Abstract
With the emergence of large multicenter trials over the past 20 years, the numbers of investigators involved and publications resulting from each study have grown exponentially. An efficient, fair, and effective way to establish authorship on study-related manuscripts could diminish conflict among the investigators and help ensure robust and timely dissemination of study results. This article describes a process developed by the investigators in the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial (ClinicalTrials.gov registration number: NCT00047437) to establish authorship of the manuscripts describing the baseline characteristics, study design, and trial outcomes in an equitable and transparent manner based on objective, quantifiable contributions to the study as a whole. The HF-ACTION investigators developed a scoring system that assigned points to investigators by using the criteria established for enrollment, adherence to the exercise program, data completion, committee service, and other trial efforts. The scoring system has been successfully implemented for baseline manuscripts and has allowed many investigators to participate in the HF-ACTION publication process.
In academic medicine, promotion and research funding are generally based on one’s academic record, with a steadily growing number of peer-reviewed publications on the curriculum vitae being a common measure of career achievement. This pressure can lead to inappropriate designation of authorship on a manuscript as an “honor” or a “gift,” a questionable practice that can raise a red flag about potential research misconduct (1, 2). The emergence of large multicenter clinical trials over the past 20 years has added several layers of complexity—and potential conflict—to this already burdened process. With more institutions and consequently more researchers involved in planning, enrollment, and data acquisition, the list of individuals who contribute substantially, and therefore deserve authorship assignments, grows commensurately. With its 98 investigators and 82 regional centers (67 centers in the United States, 9 in Canada, and 6 in France) and 2331 participants, the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial (ClinicalTrials.gov registration number: NCT00047437) provided an ideal opportunity to devise and implement a publication plan that would accommodate the complexities of the protocol and at the same time offer an equitable distribution of authorship assignments.
We describe the process through which we constructed our model and the scoring system we devised to assign authorship among the investigators from our group. We set the following goals for our process: 1) reward individuals for their efforts in obtaining funding and organizing trial infrastructure, 2) encourage contributions to the successful conduct of the study, 3) generate new and creative ideas to maximize the use and dissemination of the trial data, 4) ensure that all contributors view the process as fair, and 5) ensure compliance with the internationally accepted guidelines for authorship established by the International Committee of Medical Journal Editors (ICMJE) (3).
The Study and Its Organization
The primary objective of the HF-ACTION trial was to establish whether patients with left ventricular systolic dysfunction and New York Heart Association class II to IV symptoms given exercise training in addition to standard care would have a 20% lower rate of death and hospitalization over 2 years than patients who received usual care alone (4). The trial incorporated the general organizational features of phase III therapeutic clinical trials with additional features that address the unique nature of its exercise intervention. This structure included a steering committee, an executive committee, a coordinating center, and 3 core laboratories—a cardiopulmonary exercise (CPX) testing core laboratory, a nuclear core laboratory, and an echocardiography core laboratory (Figure 1). The site investigators all served on the steering committee, which made the final scientific decisions for the trial. The executive committee, composed of the steering committee chairperson and vice-chairperson, representatives from the coordinating center (including the principal investigator [PI], co-PI, and statistician), and members of the National Heart, Lung, and Blood Institute (NHLBI) project office, provided day-today oversight of the trial and made decisions not requiring full steering committee approval. Appointed by the executive committee, the publications committee, which continues to be active, comprises investigators from individual sites and the coordinating center who expressed an interest in participating on the committee and whose areas of expertise reflect various aspects of the trial (such as statisticians, exercise physiologists, physicians, investigators, and behaviorists). The publications committee has 18 members, including 3 members from the coordinating center and 2 from the NHLBI project office.
Figure 1. Trial organization, as illustrated in the HF-ACTION design and rationale article.
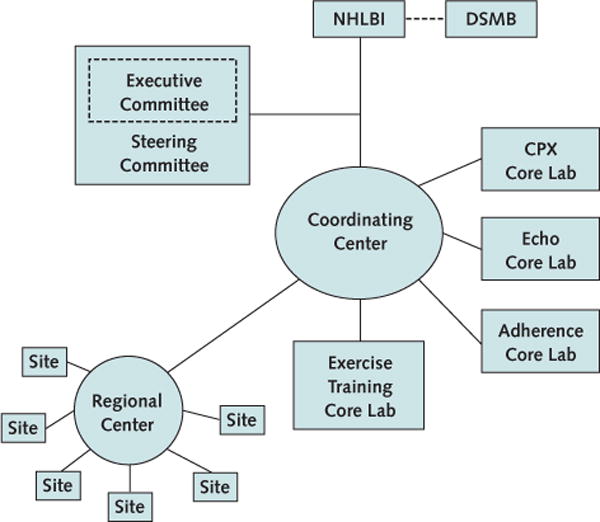
Reproduced from reference 4, with permission from Elsevier. CPX = cardiopulmonary exercise; DSMB = data and safety monitoring board; Echo = echocardiography; HF-ACTION = Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training; Lab = laboratory; NHLBI = National Heart, Lung, and Blood Institute.
