Abstract
Background
Innovative health programs for injection drug users (IDUs), such as supervised injecting facilities (SIFs), are often preceded by evaluations of IDUs’ willingness to use the service. The validity of these surveys has not been fully evaluated. We sought to determine whether measures of willingness collected prior to the opening of a Canadian SIF accurately predicted subsequent use of the program.
Methods
Data were derived from a prospective cohort of IDUs. The sample size for this study was 640 IDUs. Using multivariate logistic regression, it was assessed if a history of reporting willingness to use the program, were it available, was associated with subsequent use. In sub-analysis restricted to individuals who had a history of reported willingness, we used multivariate longitudinal analysis to identify factors associated with not attending the SIF.
Results
Among 442 IDUs, 72% of those who reported initial willingness to use a SIF later attended the program, and a prior willingness to use a SIF significantly predicted later attendance (adjusted odds ratio = 1.67). In sub-analyses restricted to those who had a history of reporting willingness to use the SIF, not using the program was predicted by not frequenting the neighborhood where the SIF was located.
Conclusion
Our findings indicate that reported willingness measures collected from IDUs regarding potential SIF program participation prior to its opening independently predicted later attendance even when variables that were likely determinants of willingness were adjusted for. These data suggest that willingness measures are reasonably valid tools for planning the delivery of health services among IDU populations.
Keywords: injection drug use, supervised injection facilities, validity of willingness measures
INTRODUCTION
Illicit drug use continues to be associated with a broad range of health and social harms and there is growing recognition that innovative interventions are needed to address these problems (1–4). However, illicit drug use, particularly injection drug use, is highly stigmatized and it can be difficult for public health programs to connect with and effectively serve this often hidden population (5–7). This poses unique challenges for public health programmers, as it is often difficult to predict whether a specific program will be accepted by drug-using communities (8–11).
One strategy that has been employed to assess the level of acceptance of innovative health programs, such as supervised injection facilities (SIFs) where injection drug users (IDUs) can bring pre-obtained illicit drugs and inject under the supervision of a nurse, has been to survey the target population and measure their willingness to use the proposed service (12–14). Behavioral willingness is considered to be distinct from behavioral intention, as willingness is typically conceived in relation to what an individual is willing to do while intention reflects what an individual plans to do (15,16). Some studies report that compared with intention measures, willingness measures are actually better predictors of behaviors (16–19). Although willingness measures have been used to determine acceptance of safer SIFs in several settings including Vancouver, Montreal, San Francisco, London, Ireland, Melbourne, and Sydney (12,20–26), the validity of these surveys among illicit drug-using populations has not been fully evaluated. To assess whether willingness measures may be effective tools for planning the delivery of public health programs for IDU populations, we sought to determine whether measures of willingness collected prior to the opening of a Canadian SIF accurately predicted later use of the program.
Methods
Data for this study were obtained from the Vancouver Injection Drug Users Study (VIDUS), an open prospective cohort that began enrolling IDUs through street outreach and self-referral in May 1996. To be eligible, participants at recruitment must reside in the Greater Vancouver Regional District, have injected illicit drugs in the previous month, and be willing and able to provide written informed consent. This study has been described in detail previously (27,28). In brief, at enrolment and on bi-annual basis, participants complete an interviewer-administered questionnaire and, after an examination by a study nurse, provide a blood sample for serologic testing. At each study visit, participants are provided with a stipend ($20 CDN) for their time. The study has received ethics approval from St. Paul’s Hospital and the University of British Columbia’s Research Ethics Board.
In the primary analysis, we assessed whether reports of willingness to use a SIF before the program opened were associated with subsequent self-reported attendance at the facility after it was established in the Downtown Eastside (DTES) of Vancouver in September 2003. Initial willingness measures were assessed during the pre-SIF period of December 2001 to May 2003. A total of 640 individuals were seen for study follow-up during the pre-SIF study period. Willingness was based on the question “If a supervised safe injection site was available, would you use it?” “Yes” responses were compared with “no” responses, and individuals who replied that they were “unsure” were assessed in sub-analyses. Attendance at the facility was measured during the post-SIF period of December 2003 to November 2005 based on the question “Have you ever used the InSite SIS?”
