Abstract
Rapid progress in the past decade with re-engineering of human plasma butyrylcholinesterase has led to enzymes that destroy cocaine so efficiently that they prevent or interrupt drug actions in the CNS even though confined to the blood stream. Over the same time window, improved gene-transfer technology has made it possible to deliver such enzymes by endogenous gene transduction at high levels for periods of a year or longer after a single treatment. This article reviews recent advances in this field and considers prospects for development of a robust therapy aimed at aiding recovering drug users avoid addiction relapse.
Enzymatic interception of cocaine
Evidence is accumulating that artificial acceleration of metabolic clearance can reduce cocaine’s reward value and might be useful in addiction therapy. Our major goal is to develop a gene-transfer delivery system that will safely provide high levels of an efficient hydrolase that blunts or eliminates cocaine actions on reward centers in the brain and aids recovering users in avoiding relapse. A key enzyme in normal cocaine metabolism is butyrylcholinesterase (BChE). BChE represents a ‘back-up’ to acetylcholinesterase in regulating synaptic acetylcholine, but has also evolved to protect against toxic plant esters such as cocaine [1]. Gorelick, 15 years ago, proposed using BChE as a rescue for cocaine overdose [2]. Unfortunately, the native human enzyme hydrolyzes cocaine only 0.1% as readily as acetylcholine and is, therefore, relatively ineffective for such a purpose. Later, however, a 1000-fold more-efficient bacterial cocaine esterase [3-5] was found to protect rats against cocaine-induced seizures [6,7] and also reduce cocaine self-administration [8]. Somewhat surprisingly, recent studies indicate that this bacterial protein is a relatively weak immunogen in rodents and that its immunogenicity can be further reduced by PEGylation [8,9]. Nonetheless, in our view, a therapeutic based on a human protein should be preferable for long-term clinical use. In particular, as computationally directed mutations of the BChE catalytic site have greatly increased this enzyme’s catalytic efficiency toward cocaine and, because this enzyme is apparently benign, the prospects for BChE-based addiction therapy look promising.
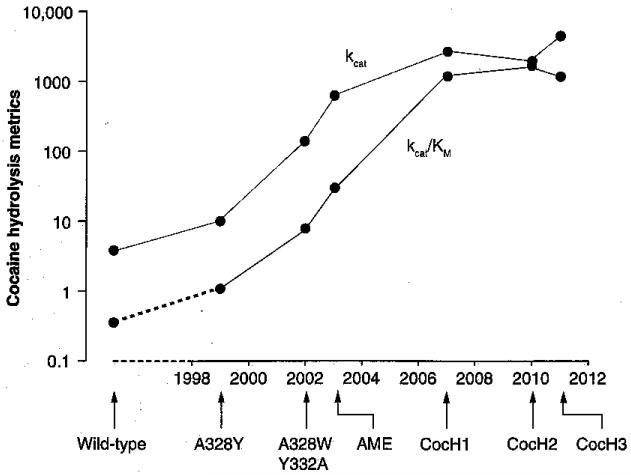
Computer-docking studies with human BChE were key steps in the process of ‘directed evolution’ toward improved cocaine hydrolysis. Much of this work was carried out by Lockridge et al., Zhan et al. and our research group at the Mayo Clinic [10-15]. We compared the docking orientation of natural (−)-cocaine with those of a biologically inactive (+)-stereoisomer that hydrolyzed approximately 2000-fold more readily [11,12]. These comparisons suggested that natural cocaine preferentially binds to the enzyme in a conformation that is suboptimal for catalysis and hinders productive reorientation. We introduced two mutations predicted to alleviate this difficulty in the active site region (A328W/Y332A). The result was a 50-fold increase in kcat (cocaine molecules hydrolyzed per min per enzyme molecule). Zhan’s group soon achieved a large further jump in catalytic power after conducting a thermodynamic analysis and designing mutations to reduce the free energy of the transition state complex [13-15]. The rational mutations of BChE ultimately led to quadruple and quintuple mutants with catalytic efficiencies up to 1700-fold higher than natural human BChE (Figure 1).
Figure 1. Directed evolution of efficient cocaine hydrolase from human butyrylcholinesterase.
Kinetic constants reported for butyrylcholinesterase (BChE) mutants designed to optimize cocaine hydrolysis are shown on a logarithmic axis against a time-line from 1999 to the present year. Most of the improvement over wild-type BChE has come from the approximately 1000-fold increases in kcat (units of min−1), but an approximately tenfold decrease in KM (micromolar units) has further enhanced catalytic efficiency (kcat/KM. These effects with cocaine are not accompanied by parallel changes in acetylcholine hydrolysis (generally poorer in the mutants than in native BChE). Mutations in the illustrated enzymes are as follows: ‘AME-CocH’ (F227A/S287G/A32SW/Y332M); ‘CocHl’ (A199S/S287G/A328W/Y332G); ‘CocH2’ (A199S/F227A/S287G/A328W/E441D); CocH3 (A199S/F227A/S287G/A328W/Y332G/E441D). Data from [10-15].
BChE-based cocaine hydrolase versus cocaine toxicity & reward-driven behavior
Our own animal work with cocaine hydrolase began with the double-mutant BChE [11,12,16]. When given to rats intravenously (iv.) before cocaine, this hydrolase reduced pressor responses, and it was also effective when given afterwards (i.e., it blunted and shortened an established hypertension) [17]. We then turned to the more-efficient enzyme, CocHl (fused to human serum albumin to prolong its plasma half-life [18]) and succeeded in aborting lethal seizures from a massive overdose (100 mg/kg, intraperitoneally [ip.]), even after major convulsions had begun [19].
In a collaborative study with Carroll and Anker [19], CocHl also had striking effects on reward-related behavior. For tests on drug seeking, rats were trained to self-administer cocaine until they maintained stable rates of lever pressing on a progressive ratio schedule of reinforcement. This behavior was then deliberately extinguished by inactivating the lever associated with reward. After 2 weeks of enforced abstinence, we introduced a classic ‘drug-primed reinstatement’ paradigm, in which deliberate exposure to a previously self-administered drug reliably evokes drug-seeking behavior. In this case, rats were given either saline or CocHl (3 mg/kg, ip.) and then, 2 h later, just before returning to the operant chamber, they received a priming injection of saline or cocaine. The result was that the enzyme prevented the increased responding that cocaine normally evokes in control or saline-pretreated rats (an average of ~40 presses on the formerly active lever) [19]. This effect was selective, that is, specific to cocaine, because amphetamine, a BChE-resistant stimulant with similar effects on synaptic monoamines, induced reinstatement responding equally well in both treatment groups.
