Abstract
Hyperactivated HER2/Neu/EGFR/RAS signaling is a major growth-promoting pathway known to drive cellular transformation and oncogenesis in breast cancers. HER2 amplification is detected in ~20% of all human breast cancer and is quite prevalent (up to 49%) in ductal carcinoma in situ (DCIS). The E3 ubiquitin ligase SIAH is considered a key downstream “gatekeeper” required for proper HER2/EGFR/RAS signal transduction. Formalin-fixed, paraffin-embedded resection specimens from 65 patients with DCIS treated with wide excision only were stained with an anti-SIAH antibody, and the percentage of tumor and normal adjacent tissue cells positive for SIAH nuclear staining were recorded. Statistical analysis was performed comparing SIAH staining in tumor cells to disease recurrence, histologic type, necrosis, hormone receptor status, and Her2/neu status, as well as nuclear grade. Correlation of SIAH expression in tumor cells with SIAH expression in normal adjacent tissue and age was also examined. Expression levels of SIAH in tumor cells was significantly higher in specimens from patients with recurrence (median = 19%) as compared to patients without recurrence (7%) (P <0.001). There was also significantly increased SIAH expression in tumors with more aggressive features including comedo morphology (13.5% in comedo vs. 7% in other histologic types, P = 0.014). No significant association was observed between SIAH expression and estrogen receptor, progesterone receptor, and Her2/neu status. There was a significant correlation between SIAH expression in tumors and normal adjacent tissue (Spearman correlation = 0.58, P <0.001) as well as between SIAH expression in normal adjacent tissue and patient age (Spearman correlation = −0.59, P <0.001). No significant correlation was identified between patient age and SIAH expression in tumors (Spearman correlation = −0.23, P = 0.067). In conclusion, SIAH may represent a useful prognostic biomarker that predicts DCIS progression to invasive breast cancer.
Keywords: SIAH, Ductal carcinoma in situ, DCIS
Introduction
Breast cancer is the most common cause of cancer in women representing 27% of all new cancer cases and is the second most common cause of cancer death in women overall after lung cancer [1]. An estimated 182,480 new cases of invasive breast cancer were diagnosed in the United States in 2008. With advances in early detection, the incidence of ductal carcinoma in situ (DCIS) has increased substantially over the last 30 years from 1% of all breast cancers in the 1970’s to 20–45% of all new cases of mammographically detected breast cancer and 10% of all breast carcinoma in the 2000’s [2-4]. Standard therapy for DCIS generally involves breast conserving surgery with or without radiation and adjuvant hormonal therapy. Ten-year cancer-specific survival for DCIS with current treatment is 97%. However, it has been argued that there is considerable overtreatment in many patients with associated morbidity from side effects as well as unneeded cost [reviewed in 3]. A better understanding of the molecular events underlying the development and progression of DCIS may identify novel prognostic biomarkers that could help guide optimal treatment selection and reduce costly overtreatment and treatment-associated morbidity.
Sanders et al. [5] examined the natural history of untreated, low grade, noncomedo DCIS and showed that 39.3% of these patients developed invasive breast cancer in the same quadrant as the initial biopsy with most events occurring within 10–15 years but with some as late as 23–42 years. Nearly half of the patients who developed invasive breast cancer died of metastatic disease 1–7 years after diagnosis [5]. The results of this study suggest that some patients can survive several years with DCIS without progression while others will develop life-threatening invasive carcinoma. Stratifying these patients using prognostic tumor markers may help to prevent both under and over treatment of biopsy identified DCIS.
SIAHs are the human homologs of Seven-In-Absentia (SINA), an evolutionarily conserved RING finger E3 ubiquitin ligase and essential downstream component of the Drosophila RAS signaling pathway—a critical “gatekeeper” required for proper RAS signal transduction [6, 7]. Two homologues, SIAH1 and SIAH 2, exist in humans, and have been shown to play a role in several pathways including those involved in response to DNA damage, the hypoxic response, estrogen signaling, inflammation, and RAS signaling [reviewed in 8]. SIAH acts as an essential downstream signaling component required for proper EGFR/HER2 and RAS signaling [9, 10]. Additionally, data from our laboratory suggests that SIAH is a downstream “gatekeeper” required for HER2/RAS-mediated tumorigenesis and metastasis in human breast cancer (Park et al. manuscript in preparation). These studies provide an initial glimpse into the significance of SIAH-dependent proteolysis in the ERBB/RAS signaling pathway during tumor progression and metastasis in human breast cancers. Understanding SIAH expression and regulation has the potential to provide novel anti-HER2/Neu/RAS and anti-cancer therapies and contribute to our understanding of the pivotal function of SIAH-dependent proteolysis in the genesis, progression, and metastasis of human breast cancer [9, 10]. To date, there are few studies addressing SIAH expression in invasive breast carcinoma. One group found that SIAH expression is down regulated in breast cancer brain metastases [11] while others found that SIAH expression is upregulated in infiltrating ductal and medullary carcinomas, except in tumors with aberrant β-catenin localization where SIAH expression is down regulated [12].