Our primary analysis sought to determine whether there was a significant relationship between our main dependent variable of interest (attendance at the SIF) and our primary independent variable (prior report of willingness to attend a SIF). To consider this association while evaluating potential confounders, we a priori selected a range of secondary explanatory independent variables hypothesized to be associated with both attendance and initial willingness to attend based on previous research (12,20,29). Secondary explanatory factors included age (younger than 39 years of age vs. older); gender (female vs. male); unstable housing, defined as living in a single occupancy room in a hotel, a treatment or recovery house, jail, shelter or hostel, or having no fixed address for the last 6 months (yes vs. no); frequent exposure to the DTES, which is Vancouver’s well-described drug use epicenter and where the Vancouver SIF is situated (20), defined as residing in or visiting the DTES at least 2–3 times per week (yes vs. no); daily cocaine injection (yes vs. no); daily heroin injection (yes vs. no); daily crack cocaine smoking (yes vs. no); non-fatal overdose (yes vs. no); and using injection drugs in public locations, such as city streets, parks, and alleys (yes vs. no). All drug use and behavioral variables refer to the previous 6-month period and were measured at participants first study visit during our study period.
As a first step, we compared baseline characteristics stratified by attendance at the Vancouver SIF. We used Pearson’s χ2-test for dichotomous variables and the Mann–Whitney test for continuous variables. We were primarily concerned with identifying whether there was an independent relationship between just two variables (attendance at the SIF and prior report of willingness to attend a SIF). To address this, we used a backward selection process with automated procedures, previously described by Maldonado and Greenland (30) and Rothman and Greenland (31), which is specific to fitting multivariate models in these instances. Specifically, we began by including all variables in a fixed model. We subsequently generated a series of confounding models by removing secondary variables one at a time. For each of these models, we assessed the relative change in the coefficient for our primary independent variable of interest (prior willingness to use a SIF). The secondary variable that resulted in the smallest absolute relative change in the coefficient of “prior willingness to use a SIF” was then removed. This approach allowed us to identify the secondary variables that had the strongest influence on the coefficient for our primary variable of interest. Using this automated procedure, secondary variables continued to be removed until the smallest relative change in the coefficient of “prior willingness to use a SIF” exceeded 5% of the value of the coefficient. The final model included prior willingness to use a SIF and all remaining secondary explanatory variables.
To further explore the relationship between initial willingness and later use of a SIF, we conducted a number of sub-analyses. First, among individuals who reported that they had not attended the SIF, we assessed rates of non-injection drug use in the past 6 months during the post-SIF period, as well as infrequent exposure to the neighborhood where the supervised injection site was located. Infrequent exposure was defined as not residing in the DTES and visiting the neighborhood less than monthly. We then sought to identify factors associated with not attending the SIF among participants who initially reported willingness to use the facility. Factors that we hypothesized might be associated with not attending the Vancouver SIF included: age (younger than 39 years of age vs. older); gender (female vs. male); infrequent exposure to the DTES (yes vs. no); infrequent cocaine injection (< daily vs. ≥ daily); infrequent heroin injection (< daily vs. ≥ daily); being recently incarcerated (yes vs. no); and recently being involved in any kind of addiction treatment program (yes vs. no). All variables, including our outcome of interest, refer to behaviors in the previous 6 months.
Since for this analysis we were interested in identifying multiple factors that might be associated with not using the SIF, we did not use the previous model-building protocol which is designed to adjust for confounding and determine whether there is an independent relationship between just two factors of interest. Another distinguishing feature of this sub-analysis was that it focused on the post-SIF follow-up period of 24 months, and we had multiple observations per person for factors potentially associated with not using the SIF as well as serial measures for each subject. Therefore, to determine factors associated with our outcome of interest throughout the entire 24-month follow-up period we used generalized estimating equations (GEE) for binary outcomes with logit link for the analysis of correlated data (32). These methods provided standard errors adjusted by multiple observations per person using an exchangeable correlation structure. With this approach, data from every participant follow-up visit were considered in this analysis. Missing data were addressed through the GEE mechanism which uses the all available pairs method to encompass the missing data from dropouts or intermittent missing. All non-missing pairs of data are used in the estimators of the working correlation parameters.
As a first step, GEE univariate analyses were conducted to obtain unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) for variables of interest. In order to adjust for potential confounding, all variables that were p < .05 in GEE univariate analyses were entered into a multivariate logistic GEE model. All statistical analyses were performed using SAS software version 9.1 (SAS, Cary, NC, USA). All p-values are two sided.