Delivery of enzyme by viral gene transfer
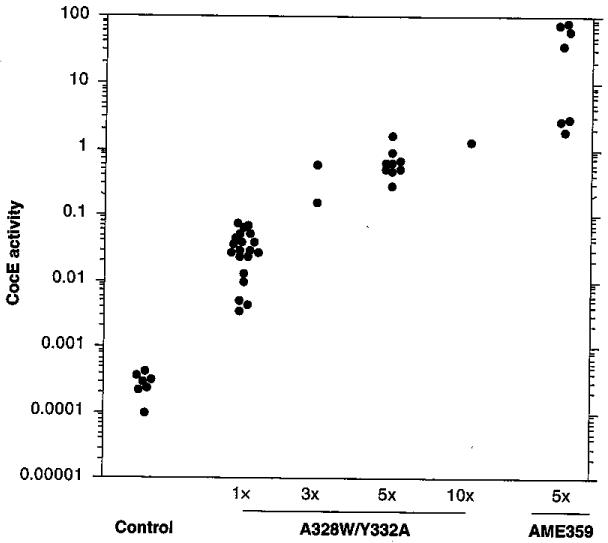
The behavioral findings with enzyme-treated rats suggested that therapies based on cocaine hydrolase might be effective treatments for cocaine abuse. However, even native BChE has only a 1-week half-life in humans [20]. To avoid the expense of pure recombinant protein in gram quantities over prolonged treatment intervals, we have been exploring cocaine hydrolase gene transfer. Our early experiments [21] used El-deleted (El−) adenoviral vectors (ADs) with mutant BChE cDNA sequences driven by a cytomegalovirus promoter [22]. This construct transduced high levels of transgene. At peak expression, 5–7 days after treatment with 2.2 × 109 plaque-forming units of vector encoding a double-mutant BChE (A328W, Y332A), cocaine hydrolase activity in rat plasma was 3000-times above its initial level [21]. In later experiments with the more efficient AME mutant [18,23], plasma cocaine hydrolase activity rose by 50,000-fold, to a geometric mean of approximately 600 mU/ml (Figure 2).
Figure 2. Adenoviral transduction of butyrylcholinesterase-based cocaine hydrolase.
Enzyme transduction versus vector dose. Rat plasma samples were collected 5 days after injection of Ad5-cytomegalovirus-cocaine esterase or Ad5-cytomegalovirus-AME359, Vector doses are shown as multiples of the ‘standard’, which was 2.2 × 109 plaque-forming units delivered through the tail vein. Data points represent mean cocaine hydrolase activity (‘cocaine esterase activity’) in duplicate assays from each individual rat, graphed on a logarithmic scale. Note the million-fold range of recorded activities [21].
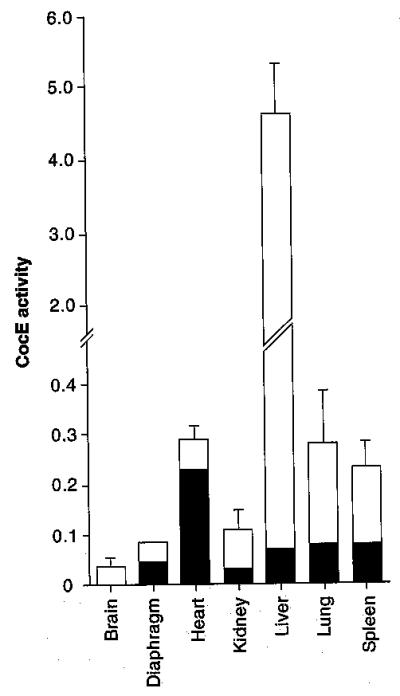
Enzyme transduction was mostly confined to liver (Figure 3). This conclusion was based partly on relative levels of cocaine hydrolase. Liver showed 20-fold greater enzyme activity than any other tissue, 100-fold more than brain, and PCR amplification of enzyme RNA was unable to detect any signal from extrahepatic sources [21]. Therefore, trace levels of activity in spleen, heart, kidney, lung, brain and skeletal muscle probably reflected enzyme in plasma retained despite saline perfusion before harvest. Our more recent QPCR results, however, indicate that significant amounts of vector DNA can be detected in Spleen [Geng L, Brimijoin S, Unpublished Data] . Thus, it remains possible that some hydrolase is, in fact, transduced in that tissue.
Figure 3. Cocaine hydrolase in tissues before and after viral transduction.
White columns are cocaine-hydrolyzing activities (mean ± SEM) in four rats sampled 5 days after treatment with E1-deleted adenoviral vectors and a cytomegalovirus vector carrying cDNA for double-mutant human BChE (A328W, Y332A). Shaded columns are activities in untreated controls (p < 0.05). Data from [21].
Immunologic reactions to transgene & vector
Unfortunately, the impressive enzyme transduction by El-AD was associated with immune responses that effectively terminated transgene expression within 10 days, returning plasma cocaine hydrolase activity to pretreatment levels. That outcome was directly in line with established data regarding these ADs [24]. Further investigations showed that the immune response involved antibodies directed toward the human transgene and probably viral coat proteins as well.
The initial results [Gao Y, Brimijoin S, Unpublished Data] revealed that plasma drawn 10 days after vector treatment not only lacked cocaine hydrolase activity but would strongly inhibit the same activity in samples drawn earlier from the same rats (at peak transgene expression), and that of the purified enzyme, in vitro. This finding is evidence for the presence of ‘neutralizing antibodies’. Subsequently, we found that pre-adsorbing plasma samples with monoclonal mouse anti-rat IgG or with anti-rat IgM would decrease the inhibitory effect, while adsorption with both reagents removed nearly all of the neutralizing ability. Hence, it appeared that the treated animals generated antibodies of both classes that interacted in some fashion with the active site of human BChE to prevent substrate binding or otherwise impair catalytic function.
Cellular immunity also seemed to be involved. In particular, it was likely that cytotoxic T cells were stimulated to attack hepatocytes (and other cells) harboring the vector construct and generating gene product. We did not assess cellular immunity directly, but were able to establish two key facts. First, at the 10-day time point, the number of hepatocytes staining intensely for BChE activity, or immunostaining for human BChE protein, was sharply lower than at 4 days, or near zero. Second, transgene mRNA levels in liver extracts fell to an equally great degree. A parsimonious hypothesis to explain this picture is that, with viral coat proteins serving as adjuvant, the immune system responded to vector in multiple ways that led to loss, not only of the transgene product, but also of the transducing hepatocytes and their episomal vector.
Improved long-term hydrolase delivery by helper-dependent AD
In a search for more stable transduction we turned to a new helper-dependent (hd) AD (hdAD) developed by R Parks, P Ng and others [25-27]. Since its viral coat is similar to that of the El-AD, the initial immune response is also similar, but because hdAD lacks DNA for all viral proteins it is far less prone to provoke chronic immunologic reaction. Much of our work now relies, in collaboration with Parks, on hdAD vectors. These vectors represent a great advance over first-generation AD vectors with simple El region deletions. Soon after they were introduced their impressive ability to sustain long-term transduction became evident. Using these vectors, it was possible to obtain high circulating levels of human α antitrypsin for over 1 year in baboons [24] and lifetime (~2.5 year) expression of the ApoE gene in genetically deficient mice [28].
