highly-conserved member of the coldshock domain protein superfamily that is capable of binding either DNA (leading to the alternative names DNA-binding protein B and Y-box binding protein 1) or RNA; it plays a role in functions ranging from transcriptional regulation and DNA repair to translational regulation and RNA stabilization (Didier et al., 1988; Kohno et al., 2003). Our laboratory has previously reported that NSEP1 is a SECIS-binding protein, based on its identification by screening an expression library with RNA from the human GPx1 SECIS element, demonstration of SECIS RNA gel shifts by purified recombinant NSEP1, and “supershift” of endogenous SECIS-binding complexes from COS-1 cell extracts by an anti-NSEP1 polyclonal antibody (Shen et al., 1998). Fagegalier et al. also identified NSEP1 in a yeast three-hybrid screening procedure using the SECIS element from rat GPx1. However, they concluded that it is not a bona fide SECIS-binding protein because they did not detect any SECIS RNA gel shift by their NSEP1 cDNA clone expressed in rabbit reticulocyte lysates, not any supershift when HA-tagged, in vitro translated DPBP and anti-HA antibody were both added to mammalian S100 extract and labeled SECIS RNA (Fagegaltier et al., 2000a). Thus, contradictory evidence has argued for and against a role for NSEP1 in SECIS binding and selenoprotein translation.
Our current studies sought to resolve this controversy, first by investigating whether NSEP1 interacts closely with SECIS elements within intact cells. After reversible in vivo cross-linking and ribonucleoprotein immunoprecipitation, mRNA encoding the selenopro-teins GPx1 and GPx4 were found to co-precipitate with NSEP1 in both human and rat cell lines (HeLa and McArdle 7777, respectively). Beta-actin transcripts did not co-precipitate, indicating that the association did not represent non-specific RNA binding by NSEP1. Co-immunoprecipitation of the GPx1 mRNAs with NSEP1 could reflect either direct protein-RNA interaction or an indirect association through one or more intermediary elements, such as an RNA-binding protein that also binds NSEP1. The two models can not be distinguished by the current data, but the direct binding modelismore consistent with our previous demonstration that purified recombinant NSEP1 (termed DNA-binding protein B in our previous publications) produces an electro-mobility gel shift when incubated with RNA sequences from the GPx1 SECIS element (Shen et al., 1998).
The association of human GPX1 mRNA with NSEP1 appears to be largely SECIS-dependent, as indicated by the loss of co-immunoprecipitation of constructs with deletions of the entire SECIS or deletion of sequences from the basal stem and conserved bulge of the stem-loop structure (Fig. 2). The low level of immunoprecipitation of the transcript with a deletion of the distal stem and terminal loop of the structure suggests that NSEP1 remains capable of binding, at lower affinity, to functional elements in the residual basal stem of the SECIS, which has been shown to provide a recognition site for SBP2 and other SECIS-binding factors (Shen et al., 1995; Copeland et al., 2001). The loss of 75Se incorporation into epitope tagged GPx1 (Fig. 5) confirms our previous demonstration that major disruptions of SECIS structure ablate its function in selenoprotein translation (Shen et al., 1995).
To test the functional importance of this interaction on selenoprotein translation, we used small inhibitory RNAs (siRNAs) to reduce the NSEP1 content of tissue culture cells and then examined the effect of that reduction on the expression of reporter genes. In these fusion construct, a UGA codon (derived from the cDNA encoding either GPx4 or selenoprotein P) has been interposed between an upstream LacZ cDNA and a downstream firefly luciferase cDNA. Thus, measurement of luciferase activity provides a sensitive and quantitative assay for translational read-through at the UGA codon. That process has been shown previously to depend entirely upon the presence and function of one or more SECIS elements in the 3′-untranslated region of each reporter construct (Kollmus et al., 1996; Nasim et al., 2000).
In the present studies, co-transfection of any of three siRNAs reduced NSEP1 content by approximately 50%.
Greater inhibition of DPBP expression was not possible with this relatively long-lived and abundant protein. Nonetheless, the siRNA effect was sufficient to significantly reduce luciferase activity, indicating inhibition of read-through at the UGA codon of the reporter construct. Together, the RIP and siRNA silencing assays demonstrate that NSEP1 is an authentic SECIS binding protein that is involved in the translation of selenoproteins in mammalian cells.