RESULTS
In the pre-SIF period 344 (54%) participants reported being willing to use a SIF, 256 (40%) reported being unwilling, and 40 (6%) were unsure (see Figure 1). Among the “unsure” group 11 (28%) were not seen for study follow-up during the post-SIF study period, and of the remaining 29 “unsure” individuals, 18 (62%)subsequently used the facility. Among the 600 participants who reported either being willing or unwilling to use a SIF, 158 (70 and 88, respectively) were not seen for study follow-up during the post-SIF study period and were therefore excluded from further analyses. Those lost to follow-up were significantly less likely to report being willing to use a SIF (p < .001). The remaining 442 participants were included in our primary comparison of those that reported yes versus no willingness. Among the 274 participants within this group who reported being initially willing to use a SIF, 198 (72%) later reported attending the SIF, while 91 (54%) of those who were initially unwilling later reported attending the SIF. The characteristics of the study sample stratified by reported attendance at the SIF are presented in Table 1. The univariate analyses of behavioral and socio-demographic variables are also presented in Table 1. Initial willingness to use a SIF was significantly associated with later use of the facility (OR = 2.20, 95% CI: 1.47–3.30). The results of the final multivariate logistic regression are shown in Table 2. Our primary explanatory variable, initial willingness to use a SIF, remained independently associated with attending the SIF (adjusted OR (AOR) = 1.67, 95% CI: 1.09–2.55). Unstable housing (AOR = 1.54, 95% CI: 1.01–2.34) and using injection drugs in public were also independently associated with using the SIF (AOR = 2.35, 95% CI: 1.46–3.77). In sub-analyses, we found that among participants who did not attend the SIF, 31 (19%) reported at some point during the post-SIF study period that they had not injected drugs in the previous 6 months. Similarly, during the same period and among the same group, 32 (21%) individuals reported infrequent exposure to the DTES.
FIGURE 1.
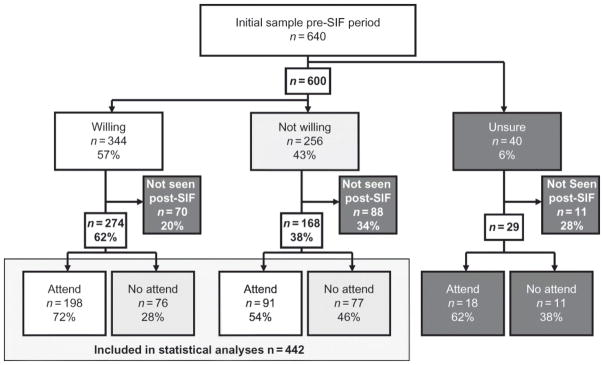
Study sample.
Note: SIF, supervised injection facility.
TABLE 1.
Univariate analyses of study population stratified by attendance at Vancouver’s SIF (n = 442).
| Characteristic1 | Attended SIF | Univariate | ||
|---|---|---|---|---|
| Yes (n= 289), n (%) | No (n= 153), n (%) | OR (95% CI) | p-Value | |
| Prior willingness to use SIF | ||||
| Yes | 198(69) | 76(50) | 2.20(1.47–3.30) | <.001 |
| No | 91 (31) | 77(50) | ||
| Younger than 39 years of age2 | ||||
| Yes | 160(55) | 60 (39) | 1.92(1.29–2.86) | <.001 |
| No | 129(45) | 93(61) | ||
| Female gender | ||||
| Yes | 122 (42) | 64 (42) | 1.02(0.68–1.51) | .938 |
| No | 167(58) | 89(58) | ||
| Unstable housing2,3 | ||||
| Yes | 162(56) | 64 (42) | 1.77(1.19–2.64) | .005 |
| No | 127 (44) | 89(58) | ||
| Frequent exposure to DTES2 | ||||
| Yes | 163(56) | 77(50) | 1.28(0.86–1.89) | .223 |
| No | 126 (44) | 76(50) | ||
| Daily cocaine injection2 | ||||
| Yes | 94 (33) | 31 (20) | 1.90(1.19–3.02) | .007 |
| No | 195(67) | 122(80) | ||
| Daily heroin injection2 | ||||
| Yes | 95 (33) | 24 (16) | 2.63(1.60–4.34) | <.001 |
| No | 194(67) | 129(84) | ||
| Daily crack use2 | ||||
| Yes | 153(53) | 57 (37) | 1.89(1.27–2.83) | .002 |
| No | 136(47) | 96(63) | ||
| Overdose(non-fatal)2 | ||||
| Yes | 21 (7) | 1 (1) | 11.91(1.59–89.42) 016 | |
| No | 268(93) | 152(99) | ||
| Public injecting2 | ||||
| Yes | 142(49) | 36 (24) | 3.14(2.02–4.87) | <.001 |
| No | 147(51) | 117(76) | ||
Notes: SIF, supervised injection facility; OR, odds ratio; CI, confidence interval; DTES, Downtown Eastside of Vancouver, which is the neighborhood where the SIF is located.