Our own experience is in good agreement with previous findings. The hdAD vector, with an ApoE promoter, transduced substantial quantities of AME cocaine hydrolase, and for drastically longer periods of time than the El-AD-cytomegalovirus vector. Since the tissue tropism of an hdAD is determined by the nature of the viral capsid [29,30], which was essentially identical to that of the first-generation vector, liver was again the main focus of enzyme transduction. In early experiments with a very small number of rats, peak plasma enzyme activity 2 weeks after vector injection (1011 particles) was approximately 300 mU/ml, or eight-times the BChE levels typical for human plasma. This value is approximately half that typically seen with the El-AD but, in more recent experiments with larger sample sizes peak levels of cocaine, hydrolase activity after the same dose of hdAD vector have averaged closer to 1000 mU/ml [Gao Y, Brimijoin S, Unpublished Data]. High-level enzyme expression typically persists for several months. Even at 1 year, the longest time tested so far, cocaine hydrolase activity in the first group of treated rats was still 20% of initial peak levels [31].
We considered it worthwhile to establish the in vivo turnover of transduced enzyme 9 months after vector delivery as an index of protein stability. For that purpose, we gave the vector-treated rats an injection of iso-OMPA (3 mg/kg, ip.), an organophosphate that in correct doses will irreversibly and selectively inhibit BChE by 98–99% with less than 5% inactivation of AChE [32]. Inhibitor-treated rats showed no overt reactions to this agent (in particular, no indications of cholinergic toxicity, such as salivation, diarrhea, or impaired locomotion). By measuring the quasi-exponential recovery of cocaine hydrolase activity in the animals over a 5-day period, we deduced a plasma half-life of 60 h for the expressed transgene [31]. This recovery was attributed entirely to de novo protein synthesis, since there was no spontaneous reactivation of enzyme in vitro during the same time interval. The 60-h estimated half-life was actually greater than the value estimated for native BChE in rats that received no vector. Therefore, the expressed enzyme remained very stable in some rats, even after 9 months of transduction, when trace amounts of anti-BChE antibody could be detected in many of the plasma samples.
Since the expressed protein was identical in the experiments with El-AD and hdAD, it is not immediately obvious why immune reactions to the transgene were so much weaker with the latter vector. A likely explanation hinges on the fact that hdAD is itself inherently less immunogenic than El-AD and, in the absence of acute immune reactions to viral proteins, the human BChE is less readily targeted. We speculate that rats’ subdued immune response to BChE also reflects the enzyme’s heavy glycosylation, up to 25% of total mass [33-36]. This carbohydrate coating, when generated in the host, may partly mask foreign amino acid epitopes from the immune system. That cannot be the whole explanation, however, for at least two reasons. First, both vectors should express glycosylated BChE similarly. Second, unlike rats, mice typically show anti-BChE antibodies within 2 weeks of viral transduction of the AME hydrolase by hdAD vector, and loss of the hydrolase activity follows rapidly [Gao Y, Brimijoin S, Unpublished Data].
By contrast, in humans, immunological reactions to BChE-based cholinesterases should pose less of a problem. Subjects exposed to native human BChE would likely show no immune response at all, except for the very few individuals who naturally lack this enzyme [37,38]. The altered residues that enhance cocaine hydrolase activity in re-engineered BChE should be poorly antigenic, because they reside within the catalytic gorge. To confirm this hypothesis we produced a murine BChE with comparable mutations and introduced it into mice with an adeno-associated viral vector (AAV) type 2/8. The preliminary data show that such enzyme persists in plasma for months with no detectable antibody response. Thus, mutating these sites in a conspecific enzyme appears not to confer immunogenicity. We anticipate a similar outcome in the human case.
We also discount the likelihood that BChE’s ability to hydrolyze acetylcholine will cause problems with cholinergic neurotransmission, First, all tested BChE-based hydrolases with enhanced activity towards cocaine show reduced activity against acetylcholine. In our experiments to date, the transgene product is most abundant in liver and plasma [21], while AChE is densely concentrated at nerve endings [39], reaching levels of activity many hundredfold above those achievable by transduced BChE diffusing in from the circulation. Furthermore, Saxena and co-workers have shown that mice and guinea pigs tolerate doses of human BChE that raise plasma enzyme levels 50- to 100-fold with no clinical evidence of dysfunction or tissue pathology [40,41]. Finally, unpublished results of human studies sponsored by the US Department of Defense [Cerasoli D, Usamricd, Pers. Comm.] have indicated no adverse effects or physiological alterations even after large BChE doses (nearly 1 g per subject), which raised plasma cholinesterase levels at least 12-fold. Overall, these results support the conclusion that exogenously administered BChE is physiologically inert.
Behavioral effects of vector-delivered cocaine hydrolase
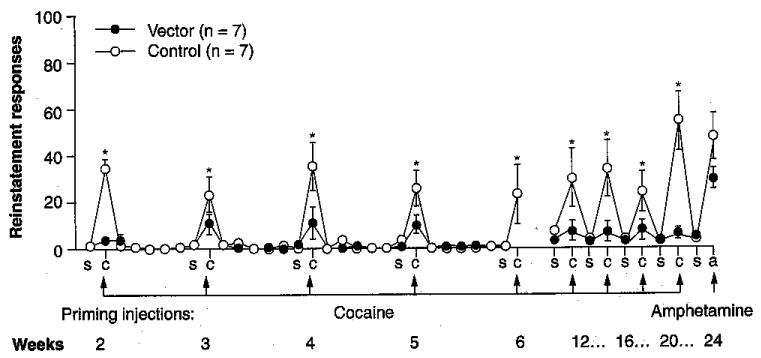
The crucial questions regarding the proposed therapeutic gene transfer are: can such a procedure suppress drug-seeking behavior, and can the suppression last long enough to be useful? Recent experiments using hdAD vector encoding the AME enzyme and ApoE promoter, carried out with the behavioral group of Marilyn Carroll at University of Minnesota (USA), have provided strongly positive answers [42]. Rats were first allowed to develop stable lever pressing for cocaine reward on a progressive ratio schedule of reinforcement, and were then given hydrolase vector through the tail vein (1011 viral particles per rat). After 2 weeks of enforced abstinence they returned to the operant chamber, in which normal cues were still present but the reinforcement lever was no longer active. Initial reinstatement tests consisted of 8 consecutive days in which saline or cocaine (5, 10 and 15 mg/kg) was given as the experimental session began. For the next 4 weeks, the rats were subjected to ip. injections of saline or cocaine (20 mg/kg) on 2 successive days. This pattern was then repeated monthly. Control animals (‘empty vector’ or no vector treatment) responded robustly to the cocaine-priming injection but not to saline. In comparison, rats given AME-transducing vector showed the same low level of responding to cocaine as to saline. Both groups responded equally well to the non-hydrolyzed priming agent, amphetamine. In other words, the hydrolase gene transfer selectively blocked drug-primed reinstatement just as did injections of purified enzyme protein. Moreover, the effect lasted 6 months after a single treatment (Figure 4). Such findings raise the possibility that a similar approach in humans could help recovering addicts to bridge the period during which relapse into addiction is often triggered by a single encounter with drug.