NSEP1, as a highly versatile protein involved in varied transcriptional and translational functions, probably plays a supporting role in the selenoprotein translation complex. Other members of this quaternary ribonucleoprotein array include mSelB (Tujebajeva et al., 2000; Fagegaltier et al., 2000b), the mammalian homolog of the bacterial selenocysteine-specific elongation factor SelB, and SBP2, a protein with domains capable of SECIS recognition, ribosome binding, and in vitro selenocysteine incorporation (Copeland et al., 2000, 2001; Lescure et al., 2002; Driscoll and Copeland, 2003). SBP2 interacts with mSelB in transfected cells (Tujebajeva et al., 2000) and elutes with an apparent molecular mass of ∼500 kDa in gel filtration chromatography (Copeland and Driscoll, 1999), suggesting that it participates in a large functional complex.
NSEP1 might function either in direct support of the translation process or as an mRNA stabilizing element. In interactions with other mRNA stem-loop structures, NSEP1 has been shown to complex with other RNA-binding proteins to affect translational control of gene expression. For example, it partners with iron-regulatory protein 2 and as many as five ribosomal proteins to regulate ferritin mRNA translation through interaction with the iron-responsive element in the 5′-untranslated region (Ashizuka et al., 2002). In addition, NSEP1 interacts with other proteins binding to 3′-untranslated region elements to stabilize mRNA and thus increase expression of transcripts encoding renin, interleukin-2, and granulocyte-macrophage colony-stimulating factor (Chen et al., 2000; Capowski et al., 2001; Skalweit et al., 2003). The interleukin-2 stabilizing ribonucleoprotein complex also includes nucleolin (Chen et al., 2000), which we have previously identified as another possible SECIS-binding protein (Wu et al., 2000). Thus, we speculate that NSEP1 participates in selenoprotein translation not only by binding to the SECIS element, but also stabilizing the ribonucleoprotein complex to prevent “nonsense”-mediated mRNA decay and permit selenocysteine incorporation rather than termination at the UGA codon.
ACKNOWLEDGMENTS
We thank Carolyn Padden for expert technical assistance and Antonio Condino-Neto, MD, PhD, for help with statistical analysis.
Contract grant sponsor: National Research Initiative of the USDA Cooperative State Research, Education and Extension Service; Contract grant number: 2001-35200-10692; Contract grant sponsor: John H. Pierce Pediatric Oncology Research Fund.
LITERATURE CITED
- Ashizuka M, Fukuda T, Nakamura T, Shirasuna K, Iwai K, Izumi H, Kohno K, Kuwano M, Uchiumi T. Novel translational control through an iron-responsive element by interaction of multifunctional protein YB-1 and IRP2. Mol Cell Biol. 2002;22:6375–6383. doi: 10.1128/MCB.22.18.6375-6383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C, Heider J, Böck A. Interaction of translation factor SELB with the formate dehydrogenase H selenopeptide mRNA. Proc Natl Acad Sci USA. 1993;90:4181–4185. doi: 10.1073/pnas.90.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD, Harney JW, Larsen PR. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature. 1991a;353:273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Banu L, Larsen PR. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991b;349:438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Banu L, Harney JW, Larsen PR. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993;12:3315–3322. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya GK, Johnson RA. Nonparametric inference. In: Bhatta-charyya GK, Johnson RA, editors. Statistical concepts and methods. Singapore: John Wiley. 1977:505–547. [Google Scholar]
- Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Burk RF, Hill KE. Regulation of selenoproteins. Annu Rev Nutr. 1993;13:65–81. doi: 10.1146/annurev.nu.13.070193.000433. [DOI] [PubMed] [Google Scholar]