Topics and Types of Publications
The process for assigning authorship included 2 parallel processes: collection of study personnel interests in a set of standard manuscripts and development of a method for ranking site personnel to assign authors (Figures 2 and 3). The publications committee determined that many manuscripts on standard topics within a trial would probably emerge, including manuscripts describing the baseline characteristics of the participants, as well as manuscripts on other trial-specific topics (such as core laboratory functions and protocol adherence). After approval by the executive committee, the list of proposed baseline manuscripts was circulated to all of the study sites. Principal investigators, coinvestigators, and participating site staff (for example, exercise physiologists or study coordinators) were asked to identify 5 manuscript topics in which they were interested and to rank these choices on a scale from 1 to 5, where a score of 1 represented the greatest interest. A writing group was formed for each manuscript on the basis of the level of interest expressed and the individuals who expressed that interest. We agreed that each writing group would comprise a lead author, 3 senior authors (second, third, and last on the byline), and 3 or more coauthors.
Figure 2. Flow chart outlining the process and tasks developed by the executive and publications committee for establishing authorship in HF-ACTION.
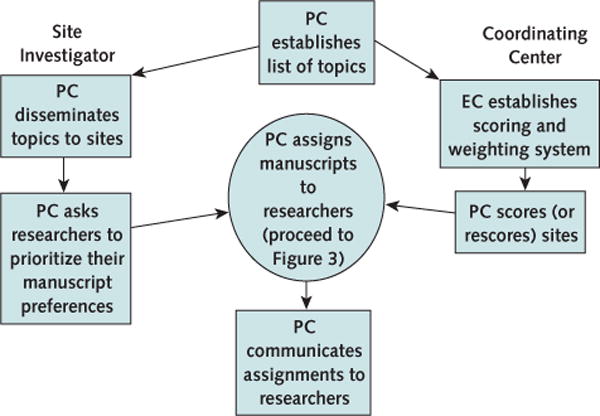
EC = executive committee; HF-ACTION = Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training; PC = publications committee.
Figure 3. Assignment of authorship by the publications committee in HF-ACTION.
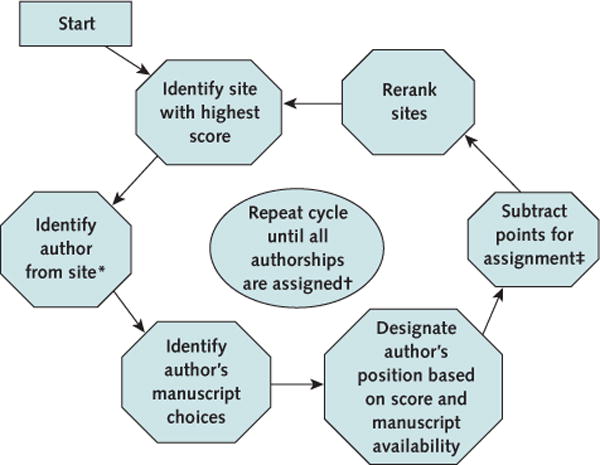
HF-ACTION = Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training.
* Authors include principal investigator, co–principal investigator, and other study personnel.
† Assign available positions: lead author (first author), senior author (second, third, or last), and coauthor (all other positions).
‡ Points for assignment are as follows: lead author = 150 points; senior author = 100 points; and coauthor = 50 points.
The initial list of topics from the publications committee included a total of 39 potential baseline manuscripts (Appendix Table, available at www.annals.org). Several baseline topics reflected the potential topics that the committee felt would be of scientific interest. After the investigators responded with their preferences, the number of baseline manuscripts was pared down to 22 on the basis of the expressed levels of interest. Investigators who had not noted a preference after that initial distribution were then presented with the pared-down list of topics and were asked again to assess their level of interest in each of the remaining 22 topics. Authors were then assigned on the basis of the method described in the following section.
Appendix Table.
