Abstract
Currently, younger, more active patients are being offered total joint replacement (TJR) for end-stage arthritic disorders. Despite improved durability of TJRs, particle-associated wear of the bearing surfaces continues to be associated with particulate debris, which can activate monocyte/macrophages. Activated macrophages then produce pro-inflammatory factors and cytokines that induce an inflammatory reaction that activates osteoclasts leading to bone breakdown and aseptic loosening. We hypothesized that activated macrophages in tissues harvested from revised joint replacements predominantly express an M1 pro-inflammatory phenotype due to wear-particle-associated cell activation, rather than an M2 antiinflammatory phenotype. We further questioned whether it is possible to convert uncommitted monocyte/macrophages to an M2 phenotype by the addition of interleukin-4 (IL-4), or whether it is necessary to first pass through an M1 intermediate stage. Retrieved periprosthetic tissues demonstrated increased M1/M2 macrophage ratios compared to non-operated osteoarthritic synovial tissues, using immunohistochemical staining and Western blotting. Uncommitted monocyte/macrophages with/without poly-methyl-methacrylate particles were transformed to an M2 phenotype by IL-4 more efficiently when the cells were first passed through an M1 phenotype by exposure to endotoxin. Wear particles induce a pro-inflammatory microenvironment that facilitates osteolysis; these events may potentially be modulated favorably by exposure to IL-4.
Keywords: M1 M2 macrophages, Osteolysis, Total joint replacement, PMMA, IL-4
1. Introduction
Total joint replacement (TJR) is a successful operation for patients suffering from disabling arthritis and other degenerative conditions. However, wear of artificial joints occurs in association with the level of activity and duration of implantation. TJR failure is often associated with osteolysis and is a long-term complication that may require revision surgery [1–5]. Production of wear particles which are biologically active and indigestible incites an innate inflammatory reaction that may lead to periprosthetic bone loss, implant loosening and pathological fracture through osteolytic bone [4–8]. Particles are phagocytosed by monocyte/macrophage lineage cells, leading to their proliferation, differentiation, and activation [1,9,10]. These events lead to intracellular signal transduction involving activation of transcription factor NFkB and nuclear translocation, which up-regulate gene expression mechanisms for pro-inflammatory cytokines, chemokines, and other substances [5,11,12]. The end result is the disruption of the homeostatic balance between bone formation and resorption [5,11,12].
A current hypothesis suggests that macrophage activation in osteolysis may culminate in a specific phenotype, with polarization to either M1 or M2 profile, due to the undifferentiated nature of monocyte/macrophage precursors, and the microenvironment of cell activation [9,13]. Studies also suggest that there may be epigenetic control of macrophage polarization, suggesting a possible genetic predisposition for osteolysis [14–16]. Specifically, this hypothesis suggests that wear particles initiate the migration of monocyte/macrophage precursors to the local site of particle production, and subsequent differentiation and activation to a classical M1 phenotype that initially promotes acute inflammation. This acute inflammatory state overcomes the anti-inflammatory environment supported by alternatively activated M2 macrophages that normally promotes bone healing, debris scavenging, wound healing, and angiogenesis [11,12].
The cytokine production profiles of M1 and M2 macrophages differ significantly and can be used to identify different predominant populations in a specific clinical situation. M1 macrophages produce primarily pro-inflammatory mediators, including TNF-α, IL-1, IL-6, and type 1 interferon, as well as IL-12 and IL-23, with the expression of inducible nitric oxide synthase (iNOS) and HLA-DR [17–19]. In contrast, M2 macrophages produce low levels of IL-12 and pro-inflammatory cytokines. The M2 profile is characterized by increased IL-4, IL-10, and IL-13 production, and expression of CCL1, CCL18, FIZZ1, mammalian chitinase Ym1, Arginase 1, CD163, and chitotriosidase [11,12,20,21]. This differential cytokine production and receptor expression can be used to characterize which macrophages are present in a clinical situation. Once individual populations of M1 and M2 macrophages are identified, the cytokine profiles induced by pro-inflammatory stimuli such as lipopolysaccharide (LPS) or wear particles may be used to confirm the phenotype of the macrophages [22]. Lipopolysaccharide is a particularly relevant stimulus to joint replacement, as Greenfield and colleagues have demonstrated the presence of LPS in some retrieved tissues [23–26].
The overall goal of this research was to identify macrophage populations in retrieved tissues from primary total joint replacement compared to revision surgeries with wear-particle-associated inflammation. First we hypothesized that there is a higher ratio of M1/M2 macrophages in tissues harvested from patients undergoing revision joint replacement compared to synovial tissues from patients undergoing primary joint replacement. We then questioned whether it was possible to isolate and modulate macrophage populations in vitro to selectively enhance an M2 profile in response to the inflammatory stimuli LPS and polymethyl-methac-rylate (PMMA) particles by adding IL-4 to differentiate uncommitted macrophages towards a M2 phenotype [11]. We tested these hypotheses using immunohistochemistry, Western blot analysis, flow cytometry, and enzyme-linked immunosorbent (ELISA) assay of culture supernatants.
2. Materials and methods
2.1. Clinical investigation
2.1.1. Tissue collection
This research was approved by the Stanford University School of Medicine’s Administrative Panel on Human Subjects in Medical Research. Synovium was collected during primary TJR and periprosthetic tissues during revision joint replacement. Periprosthetic tissues were obtained from patients with radiographic evidence of osteolysis who underwent revision total hip or knee arthroplasty in the absence of infection (all tissues were aerobic and anaerobic culture negative). We collected synovium from nine patients, seven female and two male, average age 64.2 years old. We collected pseudomembranes from seven patients undergoing revision surgery, six females and one male, average age 58.1 years old. All revision patients received a replacement metal-on-polyethylene implant (Table 1).
Table 1.
Information on patients who received a revision surgery, with the age, sex, and type of implant revised or removed.
| Age | Sex | Implant Revised |
|---|---|---|
| 45 | M | Hip revised with cobalt/chrome stem wear on cobalt/chrome cup |
| 51 | F | Knee revised with cobalt/chrome femoral component, polyethylene insert, titanium alloy tibial component |
| 34 | F | Hip revised with titanium alloy stem, ceramic head, titanium alloy cup |
| 54 | F | Hip revised with cobalt/chrome stem with cement, loose cementless cup, cobalt/chrome head |
| 68 | F | Knee revised with loose titanium alloy tibial component with cement, cobalt/chrome femoral component |
| 57 | F | Hip revised with cemented stem, cobalt/chrome head, polyethylene cup |
| 88 | F | Hip revised for metal on polyethylene wear |
2.1.2. Immunohistochemistry
Previous studies have noted the morphological variability in cellularity and composition of tissues taken from the synovium and pseudomembranes [27–29]. To account for this variability, three to six regions of each synovium or pseudomembrane from each patient sample were selected for analysis. Synovial and periprosthetic tissues were embedded in Tissue Tek OCT compound (Sakura, Torrance, CA) and fresh frozen in liquid nitrogen and stored at −80 °C for immunohistological analysis. Serial 6 µm sections were cut with a cryostat (Minotome Plus, Triangle Biomedical Sciences, Durham, NC). Each patient had three slides made from at least three different regions for immunohistological analysis. Composition and cellularity were assessed using a hematoxylin & eosin stain (H&E). Primary antibodies directed against mouse anti-human CD68 monoclonal antibodies 1:100 (Santa Cruz Biotechnologies, Santa Cruz, CA), mouse anti-HLA-DR monoclonal antibodies 1:100 (Santa Cruz Biotechnologies), and mouse anti-human CD163 antigen monoclonal antibodies 1:100 (Santa Cruz Biotechnologies) were used [21,30–32]. Double staining was performed of CD68 and CD163, or CD68 and HLA-DR, using rat anti-mouse AlexFluor488 1:1000 (Invitrogen Inc., Carlsbad, CA) to recognize CD68 and goat anti-mouse AlexaFluor594 1:1000 to recognize CD163 or HLA-DR. The threshold for positive slides was adjusted to exclude background and non-specific staining [33].