The objective of our current study was to see if SIAH expression could predict DCIS recurrence and/or if SIAH expression was associated with other clinicopathologic factors associated with DCIS. To accomplish this objective, we examined SIAH expression in DCIS and adjacent normal tissue from patients treated with wide excision only.
Materials and methods
Patient selection
DCIS breast tissue was obtained from the surgical pathology files at Thomas Jefferson University Hospital (Philadelphia, PA) for a retrospective study with Institutional Review Board approval. A total of 65 consecutive patients who underwent surgical resection and for whom tissue was available were included in this study. Clinical and treatment information were extracted via chart review. All patients were treated with surgical excision only (no radiation or hormonal therapy) by the same surgeon (Gordon F. Schwartz, MD). Negative margins (≥10 mm) were achieved at the conclusion of excision or re-excision and removal of all suspicious calcifications was confirmed on postoperative mammography. Patients with DCIS involving more than 1 quadrant were treated by mastectomy and were excluded from this study. The date of diagnosis and recurrence were defined as the date of surgery leading to the relevant pathologic diagnosis. The presence of DCIS, invasive cancer, or absence of disease at the last follow-up was established as the study endpoint. Follow-up ranged from 53.4 to 202.1 months (median = 100.8, mean = 114.1). Patients who developed invasive ductal carcinoma within 6 months of a DCIS diagnosis were excluded because invasive ductal carcinoma was felt to be part of their original disease and not recurrence. For each case, the histological pattern (cribriform, solid, comedo, papillary, micropapillary), the presence or absence of necrosis, and nuclear grade were evaluated. Hormone receptor and HER2 status was obtained from pathology reports. This study was designed and executed as per the REMARK criteria for tumor marker studies [13].
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were cut at 4 μm and deparaffinized by usual techniques. Antigen retrieval was carried out using 1 mM EDTA (pH 8.0) in a preheated 98°C steamer for 30 min. Slides were treated with 3% H2O2 to inactivate endogenous peroxidase. Sections were incubated with mouse monoclonal anti-SIAH 24E6 monoclonal antibody [9, 10] at a 1:40 dilution for 60 min at room temperature. Visualization was carried out using DAKO’s Dual + Envision link (K4061, DAKO North America, Inc., Carpinteria, CA) followed by incubation with diaminobenzidine and counterstaining with hematoxylin. Percentage of DCIS cells and normal breast tissue positive for SIAH nuclear staining was recorded. Scoring was performed by an experienced pathologist with no prior knowledge of patient age, disease recurrence, hormone receptor, or HER2 status.
Statistical analysis
Association between SIAH expression in DCIS with recurrence, necrosis, comedo histology, estrogen receptor, progesterone receptor, and HER2 status was analyzed using the Wilcoxon two-sample test. Association between SIAH expression and nuclear grade or age group (≤40, 40–60, >60) was evaluated using the Kruskal–Wallis test. Correlation between SIAH expression in DCIS and normal breast tissue was evaluated with the Spearman correlation coefficient. Logistic regression and Receiver Operating Characteristic (ROC) curves were used to evaluate the utility of SIAH expression in DCIS and normal breast tissue for predicting disease recurrence. A P value of 0.05 was considered statistically significant for all analyses. Goodness of fit for the logistic regression models was assessed using the Hosmer and Lemeshow Test. All analyses with statistical plots were performed with SAS software Version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient ages ranged from 32 to 91 (median: 56, mean: 58.9). Of 65 patients, 10 recurred as DCIS and 7 as invasive carcinoma. Three of 7 patients with invasive recurrence had lymph node metastases. The overall recurrence rate in this study is 26.2%. None of the standard potential prognostic factors (nuclear grade, necrosis, comedo histology, ER, PR, and HER2) were significantly associated with recurrence status.
SIAH expression in DCIS was compared to recurrence status as well as various clinicopathologic factors (Table 1). Because there is no standardized scoring system for SIAH immunohistochemical staining, we chose to score based on percentage of cells positive for SIAH staining. While the percentage of SIAH-positive tumor cells varied by patient, SIAH expression was consistently confined to the nucleus of tumor cells (Figs. 1, 2). SIAH expression was also present in a subset of normal luminal cells in the terminal duct lobular unit (Fig. 1). No SIAH staining was detected in myoepithelial cells or stromal tissue.