List of Publications Distributed to Investigators and Their Staff and List of Topics With Insufficient Interest
| Publications distributed to investigators and their staff |
|---|
| Relationship between age and CPX: |
| Lead author: PI from site 9 |
| Senior authors: PI from site 108, PI from site 306, PI from site 322 |
| Contributing authors: NPI from site 302, statistician, NHLBI-PO |
| Relationship between sex and CPX: |
| Lead author: PI from site 109 |
| Senior authors: PI from site 208, PI from site 215, PI from site 216 |
| Contributing authors: NPI from site 107, PI from site 102, PI from site 207 |
| Relationship between race and CPX: |
| Lead author: PI from site 304 |
| Senior authors: NHLBI-PO, statistician, PI from site 211 |
| Contributing authors: NPI from CC, NPI from site 208, NPI from site 102 |
| Relationship between BMI and CPX: |
| Lead author: PI from site 104 |
| Senior authors: PI from site 411, statistician, PI from site 324 |
| Contributing authors: NPI from site 107, NPI from site 102, NPI from site 101 |
| Relationship between functional class and CPX: |
| Lead author: PI from site 337 |
| Senior authors: NPI from site 107, PI from site 209, PI from site 203 |
| Contributing authors: PI from site 313, PI from site 344, PI from site 302 |
| Relationship between diabetes and CPX: |
| Lead author: PI from site 303 |
| Senior authors: PI from site 107, PI from site 105, PI from site 335 |
| Contributing authors: PI from site 341, PI from site 308, PI from site 340 |
| Relationship between renal function and CPX: |
| Lead author: PI from site 301 |
| Senior authors: NPI from site 206, PI from site 302, NPI from site 104 |
| Contributing authors: statistician, NPI from CC |
| Relationship between depression and CPX: |
| Lead author: PI from site 403 |
| Senior authors: PI from site 216, PI from site 306, PI from CC |
| Contributing authors: NPI from site 101, NHLBI-PO, PI from site 344 |
| Relationship of QRS duration and CRT to CPX: |
| Lead author: NPI from CC |
| Senior authors: NPI from site 209, coinvestigator from CC, PI from site 206 |
| Contributing authors: PI from site 319, PI from site 103, PI from site 111 |
| Relationship between QOL and CPX: |
| Lead author: NPI from EQOL |
| Senior authors: PI from site 109, PI from site 337, NHLBI-PO |
| Contributing authors: statistician, NPI from site 218, NPI from CC |
| Relationship between echocardiography results and CPX: |
| Lead author: PI from echocardiography core laboratory |
| Senior authors: PI from site 405, PI from site 111, PI from site 324 |
| Contributing authors: NHLBI-PO, PI from site 108, PI from site 304 |
| Relationship between nuclear imaging and CPX: |
| Lead author: PI from nuclear core laboratory |
| Senior author: NPI from CC, PI from site 331, NPI from nuclear core laboratory |
| Contributing authors: NPI from nuclear core laboratory, statistician, PI from site 336 |
| Prevalence and determination of chronotropic incompetence: |
| Lead author: PI from site 103 |
| Senior authors: PI from site 411, PI from site 105, PI from site 107 |
| Contributing author: NPI from site 218, NPI from site 209, NPI from site 105 |
| Performing maximal CPX testing in a CHF population: |
| Lead author: PI from site 107 |
| Senior authors: PI from site 404, PI from site 406, PI from site 308 |
| Contributing authors: PI from site 342, NPI from site 209, PI from CPX core laboratory |
| Relationship between 6-min walk and CPX: |
| Lead author: PI from site 102 |
| Senior authors: PI from site 103, PI from site 406, PI from site 109 |
| Contributing authors: NPI from site 107, PI from site 203, PI from site 322, NPI from EQOL |
| Relationship between age and biomarkers: |
| Lead author: PI from site 108 |
| Senior authors: NPI from CC, PI from biomarker core laboratory, PI from site 307 |
| Contributing authors: NHLBI-PO, PI from site 322, statistician |
| Relationship between functional class/CPX and biomarkers: |
| Lead author: PI from biomarker core laboratory |
| Senior authors: PI from site 213, NPI from site 310, PI from CC |
| Contributing authors: PI from site 201, PI from site 212, NPI from CC |
| Relationship between anemia and biomarkers: |
| Lead author: PI from site 201 |
| Senior authors: statistician, NPI from CC, PI from site 313 |
| Contributing author: PI from site 213 |
| Relationship between depression and biomarkers: |
| Lead author: PI from CC |
| Senior authors: NPI from CC, PI from site 403, PI from site 506 |
| Relationship between atrial fibrillation and biomarkers: |
| Lead author: PI from French CC |
| Senior authors: PI from site 505, PI from site 504, PI from site 506 |
| Contributing authors: NPI from French CC, PI from site 507, PI from site 503 |
| Relationship of echocardiographic variation and biomarkers: |
| Lead author: PI from echocardiography core laboratory |
| Senior authors: NPI from site 201, PI from site 307, PI from site 212 |
| Contributing authors: NHLBI-PO, PI from site 408, PI from site 310 |
| Relationship between resting perfusion and biomarkers: |
| Lead author: PI from nuclear core laboratory |
| Senior authors NPI from nuclear core laboratory, PI from biomarker core laboratory, NPI from nuclear core laboratory |
| Contributing authors: PI from site 346, PI from site 338, NPI from CC |
| Publication concepts without enough interest to pursue |
| Relationship between etiology and CPX |
| Relationship between anemia and CPX |
| Relationship between atrial fibrillation and CPX |
| Relationship between socioeconomic status and CPX |
| Relationship between dyssynchrony (nuclear) and CPX |
| Relationship between gene polymorphism and CPX |
| Alteration in heart rate reserve in pacers |
| Difference in CPX by center experience |
| Relationship between sex and biomarkers |
| Relationship between race and biomarker |
| Relationship between etiology and biomarker |
| Relationship between BMI and biomarker |
| Relationship between diabetes and biomarker |
| Relationship between renal function and biomarker |
| Relationship between QRS duration and biomarker |
| Relationship between CRT and biomarker |
| Relationship between QOL and biomarker |
BMI = body mass index; CC = coordinating center; CHF = congestive heart failure; CPX = cardiopulmonary exercise; CRT = cardiac resynchronization therapy; EQOL = Economic and Quality of Life Core; NHLBI-PO = National Heart, Lung, and Blood Institute project officer; NPI = non–principal investigator (any site personnel involved in study other than the PI); PI = principal investigator; QOL = quality of life.