Figure 4. Sustained blockade of reinstatement.
Drug-primed resumption of drug-seeking (reinstatement behavior) in rodents is one possible model of addiction relapse in humans. This model was used to assess the potential of hydrolase gene transfer to reduce the risk of such relapse. Rats trained to self-administer cocaine were put into forced abstinence for 2 weeks to extinguish lever-pressing behavior and were then given a single injection of helper-dependent adenoviral vector encoding AME cocaine hydrolase (1011 viral particles, intravenously), ‘empty vector7#x2019; or saline. After a further 2 weeks, they received ‘priming injections’ of saline, cocaine or amphetamine and were returned to the operant chambers. Unprotected animals (saline or empty vector) responded robustly on the formerly active lever after cocaine priming but the animals given cocaine hydrolase vector failed to respond significantly over the entire 6-month period of observation. The same rats, however, did respond to priming with amphetamine (not a substrate for cocaine hydrolase). These findings are consistent with our hypothesis that hydrolase gene transfer may be able to generate long-term resistance to cocaine-induced craving by acting selectively to reduce that drug’s access to the brain.
Steps toward clinical application
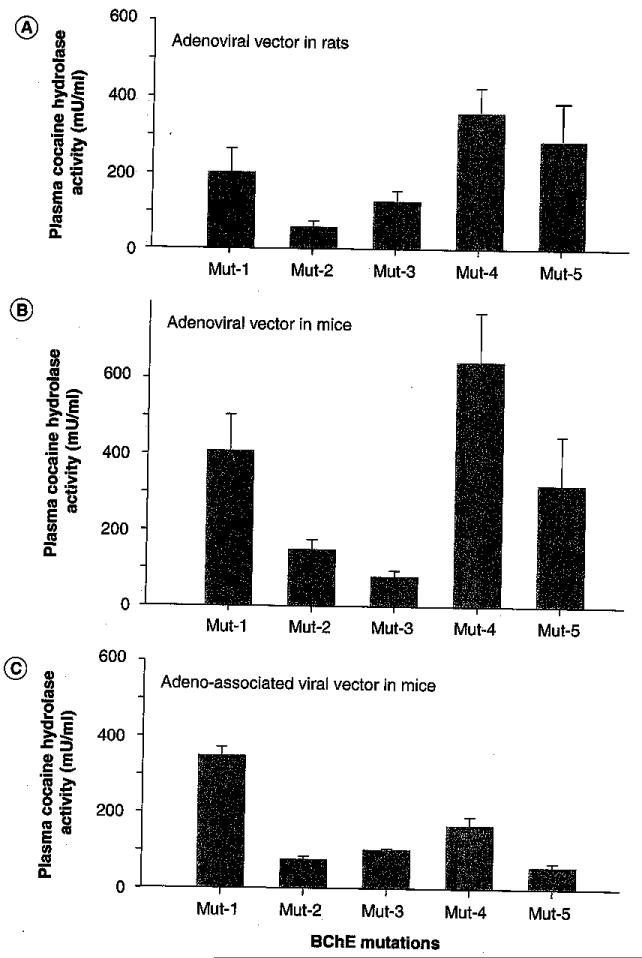
Overall, the delivery of engineered BChE cocaine hydrolases by viral gene transfer for clinical application appears feasible. However, in order to maximize the therapeutic index, it is important to identify constructs that drive cocaine metabolism to the highest possible final level with a given dose of vector and a given load of transgene. The best enzyme for cocaine interception would exhibit not only high maximal velocity but also a substrate affinity that permits rapid catalysis at drug concentrations likely to be encountered in vivo. Plasma cocaine concentrations associated with reward phenomena are fairly low, approximately 1–3 μM [43,44], and the KM value for a therapeutically useful hydrolase should ideally fall in the same range. As outlined above, it appears that recent mutational efforts with human BChE have neared that goal or may already have reached it, measured by the yardstick of catalytic efficiency. However, in vivo stability of the expressed protein is also important. Even if the most catalytically effective cocaine hydrolases have already been identified, they may not all be equally durable. Tests of the most promising new BChE mutants in different vector platforms are now underway and it will soon be possible to determine which construct in which vector leads to the greatest enzyme activity generated and maintained in vivo. Our current results with standard El-AD vector (Figure 5) suggest that vector delivery of further-enhanced BChE mutants will generate plasma cocaine hydrolase activities greater than those we previously reported.
Figure 5. In vivo transduction of multiple cocaine hydrolases by different viral vectors.
Mice and rats were given type 5 E-1-deleted adenoviral vectors or 2/8-AAV vectors with cytomegalovirus promoters at respective doses of 1010 and 3 × 1010 viral particles, intravenously. Plasma cocaine hydrolase activities (shown) were examined at time of peak expression, approximately 5 days after treatment. Tested BChE variants were: Mutant-1 (A199S/F227A/S287G/A328W/Y332G); Mutant-2 (F227A/S287G/A328W/Y332G); Mutant-3 (A199S/S287G/A328/Y332G) = CocHl; Mutant-4 (A199S/F227A/S287G/A328W/Y332G/E441D = CocH3; and Mutant-5 (F227A/S287G/A328W/Y332M) = AME [L Geng, y gao, S Brimijoin, Unpublished Data] .
The vector platform together with the promoter and enhancer sequences is as important as the enzyme since it determines the tissue locus of enzyme transduction, the level of transduced enzyme and the duration of transduction. At present, we see two viable candidates for vector of choice for cocaine hydrolase gene transfer: hdAD, serotype 5 [45-47] and AAV with dual serotype 2/8 [48,49] These two systems lack coding DNA for any of the proteins needed to produce a viral coat or replicate the viral genome: hdAD because of molecular engineering and AAV as a naturally hd parvovirus [50]. Both of these replication-incompetent vectors enter target cells (with these particular serotypes, primarily hepatocytes), by binding to unique surface proteins and inserting DNA that becomes incorporated as stable episomal elements within the nucleus (not integrating with the host chromosome).