2.1.3. Western blotting
Total cellular protein was extracted using Tri-Reagent Trizol (Invitrogen Inc.) for tissue dissolution followed by sequential precipitation steps in accordance with the manufacturer’s protocol. Following solubilization, proteins were quantified using optical density at 280 nm using a Nanodrop quantification system (Thermo Scientific, Waltham, MA) and subsequently equal amounts of proteins were loaded into each well (50 µg per well) and electro-phoresed on a 4–20% gradient SDS Tris–glycine gel. The separated proteins were then transferred onto PVDF membranes (Invitrogen Inc.). Primary antibodies directed against mouse anti-human CD68 monoclonal antibodies (Santa Cruz Biotechnologies) 1:500, mouse anti-HLA-DR monoclonal antibodies (Santa Cruz Biotechnologies) 1:500, and mouse anti-human CD163 antigen monoclonal antibodies (Santa Cruz Biotechnologies) 1:500 were used. Detection of CD68, HLA-DR, and CD163 was then performed and visualized using an ECL detection system and hyperfilm chemiluminescence [34]. Images were analyzed using Image J Software (National Institutes of Health, USA) for densitometry analysis and presented as a percentage of the total size of all the measured peaks.
2.2. In vitro cell culture
2.2.1. Cell harvesting
The animal protocols were approved by our Institutional Review Board and Administrative Panel on Laboratory Animal Care.
To harvest macrophages, bone marrow was collected from the femora of 10 C57Bl male mice aged 8–12 weeks old (Jackson Laboratories). The mice were sacrificed with CO2 gas, and the animals were sterilized by placing it in 70% ethanol three times for 5 min each. While maintaining a sterile technique, the femora were surgically removed. Using a syringe and a 25-gauge needle, the bone marrow was flushed by injecting 5 ml basal medium (RPMI medium supplemented with 10% fetal bovine serum, 100 IU ml−1 penicillin, 100 µg ml−1 streptomycin (Invitrogen Inc.)) through the marrow cavity into a 50 ml centrifuge tube. The cells were spun down and re-suspended with ice-cold red blood cell lysis buffer (Invitrogen Inc.) for 2 min followed by basal medium. The cells were spun down again and re-suspended in basal medium with 30% leukocyte-conditioned medium (LCM) v/v and 5 ng mr−1 macrophage colony stimulating factor (M-CSF), counted with a hemocytometer and re-plated in T-175 culture flasks at a concentration of 1.2 × 108 per flask [34]. Cells were allowed to expand in 37 °C incubator with 5% CO2 until 75% confluence was reached (~1 week), with medium changed every 3 days to remove nonadherent cells. The cells were lifted with 0.25% Trypsin/EDTA (Invitrogen Inc.), washed with basal medium, and re-suspended in augmented basal medium. Four groups were defined: (1) 30% LCM only, (2) 30% LCM with LPS at 1 µg ml−1, (3) 30% LCM with IL-4 at 20 ng ml−1, and (4) 30% LCM with LPS at 1 µg ml−1 followed by administration of LPS with IL-4 at 20 ng ml−1 after 3 days. The medium was changed on day 10, and IL-4 was added in addition to LPS to the appropriate group. On day 14, the cells were lifted using 0.25% Trypsin/EDTA and centrifuged at 1000 g for 7 min. Cell culture supernatants were saved for analysis of cytokine production using ELISA detection systems. We re-suspended cells in a small volume of culture medium and counted viable cells using a hemocytometer [11,35]. We created cell smears using ~50,000 cells. The smears were allowed to air-dry for 15 min, and then paraformaldehyde was added for 20 min. The cell smears were subsequently analyzed using immunohistochemistry.
2.2.2. PMMA particle administration in vitro
Macrophages were harvested and plated according to the above protocol. PMMA particles, ranging in diameter from 1 to 10 µm (mean 6.0 ±1.8 µm), were purchased from Polysciences (Warrington, PA). These particles have been used by our group and others in numerous in vitro and in vivo studies because they are commercially available and well documented for their ability to activate macrophages to release pro-inflammatory cytokines in vitro [2,36]. Additionally, PMMA particles are of clinical interest because the prevalence of cemented total knee and hip replacements that wear and generate debris is still a major clinical problem [37,38]. PMMA particles were sterilized by incubation in 70% ethanol three times for 10 min each followed by an overnight incubation with shaking. The particles were then washed three times in phosphate buffered saline (PBS). The absence of endotoxin was confirmed by Limulus Amoebocyte Lysate assay (Biowhittaker, Walksville, MD). Particles were added at a concentration of 0.15 vol.% to each T-175 culture flask at day 7 [39–41]. Five groups were defined: (1) 30% LCM only, (2) 30% LCM with PMMA particles 0.15 vol.%, (3) 30% LCM with PMMA particles 0.15 vol.% followed by administration of particle with IL-4 at 20 ng mr−1 after 3 days, (4) 30% LCM with PMMA particles 0.15 vol.% and LPS at 1 µg ml−1, and (5) 30% LCM with PMMA particles 0.15 vol.% and LPS at 1 µg ml−1 followed by administration of particles and LPS with IL-4 at 20 ng ml−1 after 3 days. After 14 days, macrophages were harvested and analyzed according to the above protocol.
2.2.3. ELISA
ELISAs were used to assess to the cytokine production by M1 and M2 macrophages upon stimulation by LPS and IL-4. We assessed cytokine production of TNF-α, IL-10, and IL-1ra (R&D Systems, Minneapolis, MN) using the protocol defined by each individual R&D kit.
2.2.4. Flow cytometry
Flow cytometry was used to sort macrophage populations and obtain a quantitative assessment of the cells present after stimulation with LPS, IL-4, and PMMA particles. Fluorescence activated cell-sorting (FACS) analysis of purified macrophages was performed and gated to isolate populations of M1 macrophages and M2 macrophages using the cell surface markers iNOS (M1 marker) and Ym1 (M2 marker) respectively, as well as propidium iodide (PI) staining for dead cells. The cells were incubated with unconjugated primary antibodies to CD68 (AbD Serotec, Raleigh, NC), iNOS (Abcam, Cambridge, MA), and Ym1 (Stem Cell Technologies, Vancouver BC, Canada) at a concentration of 1:100 for 20 min, followed by incubation with AlexaFluor 488 and 647 at a concentration of 1:800 for 20 min. During FACS sorting, forward and side scatter gates were set to include all viable cells which were identified using PI staining [42].
2.3. Statistical analysis
Data used for statistical analysis was first assessed using a Kolmogorov–Smirnov test to ensure normality and Gaussian distribution using Prism 4.1 (Graphpad Software, La Jolla, CA). The statistical analysis for immunohistologic images in part 1 utilized an unpaired t-test. The statistical analysis for Western blotting data utilized an unpaired t-test, which was conducted by Prism 4.1. The statistical analysis for ELISA data utilized a one-way analysis of variance to test for significance and a Neuman Keuls post-hoc test to compare individual groups with p < 0.05 taken as significant. Data were reported as mean ± standard error. A p value <0.05 was chosen as the threshold of significance.