At the time we were preparing this article, 14 baseline manuscripts were at various stages of the development and publication process. Among these 14 manuscripts, all but the manuscript describing the trial design, recently published (4), were assigned authors on the basis of the system described herein. The authors of the design manuscript were designated to be those involved in the development of the study and submission of the primary grant to the NHLBI. The other exceptions were the 4 protocol-specific primary analyses (2 of which are now published [5, 6[): the intention-to-treat analysis of the primary outcome; the on-treatment analysis of the primary outcome; and the economic and quality-of-life analyses, which were submitted by a representative group of investigators. We are currently establishing the assignment of process for secondary outcome manuscripts.
Author Assignments
The publications committee was responsible for reviewing proposals and assigning authorship equitably with respect to each potential author’s contribution to the trial overall and the contribution of the potential author’s site. Author contribution was based on site-specific metrics that we specified and converted into a score. Site metrics used to reflect site contribution were 1) enrollment; 2) adherence to the exercise regimen; 3) data completion and submission to the coordinating center; and 4) other trial efforts, such as serving on active trial committees (for example, clinical end points or publications committee) or overseeing operations of 1 of the core laboratories.
In brief, the enrollment component of the score was based on the number of participants registered at each site. Adherence, long recognized as a critical component of exercise training, was based on data submitted by each study site, including median exercise minutes per week and median exercise training intensity measured as percentage of heart rate reserve during supervised training and home-based training at 6 and 9 months. The data completion component was a composite of several missing data rates. Both adherence and data completion were normalized to the mean of all study sites. Finally, the other trial effort score was a way to recognize trial activity independent of site-based effort, as previously described. The composite adherence and data completion scores were rescaled to have the same mean and standard deviation as those used for the enrollment score.
We also recognized that other key authorship assignments would involve people who were needed for a particular area of expertise and for whom these scores were not necessarily applicable, such as biostatisticians and specialists in imaging and biomarker analysis. We accounted for these situations in the assignment of writing groups. The executive committee assured that a statistician participated on all manuscripts that described trial results, and the publications committee established that key statisticians working directly on a manuscript would be considered coauthors, with the order of authorship itself determined by the individual writing group. Coordinating center personnel (that is, the PI and coinvestigators) were assigned according to interest and level of participation. Because these persons could not generate the necessary metrics for a score, the executive committee approved their participation as authors. Staff at the NHLBI were considered for authorship assignments in the same manner as the coordinating center personnel.
If an investigator had a publication concept that was not included in the initial list of baseline or outcome manuscript ideas but might be considered a post hoc analysis, the investigator had the opportunity to submit a de novo publication proposal to the publications committee. If the committee deemed that the concept warranted publication, the investigator was appointed lead author and established the members of the writing group, pending approval by the publications committee. To maintain an equal distribution of authorship among sites, we established that no site could have more than 1 lead author for any baseline manuscript proposed by the publications committee.
Scoring
Weighting factors of 1.7 and 1.5 were applied for enrollment and data completion, respectively, in determining the score for baseline manuscripts. Adherence was weighted with a factor of 1.3. We assigned higher weighting factors to enrollment and data completion to highlight that all the data used for these manuscripts relied completely on a participant being enrolled and on the coordinating center receiving good-quality data at baseline, rather than from follow-up data or adherence. The steering committee approved this scoring method approximately 1 year before the end of enrollment.