Challenges to clinical application
Despite the encouraging results to date, serious challenges to clinical application remain. The greatest of these is a risk of tissue toxicity, particularly in liver, reflecting host responses to the transiently exposed capsid proteins of the delivered vector. These proteins, required for initial cell penetration and targeting the liver, evoke a rapid, dose-related and potentially severe attack from the innate host immune system [51,52]. Hepatocytes are particularly vulnerable during the process of vector uptake. This reaction is equivalent to the one provoked by early generation El’AD vectors and suspected as the primary cause of the fatal outcome in the very first clinical trial of AD gene therapy [53]. Since the immune response is transient and limited to the period during which viral capsid proteins remain in situ, the problem may be susceptible to mitigation by temporary immune suppression. In fact, although multiple factors must come into play, it has proved possible to reduce the innate toxicity of hdAD in mice by pretreatment with the anti-inflammatory steroid, dexamethasone [54]. In this regard, however, our preliminary histo-pathology studies show that hdAD transduction of CocH is not accompanied by liver damage in rats or mice, as evidenced by histopathology or measures of alanine transaminase activity in plasma. Furthermore, the vector treatment offers significant protection against cocaine-induced hepatoxicity. In other words, liver samples of rats and mice transduced with CocH by hdAD treatment and later exposed to large doses of cocaine contrast with those from unprotected animals in showing greatly reduced evidence of centrilobular necrosis and other pathologic signs. Nonetheless, concerns regarding clinical use of hdAD vectors dictate that alternatives to hdAD vectors should also be thoroughly investigated.
The principal alternative to hdAD in gene therapy for cocaine abuse is AAV. AAVs have seen limited applications until recently, because of their tight limits on cloning capacity. Practically speaking, the length of DNA sequences that can be introduced as a transgene payload is less than 3.5 kB [55]. A second problem is that infections with wild-type AAV are prevalent in the human population and the resulting immunity prevents effective use of vectors with the same serotype [51]. Third, AAVs generally show lower transduction efficiency than El AD [56] and, presumably, hdAD. On the positive side, wild-type AAV has never been associated with human illness [57], and AAVs have proved relatively benign in animal studies [58,59]. As a result, and despite the drawbacks just noted, over a dozen clinical trials have been completed with AAVs, focusing on multiple diseases such as a 1 antitrypsin deficiency [60], hemophilia B [61], cystic fibrosis [62], Parkinson’s disease [63], heart failure [64] and rheumatoid arthritis [65], among others. More are ongoing or pending. Rodent studies on transducing cocaine hydrolase with AAV-2/8 and a human ApoE promoter are in the early stages in our laboratory. To date, these constructs have been yielding less plasma cocaine hydrolase activity than the equivalent AD or hdAD constructs (Figure 5). Performance may improve as we test the effects of alternate intronic sequences and self-complementary design to avoid problems with second strand DNA synthesis [66]. If so, the stage will be set for a fair comparison between two promising vector platforms, in which the primary standard will be delivery of the greatest drug metabolizing power with the least risk of adverse effect.
Selecting locus of expression
One factor in the choice of gene-transfer vector is the desired locus of transgene expression. The actual locus of expression is strongly influenced by the general nature of the parent virus and its particular serotype [67], as well as by the promoter sequence incorporated to drive transgene expression [68,69]. To impact a behavioral problem like drug addiction there are two logical sites for expression. One is the CNS itself. The other is a peripheral site involved in filtering the blood or suited to releasing transgene into plasma, where it can intercept or destroy the drug before it reaches the CNS targets. Among the vector candidates for such therapies, none are likely to drive substantial gene expression in the brain unless centrally administered, because most viral particles lack access to brain parenchyma. Stereotaxic delivery can bypass that problem and is properly considered for treatment of fatal illness, such as malignant glioblastoma or progressive and irreversible conditions, such as Parkinson’s disease, but such measures appear excessive for a behavioral disorder. Fortunately, our rat data indicate that, by at least one molecular measure, transduction of cocaine hydrolase after iv. injection is at least as effective as transduction in neostriatal reward nuclei after stereotaxic injection. Thus, vector delivered systemically before a week-long series of twice-daily cocaine exposures prevented induction of δ-FosB throughout the caudate nucleus, whereas intracerebral delivery protected only neurons close to the injection site [31].
For systemic gene transduction, there are few promising target tissues. Muscle is an accessible, large-mass tissue that may be well suited to provide replacement for a deficient protein that is not required in large quantities (e.g., a clotting factor). However, for high levels of expression and to intercept drugs entering the circulation, liver seems a better choice. Liver receives a large blood flow, approximately 500 ml/m2 per min [70], or approximately 20% of total cardiac output. Therefore, within a few circulation times, liver can interact with nearly all blood-borne molecules. Liver is readily targeted by vectors derived from type-5 AD. Liver is also the natural source for BChE, which, after protein engineering, has shown such promise as a means of degrading cocaine en route to brain. Thus, liver-transduced cocaine hydrolase is well placed for efficiency in cocaine metabolism. Furthermore, much of the newly synthesized BChE is secreted into the circulation, where it remains with a half-life of many days, able to destroy cocaine on contact.
If the liver indeed is to be considered as the tissue target of choice for present purposes, priority should be given to avoiding immunotoxicity. Recent findings have indicated that, under certain circumstances, type 2 AAVs can elicit capsid-specific cytotoxic T lymphocytes capable of destroying transduced hepatocytes in tissue culture [71]. Fortunately, the in vivo effects resulting from systemic iv. delivery of vector appear to be modest, and potential countermeasures have been identified [72]. Nonetheless, going forward, caution is warranted.
Desirable therapeutic window
It should not be necessary to provide permanent gene transduction in order to treat cocaine addiction effectively. However, at least 6 months of effective drug interception is essential, and a period of 2-3 years may be needed to achieve full cessation of drug intake and to avoid relapse. This timeframe would not require agents such as lentiviral vectors that incorporate permanently into genomic DNA (with attendant risk of disrupting oncogenes). The hd ‘gutted’ AD or AAV considered above should be sufficient because they show long persistence as episomal elements, even though they do not propagate from cell to cell. However, they are usually lost during mitosis because they lack elements needed to replicate the viral DNA and segregate it to daughter cells [73]. Fortunately, for present purposes, although liver cells are not immortal they turn over slowly. Even in rats and mice, whose hepatocytes exhibit a half-life of 1 year or less [74], effective levels of transgene remain for many months after initial vector treatment as already noted. Life spans for hepatocytes in adult human liver are estimated to lie between 300 and 500 days [75]. Therefore, if cell division or cell death is the primary mechanism for loss of vector DNA, one could expect a therapeutic window on the order of 1 or 2 years after a successful hepatic transduction.
Conclusion
There is a real prospect of developing therapies for cocaine abuse based upon the idea of pharmacologic interception’. We consider that long-term delivery of a physiologically benign cocaine hydrolase by gene transfer could provide extended protection against relapse during the period when drug memories and cravings are most intense. As we have argued elsewhere [76], this gene-transfer approach might be combined with an anti-cocaine vaccine because these two agents should constitute a self-regenerating system, in which antibody absorbs an initial wave of drug, while enzyme destroys the free molecules, unloads the immunoglobulin-bound portion and resets the system. In our ongoing animal work, combinations of enzyme and antibody are proving more effective than either agent alone in reducing cocaine-induced locomotor activity and hepatotoxicity [Gao Y, Carroll ME, Anker JJ, Unpublished Data] . It will soon be possible to determine if the same is true in animal models of drug-seeking behavior.