- Burk RF, Hill KE. Selenoprotein P. A selenium-rich extracellular glycoprotein. J Nutr. 1994;124:1891–1897. doi: 10.1093/jn/124.10.1891. [DOI] [PubMed] [Google Scholar]
- Böck A, Forchhammer K, Heider J, Baron C. Selenoprotein synthesis: An expansion of the genetic code. Trends Biochem Sci. 1991;16:463–467. doi: 10.1016/0968-0004(91)90180-4. [DOI] [PubMed] [Google Scholar]
- Capowski EE, Esnault S, Bhattacharya S, Malter JS. Y box-binding factor promotes eosinophil survival by stabilizing granulocyte-macrophage colony-stimulating factor mRNA. J Immunol. 2001;167:5970–5976. doi: 10.4049/jimmunol.167.10.5970. [DOI] [PubMed] [Google Scholar]
- Chambers I, Frampton J, Goldfarb P, Affara N, McBain W, Harrison PR. The structure of the mouse glutathione peroxidase gene: The selenocysteine in the active site is encoded by the termination codon, TGA. EMBO J. 1986;5:1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Gherzi R, Andersen JS, Gaietta G, Ju¨rchott K, Royer HD, Mann M, Karin M. Nucleolin and YB-1 are required for JNK-mediated interleukin-2 mRNA stabilization during T-cell activation. Genes Dev. 2000;14:1236–1248. [PMC free article] [PubMed] [Google Scholar]
- Chu F-F, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione perox-idase, GSHPx-GI. J Biol Chem. 1993;268:2571–2576. [PubMed] [Google Scholar]
- Copeland PR, Driscoll DM. Purification, redox sensitivity, and RNA binding properties of SECIS-binding protein 2, a protein involved in selenoprotein biosynthesis. J Biol Chem. 1999;274:25447–25454. doi: 10.1074/jbc.274.36.25447. [DOI] [PubMed] [Google Scholar]
- Copeland PR, Fletcher JE, Carlson BA, Hatfield DL, Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland PR, Stepanik VA, Driscoll DM. Insight into mammalian selenocysteine insertion: Domain structure and ribosome binding properties of Sec insertion sequence binding protein 2. Mol Cell Biol. 2001;21:1491–1498. doi: 10.1128/MCB.21.5.1491-1498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond AM, Soon Choi I, Crain PF, Hashizume T, Pomerantz SC, Cruz R, Steer CJ, Hill KE, Burk RF, McCloskey JA, Hatfield DL. Dietary selenium affects methylation of the wobble nucleoside in the anticodon of selenocysteine tRNA[Ser]Sec. J Biol Chem. 1993;268:14215–14223. [PubMed] [Google Scholar]
- Didier DK, Schiffenbauer J, Woulfe SL, Zacheis M, Schwartz BD. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci USA. 1988;85:7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- Esworthy RS, Chu F-F, Paxton RJ, Akman S, Doroshow JH. Characterization and partial amino acid sequence of human plasma glutathione peroxidase. Arch Biochem Biophys. 1991;286:330–336. doi: 10.1016/0003-9861(91)90048-n. [DOI] [PubMed] [Google Scholar]
- Fagegaltier D, Hubert N, Carbon P, Krol A. The selenocysteine insertion sequence binding protein SBP is different from the Y-box protein dbpB. Biochimie. 2000a;82:117–122. doi: 10.1016/s0300-9084(00)00192-9. [DOI] [PubMed] [Google Scholar]
- Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 2000b;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JE, Copeland PR, Driscoll DM, Krol A. The selenocysteine incorporation machinery: Interactions between the SECIS RNA and the SECIS-binding protein SBP2. RNA. 2001;7:1442–1453. [PMC free article] [PubMed] [Google Scholar]
- Forchhammer K, Leinfelder W, Böck A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature. 1989;342:453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, Jeang KT, Stadtman TC. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc Natl Acad Sci USA. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundner-Culemann E, Martin GWIII, Harney JW, Berry MJ. Two distinct SECIS structures capable of directing selenocysteine incorporation in eukaryotes. RNA. 1999;5:625–635. doi: 10.1017/s1355838299981542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KE, Burk RF. Selenoprotein P: Recent studies in rats and in humans. Biomed Environ Sci. 1997;10:198–208. [PubMed] [Google Scholar]