The authorship score for the outcomes manuscripts was based on the same 4 components used for the baseline manuscripts. However, whereas the baseline manuscripts seemed to warrant higher weighting factors for enrollment and data completion, the outcomes manuscripts were assigned a higher weighting factor for adherence by the steering committee (composed of the PIs at all sites) to create an additional incentive for the sites to retain participants during follow-up and to ensure timely completion and transmission of study data forms. The steering committee approved the scoring method for the outcomes manuscripts and relayed its decision to the collaborators at their respective clinical sites approximately 1 year before the completion of the study.
The scores for enrollment, adherence, data collection, and other trial effort were summed, and each score was rescaled by adding 150 points so that no scores would be negative. Each site was ranked on the basis of this final score (Table). Beginning with the highest-ranked site, we established the authors and the order of authorship by using the choice of manuscript given by that site’s PI first and then proceeding to other study personnel if available (Figure 3). The PI at a site also could offer his or her authorship position to another qualified collaborator (for example, a Fellow) at the site. If the next manuscript, in order by preference, did not have a lead author, the PI or selecting site author was designated as the lead author. If all lead authorship positions were taken, the PI or selected author was designated for 1 of the 3 senior author positions following the same process that was used for lead authorship. If all senior author positions were taken from the PI’s ranked choices, the PI (or site author) was selected as a coauthor. Thus, within the HF-ACTION system for authorship assignment, a higher level of authorship took precedence over the investigator’s ranking of individual manuscripts. Unless communicated differently by the PI, authorship selection from a site followed the order of PI, coinvestigator, and study coordinator.
Table.
HF-ACTION Site Scores and Rank Before Authorship Assignment
| Site Number | Enrollment* | Adjusted Enrollment* | Adherence | Data Completion | Other Trial Effort | Total Score† | Rank |
|---|---|---|---|---|---|---|---|
| 101 | 43 | 73.1 | 55.8 | 12.6 | 25 | 317 | 27 |
| 102 | 56 | 95.2 | 28.3 | 67.9 | 100 | 441 | 8 |
| 103‡ | 94 | 159.8 | 55.2 | 68.7 | 125 | 559 | 4 |
| 104 | 49 | 83.3 | 42.0 | 65.9 | 25 | 366 | 14 |
| 105 | 67 | 113.9 | 45.0 | 68.8 | 25 | 403 | 12 |
| 106 | 57 | 96.9 | 28.7 | 14.8 | 50 | 340 | 22 |
| 107‡ | 109 | 185.3 | 45.8 | 64.9 | 400 | 846 | 1 |
| 108 | 114 | 193.8 | 24.2 | 58.7 | 125 | 552 | 5 |
| 109‡ | 106 | 180.2 | 37.8 | 71.1 | 275 | 714 | 3 |
| 110 | 63 | 107.1 | 25.6 | 62.9 | 0 | 346 | 20 |
| 111 | 40 | 68 | 11.3 | 12.5 | 25 | 267 | 49 |
| 201 | 41 | 76.7 | 21.4 | 29.4 | 150 | 428 | 9 |
| 202 | 25 | 46.8 | 26.7 | 73.8 | 0 | 297 | 33 |
| 203 | 37 | 69.2 | 36.7 | 70.1 | 25 | 351 | 19 |
| 204 | 7 | 13.1 | 18.2 | −112.8 | 150 | 218 | 71 |
| 205 | 6 | 11.2 | 6.5 | 63.8 | 75 | 307 | 31 |
| 206 | 55 | 102.9 | 39.0 | 70.7 | 0 | 363 | 18 |