Gene-transfer technology is still maturing, and we envision other applications to the therapy of drug addiction, beyond those directed simply at blocking drug access to brain. In a recent review article, Thome et al. stated that there is no indication that gene therapy can be applied in psychiatric patients any time soon, but also noted several promising developments in experimental neuroscience that foreshadow applications in a variety of disorders, including drug addiction [77]. Of interest in this regard are studies by Neumaier and colleagues demonstrating that over-expression of specific subtypes of serotonin receptors in the rat nucleus accumbens can selectively enhance cocaine rewarding properties (5HT1B) or reduce them (5HT6) [78,79]. Others have been able to modulate behavioral responses to cocaine through viral-mediated expression of ERK2 in the ventral tegmental area [so]. These and related approaches seem well worth pursuing in the future.
Future perspective
Though the road ahead may be rocky, we are bold enough to predict that gene transfer of agents that bind or destroy addictive drugs in plasma, especially cocaine, will progress to the point of clinical trial within the next decade. We do not expect a ‘magic bullet’ that will eliminate drug craving and provide effortless recovery without risk of further relapse. Nevertheless, on the assumption that the basic methods of gene transfer will continue to advance in the meanwhile, providing platforms with reduced risk and greater tolerance, we think that such treatments will enable many individuals to regain drug-free lives.
Executive summary.
Enzymatic interception of cocaine
■Molecular engineering of human plasma cholinesterase (butyrylcholinesterase) has increased its catalytic efficiency with cocaine by nearly 2000-fold.
BChE-based cocaine hydrolase versus cocaine toxicity & reward-driven behavior
■Re-engineered butyrylcholinesterase given to rats will prevent and reverse toxicity from lethal cocaine overdose and reduce drug-seeking behavior in a model of addiction relapse.
Delivery of enzyme by viral gene transfer
■It proved possible to raise levels of circulating cocaine hydrolase activity in rat plasma by a factor of 50,000-fold after injection of a classic adenoviral gene transfer vector.
Immunologic reactions to transgene & vector
■Classic adenoviral vectors provoke immune responses to viral coat;.protein and to the encoded transgene. Such reactions can be deleterious to the host and they radically shorten the duration of effective gene transduction.
Improved long-term delivery of enzyme by viral gene transfer
■With the aid of modern helper-dependent adenoviral vector it is now possible to sustain transduction of cocaine hydrolase at high levels in rats for a year or more after a single treatment.
Behavioral effects of vector-delivered cocaine hydrolase
■Unpublished data from collaborative studies in rodent models demonstrate that hydrolase gene transfer has the potential to provide permanent protection against relapse into drug seeking after a re-encounter with previously self-administered drug.
Steps toward clinical application
■Much work remains to establish the safety and efficacy of cocaine hydrolase gene therapy in human beings. A principle concern will be to reduce or avoid potential toxicity from innate host immune responses to vector coat protein. Both helper-dependent adenovirus and adeno-associated virus vectors deserve further exploration.
Challenges to clinical application
■An important challenge is to identify a vector platform capable of sustaining high-level transduction of a therapeutic drug-intercepting protein for many months without noticeable adverse effect.
Selecting locus of expression
■The liver is one obvious choice as target for expressing and secreting a blood-borne drug-intercepting enzyme, because its natural function has adapted this organ for drug metabolism. However, caution is warranted because the liver is vital and there may be real risks of treatment-induced immunologic toxicity.
Desirable therapeutic window
■Therapeutic gene transfer to prevent addiction relapse should not require permanent expression of transgene but probably does require expression that lasts at least 6 months; however, 1 or 2 years might be optimal.
Conclusion
■The authors postulate a real prospect of developing effective therapies for recovering cocaine addicts, based upon gene transfer of agents that intercept drug before it reaches the brain.
Acknowledgements
The authors gratefully acknowledge key collaborators at Mayo Clinic, USA (YP Pang, S Russell and H Sun), the University of Minnesota, USA (ME Carroll and J Anker), the University of Ottawa, Canada (R Parks), Baylor University and the Veterans Administration Medical Center in Houston, USA (T Kosten, F Orson and B Kinsey) and CoGenesys, Inc., Rockville, USA (D LaFleur).
Financial & competing interests disclosure
Financial support for the authors’ research comes from NIDA grants (R01DA23979 and DI DA31340), Previous work described was supported by the Minnesota Partnership for Medical Genomics and Biotechnology, and CoGenesys, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Key Terms
- Butyrylcholinesterase
Plasma enzyme capable of hydrolyzing acetylcholine and numerous other bioactive esters, Including cocaine (but at low efficiency).
- Cocaine hydrolase
Any enzyme capable of efficiently converting cocaine into relatively inert metabolites by breaking the ester bond.
- Adeno-associated viral vector
Gene-transfer vector based on a naturally replication-incompetent parvovirus and now approved for clinical use in an increasing number of applications.
- Gene transduction:
Process of expressing a desired protein in vivo via the cellular actions of a gene-transfer vector
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Duysen EG, Li B, Darvesh S, Lockridge O. Sensitivity of butyrylcholinesterase knockout mice to (−)-huperzine A and donepezil suggests humans with butyrylcholinesterase deficiency may not tolerate these Alzheimer’s disease drugs and indicates butyrylcholinesterase function in neurotransmission. Toxicology. 2007;233(1-3):60–69. doi: 10.1016/j.tox.2006.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick DA. Enhancing cocaine metabolism with butyrylcholinesterase as a treatment strategy. Drug Alcohol Depend. 1997;48(3):159–165. doi: 10.1016/s0376-8716(97)00119-1. ■■ Seminal concept paper drawing attention to therapeutic possibilities of natural human enzyme.
- 3.Bresler MM, Rosser SJ, Basran A, Bruce NC. Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MBl. Appl. Environ. Microbiol. 2000;66(3):904–908. doi: 10.1128/aem.66.3.904-908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen NA, de Prada P, Deng SX, et al. Crystallographic and biochemical analysis of cocaine-degrading antibody 15A10. Biochemistry. 2004;43(25):8067–8076. doi: 10.1021/bi049495l. [DOI] [PubMed] [Google Scholar]
- 5.Turner JM, Larsen NA, Basran A, et al. Biochemical characterization and structural analysis of a highly proficient cocaine esterase. Biochemistry. 2002;41(41):12297–12307. doi: 10.1021/bi026131p. [DOI] [PubMed] [Google Scholar]
- 6.Cooper ZD, Narasimhan D, Sunahara RK, et al. Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol. Pharmacol. 2006;70:1885–1891. doi: 10.1124/mol.106.025999. ■■ First published evidence that an efficient hydrolase could dramatically reduce cocaine actions in vivo.