3. Results
3.1. Clinical investigation
The results are presented in the context of two experimental research plans. The first focuses on the clinical characterization of the synovium and pseudomembrane retrieval tissues by immunohistochemistry and Western blotting. Initial studies focused on the differential expression of M1 and M2 macrophages in human synovial tissue as compared to periprosthetic tissues.
3.1.1. Immunohistochemistry
Using antibody specific staining and morphometric analysis, we found that the number of CD68 staining macrophages was comparable in both the primary synovium (72.2 ± 3.103) and revision tissues (78.3 ± 6.22), which was not statistically significant. In an analysis of the differential polarization of the macrophages into the M1 phenotype based on HLA-DR detection, there was an increased number of macrophages staining positively for HLA-DR in revision tissue compared to synovial tissues (Fig. 1a). The HLA-DR positive staining cells (M1) formed a subset of cells that also stained positively for CD68. As shown in Table 2, the ratio of M1/CD68+ cells in the synovium was 0.48 ± 0.03 compared to the ratio of M1/CD68+ cells in the pseudomembrane of 0.85 ± 0.05 with p < 0.0001. In an analysis of the differential polarization of macrophages into the M2 phenotype based on CD163 detection, although not statistically significantly (p = 0.12), there was a slightly higher proportion of M2 macrophages in the synovium (0.52 ± 0.11) compared to the revision tissues (0.34 ± 0.08) (Fig. 1b). The CD163 presenting macrophages (M2) also co-stained with CD68, confirming that they were a macrophage subset. To assess the relative distribution of M1 vs. M2 macrophages, a ratio of M1/M2 was developed for each individual patient, and the mean values were compared by a Student’s unpaired t-test. For the synovium the average M1/M2 ratio was 0.46 ± 0.04 compared to the pseudomembranes, which was 2.87 ± 0.54 (p < 0.001) (Table 2).
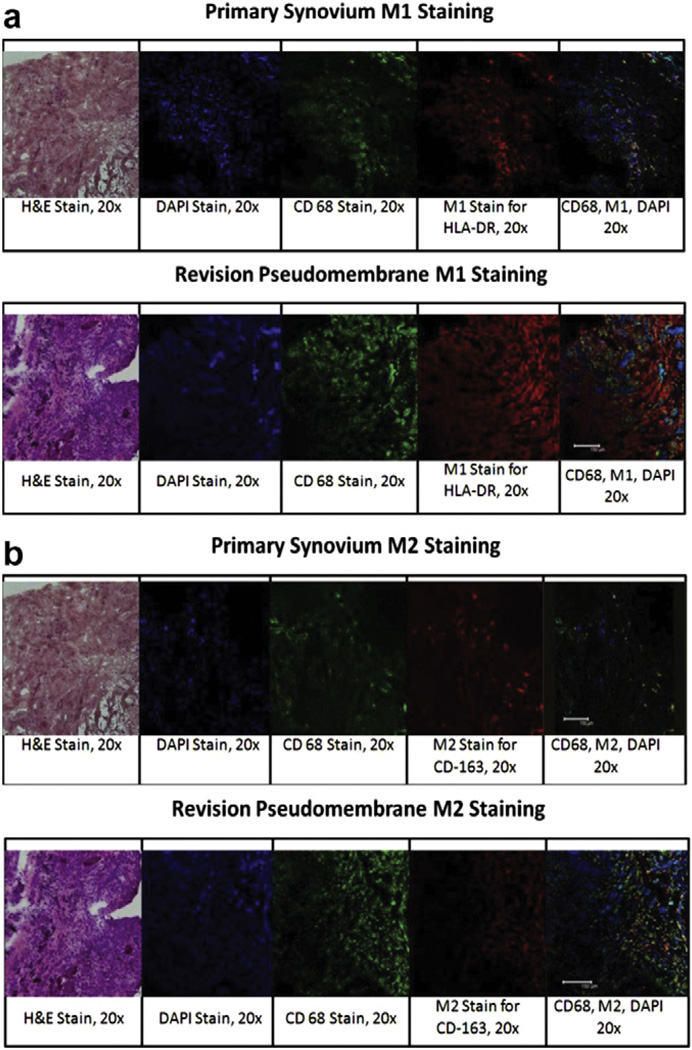
Fig. 1.
For immunohistological analysis of primary synovium and revision pseudomembranes, three to six regions of each sample were selected for analysis, and three slides were made from at least three different regions for analysis. Images are represented as H&E (×20), DAPI staining (×20), CD68 staining (×20), M1 or M2 staining (×20), and an overlay of DAPI, CD68, and M1/M2. (a) M1 staining with HLA-DR in the primary synovium compared to the revision pseudomembrane showed a higher level of HLA-DR expression in the revision pseudomembrane. (b) M2 staining with CD163 in the primary synovium compared to the revision pseudomembrane shows a higher level of CD163 expression in the primary synovium.
Table 2.
Statistical analysis of immunohistological staining of primary synovium and revision pseudomembrane.
| CD68 | HLA-DR/CD68 | CD163/CD68 | M1/M2 | |
|---|---|---|---|---|
| Primary Synovium | 72.2 ±3.103 | 0.48 ±0.03 | 0.52 ±0.11 | 0.46 ±0.04 |
| Revision Pseudomembrane | 78.3 ±6.22 | 0.85 ±0.05 | 0.34 ±0.08 | 2.87 ± 0.54 |
| P values | p<0.0001 | p = 0.1148 | p = 0.0008 |
The ratio of M1/CD68+ cells in the synovium was 0.48 ± 0.03, compared to the ratio of M1 to CD68+ cells in the pseudomembrane of 0.85 ± 0.05 with p < 0.0001. There is a slightly higher proportion of M2 macrophages in the synovium (0.52 ± 0.11) compared to the revision tissues (0.34 ± 0.08) (p = 0.115). For the synovium the average M1/M2 ratio was 0.46 ± 0.04; this was compared to that of the pseudomembranes, which was 2.87 ± 0.54, with p = 0.0008 using an unpaired t-test.
3.1.2. Western blotting
As an alternative method to validate the relative abundance of M1 and M2 cells in synovium and pseudomembranes, we used Western blotting to detect the HLA-DR and CD163 protein. We used the densitometry analysis of CD68, HLA-DR, and CD163 for each patient to obtain ratios of M1/CD68 and M2/CD68. We then compared these ratios to obtain an M1/M2 ratio. As shown in Table 3, the M1/CD68 ratio in the primary synovium was 0.25 ± 0.03 compared to 18.31 ± 11.59 in the revision pseudomembrane. The M2/CD68 ratio in the synovium was 1.20 ±0.35 compared to 1.07 ±0.35 in the revision pseudomembrane. The M1/M2 ratio in the synovium was 0.24 ± 0.04 compared to 12.95 ± 7.51 in the revision pseudomembrane, suggesting that there is a predominance of M1 macrophages in the revision pseudomembrane. There was a strong trend (p = 0.1) for both the M1/CD68 and M2/CD68 ratios in the synovium compared to the pseudomembrane.
Table 3.
Total cellular protein in the primary synovium was compared to the revision pseudomembranes using Western blotting staining for CD68, the M1 marker HLA-DR, and the M2 marker, CD163.
| Primary Synovium | Revision Pseudomembrane | |
|---|---|---|
| M1/CD68 | 0.25 ± 0.03 | 18.31 ± 11.59 |
| M2/CD68 | 1.20 ± 0.35 | 1.07 ± 0.35 |
| M1/M2 | 0.24 ± 0.04 | 12.95 ± 7.51 |
M1 and M2 ratios in the primary synovium and revision pseudomembrane assessed by Western blotting using 50 µg of protein per well. The M1/CD68 ratio in the primary synovium was 0.25 ± 0.03, compared to 18.31 ± 11.59 in the revision pseudomembrane. The M2/CD68 ratio in the synovium was 1.20 ± 0.35, compared to 1.07 ± 0.35 in the revision pseudomembrane. The M1/M2 ratio in the synovium was 0.24 ± 0.04, compared to 12.95 ± 7.51 in the revision pseudomembrane, suggesting that there is a predominance of M1 macrophages in the revision pseudomembrane.