| 207 | 4 | 7.5 | −25.7 | 51.6 | 0 | 183 | 78 |
| 208 | 48 | 89.8 | 22.1 | 35.3 | 0 | 297 | 34 |
| 209‡ | 127 | 237.5 | 40.6 | 38.6 | 300 | 767 | 2 |
| 211 | 9 | 16.8 | 7.0 | 10.9 | 0 | 185 | 76 |
| 212 | 7 | 13.1 | 8.4 | 75.5 | 0 | 247 | 55 |
| 213 | 6 | 11.2 | 29.7 | 64.4 | 0 | 255 | 52 |
| 215 | 54 | 101.0 | 15.6 | −65.6 | 25 | 226 | 68 |
| 216 | 77 | 144.0 | 52.7 | 62.5 | 0 | 409 | 10 |
| 217 | 12 | 22.4 | 13.8 | 56.4 | 50 | 293 | 36 |
| 218 | 51 | 95.4 | 35.6 | 48.2 | 0 | 329 | 25 |
| 219 | 26 | 48.6 | 36.1 | 21.3 | 0 | 256 | 51 |
| 301 | 47 | 87.9 | 24.1 | 17.5 | 0 | 279 | 43 |
| 302 | 38 | 71.1 | 60.0 | 62.4 | 0 | 343 | 21 |
| 303 | 17 | 31.8 | 24.6 | 63.6 | 0 | 270 | 46 |
| 304 | 65 | 121.6 | 16.2 | 69.5 | 50 | 407 | 11 |
| 305 | 17 | 31.8 | 27.3 | −2.3 | 0 | 207 | 73 |
| 306 | 15 | 28.1 | 64.8 | 70.3 | 50 | 363 | 17 |
| 307 | 12 | 22.4 | 66.3 | 47.6 | 0 | 286 | 39 |
| 308 | 5 | 9.4 | 8.1 | 76.2 | 0 | 243 | 57 |
| 309 | 9 | 16.8 | 59.4 | 52.3 | 0 | 278 | 44 |
| 310 | 22 | 41.1 | −6.1 | 58.9 | 0 | 244 | 56 |
| 312 | 15 | 28.1 | 138.0 | 49.6 | 0 | 366 | 15 |
| 313 | 17 | 31.8 | 16.6 | 70.7 | 0 | 269 | 47 |
| 315 | 1 | 1.9 | 0.0 | 40.7 | 50 | 243 | 58 |
| 316 | 11 | 20.6 | 43.4 | 73.5 | 0 | 287 | 38 |
| 319 | 26 | 48.6 | 11.2 | 32.7 | 0 | 243 | 59 |
| 320 | 17 | 31.8 | 14.0 | 43.9 | 0 | 240 | 62 |
| 322 | 54 | 101.0 | 12.4 | −4.0 | 25 | 284 | 40 |
| 323 | 7 | 13.1 | 57.1 | 64.1 | 0 | 284 | 41 |
| 324 | 34 | 63.6 | 65.4 | 60.0 | 0 | 339 | 23 |
| 325 | 11 | 20.6 | 11.0 | 69.1 | 0 | 251 | 53 |
| 326 | 8 | 15.0 | 10.3 | −119.7 | 0 | 56 | 82 |
| 327 | 4 | 7.5 | 7.9 | 76.2 | 0 | 242 | 61 |
| 329 | 16 | 29.9 | 56.2 | 72.1 | 0 | 308 | 29 |
| 330 | 4 | 7.5 | 17.9 | 50.6 | 0 | 226 | 69 |
| 331 | 18 | 33.7 | 61.0 | 43.5 | 0 | 288 | 37 |
| 332 | 3 | 5.6 | 1.3 | 30.2 | 0 | 187 | 75 |
| 333 | 5 | 9.4 | 20.5 | 48.8 | 0 | 229 | 67 |
| 334 | 18 | 33.7 | 43.1 | 67.6 | 0 | 294 | 35 |
| 335 | 4 | 7.5 | 31.3 | 76.2 | 0 | 265 | 50 |
| 336 | 6 | 11.2 | 35.3 | 40.7 | 0 | 237 | 63 |
| 337 | 5 | 9.4 | 51.3 | 76.2 | 100 | 387 | 13 |
| 338 | 3 | 5.6 | 35.3 | 40.7 | 0 | 232 | 65 |
| 340 | 11 | 20.6 | 60.5 | −23.7 | 0 | 207 | 72 |
| 341 | 13 | 24.3 | 49.6 | −4.6 | 0 | 219 | 70 |
| 342 | 21 | 39.3 | 71.3 | 61.8 | 0 | 322 | 26 |
| 343 | 9 | 16.8 | 44.3 | 31.2 | 0 | 242 | 60 |
| 344 | 9 | 16.8 | 40.6 | 68.8 | 0 | 276 | 45 |
| 345 | 2 | 3.7 | 23.7 | −19.5 | 0 | 158 | 80 |
| 346 | 6 | 11.2 | 24.8 | −67.6 | 0 | 118 | 81 |
| 401 | 19 | 35.5 | 58.2 | 71.1 | 50 | 365 | 16 |
| 402 | 4 | 7.5 | 81.2 | 58.5 | 0 | 297 | 32 |
| 403 | 24 | 44.9 | 24.0 | 64.4 | 50 | 333 | 24 |
| 404 | 61 | 114.1 | 54.2 | 53.1 | 150 | 521 | 6 |
| 405 | 19 | 35.5 | 18.7 | 64.6 | 0 | 269 | 48 |
| 406 | 9 | 16.8 | 80.7 | 72.4 | 150 | 470 | 7 |
| 408 | 5 | 9.4 | 19.7 | −45.1 | 50 | 184 | 77 |
| 409 | 10 | 18.7 | 55.6 | −57.3 | 0 | 167. | 79 |
| 411 | 21 | 39.3 | 63.7 | 54.1 | 0 | 307 | 30 |
| 501 | 12 | 22.4 | 23.2 | 52.2 | 0 | 248 | 54 |
| 503 | 11 | 20.6 | 15.8 | 1.5 | 0 | 188 | 74 |
| 504 | 8 | 15.0 | 43.7 | 71.4 | 0 | 280 | 42 |
| 505 | 20 | 37.4 | 62.6 | 65.2 | 0 | 315 | 28 |
| 506 | 2 | 3.7 | 35.3 | 40.7 | 0 | 230 | 66 |
| 507 | 4 | 7.5 | 35.3 | 40.7 | 0 | 233 | 64 |
HF-ACTION = Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training.