- Horwitz EM, Maloney KA, Ley TJ. A human protein containing a “cold shock” domain binds specifically to H-DNA upstream from the human gamma-globin genes. J Biol Chem. 1994;269:14130–14139. [PubMed] [Google Scholar]
- Hu ML, Tappel AL. Selenium as a sulfhydryl redox catalyst and survey of potential selenium-dependent enzymes. J Inorg Biochem. 1987;30:239–248. doi: 10.1016/0162-0134(87)80067-3. [DOI] [PubMed] [Google Scholar]
- Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. BioEssays. 2003;25:691–698. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- Kollmus H, Flohé L, McCarthy JEG. Analysis of eukaryotic mRNA structures directing cotranslational incorporation of selenocysteine. Nucleic Acids Res. 1996;24:1195–1201. doi: 10.1093/nar/24.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhrle J, Brigelius-Flohé R, Böck A, Gärtner R, Meyer O, Flohé L. Selenium in biology: Facts and medical perspectives. Biol Chem. 2000;381:849–864. doi: 10.1515/BC.2000.107. [DOI] [PubMed] [Google Scholar]
- Larsen PR, Berry MJ. Nutritional and hormonal regulation of thyroid hormone deiodinases. Annu Rev Nutr. 1995;15:323–352. doi: 10.1146/annurev.nu.15.070195.001543. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Worland PJ, Davis JN, Stadtman TC, Hatfield DL. Identification of a selenocysteyl-tRNASer in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989;264:9724–9727. [PubMed] [Google Scholar]
- Lee A, Whyte MKB, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukocyte Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- Lescure A, Allmang C, Yamada K, Carbon P, Krol A. cDNA cloning, expression pattern and RNA binding analysis of human selenocysteine insertion sequence (SECIS) binding protein 2. Gene. 2002;291:279–285. doi: 10.1016/s0378-1119(02)00629-7. [DOI] [PubMed] [Google Scholar]
- Lesoon A, Mehta A, Singh R, Chisolm GM, Driscoll DM. An RNA-binding protein recognizes a mammalian selenocysteine insertion sequence element required for cotranslational incorporation of selenocysteine. Mol Cell Biol. 1997;17:1977–1985. doi: 10.1128/mcb.17.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GW, Harney JW, Berry MJ. Selenocysteine incorporation in eukaryotes: Insights into mechanism and efficiency from sequence, structure, and spacing proximity studies of the type 1 deiodinase SECIS element. RNA. 1996;2:171–182. [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Conte D., Jr Revealing the world of RNA interference. Nature. 2004;431:338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- Nasim MT, Jaenecke S, Belduz A, Kollmus H, Flohe L, McCarthy JE. Eukaryotic selenocysteine incorporation follows a nonprocessive mechanism that competes with translational termination. J Biol Chem. 2000;275:14846–14852. doi: 10.1074/jbc.275.20.14846. [DOI] [PubMed] [Google Scholar]
- Niranjanakumari S, Lasda E, Brazas R, Garcia-Blanco MA. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods. 2002;26:182–190. doi: 10.1016/S1046-2023(02)00021-X. [DOI] [PubMed] [Google Scholar]
- Novina CD, Sharp PA. The RNAi revolution. Nature. 2004;430:161–164. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- Penalva LO, Burdick MD, Lin SM, Sutterluety H, Keene JD. RNA-binding proteins to assess gene expression states of co-cultivated cells in response to tumor cells. Mol Cancer. 2004;3:24. doi: 10.1186/1476-4598-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiet MJ, Tardif M, Braun L, Boulay F. Inhibitory effects of a dominant-interfering form of the Rho-GTPase Cdc42 in the chemoattractant-elicited signaling pathways leading to NADPH oxidase activation in differentiated HL-60 cells. Blood. 2002;100:1835–1844. doi: 10.1182/blood-2001-12-0193. [DOI] [PubMed] [Google Scholar]
- Rodan SB, Imai Y, Thiede MA, Wesolowski G, Thompson D, Bar-Shavit Z, Shull S, Mann K, Rodan GA. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961–4966. [PubMed] [Google Scholar]
- Sakura H, Maekawa T, Imamoto F, Yasuda K, Ishii S. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene. 1988;73:499–507. doi: 10.1016/0378-1119(88)90514-8. [DOI] [PubMed] [Google Scholar]