Table 1.
SIAH expression in DCIS as compared to disease recurrence and various clinicopathologic factors
| Prognostic factor | SIAH expression Median (Range) (%) | P value |
|---|---|---|
| Recurrence | < 0.001 | |
| No recurrence (N = 48) | 7 (1–40) | |
| Recurrence (N = 17) | 19 (4–40) | |
| Age | 0.144 | |
| ≤40 (N = 6) | 13 (1–40) | |
| 40–60 (N = 28) | 8 (1–25) | |
| >60 (N = 31) | 17.5 (5–40) | |
| Nuclear Grade | ||
| 1 (N = 13) | 2 (1–16) | 0.003 |
| 2 (N = 28) | 8.5 (2–30) | |
| 3 (N = 24) | 15 (3–40) | |
| Necrosis | 0.037 | |
| No necrosis (N = 35) | 8 (1–30) | |
| Necrosis present (N = 30) | 11 (2–40) | |
| Comedo | 0.014 | |
| No comedo (N = 43) | 8 (1–30) | |
| Comedo present (N = 22) | 13.5 (3–40) | |
| ER | 0.062 | |
| Negative (N = 13)* | 20 (3–40) | |
| Positive (N = 46)* | 10 (1–40) | |
| PR* | 0.476 | |
| Negative (N = 17)* | 10 (3–40) | |
| Positive (N = 42)* | 10.5 (1–40) | |
| HER2* | 0.064 | |
| Negative (N = 29)* | 9.5 (1–40) | |
| Positive (N = 29)* | 15 (2–20) |
N is the number of non-missing values. Receptor status was unavailable for some patients
Fig. 1.
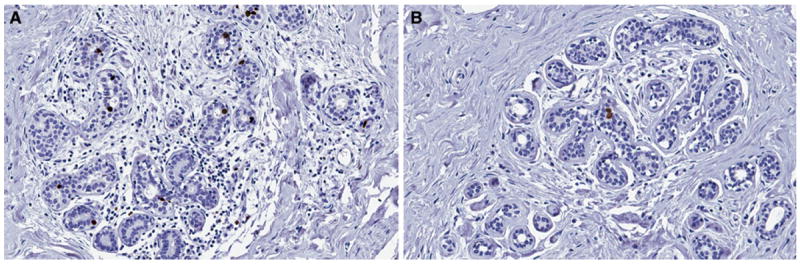
SIAH expression in normal breast tissue. a Increased SIAH expression in normal breast tissue (×200), b Low SIAH expression in normal breast tissue (×200)
Fig. 2.
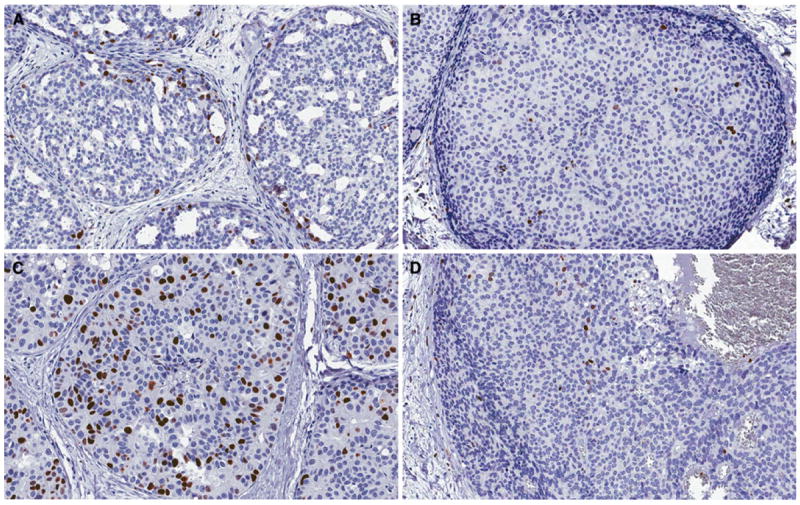
SIAH expression in DCIS. a, b Low grade DCIS, c–d High grade DCIS. a Increased SIAH expression in low grade DCIS (×200), b Low SIAH expression in low grade DCIS (×200), c Increased SIAH expression in high grade DCIS (×200), d Low SIAH expression in high grade DCIS
SIAH expression predicts DCIS progression and disease recurrence
Expression levels of SIAH were significantly higher in DCIS cases associated with recurrence (median = 19%, range = 4–40%) as opposed to DCIS with no recurrence on follow up (median = 7%, range = 1–40%) (P < 0.001). SIAH expression was significantly higher in lesions with necrosis (median = 11%, range = 2–40%) than in lesions without necrosis (median = 8%, range = 1–30%) (P = 0.037). SIAH expression was also significantly higher in lesions with comedo histology (median = 13.5%, range = 3–40%) as opposed to those without comedo histology (median = 8%, range = 1–30%) (P = 0.014). SIAH expression also increased (P = 0.003) with increasing nuclear grade (grade 1: median = 2%, range = 1–16% for grade 1; grade 2: median = 8.5%, range = 2–30%; and grade 3: median = 15%, range = 3–40%). No significant association was observed between SIAH expression and age or estrogen receptor (ER), progesterone receptor (PR), or HER2 receptor status.