3.2. In vitro cell culture
In a second approach, the experimental plan involved isolation of macrophages and culture in vitro. The primary macrophages were then stimulated with LPS and IL-4 and analyzed for the M1 and M2 polarization using immunohistochemistry and flow cytometry for cell surface markers, and ELISAs for TNF-α and IL-1ra. Based on the findings of these experiments, we then cultured macrophages and stimulated them with PMMA particles, LPS, and IL-4 to obtain a more clinically relevant picture of macrophage polarization. We assessed the macrophage polarization using flow cytometry and ELISAs.
3.2.1. Immunohistochemistry and flow cytometry with LPS and IL-4 administration
Using the immunohistochemical markers CD68, HLA-DR, and Arg1, an M2 marker, we found that uncommitted, non-stimulated CD68 positive macrophages express low levels of M1 and M2 macrophage markers (Fig. 2a). Macrophages exposed to LPS (which is known to induce an M1 profile [11,22]) showed a high level of HLA-DR expression, indicating polarization to M1 macrophages preferentially (Fig. 2b). We then modulated macrophage phenotype by exposing LPS-induced macrophages to IL-4, which is known to switch the macrophage phenotype from M1 to M2. When uncommitted, non-activated macrophages were exposed to IL-4 alone without prior LPS stimulation, there was a low expression of M2 phenotype (Fig. 2c); when LPS was administered prior to IL-4 administration, there was a higher expression of Arg1 (Fig. 2d).
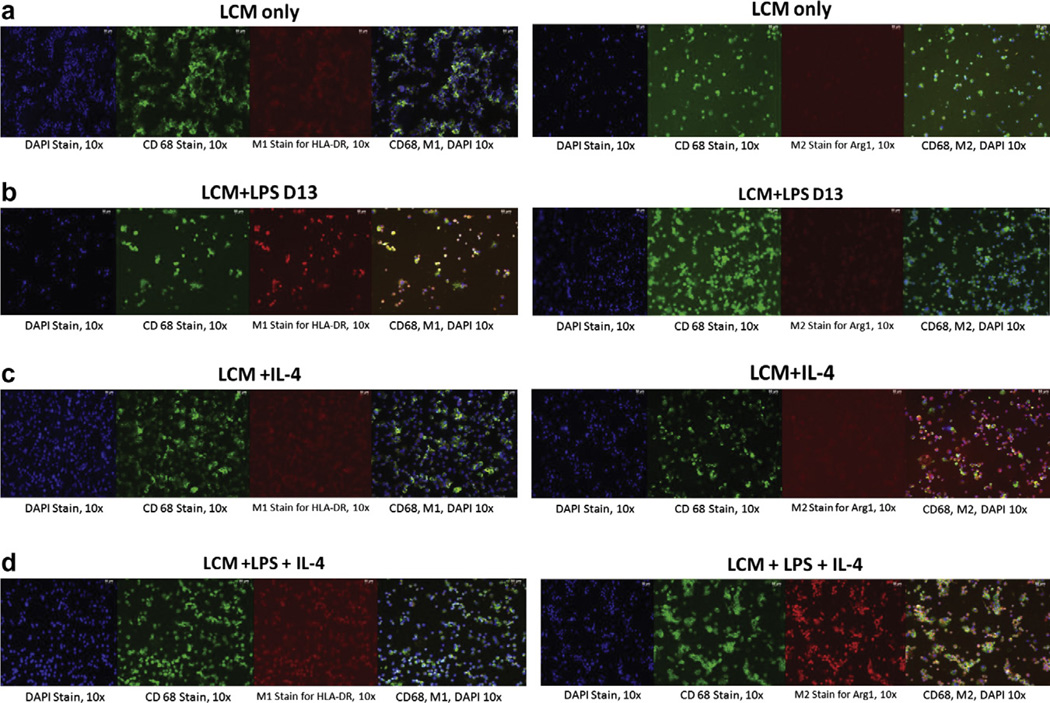
Fig. 2.
Immunohistochemical analysis of macrophages cultured in vitro stimulated with LPS and IL-4. Panels are shown as DAPI staining (× 10), CD68 staining (× 10) M1 (HLA-DR) or M2 (Arg1) staining (× 10), and an overlay of all three. (a) Macrophages stimulated with LCM only showed a low expression of both HLA-DR and Arg1. (b) Macrophages stimulated with LPS showed a high expression of HLA-DR, but a low expression of Arg1. (c) Macrophages stimulated with IL-4 showed a low expression of both HLA-DR and Arg1. (d) Macrophages stimulated with LPS for 3 days, followed by LPS with IL-4 for 3 days, showed a low expression of HLA-DR, but a high expression of Arg1.
These results were also confirmed using flow cytometry using the M1 marker iNOS and M2 marker Ym1. We found that 9.02% of macrophages in LCM expressed the M1 marker iNOS, and only 0.47% of macrophages in LCM expressed the M2 marker Ym1, demonstrating a relatively undifferentiated state (Fig. 3a). LPS stimulation increased iNOS expression in 85.1% of macrophages, whereas only 8.06% expressed Ym1 (Fig. 3b). Flow cytometry demonstrated that given IL-4 alone, only 1.96% of the macrophages expressed Ym1 (Fig. 3c); however, when the cells were first exposed to LPS for 3 days prior to IL-4 administration, 29.3% of the cells expressed Ym1 (Fig. 3d). Using PI staining, we were also able to assess that IL-4 was not toxic to cells, unlike LPS (data not shown). There was an increase in cell death in response to LPS stimulation, but there was no increase in cell death in response to IL-4 treatment alone. This suggests that macrophage polarization to the anti-inflammatory M2 phenotype is more successful if M1 rather than uncommitted, non-activated macrophages are administered IL-4.