The adjusted enrollment total differs from the enrollment total to reflect the existence of U-centers, which had an expected level of enrollment as the basis for their grants.
The total score is the sum of the scores for adjusted enrollment, adherence, data completion, and other trial effort plus 150 points to ensure that no scores were negative.
These sites have the 4 top scores and are the examples used in the text.
Points were then “spent” from the site’s overall score on the basis of authorship position: 150 points for lead authorship, 100 points for senior authorship, and 50 points for coauthorship. The sites were reranked after each assignment, as shown in Figures 2 and 3. As we moved down the list of topics, the sites with lower scores were assigned authorship positions within the remaining writing groups. To maintain an equal distribution of authorship among sites, we established that no site could have more than 1 lead author for the proposed baseline manuscripts. In addition, we made certain that the number of topics available for selection would be sufficient for all sites—even those with the lowest total scores—to receive an assignment. No site was “disqualified” because of a low score. We also realized that a few topics might be “orphaned” if no site selected them. We considered this to be an acceptable balance, favoring accommodation of all sites over the possibility that some topics might remain unselected. The Appendix Table (available at www.annals.org) includes the list of topics with insufficient interest.
The Table lists all the sites in consecutive order by number along with the score for each of the 4 components (enrollment, adherence, data completion, and other trial effort) and the total score for each site. It includes columns for enrollment and for “adjusted enrollment,” but we counted only the adjusted enrollment toward the total score. The adjusted score reflects the existence of U-centers, which are sites that received fixed grants on the basis of expected enrollment as opposed to the more common arrangement, in which sites are reimbursed on a perpatient payment schedule. Because the U-center grants had more favorable financial arrangements, we recognized the efforts of non–U-center grant recipients by increasing the enrollment numbers by 10% as approved by the executive committee. Thus, the total for each site is the sum of the values for adjusted enrollment, adherence, data completion, and other trial effort plus 150 points (so that no score would be negative).
The following examples describe the system we used to deduct points from the preassignment site totals as we moved through the assignment process. You will note from the Table that site 107 had the highest total score: 846. The PI for site 107 had indicated a top preference for the manuscript evaluating the safety of CPX testing in patients with congestive heart failure and was assigned the lead authorship of that writing group. We deducted 150 points (the amount for lead authorship) from that site’s score, making the new site score 696 and reranking that site from first to third. Site 209 was then the highest-ranked site, with a score of 767 points. The PI for site 209 had selected the baseline manuscript on the relationship between age and CPX testing and was assigned the lead authorship for that writing group. Again, we deducted 150 points from that site’s score, reducing it to 617 and moving site 107 into second place. Site 109 then became the highest-ranked site. The PI for that site had selected the baseline manuscript on the relationship between sex and CPX testing. Once again, we deducted 150 points, moving site 107 back to the top-ranked position. However, because site 107 had already been assigned lead authorship, the PI for that site was assigned a senior authorship position on the baseline manuscript evaluating the relationship between diabetes and CPX testing. Once the senior authorship position was assigned, we subtracted 100 points from the site’s score, and the site was reranked as third. The PI for the next-highest site, site 103, with a score of 559, was not assigned a lead authorship position for the baseline manuscript on the prevalence and determination of chronotropic incompetence until after study personnel for sites 209, 107, and 109 (now reranked for the second time) were assigned senior authorship positions on other baseline manuscripts. The process continued until all manuscripts had been assigned in this manner.
Larger Context
Authorship within multicenter studies has been an ongoing issue within research networks and clinical trials. Although other groups have developed authorship guidelines, recommendations based on smaller and single-center studies would have become impractical for us to implement in HF-ACTION. We sought to develop a plan that would be relatively equitable and transparent, allow for the free exchange of novel concepts among investigators, and maximize dissemination and use of the study data. We examined many models from other disciplines and incorporated aspects of these into our plan (7, 8). Among those we found especially helpful were the National Psychosis Research Framework (7) and a system developed by the CanChild Centre for Childhood Disability Research (8). One attractive feature of the CanChild model was its organization of potential authors into a grid that incorporated interest, experience, and expertise. This group of researchers also offered scenarios for early resolution of authorship problems. Also helpful was a system proposed in 1994 by Digiusto (9) that assigned points on the basis of study contribution in 1 multidisciplinary research center, although we found its assignment of point values for study activities to be rather subjective. A potential concern with both the CanChild and the Digiusto systems is the assignment of points based on financial support, supervision, and/or data collection and submission. The ICMJE states that acquisition of funding, collection of data, or general supervision alone does not justify authorship. Whether fulfilling any of these trial activities alone would give an investigator enough points to qualify for authorship within either system is unclear.