All explanatory variables measured at first study visit during study period.
Denotes activities or situations referring to previous 6 months.
Unstable housing is defined as living in a single occupancy room in a hotel, a treatment or recovery house, jail, shelter or hostel, or having no fixed address for the last 6 months.
TABLE 2.
Multivariate logistic regression analysis of factors associated with attending Vancouver’s SIF versus not attending the facility (n = 442).
| Characteristic1 | Adjusted odds ratio | (95% confidence interval) | p-Value |
|---|---|---|---|
| Prior willingness to use SIF | |||
| Yes versus no | 1.67 | (1.09–2.55) | .019 |
| Unstable housing2,3 | |||
| Yes versus no | 1.54 | (1.01–2.34) | .044 |
| Daily cocaine injection2 | |||
| Yes versus no | 1.52 | (0.93–2.48) | .095 |
| Daily heroin injection2 | |||
| Yes versus no | 1.63 | (0.95–2.81) | .076 |
| Public injecting2 | |||
| Yes versus no | 2.35 | (1.46–3.77) | <.001 |
Notes: Overdose was not considered in this model due to low-frequency counts. SIF, supervised injection facility.
All explanatory variables were measured at first study visit during study period.
Denotes activities or situations referring to previous 6 months.
Unstable housing is defined as living in a single occupancy room in a hotel, a treatment or recovery house, jail, shelter or hostel, or having no fixed address for the last 6 months.
In the sub-analysis restricted to the 274 individuals who initially reported being willing to use a SIF (see Table 3), being younger than 39 years of age, infrequent exposure to the DTES, infrequent cocaine injection, infrequent heroin injection, and engagement in any addiction treatment program were significantly associated with not using the SIF in univariate GEE analyses. In multivariate GEE analyses, infrequent exposure to the DTES (AOR = 1.89, 95% CI: 1.31–2.71), infrequent cocaine injection (AOR = 1.54, 95% CI: 1.13–2.09), and infrequent heroin injection (AOR = 2.37, 95% CI: 1.77–3.17) were significantly positively associated with not using the SIF, while being younger than 39 years of age (AOR = 0.03, 95% CI: 0.01–0.05) was significantly negatively associated with not using the SIF.
TABLE 3.
GEE analysis of factors associated with not using the SIF in the last 6 months (n = 76) versus using the facility among those who initially reported being willing to use an injection site (n = 274)
| Characteristic1 | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR(95% CI) | p-Value | AOR(95% CI) | p-Value | |
| Younger than 39 years of age | ||||
| Yes versus no | 1.65(1.18–2.29) | .003 | 1.68(1.21–2.34) | .002 |
| Gender | ||||
| Female versus male | 1.09(0.75–1.58) | .660 | ||
| Infrequent exposure to DTES2 | ||||
| Yes versus no | 1.93(1.38–2.71) | <.001 | 1.86(1.30–2.66) | <.001 |
| Infrequent cocaine injection2 | ||||
| < daily versus > daily | 1.82(1.36–2.44) | <.001 | 1.52(1.12–2.06) | .007 |
| Infrequent heroin injection2 | ||||
| < daily versus > daily | 2.80(2.11–3.71) | <.001 | 2.46(1.84–3.28) | <.001 |
| Incarceration2 | ||||
| Yes versus no | 0.78(0.59–1.09) | .141 | ||
| Any addiction treatment2 | ||||
| Yes versus no | 1.42(1.05–1.93) | .024 | 1.23(0.90–1.68) | .185 |
Notes: GEE, generalized estimating equations; SIF, supervised injection facility; OR, odds ratio; CI, confidence interval; AOR, adjusted odds ratio; DTES, Downtown Eastside of Vancouver, which is the neighborhood where the SIF is located, infrequent exposure defined as being in the neighborhood less than 2–3 times per week.