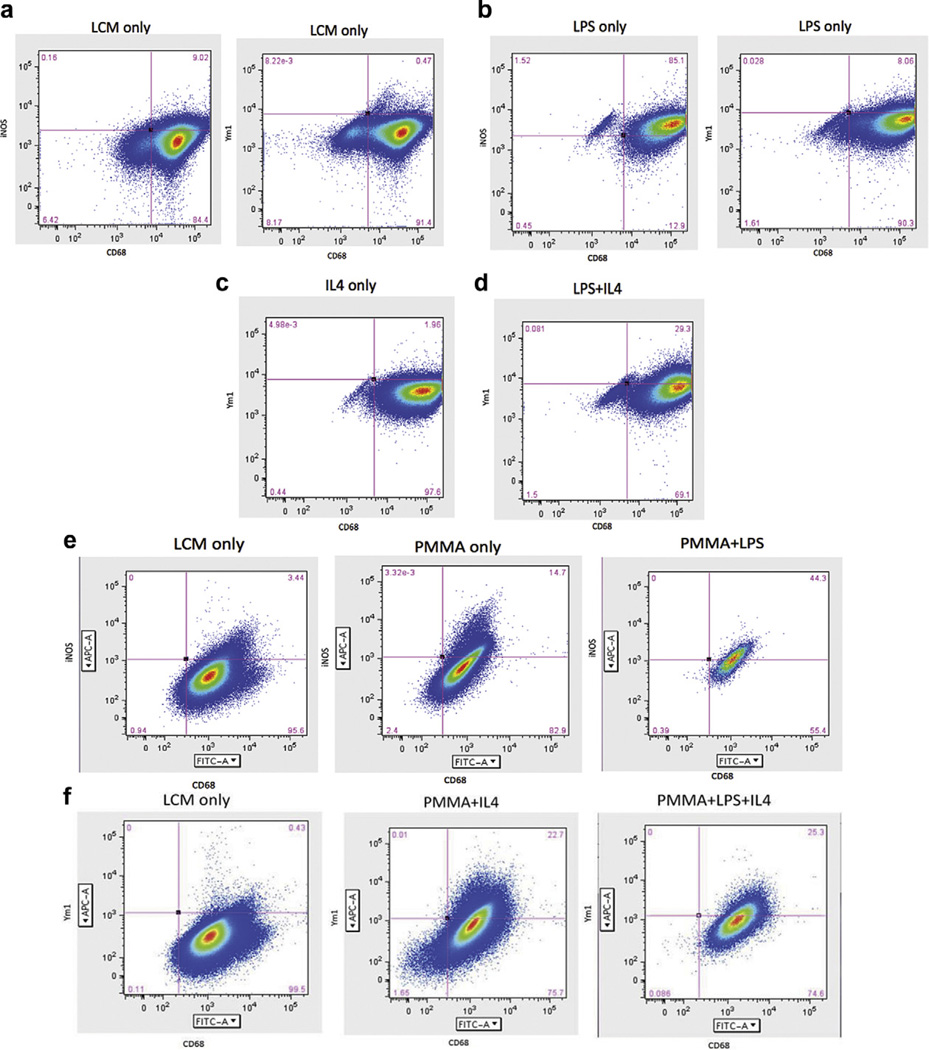
Fig. 3.
FACS analysis of macrophages cultured in vitro stimulated with LPS with/without PMMA and IL-4. Cells were gated using CD68 and either an M1 label (iNOS) or an M2 label (Ym1). (a) Macrophages stimulated with LCM sorted by CD68 and iNOS showed that LCM stimulation does not differentiate cells into M1 or M2 macrophages. (b) LPS stimulated macrophages gated for CD68 and iNOS showed that a majority of the cells differentiated into M1 macrophages. (c) IL-4 stimulation alone did not differentiate cells into M2 macrophages. (d) LPS stimulation for 3 days followed by LPS + IL-4 stimulation for 3 days resulted in an increase in M2 expression compared to IL-4 alone. (e) FACS sorting of macrophages stimulated with LCM showed an undifferentiated state. Stimulation with PMMA particles increased M1 expression. Addition of PMMA with LPS resulted in a greater increase in M1 expression. (f) FACS sorting of macrophages stimulated with LCM showed an undifferentiated state; however, stimulation with PMMA followed by IL-4 increased M2 expression. Similarly, stimulation with PMMA and LPS followed by IL-4 resulted in an increase in M2 expression. This suggests that macrophage polarization to the anti-inflammatory M2 phenotype is more successful if M1 rather than uncommitted, non-activated macrophages are administered IL-4. Both LPS and PMMA are sufficient to induce an M1 inflammatory state. Addition of IL-4 to M1 stimulated macrophages results in a conversion of 20–30% of the macrophages to an M2 phenotype.
3.2.2. Flow cytometry with PMMA, LPS, and IL-4
To further explore this finding, we next cultured murine macrophages with PMMA particles, which have been previously documented for their ability to activate macrophages to release pro-inflammatory cytokines in vitro [2,36]. We found that given LCM only, 3.44% of cells expressed the M1 marker iNOS (Fig. 3e) and only 0.43% of cells expressed the M2 marker Ym1 (Fig. 3f). PMMA stimulation alone increased the iNOS expression in the macrophage population to 14.7%; however, administration of LPS in addition to PMMA increased the iNOS expression in the macrophages to 44.3% of cells (Fig. 3e). With IL-4 stimulation, we found that PMMA particles with IL-4 resulted in a double positive Ym1 and CD68 population of 22.7%. Similarly, stimulation with PMMA and LPS followed by IL-4 resulted in a double positive population of 25.3% (Fig. 3f).
3.2.3. ELISA analysis
Finally, we examined the cell culture supernatants to determine whether macrophage polarization was reflected in the cytokine profile. We found statistically significant higher levels of TNF-α expression in supernatants from LPS stimulated macrophages (454.22 ±73.11 pgmr−1) compared to conditioned media alone (88.05 ±22.76 pg ml−1), uncommitted macrophages exposed to IL-4 stimulation alone (7.20 ±3.56 pg ml−1), or macrophages exposed to LPS followed by IL-4 (174.65 ± 25.99 pg ml−1). This suggests that LPS stimulation preferentially polarizes macrophages to an M1 profile. However, this profile can be manipulated with the addition of IL-4, which significantly decreased the secretion of TNF- α (Fig. 4a). We next examined the secretion of IL-10, which is an anti-inflammatory cytokine that is secreted by M2 macrophages, but also is induced by exposure to LPS [43–45]. We found high levels of IL-10 in response to LPS stimulation (51.42 ±5.56 pg ml−1) compared to conditioned media (17.25 ±3.01), IL-4 stimulation of uncommitted macrophages (39.98 ± 8.06 pg ml−1), and LPS exposure followed by IL-4 stimulation (27.45 ± 4.92 pg ml−1) (Fig. 4b). To more specifically examine M2 cytokine expression, we assayed the supernatants for IL-1 receptor antagonist (IL-1ra), which is thought to be up-regulated in M2 macrophages [12]. We found a statistically significant higher expression of IL-1ra in response to macrophage exposure to LPS administration (4731.35 ± 544.85 pg ml−1) compared to conditioned media alone (347.17 ± 138.61 pg mr1) and IL-4 administration alone (289.90 ± 68.90 pg ml−1). Administration of IL-4 after initial priming of the macrophages with LPS significantly increased the expression of IL-1ra (7459.75 ± 779.20 pg ml−1) compared to LPS alone (Fig. 4c). This showed that IL-4 administration alone was not sufficient to increase IL-1ra production as compared to un-activated macrophages; however, LPS alone, and LPS followed by IL-4, were able to increase IL-1ra production [46–48]. LPS followed by IL-4 increased the expression of IL-1ra more than LPS alone, suggesting that administration of IL-4 after priming with LPS was able to increase M2 macrophage polarization and subsequent expression of IL-1ra.