When tumor recurrence was separated by type (invasive carcinoma vs. DCIS; Table 2), SIAH expression was significantly higher in primary DCIS associated with invasive recurrence (median 21 vs. 15.5%; P = 0.036). Significant correlation was found between SIAH expression in DCIS and in normal breast tissue (see Table 3, Spearman correlation coefficient = 0.53, P < 0.001). In normal breast tissue, the median SIAH expression levels were higher in women who had invasive recurrence when compared to normal breast tissue adjacent to nonrecurrent DCIS (median 3 vs. 0.5%; P = 0.023).
Table 2.
Associated between SIAH expression in DICS or normal breast tissue and recurrence type
| Recurrence Status | SIAH expression in DCIS Median (Range) (%) | SIAH expression in normal breast tissue Median (Range) |
|---|---|---|
| Invasive recurrence | 21 (18–40) | 3 (0–8) |
| DCIS recurrence | 15.5 (4–40) | 1 (0–11) |
| No recurrence | 7 (1–40) | 0.5 (0–10) |
Table 3.
Correlation between SIAH expression in DCIS, normal breast tissue, and age
| Correlation coefficient | 95% confidence interval | P value | |
|---|---|---|---|
| SIAH expression in DCIS and age | −0.23 | (−0.446, −0.018) | 0.067 |
| SIAH expression in DCIS and normal breast tissue | 0.53 | (0.318, 0.681) | <0.001 |
| SIAH expression in normal breast tissue and age | −0.54 | (−0.690, −0.329) | <0.001 |
Since younger patients have an increased risk of DCIS recurrence, correlation between SAIH expression in DCIS, normal breast tissue, and patients’ age was also examined (Table 3) [14, 15]. Significant negative correlation was found between SIAH expression in normal adjacent tissue and patient age (Spearman correlation coefficient = −0.54, P < 0.001). No statistically significant correlation was found between patient age and SIAH expression in DCIS (Spearman correlation coefficient = −0.23, P = 0.067).
SIAH expression in DCIS and normal breast tissue were found to be significant predictors of disease recurrence using a univariate logistic regression model (P = 0.003 for DCIS and P = 0.013 for normal adjacent tissue). Additionally, the odds ratios associated with 1%, incremental increases in SIAH expression were determined (Table 4). A 1% increase in SIAH expression in DCIS predicts a 10% increase in odds of recurrence (odds ratio = 1.10, 95% CI: 1.03, 1.16), while a 1% increase in SIAH expression in normal breast tissue predicts a 31% increase in odds of recurrence (odds ratio = 1.31, 95% CI: 1.06, 1.62). The higher odds ratio for SIAH expression in normal breast tissue is consistent with the smaller range of SIAH expression in normal breast tissue (0–11%) as compared to DCIS (1–40%).
Table 4.
Logistic model for the rate of any recurrence
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| SIAH expression in normal breast tissue | 1.31 | (1.06, 1.62) | 0.013 |
| SIAH expression in DCIS | 1.10 | (1.03, 1.16) | 0.003 |
When SIAH expression in neoplastic and normal adjacent breast tissue was considered jointly in a multivariate logistic regression model, SIAH expression in normal breast tissue was not a significant predictor of recurrence. Therefore, reduction to a parsimonious model, which is necessary for data with a small number of recurrences, resulted in the logistic regression model including only one predictor, SIAH expression in DCIS.