Variable measures collected between December 2003 and November 2005.
Denotes activities or situations referring to previous 6 months.
DISCUSSION
Our study found that initial willingness to use a SIF was independently associated with subsequent attendance at Vancouver’s SIF, even after adjusting for other determinants of willingness. We also found that not actively injecting drugs and infrequent exposure to the neighborhood where Vancouver’s SIF is located were factors that appear to negatively influence whether individuals use a SIF following a report of being willing to use the program before it opened.
These findings are largely consistent with a broad literature suggesting that behavioral intention is a reasonable predictor of later action (16,33). Intention measures have been found to be correlated with health-related behaviors in a number of areas including adolescent smoking, illicit drug use, and sexual health (15,17,34–37). More specifically, these findings support previous studies suggesting that willingness measures are generally good predictors of future behaviors (16–19).
While our study indicates that willingness predicts future SIF use, it is also noteworthy that personal circumstances including cessation from injection drug use, lower intensity injection drug use, and infrequent exposure to the DTES appear to have an expected deterrent effect on SIF use. These effects are expected given that actively injecting drugs is a prerequisite for using the SIF, and the SIF has been shown to attract high-intensity drug injectors (38). Being an infrequent visitor to the neighborhood where the SIF was established would also be expected to reduce the likelihood that an individual would use the facility. Indeed, previous studies indicate that travel time to the SIF from where the IDU resides and purchases drugs is a significant barrier to using the injection facility (39). The association between younger age and lower likelihood of using the SIF may reflect the demographic character of the neighborhood where the SIF was established. Previous research in our study setting suggests that street-involved youth who use drugs tend to spatially separate themselves from Vancouver’s DTES neighborhood and prefer to congregate in the Downtown South area of the city (40). This distancing may partially explain why younger age was associated with not using Vancouver’s injection site despite being initially willing. In addition to the factors identified in our analysis, there are a number of other considerations that could influence whether or not an individual chooses to use a facility of this nature. For example, waiting times and operating regulations such as a ban on assisted injections could present barriers to individuals suffering from drug withdrawal symptoms or who do not have the ability to self-inject. This might hinder the ability of these individuals to use the facility despite an initial willingness or intention to use it. Clearly, situational factors are relevant in determining SIF utilization; however, we found that despite these multiple factors, willingness measures are meaningful indicators of later SIF use.
These findings have implications for the validity of willingness studies that have been conducted in other settings to assess the acceptability of establishing SIFs. For instance, a willingness study conducted among IDUs in San Francisco recently reported that 85% of local IDUs were willing to use a SIF (21). Our study suggests that policy planners in San Francisco can be confident that this measure is a good indicator of client uptake, should a SIF be established in that area.
Our findings are also relevant to the planning of other types of public health programs and services for IDU populations as they suggest that willingness measures are relatively accurate markers of a population’s intention to use a particular service. We should note that directly engaging with people who use drugs and assessing willingness prior to the establishment of a health service or program are consistent with a growing recognition of the importance of involving target populations in the planning and delivery of health and social services, particularly among vulnerable populations (6,41). Although assessing willingness is not a substitute for meaningful involvement, we suggest it can be a useful first step in engaging a target population in service design and delivery.
Our study has a number of potential limitations. First, our measures relied on self-report which can be subject to socially desirable reporting and recall bias. Most importantly, socially desirable reporting could have inflated our measure of SIF attendance, given its widespread support and acceptance among local drug users. While it would have been favorable to validate self-reported attendance against the database of registered clients of the SIF a number of data limitations prevented this. First, in an effort to build trust among local drug users and establish a low threshold service, the SIF client registry was not fully operational until 6 months after the facility opened (42). Second, the opportunity to consent to linking VIDUS participants with the SIF client registry was only offered during specific study follow-up periods. As a result not all VIDUS participants in our study sample were given the option of consenting to this process. Given the significant data limitations resulting from these factors, it was unfortunately not feasible to accurately validate self-reports with the SIF client registry.