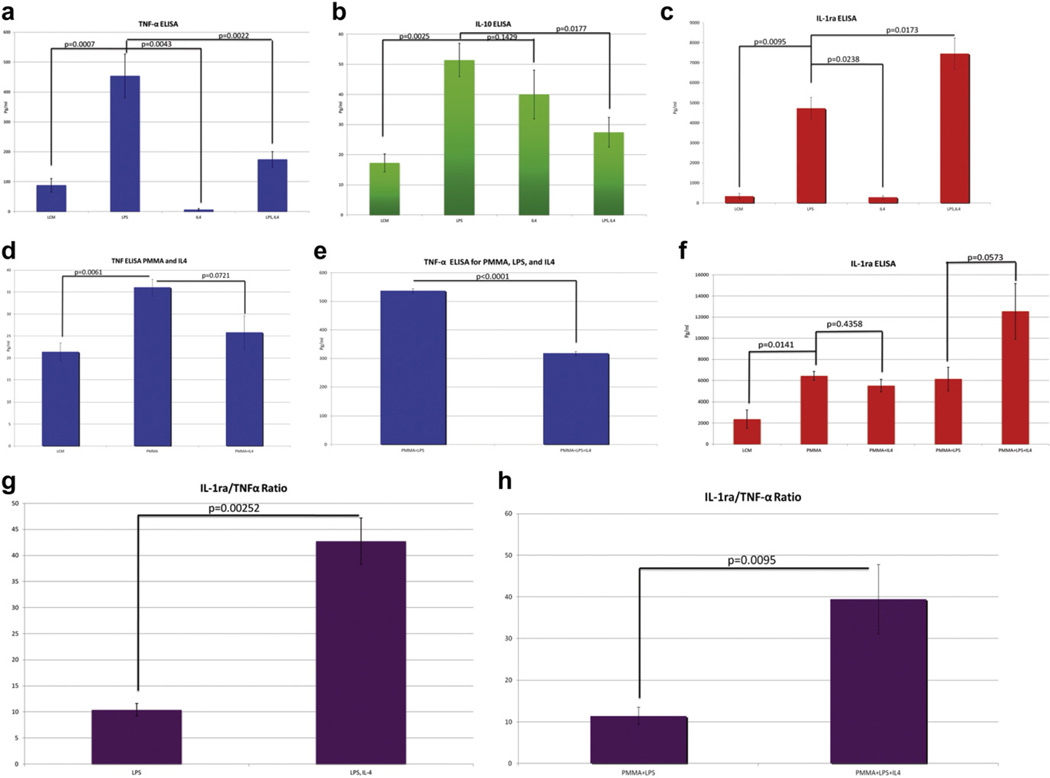
Fig. 4.
ELISA analysis was performed on cell culture supernatants taken from in vitro macrophage cultures stimulated with LPS with/without PMMA and IL-4. Analysis was performed for TNF-α, IL-10, and IL-1ra. A ratio of IL-1ra/TNF-α is also presented. (a) ELISA analysis of TNF-α in macrophages stimulated with LPS shows a higher level of section compared to conditioned media alone. This response is decreased with the addition of IL-4 after initial LPS administration. (b) ELISA analysis of IL-10 in macrophages stimulated with LPS shows a high level of secretion. This may be due to LPS induction of IL-10 production, which has been found to be a well-established effect. (c) ELISA analysis of IL-1ra in macrophages stimulated with LPS shows a high level of secretion. This response is increased by the subsequent addition of IL-4, suggesting that IL-4 administration following LPS priming may preferentially polarize macrophages towards M2, increasing the expression of IL-1ra. (d) ELISA analysis of TNF-α in macrophages stimulated with PMMA particles shows a significantly high level of section. This response is decreased with the addition of IL-4 after initial PMMA administration. (e) ELISA analysis of TNF-α in PMMA stimulated macrophages shows a significantly higher level of TNF-α release in response to PMMA particles with LPS administration. This response is decreased with the addition of IL-4 after initial PMMA and LPS priming. (f) ELISA analysis of IL-1ra macrophages stimulated with PMMA and PMMA with LPS priming prior to IL-4 administration shows a significantly high level of IL-1ra release. (g) The ratio of IL-1ra/TNF-α in the LPS only group was 1.20, whereas with LPS followed by IL-4, the IL-1ra/TNF-α increased to 4.46. This shows that the increase in IL-1ra expression is not based on TNF-α stimulation alone. (h) The ratio of IL-1ra/TNF-α in the PMMA with LPS group was 11.44, whereas with PMMA and LPS followed by IL-4, the IL-1ra/TNF-α increased to 39.47, showing that IL-1ra expression was increased beyond what would be expected if it was solely related to TNF-α release.
We also performed ELISA analysis for TNF-α and IL-1ra following PMMA particle administration. We found that LCM stimulation alone resulted in a TNF-α expression level of 21.39 ± 2.03 pg ml−1. PMMA administration increased the TNF-α expression to 36.06 ± 1.89 pg ml−1, which was statistically different from LCM administration alone. IL-4 administration after PMMA excitation decreased the TNF-α expression to 25.83 ±3.76 pg ml−1, which had a strong trend towards significance compared to PMMA particles alone (Fig. 4d). However, LPS administration in conjunction with PMMA resulted in a very significant increase in TNF-α to 536.67 ± 7.99 pg ml−1, which was significantly different from the groups without LPS administration. However, IL-4 administration following PMMA and LPS excitation was able to decrease the level of TNF-α expression to 317.89 ± 6.99 pg ml−1, which was significantly lower than PMMA and LPS alone (Fig. 4e).
ELISA analysis of IL-1ra was also performed. We found that LCM stimulation alone resulted in an IL-1ra expression level of 2365.93 ± 884.50 pg ml−1. PMMA administration increased this to 6442 ±418.16 pg ml−1. IL-4 administration following PMMA excitation resulted in an IL-1ra expression level of 5514.33 ± 599. 51 pg ml−1, which was not significantly from PMMA alone. PMMA administration with LPS resulted in an IL-1ra expression of 6138.46 ± 1114.21 pg ml−1, compared to 12546.27 ±2638.55 pg ml−1 following IL-4 administration after LPS and PMMA (Fig. 4f). Although this is not statistically significant, this is a very strong trend showing that IL-4 administration was able to increase the expression of IL-1ra following LPS and PMMA administration.
3.2.4. Analysis of ELISA IL-1ra to TNF-α ratios
We also looked at the ratio of IL-1ra to TNF-α expression, since we hypothesized that relative to the TNF-α expression level, the IL-1ra expression may be higher than predicted if only M1 macrophages were present. We found that in the first experiment, for the LPS only group, the IL-1ra/TNF-a level was 1.20, whereas with LPS followed by IL-4, the IL-1ra/TNF-α was 4.46, which was statistically significant at p = 0.00252 using an un-paired two-tailed t-test (Fig. 4g). Additionally, in the second experiment, the ratio of IL-1ra/TNF-α expression for the PMMA with LPS group was 11.44 compared to PMMA with LPS and IL-4 administration, which had an IL-1ra/TNF-α ratio of 39.47 (p = 0.0095) (Fig. 4h). This helped to confirm that IL-4 administration following LPS increased IL-1ra due to the presence of M2 macrophage secretion, and not based on TNF-α stimulation alone. This suggests that macrophage differentiation may occur in a step-wise fashion from M0 to M1 to M2 macrophages, with M2 macrophages requiring activation and subsequent stimulation to differentiate.
4. Discussion
The purpose of this study was to determine whether wear particles associated with joint replacements influenced macrophage polarization. The experimental plan used human tissue retrievals and in vitro studies with isolated murine monocyte/macrophages. Although the revision surgery cases represent a variety of implant and wear debris types, which may be seen as a weakness of the study, as all cases demonstrated the same principles of macrophage polarization in response to wear debris, this gives generality to the study and conclusions made. Additionally, variability in his-tomorphology of tissues harvested from different anatomical areas of revision interfaces has been noted [27–29,33,49]. In this study, the results may reflect the cellularity and composition of retrieved tissues that may vary depending on the sampling. However, our protocol selected similar anatomical regions of interest among the individual cases to minimize potential sampling error.
In the retrieved tissues, we used morphological analysis of the cells using an H&E stain to verify the phenotype of macrophages. By doing so, we accounted for nonspecific binding with the surface markers used for designation of the M1 and M2 phenotype. In vitro, we used leukocyte-conditioned medium as well as M-CSF to preferentially select for macrophage differentiation. We used CD68 to broadly identify monocyte/macrophage lineage cells to ensure that the M1 and M2 surface marker expressions were indeed macrophages. We recognize that the M1 marker HLA-DR is also expressed by other cell types [50], and is a human antigen that has cross-reactivity in mice, but may not be as specific in a murine model. The M2 marker CD163 is also expressed by other cell types [51]; however, Ym1 seems to be specific to alternatively activated macrophages. Thus, many of the markers used in our studies are expressed by other cell populations; however, by using an H&E stain for the retrieved tissues and stimulating the in vitro cultures with LCM and MCSF, we believe we have controlled for some of the cross-reactivity of the M1 and M2 surface markers. Additionally, the in vitro studies are also subject to contamination with endotoxin, which would lead to macrophage activation and over-expression of the M1 phenotype. We mitigated this possibility by maintaining a strict sterile experimental technique and measuring endotoxin levels of particles and reagents using the Limulus Amebocyte Lysate Kit (BioWhittaker, Walkersville, MD).