- Schuckelt R, Brigelius-Flohé R, Maiorino M, Roveri A, Reumkens J, Strassburger W, Ursini F, Wolf B, Flohé L. Phospholipid hydroperoxide glutathione peroxidase is a seleno- enzyme distinct from the classical glutathione peroxidase as evident from cDNA and amino acid sequencing. Free Radic Res Commun. 1991;14:343–361. doi: 10.3109/10715769109093424. [DOI] [PubMed] [Google Scholar]
- Shen Q, Chu F-F, Newburger PE. Sequences in the 3′untranslated region of the human cellular glutathione peroxidase gene are necessary and sufficient for selenocysteine incorporation at the UGA codon. J Biol Chem. 1993;268:11463–11469. [PubMed] [Google Scholar]
- Shen Q, Leonard JL, Newburger PE. Structure and function of the selenium translation element in the 3′untranslated region of human cellular glutathione peroxidase mRNA. RNA. 1995;1:519–525. [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wu R, Leonard JL, Newburger PE. Identification and molecular cloning of a human selenocysteine insertion sequence-binding protein. A bifunctional role for DNA-binding protein B. J Biol Chem. 1998;273:5443–5446. doi: 10.1074/jbc.273.10.5443. [DOI] [PubMed] [Google Scholar]
- Shockett PE, Schatz DG. Diverse strategies for tetracycline-regulated inducible gene expression. Proc Natl Acad Sci USA. 1996;93:5173–5176. doi: 10.1073/pnas.93.11.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalweit A, Doller A, Huth A, Kahne T, Persson PB, Thiele BJ. Posttranscriptional control of renin synthesis: Identification of proteins interacting with renin mRNA 3′-untranslated region. Circ Res. 2003;92:419–427. doi: 10.1161/01.RES.0000059300.67152.4E. [DOI] [PubMed] [Google Scholar]
- Stadtman TC. Selenium biochemistry. Annu Rev Biochem. 1990;59:111–127. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- Stadtman TC. Selenocysteine. Annu Rev Biochem. 1996;65:83–100. doi: 10.1146/annurev.bi.65.070196.000503. [DOI] [PubMed] [Google Scholar]
- Sunde RA. Molecular biology of selenoproteins. Annu Rev Nutr. 1990;10:451–474. doi: 10.1146/annurev.nu.10.070190.002315. [DOI] [PubMed] [Google Scholar]
- Thanbichler M, Bock A. The function of SECIS RNA in translational control of gene expression in Escherichia coli. EMBO J. 2002;21:6925–6934. doi: 10.1093/emboj/cdf673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M, Böck A. The function of SECIS RNA in translational control of gene expression in Escherichia coli. EMBO J. 2002;21:6925–6934. doi: 10.1093/emboj/cdf673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tujebajeva RM, Copeland PR, Xu X-M, Carlson BA, Harney JW, Driscoll DM, Hatfield DL, Berry MJ. Decoding apparatus for eukaryotic selenocyteine insertion. EMBO Reports. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendeland SC, Beilstein MA, Yeh JY, Ream W, Whanger PD. Rat skeletal muscle selenoprotein W: cDNA clone and mRNA modulation by dietary selenium. Proc Natl Acad Sci USA. 1995;92:8749–8753. doi: 10.1073/pnas.92.19.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak R, Carbon P, Krol A. An essential non-Watson-Crick base pair motif in 3′UTR to mediate selenoprotein translation. RNA. 1998;4:74–84. [PMC free article] [PubMed] [Google Scholar]
- Wilting R, Vamvakidou K, Böck A. Functional expression in Escherichia coli of the Haemophilus influenzae gene coding for selenocysteine-containing selenophosphate synthetase. Arch Microbiol. 1998;169:71–75. doi: 10.1007/s002030050542. [DOI] [PubMed] [Google Scholar]
- Wu R, Shen Q, Newburger PE. Recognition and binding of the human selenocysteine insertion sequence by nucleolin. J Cell Biochem. 2000;77:507–516. doi: 10.1002/(sici)1097-4644(20000601)77:3<507::aid-jcb15>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Zinoni F, Heider J, Böck A. Features of the formate dehydrogenase mRNA necessary for decoding of the UGA codon as selenocysteine. Proc Natl Acad Sci USA. 1990;87:4660–4664. doi: 10.1073/pnas.87.12.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]