Another potential limitation of our study is the generalizability of our findings. The VIDUS is not a randomized sample of IDUs and may not be reflective of other drug user populations. It is, however, believed to be representative of IDUs in the community (43,44).
CONCLUSIONS
In summary, we found that individuals who indicated that they were willing to use a SIF were more likely to later attend the Vancouver SIF once it was opened, even after we adjusted for factors expected to be associated with willingness. These data suggest that willingness measures may be valid tools for planning the delivery of health services among IDU populations and should be considered by future health program planners.
Acknowledgments
We thank the study participants for their contribution to the research, as well as current and past researchers and staff. We would specifically thank Deborah Graham, Tricia Collingham, Carmen Rock, Peter Vann, Caitlin Johnston, Steve Kain, Danny Kain, and Calvin Lai for their research and administrative assistance. The study was supported by the US National Institutes of Health (R01DA011591 and R01DA021525) and the Canadian Institutes of Health Research (MOP–79297, RAA–79918). Thomas Kerr is supported by the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research. Kora DeBeck is supported by a Michael Smith Foundation for Health Research Senior Graduate Trainee Award and a Canadian Institutes of Health Research Doctoral Research Award. Julio Montaner has received an Avant-Garde Award (DP1DA026182) from the National Institute of Drug Abuse, US National Institutes of Health.
Footnotes
Author contributions:
KD, TK, JM, and EW were responsible for study design; CL conducted the statistical analyses; KD prepared the first draft of the analysis; TK, JB, JM, and EW contributed to the main content and provided critical comments on the final draft. All authors approved the final manuscript.
Declaration of Interest
Dr. Julio Montaner has received grants from, served as an ad hoc advisor to, or spoke at various events sponsored by Abbott, Argos Therapeutics, Bioject Inc., Boehringer Ingelheim, Bristol-Myers Squibb (BMS), Gilead Sciences, GlaxoSmithKline, Hoffmann-La Roche, Janssen-Ortho, Merck Frosst, Pfizer, Schering, Serono Inc., TheraTechnologies, Tibotec, and Trimeris. The authors declare no other competing interests.
References
- 1.DeBeck K, Wood E, Montaner J, Kerr T. Canada’s 2003 renewed drug strategy – An evidence-based review. HIV AIDS Policy Law Rev. 2006;11(2–3):1, 5–12. [PubMed] [Google Scholar]
- 2.Reuter P, Caulkins J. Redefining the goals of national drug policy: Recommendations from a working group. Am J Public Health. 1995;85(8 Pt 1):1059. doi: 10.2105/ajph.85.8_pt_1.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes T. ‘The risk environment’: A framework for understanding and reducing drug-related harm. Int J Drug Policy. 2002;13(2):85–94. [Google Scholar]
- 4.Wood E, Stoltz JA, Zhang R, Strathdee SA, Montaner JSG, Kerr T. Circumstances of first crystal methamphetamine use and initiation of injection drug use among high-risk youth. Drug Alcohol Rev. 2008;27(3):270–276. doi: 10.1080/09595230801914750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowden D, Dorsey P, Bullman S, Lestina R, Han C, Herrell J. HIV outreach for hard-to-reach populations: A cross-site perspective. Eval Program Plann. 1999;22(3):251–258. doi: 10.1016/s0149-7189(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 6.Vlahov D, Coady MH, Ompad DC, Galea S. Strategies for improving influenza immunization rates among hard-to-reach populations. J Urban Health. 2007;84(4):615–631. doi: 10.1007/s11524-007-9197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platt L, Wall M, Rhodes T, Judd A, Hickman M, Johnston LG, Renton A, Bobrova N, Sarang A. Methods to recruit hard-to-reach groups: Comparing two chain referral sampling methods of recruiting injecting drug users across nine studies in Russia and Estonia. J Urban Health. 2006;83(6 Suppl):i39–i53. doi: 10.1007/s11524-006-9101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood E, Kerr T, Montaner J, Strathdee S, Wodak A, Hankins C, Schechter M, Tyndall M. Rationale for evaluating North America’s first medically supervised safer-injecting facility. Lancet Infect Dis. 2004;4(5):301–306. doi: 10.1016/S1473-3099(04)01006-0. [DOI] [PubMed] [Google Scholar]