The inclusion of an adherence score is unique to HF-ACTION and reflects the features that differentiate it from other trials evaluating a drug, device, or procedural intervention. Lifestyle interventions, such as exercise training, require substantial site and enrollee effort to maintain the exposure to the therapy being evaluated. The adherence score for this kind of intervention should be individualized to the type of treatment under evaluation. Interventions that require intense, site-based effort should provide rewards for above-average performance, as was done in the HF-ACTION scoring system.
The authorship system we devised for HF-ACTION has a few limitations that should be noted. One limitation is that the guidelines for baseline manuscripts were established after enrollment had begun. Consequently, sites did not have the maximum amount of time to achieve the best results for enrollment, adherence, and data completion and to maximize their participation in manuscript generation. In addition, the data completion formula did not include a benchmark for the quality of data being provided. We considered including the number of queries each center created but decided against it because of the potential for complications.
Finally, we must acknowledge that assignment of authorship is only the beginning of the process for producing manuscripts and disseminating results from study data. The assignment process needs to be partnered with other elements, including efficient and effective production of statistical output, maintenance of production timelines, and review of the final manuscript before journal submission.
In many studies, particularly those with limited funding, authorship is seen as true academic currency that can be used to promote strong participation within the study. To maximize this potential and to motivate investigators and study site personnel, the process for authorship selection must be transparent and equitable and should be established early in the trial. In a large multicenter clinical trial, it is easy to downplay the contribution of individual centers, but the sites’ effort in enrolling participants, following them on the basis of the protocol, and completing case report forms is invaluable. By creating a mechanism that recognizes this effort and meets the requirements established by ICMJE for authorship, all members of the investigational team have an opportunity to participate in the dissemination of results. The HF-ACTION model for authorship meets these goals.
The model used in the HF-ACTION study provided a transparent and equitable way to include all study personnel in the process of disseminating the results of the study. This is in sharp contrast to the traditional methods used in the past, which relied heavily on an executive committee for both lead authorship and coauthorship. By including a broad number of investigators, we have been able to harness the energies of many individuals in producing several manuscripts. For investigators considering a similar approach, it is critical to select key components of the study that will be required for the trial’s success and to outline the methods by which these components will be used to assign study personnel to manuscripts. However, with regard to weighting of individual components, such as enrollment and adherence, our decisions reflected the nature of our study and were not intended to be a template for others to follow. We hope that the methods outlined in this article will provide a helpful model for future studies to develop authorship systems that increase participation in the clinical research endeavor and enhance publication of critical trial results.
Acknowledgments
The authors thank Sue Russell, MFA, for her assistance in developing, editing, and submitting this manuscript for publication.
Grant Support: By the National Heart, Lung, and Blood Institute, National Institutes of Health, for the HF-ACTION trial (NIH/NHLBIU01HL063747).
Footnotes
Potential Financial Conflicts of Interest: None disclosed.
References
- 1.Horton R, Smith R. Time to redefine authorship [Editorial] BMJ. 1996;312:723. doi: 10.1136/bmj.312.7033.723. [PMID: 8605447] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith R. Time to face up to research misconduct [Editorial] BMJ. 1996;312:789–90. doi: 10.1136/bmj.312.7034.789. [PMID: 8608272] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Committee of Medical Journal Editors. Uniform Requirements for Manuscripts Submitted to Biomedical Journals. 2008 Oct; Updated. Accessed at www.icmje.org on 20 April 2009. [PMC free article] [PubMed]
- 4.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. HF-ACTION Trial Investigators Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [PMID: 17239677] [DOI] [PubMed] [Google Scholar]
- 5.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. HF-ACTION Investigators Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [PMID: 19351941] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [PMID: 19351942] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker A, Powell RA. Authorship. Guidelines exist on ownership of data and authorship in multicentre collaborations [Letter] BMJ. 1997;314:1046. doi: 10.1136/bmj.314.7086.1046. [PMID: 9112869] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourbonniere MC, Russell DJ, Goldsmith CH. Authorship issues: one research center’s experience with developing author guidelines. Am J Occup Ther. 2006;60:111–7. doi: 10.5014/ajot.60.1.111. [PMID: 16541990] [DOI] [PubMed] [Google Scholar]
- 9.Digiusto E. Equity in authorship: a strategy for assigning credit when publishing. Soc Sci Med. 1994;38:55–8. doi: 10.1016/0277-9536(94)90299-2. [PMID: 8146715] [DOI] [PubMed] [Google Scholar]