- 7.Collins GT, Brim RL, Narasimhan D, et al. Cocaine esterase prevents cocaine-induced toxicity and the ongoing intravenous self-administration of cocaine in rats. J. Pharmacol. Exp. Ther. 2009;331(2):445–455. doi: 10.1124/jpet.108.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins GT, Carey KA, Narasimhan D, et al. Amelioration of the cardiovascular effects of cocaine in rhesus monkeys by a long-acting mutant form of cocaine esterase. Neuropsychopharmacology. 2011;36(5):1047–1059. doi: 10.1038/npp.2010.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko MC, Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Woods JH. Effects of cocaine esterase following its repeated administration with cocaine in mice. Drug Alcohol Depend. 2009;101(3):202–209. doi: 10.1016/j.drugalcdep.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie W, Altamirano CV, Barrels CF, Speirs RJ, Cashman JR, Lockridge O. An improved cocaine hydrolase: the A328Y mutant of human butyrylcholinesterase is 4-fold more efficient. Mol. Pharmacol. 1999;55(1):83–91. doi: 10.1124/mol.55.1.83. ■■ Initial report demonstrating feasibility of increasing the catalytic efficiency of human cholinesterase toward cocaine.
- 11.Sun H, Shen ML, Pang YP, Lockridge O, Brimijoin S. Cocaine metabolism accelerated by a re-engineered human butyrylcholinesterase. J. Pharmacol. Exp. Ther. 2002;302(2):710–716. doi: 10.1124/jpet.302.2.710. [DOI] [PubMed] [Google Scholar]
- 12.Sun H, El Yazal J, Lockridge O, Schopfer LM, Brimijoin S, Pang YP. Predicted Michaelis-Menten complexes of cocaine-butyrylcholinesterase. Engineering effective butyrylcholinesterase mutants for cocaine detoxication. J. Biol. Chem. 2001;276(12):9330–9336. doi: 10.1074/jbc.M006676200. □ Provided initial theoretical basis to improve butyrylcholinesterase for cocaine hydrolysis.
- 13.Pan Y, Gao D, Yang W, Cho H, Zhan CG. Free energy perturbation (FEP) simulation on the transition states of cocaine hydrolysis catalyzed by human butyrylcholinesterase and its mutants. J. Am. Chem. Soc. 2007;129(44):13537–13543. doi: 10.1021/ja073724k. ■■ Presents a brilliant theoretical analysis that led to the identification of a true high-efficiency cocaine hydrolase based on human plasma cholinesterase.
- 14.Yang W, Xue L, Fang L, Chen X, Zhan CG. Characterization of a high-activity mutant of human butyrylcholinesterase against (−)-cocaine. Chem. Biol. Interact. 2010;187(1-3):148–152. doi: 10.1016/j.cbi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue L, Ko MC, Tong M, et al. Design, preparation, and characterization of high-activity mutants of human butyrylcholinesterase specific for detoxification of cocaine. Mol. Pharmacol. 2011;79(2):290–297. doi: 10.1124/mol.110.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun H, Pang Y-P, Lockridge O, Brimijoin S. Re-engineering butyrylcholinesterase as a cocaine hydrolase. Mol. Pharmacol. 2002;621:220–224. doi: 10.1124/mol.62.2.220. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J. Pharmacol. Exp. Ther. 2004;310:1046–1052. doi: 10.1124/jpet.104.068122. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem. Biol. Interact. 2008;175(1-3):83–87. doi: 10.1016/j.cbi.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brimijoin S, Gao Y, Anker JJ, et al. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology. 2008;33(11):2715–2725. doi: 10.1038/sj.npp.1301666. ■ First demonstration that re-engineered cholinesterase could favorably affect drug-seeking behavior in an animal model of cocaine-addiction relapse.
- 20.Ostergaard D, Viby-Mogensen J, Hanel HK, Skovgaard LT. Half-life of plasma cholinesterase. Acta Anaesthesiol. Scand. 1988;32(3):266–269. doi: 10.1111/j.1399-6576.1988.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Atanasova E, Sui N, Pancook JD, Watkins JD, Brimijoin S. Gene transfer of cocaine hydrolase suppresses cardiovascular responses to cocaine in rats. Mol. Pharmacol. 2005;67(1):204–211. doi: 10.1124/mol.104.006924. [DOI] [PubMed] [Google Scholar]
- 22.Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy. Curr. Gene Ther. 2004;4(1):89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- 23.Pancook JD, Pecht G, Ader M, Mosko M, Lockridge O, Watkins JD. Application of directed evolution technology to optimize the cocaine hydrolase activity of human butyrylcholinesterase. FASEB J. 2003;17:A565. [Google Scholar]
- 24.Morral N, O’Neal W, Rice K, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl Acad. Sci. USA. 1999;96(22):12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitani K, Graham FL, Caskey CT, Kochanek S. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc, Natl Acad. Sci. USA. 1995;92(9):3854–3858. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and β-galactosidase. Proc. Natl Acad. Sci. USA. 1996;93(12):5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl Acad. Sci. USA. 1996;93(24):13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim 1H, Jozkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc. Natl Acad. Sci. USA. 2001;98(23):13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunetti-Pierri N, Nichols TC, McCorquodale S, et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum. Gene Ther. 2005;16(7):811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- 30.Muruve DA, Cotter MJ, Zaiss AK, et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 2004;78(11):5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y, Brimijoin S. Lasting reduction of cocaine action in neostriatum – a hydrolase gene therapy approach. J. Pharmacol. Exp. Ther. 2009;330(2):449–457. doi: 10.1124/jpet.109.152231. ■ Paved the way towards a practical gene therapy for cocaine addiction by showing that a single injection of helper-dependent vector encoding cocaine hydrolase leads to semi-permanent enzyme expression and long-term blockade of cocaine-driven molecular plasticity in brain.