- 9.McCoy CB, Metsch LR, Chitwood DD, Miles C. Drug use and barriers to use of health care services. Subst Use Misuse. 2001;36(6):789–804. doi: 10.1081/ja-100104091. [DOI] [PubMed] [Google Scholar]
- 10.Strathdee SA, Palepu A, Cornelisse PGA, Yip B, O’Shaughnessy MV, Montaner JSG, Schechter M, Hogg R. Barriers to use of free antiretroviral therapy in injection drug users. J Am Med Assoc. 1998;280(6):547. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 11.Edlin BR, Kresina TF, Raymond DB, Carden MR, Gourevitch MN, Rich JD, Cheever L, Cargill V. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40(Suppl 5):S276–S285. doi: 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood E, Kerr T, Spittal PM, Li K, Small W, Tyndall MW, Hogg R, O’Shaughnessy M. The potential public health and community impacts of safer injecting facilities: Evidence from a cohort of injection drug users. JAIDS. 2003;32(1):2. doi: 10.1097/00126334-200301010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Wood E, Tyndall M, Montaner J, Kerr T. Summary of findings from the evaluation of a pilot medically supervised safer injecting facility. CMAJ. 2006;175(11):1399–1404. doi: 10.1503/cmaj.060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyne-Beasley T, Ford CA, Waller MW, Adimora AA, Resnick MD. Sexually active student’s willingness to use school-based health centers for reproductive health care services in North Carolina. Ambul Pediatr. 2003;3(4):196–202. doi: 10.1367/1539-4409(2003)003<0196:saswtu>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Myklestad I, Rise J. Predicting willingness to engage in unsafe sex and intention to perform sexual protective behaviors among adolescents. Health Educ Behav. 2007;34(4):686–699. doi: 10.1177/1090198106289571. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons FX, Gerrard M, Blanton H, Russell DW. Reasoned action and social reaction: Willingness and intention as independent predictors of health risk. J Pers Soc Psychol. 1998;74(5):1164–1180. doi: 10.1037//0022-3514.74.5.1164. [DOI] [PubMed] [Google Scholar]
- 17.Litchfield R, White K. Young adults’ willingness and intentions to use amphetamines: An application of the theory of reasoned action. E J Appl Psychol. 2006;2(1):45–51. [Google Scholar]
- 18.Gerrard M, Gibbons FX, Stock ML, Lune LS, Cleveland MJ. Images of smokers and willingness to smoke among African American pre-adolescents: An application of the prototype/willingness model of adolescent health risk behavior to smoking initiation. J Pediatr Psychol. 2005;30(4):305–318. doi: 10.1093/jpepsy/jsi026. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons FX, Gerrard M, Lane DJ. A social reaction model of adolescent health risk. In: Suls JM, Wallston KA, editors. Social Psychological Foundations of Health and Illness. Oxford: Blackwell; 2003. p. 107. [Google Scholar]
- 20.Kerr T, Wood E, Small D, Palepu A, Tyndall MW. Potential use of safer injecting facilities among injection drug users in Vancouver’s Downtown Eastside. CMAJ. 2003;169(8):759. [PMC free article] [PubMed] [Google Scholar]
- 21.Kral AH, Wenger L, Carpenter L, Wood E, Kerr T, Bourgois P. Acceptability of a safer injection facility among injection drug users in San Francisco. Drug Alcohol Depend. 2010;110(1–2):160–163. doi: 10.1016/j.drugalcdep.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt N, Lloyd C, Kimber J, Tompkins C. Public injecting and willingness to use a drug consumption room among needle exchange programme attendees in the UK. Int J Drug Policy. 2007;18(1):62–65. doi: 10.1016/j.drugpo.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 23.O’Shea M. Introducing safer injecting facilities (SIFs) in the Republic of Ireland: ‘Chipping away’ at policy change. Drugs Educ Prev Policy. 2007;14(1):75–88. [Google Scholar]
- 24.Fry CL. Injecting drug user attitudes towards rules for supervised injecting rooms: Implications for uptake. Int J Drug Policy. 2002;13(6):471–476. [Google Scholar]