Survival ROC curves
A ROC curve was created to further assess the use of SIAH expression in DCIS as a predictor for disease recurrence. In the absence of the standard categorical grading system for SIAH expression, our study aimed to propose a meaningful cutoff to classify SIAH expression as “negative” vs. “positive” for the purpose of predicting recurrence. The ROC curve methodology is specifically designed to address the question of optimal cutoffs. The Area Under the Curve (AUC) for recurrence was used to evaluate the performance of SIAH expression as a novel predictor in the model. The ROC curve based on SIAH expression in DCIS had an area under the curve of 0.79 (Fig. 3). A candidate optimal cutoff of 15% (“negative” SIAH expression ≤ 15% and “positive” SIAH > 15%) corresponds to 28% probability of recurrence (labeled on the ROC curve as 0.28) and yields 76% sensitivity (13 of 17 correctly predicted recurrences; 95% CI: 50–93%) and 79% specificity (38 of 48 correctly predicted non-recurrences; 95% CI: 65–90%). This candidate optimal cutoff would need to be validated in future larger studies utilizing automated technology.
Fig. 3.
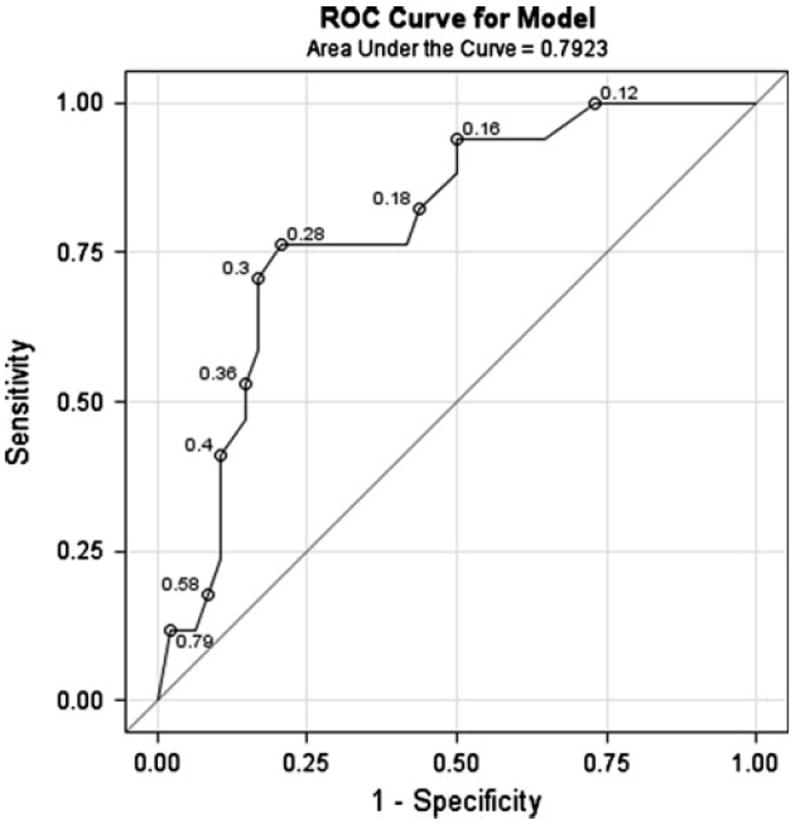
Receiver operating characteristic (ROC) curve for SIAH expression in DCIS as a predictor for disease recurrence. The area under the curve is 0.79. A candidate optimal cutoff of 15% corresponds to 28% probability of recurrence and yields 76% sensitivity and 79% specificity
Discussion
In the current study, we examined SIAH expression in primary DCIS treated with wide excision only and in adjacent normal breast tissue. We found that increased SIAH expression in DCIS was correlated with subsequent disease recurrence and increased risk of progression. Increased SIAH expression was also correlated with more aggressive features including comedo histology and increasing nuclear grade. However, aggressive features alone were not significantly associated with disease recurrence. We feel this potential discrepancy is not due to size of the patient cohort since other studies with larger numbers of patients have also found a lack of correlation between DCIS with aggressive features and increased recurrence in patients treated with wide excision only [16]. Rather, we feel that SIAH has a relatively strong predictive value that is evident in the current study population.
SIAH expression in DCIS was positively correlated with expression levels in normal breast tissue with younger patients having higher SIAH expression in normal adjacent breast tissue. In a univariate logistic regression analysis, SIAH expression in DCIS and normal adjacent tissue were both able to predict recurrence. A multivariate analysis controlling for age, necrosis, comedo, and histologic subtype was not possible due to the small number of recurrences [17, 18]. Additionally, a ROC curve using 15% as a cutoff for positive SIAH staining resulted in a sensitivity of 76% and a specificity of 79% for disease recurrence. These data suggest that SIAH may be a potential biomarker for disease recurrence in patients with DCIS. In the future, we hope to expand our study to validate our findings in patients treated with radiation and/or tamoxifen in addition to surgery.