Since metal-on-UHWMPE implants account for at least 50–70% of the bearing surfaces used in TJR in the USA [52,53], wear and the biological consequences of wear debris on the implant survival are critical to implant longevity [54]. Previously, it was thought that macrophage response to wear particles involved phagocytosis and cytokine production, leading to a pro-inflammatory state and osteolysis. Macrophage polarization towards an M1 phenotype in response to wear debris following TJR contributes to periprosthetic osteolysis and subsequent loosening of the implant initially; we hypothesize that these events may be potentially manipulated towards an M2 response, promoting bone healing and new bone formation. We found a higher expression of M1 macrophages in the revision tissues, compared to synovium from patients undergoing joint replacement. This is consistent with the hypothesis that particles produced from wear of prosthetic joints activates macrophages and polarizes them to an M1 profile. This activates a cascade of pro-inflammatory factor release, which contributes to inflammation, osteolysis and potentially loosening of the implant [6]. The ability to polarize this response to wear particles towards an anti-inflammatory phenotype with a predominance of M2 macrophages could help to mitigate these adverse events.
Using in vitro cell culture, we examined whether polarization of macrophages directly from an M0 to an M2 phenotype is more efficient, or whether it is necessary to go through an M1 intermediary stage. Using immunohistochemistry and flow cytometry, we found that IL-4 administration alone to M0 macrophages was not sufficient to polarize the macrophages towards an M2 profile. However, by priming the macrophages with LPS and/or PMMA particles to induce an M1 profile, we could then administer IL-4 to switch some of the M1 macrophages to M2.We also found that both PMMA and LPS can polarize macrophages towards an M1 phenotype, and IL-4 administration is sufficient in both cases to increase polarization of M1 to M2 macrophages. This was reflected in the cytokine profile of IL-1ra, an anti-inflammatory cytokine, which was more highly expressed following PMMA/LPS and IL-4 administration.
The in vitro studies combined with the findings of our tissue retrieval investigation suggest that in the future it may be possible to manipulate macrophage polarization and subsequent inflammatory reaction that occurs in response to wear debris particles. Our tissue retrieval studies found a predominance of M1 macrophages in response to wear particles, and our in vitro study found that we are able to selectively polarize M1 macrophages towards an M2 phenotype with IL-4. Thus, our study has implications for the use of a biological agent, such as IL-4, to manipulate and control the inflammatory reaction that occurs in response to wear particle production. Studies by Badylak et al. [55] have also shown that acellular scaffolds may elicit a predominantly M2 type response and constructive remodeling in tissue defects as compared to cellular scaffolds, which resulted in a predominantly M1 response and deposition of dense connective tissue and/or scarring. We found that a higher M2 profile could be achieved through an M1 intermediary. The combination of both IL-4 administration and an acellular scaffold may be an avenue of exploration in the future as the particle production in the joint following TJR would induce M1 macrophages, which could then interact with both the scaffold and IL-4 and differentiate into M2 macrophages. However, the IL-4 delivery will be crucial as IL-4 antagonists are currently under investigation for clinical use in asthma [56,57]. Thus, it will be imperative to create a scaffold, stent, or other method for delivery to ensure that a local reaction ensues rather than a systemic response. At the local level, in osteolysis, IL-4 may help to promote tissue healing, angiogenesis, and constructive remodeling rather that periprosthetic osteolysis and implant loosening, potentially reducing the need for revision surgeries. In future studies, we will also translate these in vitro studies to an in vivo model of particle-induced inflammation and osteolysis, to assess whether the addition of IL-4 can diminish these adverse events [43–45].
Acknowledgements
This research was supported by the Ellenburg Chair in Surgery at Stanford University and the Medical Scholars Program at Stanford University School of Medicine. We would like to thank Dr Nid-hi Bhutani, Matt Decker, Subbus Dhulipala, and Andrew Peterman for their contributions.
Footnotes
Appendix A. Figures with essential colour discrimination
Certain figures in this article, particularly Figures 1–4, are difficult to interpret in black and white. The full colour images can be found in the on-line version, at http://dx.doi.org/10.1016/j.actbio.2012.03.042.
References
- 1.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. Hss J. 2006;2:102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, et al. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–116. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- 3.Chiu R, Smith KE, Ma GK, Ma T, Smith RL, Goodman SB. Polymethylmethacrylate particles impair osteoprogenitor viability and expression of osteogenic transcription factors Runx2, osterix, and Dlx5. J Orthop Res. 2010;28:571–577. doi: 10.1002/jor.21035. [DOI] [PubMed] [Google Scholar]
- 4.Chiu R, Ma T, Smith RL, Goodman SB. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J Biomed Mater Res A. 2009;89:242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- 5.Ma T, Goodman SB. Biological effects of wear debris from joint arthroplasties. In: Wnek GE, Bowlin GL, editors. Encyclopedia of biomaterials and biomedical engineering. London: Informa Healthcare; 2008. [Google Scholar]
- 6.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Marshall A, Ries MD, Paprosky W. How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J Am Acad Orthop Surg. 2008;16(Suppl. 1):S1–S16. doi: 10.5435/00124635-200800001-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wooley PH, Schwarz EM. Aseptic loosening. Gene Ther. 2004;11:402–407. doi: 10.1038/sj.gt.3302202. [DOI] [PubMed] [Google Scholar]
- 9.Purdue PE. Alternative macrophage activation in periprosthetic osteolysis. Autoimmunity. 2008;41:212–217. doi: 10.1080/08916930701694626. [DOI] [PubMed] [Google Scholar]
- 10.Ren PG, Lee SW, Biswal S, Goodman SB. Systemic trafficking of macrophages induced by bone cement particles in nude mice. Biomaterials. 2008;29:4760–4765. doi: 10.1016/j.biomaterials.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho VW, Sly LM. Derivation and characterization of murine alternatively activated (M2) macrophages. Methods Mol Biol. 2009;531:173–185. doi: 10.1007/978-1-59745-396-7_12. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuch O, Akira S. Epigenetic control of macrophage polarization. Eur J Immunol. 2011;41:2490–2493. doi: 10.1002/eji.201141792. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson JM, Wilson AG, Stockley I, Scott IR, Macdonald DA, Hamer AJ, et al. Variation in the TNF gene promoter and risk of osteolysis after total hip arthroplasty. J Bone Miner Res. 2003;18:1995–2001. doi: 10.1359/jbmr.2003.18.11.1995. [DOI] [PubMed] [Google Scholar]
- 16.Gordon A, Southam L, Loughlin J, Wilson AG, Stockley I, Hamer AJ, et al. Variation in the secreted frizzled-related protein-3 gene and risk of osteolysis and heterotopic ossification after total hip arthroplasty. J Orthop Res. 2007;25:1665–1670. doi: 10.1002/jor.20446. [DOI] [PubMed] [Google Scholar]