- 25.Fry C, Fox S, Rumbold G. Establishing safe injecting rooms in Australia: Attitudes of injecting drug users. Aust N Z J Public Health. 2008;23(5):501–504. doi: 10.1111/j.1467-842x.1999.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Beek I, Gilmour S. Preference to have used a medically supervised injecting centre among injecting drug users in Kings Cross, Sydney. Aust N Z J Public Health. 2000;24(5):540–542. doi: 10.1111/j.1467-842x.2000.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 27.Wood E, Tyndall MW, Spittal PM, Li K, Kerr T, Hogg RS, Montaner J, O’Shaughnessy M, Schechter M. Unsafe injection practices in a cohort of injection drug users in Vancouver: Could safer injecting rooms help? CMAJ. 2001;165(4):405–410. [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr T, Marshall A, Walsh J, Palepu A, Tyndall M, Montaner J, Hogg R, Wood E. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17(5):539–549. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 29.Tyndall M, Kerr T, Zhang R, King E, Montaner J, Wood E. Attendance drug use patterns, and referrals made from North America’s first supervised injection facility. Drug Alcohol Depend. 2006;83(3):193–198. doi: 10.1016/j.drugalcdep.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado G, Greenland S. Simulation study of confounder selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S. Modern Epidemiology. New York: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 32.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13. [Google Scholar]
- 33.Armitage CJ, Conner M. Efficacy of the theory of planned behaviour: A meta-analytic review. Br J Soc Psychol. 2001;40(Pt 4):471–499. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- 34.Hukkelberg SS, Dykstra JL. Using the prototype/willingness model to predict smoking behaviour among Norwegian adolescents. Addict Behav. 2009;34(3):270–276. doi: 10.1016/j.addbeh.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Albarracin D, Johnson BT, Fishbein M, Muellerleile PA. Theories of reasoned action and planned behavior as models of condom use: A meta-analysis. Psychol Bull. 2001;127(1):142–161. doi: 10.1037/0033-2909.127.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheeran P, Orbell S. Do intentions predict condom use? Meta-analysis and examination of six moderator variables. Br J Soc Psychol. 1998;37(Pt 2):231–250. doi: 10.1111/j.2044-8309.1998.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 37.Fisher WA, Fisher JD, Rye BJ. Understanding and promoting AIDS-preventive behavior: Insights from the theory of reasoned action. Health Psychol. 1995;14(3):255–264. doi: 10.1037//0278-6133.14.3.255. [DOI] [PubMed] [Google Scholar]
- 38.Wood E, Tyndall M, Li K, Lloyd-Smith E, Small W, Montaner J, Kerr T. Do supervised injecting facilities attract higher-risk injection drug users? Am J Prev Med. 2005;29(2):126–130. doi: 10.1016/j.amepre.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Petrar S, Kerr T, Tyndall MW, Zhang R, Montaner JS, Wood E. Injection drug user’s perceptions regarding use of a medically supervised safer injecting facility. Addict Behav. 2007;32(5):1088–1093. doi: 10.1016/j.addbeh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Fast D, Shoveller J, Shannon K, Kerr T. Safety and danger in downtown Vancouver: Understandings of place among young people entrenched in an urban drug scene. Health Place. 2010;16(1):51–60. doi: 10.1016/j.healthplace.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jürgens R. Nothing about Us without Us: Greater, Meaningful Involvement of People Who Use Illegal Drugs: A Public Health, Ethical and Human Rights Imperative. Toronto, ON, Canada: Canadian HIV/AIDS Legal Network; 2005. [Google Scholar]
- 42.Wood E, Kerr T, Lloyd-Smith E, Buchner C, Marsh D, Montaner J, Tyndall M. Methodology for evaluating Insite: Canada’s first medically supervised safer injection facility for injection drug users. Harm Reduct J. 2004;1(9):1–5. doi: 10.1186/1477-7517-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyndall MW, Craib KJP, Currie S, Li K, O’Shaughnessy MV, Schechter MT. Impact of HIV infection on mortality in a cohort of injection drug users. JAIDS. 2001;28(4):351. doi: 10.1097/00126334-200112010-00008. [DOI] [PubMed] [Google Scholar]
- 44.DeBeck K, Kerr T, Li K, Fischer B, Buxton J, Montaner J, Wood E. Emergence of crack cocaine smoking as a risk factor for HIV seroconversion among injection drug users in Vancouver, Canada. CMAJ. 2009;181(9):585–589. doi: 10.1503/cmaj.082054. [DOI] [PMC free article] [PubMed] [Google Scholar]