Numerous biomarkers have been investigated for risk stratification of patients with DCIS. Elevated Ki-67 levels, p53 mutations, and HER2 amplification are known to be associated with increased nuclear grade and necrosis which have also been associated with disease recurrence and progression [reviewed in 3]. p53 mutations and HER2 amplification have been seen in 25 and 30% of DCIS, respectively. Other cell cycle markers have also been studied including p21, p27, and cyclin D1 [reviewed in 3]. Despite these studies, no single biomarker has been identified to guide proper and effective therapies.
Another group, Tsikitis et al., has suggested a new classification scheme based on the combination of molecular and histologic characteristics of DCIS [19]. The histological features of aggressive lesions include comedo necrosis, high nuclear grade, and negative hormone receptor status while the molecular features include increased Ki-67, p53 mutation, HER2 amplification, increased COX2 expression, loss of heterozygosity at 11q13, and increased angiogenesis [19]. Less aggressive DCIS has lower Ki-67, normal p53, no HER2 amplification, and deletion of chromosome 16. They suggested that their classification scheme helps separate patients who will develop invasive breast cancer within a short period of time (2–5 years) necessitating more aggressive treatment from those who will develop invasive breast cancer over a longer period of time (10–20 years) [19].
In the current study, SIAH expression was found to be correlated with nuclear grade and comedo histology. However, more importantly, we were able to show an increase in the odds ratio for recurrence with increased SIAH expression in DCIS as well as normal breast tissue. Despite the correlation of SIAH expression with more aggressive tumor biology, the exact mechanism of increased SIAH expression in tumorigenesis is unknown. However, increased SIAH expression has been seen in both neoplastic and non-neoplastic proliferating cells [9, 10]. Therefore, it is not surprising that SIAH expression is associated with more aggressive lesions given that these lesions also tend to have an increased mitotic index, i.e., increased Ki-67.
Paradoxically, SIAH was initially thought to be a tumor suppressor gene [reviewed in 8]. However, recent studies have shown that decreased SIAH function results in inhibition of tumor growth [9, 10, reviewed in 8]. Indeed, recent studies using anti-SIAH molecules (shRNA knock down and dominant negative SIAH) have shown that SIAH deficiency significantly impedes lung and pancreatic tumor growth in vitro and in vivo [9, 10]. Also, Bowtell and Möller found that SIAH expression was strongly upregulated in ER-positive breast tumors arguing against a role for SIAH as a tumor suppressor [unpublished observations, reviewed in 8]. In contrast, we found no correlation between SIAH expression and ER expression in this study.
Interestingly, we also found an inverse correlation between SIAH expression in normal breast tissue and patient age (Spearman correlation = −0.54, P < 0.001) as well as a trend toward an inverse correlation between SIAH expression in DCIS and patient age (Spearman correlation = −0.23, P = 0.067). It has long been known that DCIS in younger patients has a higher risk of recurrence. In a recent study of 657 patients, Collins et al. found that DCIS in young women was more likely to be symptomatic and more extensive at presentation and was also more likely to involve cancerization of the lobules. Interestingly, there was no correlation found with histologic features known to be associated with more aggressive disease such as high nuclear grade, comedo necrosis, or architectural pattern. Collins et al. also interrogated currently used molecular markers (ER, PR, and Her-2) and found no correlation with age. They propose that more aggressive disease may be related to less screening in younger patients which causes lesions to become more extensive at presentation [14].
Another study addressing molecular markers and histologic features of DCIS in relation to age showed a trend toward higher HER2 expression in younger patients with DCIS. They also showed a correlation of HER2 expression with high-grade lesions, ER and PR negativity, and increased Ki-67. However, due to a small number of patients examined, the study was unable to show a statistically significant difference in the presentation of these aggressive features in DCIS in younger patients [15]. Again, this study failed to conclusively tie currently used molecular markers to lesions that are more aggressive, i.e., DCIS in younger patients. Our study demonstrated a significant correlation between increased SIAH expression in normal breast tissue adjacent to DCIS lesions and younger patient age suggesting an overall difference in breast tissue biology in younger and older patient populations which may predispose younger patients to the development of more aggressive DCIS.