- 17.Sharda DR, Yu S, Ray M, Squadrito ML, De Palma M, Wynn TA, et al. Regulation of macrophage arginase expression and tumor growth by the ron receptor tyrosine kinase. J Immunol. 2011;187:2181–2192. doi: 10.4049/jimmunol.1003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 19.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raes G, Noel W, Beschin A, Brys L, de Baetselier P, Hassanzadeh GH. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev Immunol. 2002;9:151–159. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CS, Chen FH, Hong JH, Jiang PS, Huang HL, Wang CC, et al. Functional phenotype of macrophages depends on assay procedures. Int Immunol. 2008;20:215–222. doi: 10.1093/intimm/dxm137. [DOI] [PubMed] [Google Scholar]
- 23.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, et al. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 24.Bi Y, Collier TO, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin mediates biological responses of titanium particles without stimulating their phagocytosis. J Orthop Res. 2002;20:696–703. doi: 10.1016/S0736-0266(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM. Does endotoxin contribute to aseptic loosening of orthopedic implants? J Biomed Mater Res B Appl Biomater. 2005;72:179–185. doi: 10.1002/jbm.b.30150. [DOI] [PubMed] [Google Scholar]
- 26.Nalepka JL, Lee MJ, Kraay MJ, Marcus RE, Goldberg VM, Chen X, et al. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clin Orthop Relat Res. 2006;451:229–235. doi: 10.1097/01.blo.0000224050.94248.38. [DOI] [PubMed] [Google Scholar]
- 27.Goodman SB, Knoblich G, O’Connor M, Song Y, Huie P, Sibley R. Heterogeneity in cellular and cytokine profiles from multiple samples of tissue surrounding revised hip prostheses. J Biomed Mater Res. 1996;31:421–428. doi: 10.1002/(SICI)1097-4636(199607)31:3<421::AID-JBM17>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Goodman SB, Lind M, Song Y, Smith RL. In vitro, in vivo, and tissue retrieval studies on particulate debris. Clin Orthop Relat Res. 1998;352:25–34. [PubMed] [Google Scholar]
- 29.Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80:531–539. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. The tissue microlocalisation and cellular expression of CD163, VEGF, HLA-DR, iNOS, and MRP 8/14 is correlated to clinical outcome in NSCLC. PLoS One. 2011;6:e21874. doi: 10.1371/journal.pone.0021874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuentes-Duculan J, Suarez-Farinas M, Zaba LC, Nograles KE, Pierson KC, Mitsui H, et al. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Invest Dermatol. 2010;130:2412–2422. doi: 10.1038/jid.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spanogle JP, Miyanishi K, Ma T, Epstein NJ, Smith RL, Goodman SB. Comparison of VEGF-producing cells in periprosthetic osteolysis. Biomaterials. 2006;27:3882–3887. doi: 10.1016/j.biomaterials.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 34.da Silva RP, Gordon S. Phagocytosis stimulates alternative glycosylation of macrosialin (mouse CD68), a macrophage-specific endosomal protein. Biochem J. 1999;338(Pt 3):687–694. [PMC free article] [PubMed] [Google Scholar]
- 35.Davies JQ, Gordon S. Isolation and culture of murine macrophages. Methods Mol Biol. 2005;290:91–103. doi: 10.1385/1-59259-838-2:091. [DOI] [PubMed] [Google Scholar]
- 36.Trindade MC, Nakashima Y, Lind M, Sun DH, Goodman SB, Maloney WJ, et al. Interleukin-4 inhibits granulocyte-macrophage colony-stimulating factor, interleukin-6, and tumor necrosis factor-alpha expression by human monocytes in response to polymethylmethacrylate particle challenge in vitro. J Orthop Res. 1999;17:797–802. doi: 10.1002/jor.1100170602. [DOI] [PubMed] [Google Scholar]
- 37.Goodman S. Wear particulate and osteolysis. Orthop Clin North Am. 2005;36:41–48. doi: 10.1016/j.ocl.2004.06.015. vi. [DOI] [PubMed] [Google Scholar]
- 38.Salvati EA, Evans BG, Betts F, Doty SB. Particulate debris in cemented total hip replacement. Instr Course Lect. 1994;43:277–288. [PubMed] [Google Scholar]
- 39.Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue Eng. 2007;13:2311–2320. doi: 10.1089/ten.2006.0423. [DOI] [PubMed] [Google Scholar]
- 40.Huang Z, Ren PG, Ma T, Smith RL, Goodman SB. Modulating osteogenesis of mesenchymal stem cells by modifying growth factor availability. Cytokine. 2010;51:305–310. doi: 10.1016/j.cyto.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z, Ma T, Ren PG, Smith RL, Goodman SB. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J Biomed Mater Res A. 2010;94:1264–1269. doi: 10.1002/jbm.a.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW. Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry. Ann Rheum Dis. 2004;63:774–784. doi: 10.1136/ard.2003.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pengal RA, Ganesan LP, Wei G, Fang H, Ostrowski MC, Tridandapani S. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol Immunol. 2006;43:1557–1564. doi: 10.1016/j.molimm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chanteux H, Guisset AC, Pilette C, Sibille Y. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir Res. 2007;8:71. doi: 10.1186/1465-9921-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hart PH, Ahern MJ, Smith MD, Finlay-Jones JJ. Comparison of the suppressive effects of interleukin-10 and interleukin-4 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Immunology. 1995;84:536–542. [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh CB, Wewers MD. Cytokine-induced interleukin-1 receptor antagonist release in mononuclear phagocytes. Am J Respir Cell Mol Biol. 1994;10:521–525. doi: 10.1165/ajrcmb.10.5.8179914. [DOI] [PubMed] [Google Scholar]
- 48.Janson RW, Hance KR, Arend WP. Production of IL-1 receptor antagonist by human in vitro-derived macrophages. Effects of lipopolysaccharide and granulocyte-macrophage colony-stimulating factor. J Immunol. 1991;147:4218–4223. [PubMed] [Google Scholar]
- 49.Goodman SB, Huie P, Song Y, Lee K, Doshi A, Rushdieh B, et al. Loosening and osteolysis of cemented joint arthroplasties. A biologic spectrum. Clin Orthop Relat Res. 1997;337:149–163. doi: 10.1097/00003086-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura H, Saji H, Aute I, Kawasaki N, Hosaka M, Ogata A, et al. Peripheral leukocytes with HLA-DR+/CD8− phenotype are associated with prognosis in patients with lung cancer. Anticancer Res. 2003;23:4149–4152. [PubMed] [Google Scholar]
- 51.Maniecki MB, Moller HJ, Moestrup SK, Moller BK. CD163 positive subsets of blood dendritic cells: the scavenging macrophage receptors CD163 and CD91 are coexpressed on human dendritic cells and monocytes. Immunobiology. 2006;211:407–417. doi: 10.1016/j.imbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 53.Bozic KJ, Ong K, Lau E, Kurtz SM, Vail TP, Rubash HE, et al. Risk of complication and revision total hip arthroplasty among Medicare patients with different bearing surfaces. Clin Orthop Relat Res. 2010;468:2357–2362. doi: 10.1007/s11999-010-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibon E, Ma T, Ren PG, Fritton K, Biswal S, Yao Z, et al. Selective inhibition of the MCP-1-CCR2 ligand–receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles. J Orthop Res. 2012;30:547–553. doi: 10.1002/jor.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burmeister Getz E, Fisher DM, Fuller R. Human pharmacokinetics/ pharmacodynamics of an interleukin-4 and interleukin-13 dual antagonist in asthma. J Clin Pharmacol. 2009;49:1025–1036. doi: 10.1177/0091270009341183. [DOI] [PubMed] [Google Scholar]
- 57.Barnes PJ. Cytokine modulators as novel therapies for airway disease. Eur Respir J Suppl. 2001;34:67s–77s. doi: 10.1183/09031936.01.00229901. [DOI] [PubMed] [Google Scholar]