- 32.Heffron PF. Actions of the selective inhibitor of cholines terase tetramonoisopropyl pyrophosphortetramide on the rat phrenic nerve-diaphragm preparation. Br. J. Pharmacol. 1972;46(4):714–724. doi: 10.1111/j.1476-5381.1972.tb06896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.La Du BN, Lockridge O. Molecular biology of human serum cholinesterase. Fed. Proc. 1986;45(13):2965–2969. [PubMed] [Google Scholar]
- 34.Nachon F, Nicolet Y, Viguie N, Masson P, Fontecilla-Camps JC, Lockridge O. Engineering of a monomeric and low-glycosylated form of human butyrylcholinesterase: expression, purification, characterization and crystallization. Eur. J. Biochem. 2002;269(2):630–637. doi: 10.1046/j.0014-2956.2001.02692.x. [DOI] [PubMed] [Google Scholar]
- 35.Lockridge O, Eckerson HW, La Du BN. Interchain disulfide bonds and subunit organization in human serum cholinesterase. J. Biol. Chem. 1979;254(17):8324–8330. [PubMed] [Google Scholar]
- 36.Lockridge O, Battels CF, Vaughan TA, Wong CK, Norton SE, Johnson LL. Complete amino acid sequence of human serum cholinesterase. J. Biol. Chem. 1987;262(2):549–557. [PubMed] [Google Scholar]
- 37.Scott EM. Inheritance of two types of deficiency of human serum cholinesterase. Ann. Hum. Genet. 1973;37(2):139–143. doi: 10.1111/j.1469-1809.1973.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 38.Hodgkin W, Giblett ER, Levine H, Bauer W, Motuisky AG. Complete pseudocholi nes terase deficiency: genetic and immunologic characterization. J. Clin. Invest. 1965;44:486–493. doi: 10.1172/JCI105162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silver A. The Biology of Cholinesterases. North-Holland Publishing Co.; Amsterdam, The Netherlands: 1974. [Google Scholar]
- 40.Saxena A, Sun W, Fedorko JM, Koplovitz I, Doctor BP. Prophylaxis with human serum butyrylcholinesterase protects guinea pigs exposed to multiple lethal doses of soman or VX. Biochem. Pharmacol. 2011;81(1):164–169. doi: 10.1016/j.bcp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Saxena A, Sun W, Luo C, Doctor BP. Human serum butyrylcholinesterase: in vitro and in vivo stability, pharmacokinetics, and safety in mice. Chem. Biol. Interact. 2005;157-158:199–203. doi: 10.1016/j.cbi.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Anker JJ, Brimijoin S, Gao Y, et al. Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol. Psychiatry. 2012 doi: 10.1016/j.biopsych.2011.11.014. DOI: 10.1016/]. biopsych.2011.11.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jufer RA, Walsh SL, Cone EJ. Cocaine and metabolite concentrations in plasma during repeated oral administration: development of a human laboratory model of chronic cocaine use. J. Anal. Toxicol. 1998;22(6):435–444. doi: 10.1093/jat/22.6.435. [DOI] [PubMed] [Google Scholar]
- 44.Walsh SL, Stoops WW, Moody DE, Lin SN, Bigelow GE. Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics. Exp. Clin. Psychopharmacol. 2009;17(4):205–216. doi: 10.1037/a0016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jozkowicz A, Dulak J. Helper-dependent adenoviral vectors in experimental gene therapy. Acta Biochim. Pol. 2005;52(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- 46.Brunetti-Pierri N, Ng P. Helper-dependent adenoviral vectors for liver-directed gene therapy. Hum. Mol. Genet. 2011;20(R1):R7–R13. doi: 10.1093/hmg/ddr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vetrini F, Ng P. Liver-directed gene therapy with helper-dependent adenoviral vectors: current state of the art and future challenges. Curr. Pharm. Design. 2011;17(24):2488–2499. doi: 10.2174/138161211797247532. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Wang H, Morizono H, et al. Sustained correction of OTC deficiency in spf(ash) mice using optimized self-complementary AAV2/8 vectors. Gene Ther. doi: 10.1038/gt.2011.111. doi:10.1038/gt.2011.111 (2011) (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12(5):341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 50.Berns KI, Hauswirth WW. Adeno-associated viruses. Adv. Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- 51.Muruve DA. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004;15(12):1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 52.Brunetti-Pierri N, Palmer DJ, Beaudf, et al. Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004;15(1):35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- 53.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80(1-2):148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Seregin SS, Appledorn DM, McBride AJ, et al. Transient pre treatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol. Ther. 2009;17(4):685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 1996;7(17):2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 56.Wang AY, Peng PD, Ehrhardt A, Storm TA, Kay MA. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum. Gene Ther. 2004;15(4):405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- 57.Kaplitt MG, Leone P, Samulski RJ, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994;8(2):148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 58.Koornneef A, Maczuga P, van Logtenstein R, et al. Apolipoprotein B knockdown by AAV-delivered shRNA lowers plasma cholesterol in mice. Mol. Ther. 2011;19(4):731–740. doi: 10.1038/mt.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi SH, Lee HC. Long-term, antidiaberogenic effects of GLP-1 gene therapy using a double-stranded, adeno-associated viral vector. Gene Ther. 2011;18(2):155–163. doi: 10.1038/gt.2010.119. [DOI] [PubMed] [Google Scholar]
- 60.Brandy ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAVl-AAT gene therapy. Proc. Natl Acad. Sci. USA. 2009;106(38):16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 62.Wagner JA, Messner AH, Moran ML, et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope. 1999;109(2 Pt 1):266–274. doi: 10.1097/00005537-199902000-00017. [DOI] [PubMed] [Google Scholar]
- 63.Christine CW, Starr PA, Larson PS, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73(20):1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaski BE, Jessup ML, Mancini DM, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human Phase I/II clinical trial. J. Card. Fail. 2009;15(3):171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mease PJ, Wei N, Fudman EJ, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a Phase I/II study. J. Rheumatol. 2010;37(4):692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- 66.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 67.Michelfelder S, Trepel M. Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv. Genet. 2009;67:29–60. doi: 10.1016/S0065-2660(09)67002-4. [DOI] [PubMed] [Google Scholar]
- 68.Gill DR, Pringle IA, Hyde SC. Progress and prospects: the design and production of plasmid vectors. Gene Ther. 2009;16(2):165–171. doi: 10.1038/gt.2008.183. [DOI] [PubMed] [Google Scholar]
- 69.Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv. Drug Deliv. Rev. 2009;61(7-8):554–571. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Leevy CM, Mendenhall CL, Lesko W, Howard MM. Estimation of hepatic blood flow with indocyanine green. J. Clin. Invest. 1962;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J. Clin. Invest. 2009;119(6):1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nathwani AC, Gray JT, Mcintosh J, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109(4):1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kreppel F, Kochanek S. Long-term transgene expression in proliferating cells mediated by episomally maintained high-capacity adenovirus vectors. J. Virol. 2004;78(1):9–22. doi: 10.1128/JVI.78.1.9-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuntz E, Kuntz H-D, editors. Hepatology: Textbook and Atlas: History, Morphology, Biochemistry, Diagnostics, Clinic, Therapy. Springer; Heidelberg, NY, USA: 2008. [Google Scholar]
- 75.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 76.Gao Y, Orson FM, Kinsey B, Koscen T, Brimijoin S. The concept of pharmacologic cocaine interception as a treatment for drug abuse. Chem. Biol. Interact. 2010;187(1-3):421–424. doi: 10.1016/j.cbi.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thome J, Hassler F, Zachariou V. Gene therapy for psychiatric disorders. World J. Biol. Psychiatry. 2011;12(Suppl. 1):16–18. doi: 10.3109/15622975.2011.601927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferguson SM, Mitchell ES, Neumaier JF. Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol. Psychiatry. 2008;63(2):207–213. doi: 10.1016/j.biopsych.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 79.Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr. Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J. Neurosci. 2002;22(24):10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iniguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolanos-Guzman CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav. Brain Res. 2010;214(2):460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]