Another biomarker, Enhancer of Zeste 2 (EZH2), a Polycomb family of transcription repressors, has also been shown to have an increased expression in normal breast tissue adjacent to atypical ductal hyperplasia and DCIS [20, 21]. The group that discovered this relationship was able to show that this was not simply a “field effect” but rather was a biomarker for breast tissue at risk for the development of invasive carcinoma. This relationship was demonstrated by increased EZH2 expression in benign breast biopsies from patients that later developed carcinoma as well as in morphologically normal mammary tissue from prophylactic mastectomy specimens of patients with BRCA1 mutations. Interestingly, like SIAH, EZH2 expression is increased with increasing nuclear grade and comedo-type histology. The authors of this study suggest that EZH2 could be used as a novel biomarker to predict which patients are at increased risk for the development of invasive breast carcinoma [20, 21].
Recently, Chen et al. examined the molecular profile of invasive ductal carcinoma (IDC) and compared it to normal adjacent breast tissue using microarray analysis [22]. They found that a subset of normal adjacent breast tissue had a molecular signal similar to invasive ductal carcinoma despite the fact that there was no morphological evidence of carcinoma or other premalignant conditions such as DCIS or atypical ductal hyperplasia. The genes comprising the molecular signal for invasive ductal carcinoma were primarily those involved in cell adhesion and proliferation. Interestingly, genes involved in proliferation were over-represented in the molecular signal of the normal adjacent breast tissue with an invasive ductal carcinoma-like signature suggesting that genes involved in proliferation are important early in the development of breast carcinoma [22]. Another group has found that SIAH expression is associated with proliferating cells [9, 10]. Therefore, increased SIAH expression may be a biomarker for cells with a more “proliferative” gene expression profile. This is consistent with the function of SIAH as an essential signaling module downstream of the RAS signaling pathway that drives cell proliferation, tumor growth, and metastasis. Also, the active stromal or adjacent pre-malignant tissues may be primed to facilitate tumor progression and invasion or are at an increased risk for the development of primary or recurrent carcinoma. Indeed, in our study, we found that a 1% increase in SIAH expression in DCIS lesions results in a 10% increase in the odds of recurrence while a 1% increase in SIAH expression in normal adjacent tissue results in a 31% increase in the odds of recurrence.
In summary, we evaluated SIAH expression in a cohort of 65 DCIS patients who underwent surgical resection only for local disease and with extensive follow-up data. We found that SIAH expression alone is a good predictor of disease recurrence in DCIS patients with a sensitivity of 76% and a specificity of 79%. It is conceivable that the use of SIAH expression in combination with other biomarkers such as EZH2 may further increase overall specificity and sensitivity of these novel prognostic biomarkers thereby allowing us to more accurately identify those patients at the highest risk for development and/or recurrence of breast carcinoma. This will allow for use of more aggressive treatments in the DCIS population with poor prognosis and may prevent overtreatment in patients with highly favorable prognosis.
Acknowledgments
A.K.W. was supported by the Susan G. Komen Career Catalyst Grant.
Contributor Information
Kathryn C. Behling, Email: kathryn.behling@jeffersonhospital.org, Department of Pathology, Thomas Jefferson University and Kimmel Cancer Center, Philadelphia, PA 19107, USA.
Amy Tang, Departments of Surgery and Biochemistry and Molecular Biology, Mayo Clinic Cancer Center, Mayo Clinic College of Medicine, Rochester, MN, USA.
Boris Freydin, Department of Pharmacology and Experimental Therapeutics, Thomas Jefferson University and Kimmel Cancer Center, Philadelphia, PA 19107, USA.
Inna Chervoneva, Department of Pharmacology and Experimental Therapeutics, Thomas Jefferson University and Kimmel Cancer Center, Philadelphia, PA 19107, USA.
Sameep Kadakia, Department of Pathology, Thomas Jefferson University and Kimmel Cancer Center, Philadelphia, PA 19107, USA.
Gordon F. Schwartz, Department of Surgery, Thomas Jefferson University and Kimmel Cancer Center, Philadelphia, PA 19107, USA
Hallgeir Rui, Department of Cancer Biology, Thomas Jefferson University and Kimmel Cancer Center, Philadelphia, PA 19107, USA.
Agnieszka K. Witkiewicz, Department of Pathology, Thomas Jefferson University and Kimmel Cancer Center, Philadelphia, PA 19107, USA
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Virnig BA, Tuttle TM, Shamliya T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 3.Sakorafas GH, Farley DR, Peros G. Recent advances and current controversies in the management of DCIS of the breast. Cancer Treat Rev. 2008;34(6):483–497. doi: 10.1016/j.ctrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela M, Julian TB. Ductal carcinoma in situ: biology, diagnosis, and new therapies. Clin Breast Cancer. 2007;7:676–681. [PubMed] [Google Scholar]
- 5.Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 6.Carthew RW, Rubin GM. Seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- 7.Tang AH, Neufeld TP, Kwan E, Rubin GM. PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- 8.House CM, Möller A, Bowtell DD. Siah proteins: novel drug targets in the Ras and hypoxia pathways. Cancer Res. 2009;69(23):8835–8838. doi: 10.1158/0008-5472.CAN-09-1676. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt RL, Park CH, Ahmed AU, Gundelach JH, Reed NR, Cheng S, Knudsen BE, Tang AH. Inhibition of RAS-mediated transformation and tumorigenesis by targeting the downstream E3 ubiquitin ligase seven in absentia homologue. Cancer Res. 2007;67(24):11798–11810. doi: 10.1158/0008-5472.CAN-06-4471. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed AU, Schmidt RL, Park CH, Reed NR, Hesse SE, Thomas CF, Molina JR, Deschamps C, Yang P, Aubry MC, Tang AH. Effect of disrupting seven-in-absentia homolog 2 function on lung cancer cell growth. J Natl Cancer Inst. 2008;100(22):1606–1629. doi: 10.1093/jnci/djn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmieri D, Fitzgerald D, Shreeve SM, Hua E, Bronder JL, Weil RJ, Davis S, Stark AM, Merino MJ, Kurek R, Mehdom HM, Davis G, Steinberg SM, Meltzer PS, Aldape K, Steeq PS. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol Cancer Res. 2009;7(9):1438–1445. doi: 10.1158/1541-7786.MCR-09-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roh MS, Hong SH, Jeong JS, Kwon HC, Kim MC, Cho SH, Yoon JH, Hwang TH. Gene expression profiling of breast cancers with emphasis of beta-catenin regulation. J Korean Med Sci. 2004;19(2):275–282. doi: 10.3346/jkms.2004.19.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23(36):9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 14.Collins LC, Achacoso N, Nekhlyudov L, Fletcher SW, Haque R, Quesenberry CP, Jr, Puligandla B, Alshak NS, Goldstein LC, Gown AM, Schnitt SJ, Habel LA. Relationship between clinical and pathologic features of ductal carcinoma in situ and patient age: an analysis of 657 patients. Am J Surg Pathol. 2009;33(12):1802–1808. doi: 10.1097/pas.0b013e3181b7cb7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues NA, Dillon D, Carter D, Parisot N, Haffty BG. Differences in the pathologic and molecular features of intraductal breast carcinoma between younger and older women. Cancer. 2003;97(6):1393–1403. doi: 10.1002/cncr.11204. [DOI] [PubMed] [Google Scholar]
- 16.Cornfield DB, Palazzo JP, Schwartz GF, Goonewardene SA, Kovatich AJ, Chervoneva I, Hyslop T, Schwarting R. The prognostic significance of multiple morphologic features and biologic markers in ductal carcinoma in situ of the breast: a study of a large cohort of patients treated with surgery alone. Cancer. 2004;100(11):2317–2327. doi: 10.1002/cncr.20260. [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied logistic regression. 2. Wiley: New York; 2000. pp. 346–347. [Google Scholar]
- 18.Peduzzi PN, Concato J, Kemper E, Holford TR, Feinstein A. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;99:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 19.Tsikitis VL, Chung MA. Biology of ductal carcinoma in situ classification based on biologic potential. Am J Clin Oncol. 2006;29(3):305–310. doi: 10.1097/01.coc.0000198740.33617.2f. [DOI] [PubMed] [Google Scholar]
- 20.Ding L, Kleer CG. Enhancer of Zeste 2 as a marker of preneoplastic progression in the breast. Cancer Res. 2006;66(19):9352–9355. doi: 10.1158/0008-5472.CAN-06-2384. [DOI] [PubMed] [Google Scholar]
- 21.Ding L, Erdmann C, Chinnaiyan AM, Merajver SD, Kleer CG. Identification of EZH2 as a molecular marker for a pre-cancerous state in morphologically normal breast tissues. Cancer Res. 2006;66(8):4095–4099. doi: 10.1158/0008-5472.CAN-05-4300. [DOI] [PubMed] [Google Scholar]
- 22.Chen DT, Nasir A, Culhane A, Venkataramu C, Fulp W, Rubio R, Wang T, Agrawal D, McCarthy SM, Gruidl M, Bloom G, Anderson T, White J, Quackenbush J, Yeatman T. Proliferative genes dominate malignancy-risk gene signature in histologically-normal breast tissue. Breast Cancer Res Treat. 2010;119(2):335–346. doi: 10.1007/s10549-009-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


