Abstract
Cadmium (Cd) in soil is enriched through several leaky management agricultural practices and natural resources. Cd enriched soil is inevitable cause of nutritional stress besides Cd induced toxicity symptoms and physiological malfunctions. Redox signals shift toward oxidative stress which accelerates cellular damage and elicits defense mechanism at the cost of growth. Plants get enriched with this toxic, abundant and undesirable element through ‘mineral uptake system’ non-specifically. Different components and pathways have been marked cooperating in cellular sequestration and systemic localization of Cd, escaped from avoidance and efflux. Cd induced metabolic alteration led to electron leakage as ROS, reduced photosynthesis and carbon fixation. Compromised primary metabolism negatively feedbacks the plant growth, result into loss of potential crop yield.
Keywords: Cadmium, Nutrient-signaling, Responses, Sequestration, Transport, Uptake
1. Introduction to cadmium enrichment in agriculture
The non-judicial use of phosphate fertilizers, industrial and sewage waste water, mining and industrial and vehicular emissions from industries, mining and transport vehicles significantly adds the heavy metals (HMs) in agricultural soils at toxic level (Arora et al., 2008; Wuana and Okieimen, 2011), particularly the nearby urban and peri-urban farmlands. The leafy vegetables and food crops grown in such soils accumulate toxic level of these toxic metals to add them further in the trophic chain (soil–plant–animal) (Wuana and Okieimen, 2011). Among the heavy metals, Cd is the most abundant, and readily taken up toxic HM by the crop plants; therefore, of great concern (Arora et al., 2008). Its normal concentration in soil ranges from 0–1 mg/kg, while 1–3 mg/kg indicates slight contamination (Rodriguez-Flores and Rodriguez-Castellon, 1982). The Cd polluted soil may comprise 3–10 mg/kg of soil Cd (Rodriguez-Flores and Rodriguez-Castellon, 1982) to a lethal level of <100 mg/kg in sewage sludge treated soils as shown in the study by NEERI (1999–2002). Cd over-accumulate in plant (Wuana and Okieimen, 2011) with an enrichment ratio (ER) ranging from 1–10. FAO/WHO recommended maximum tolerable intake of Cd of 400–500 μg per week or equal 70 μg per day. Alternatively studies reveal that 60–80% of HM toxins found in human bodies in urban areas were the results of consuming contaminated foods rather than air pollution.
2. Cadmium causes nutrient deficiency signaling
Cd induced oxidative signals at root membranes get intensified at toxic Cd level to elicit PCD. Leakage of energy pool as reactive oxygen species (ROS) alters the growth metabolism toward defense metabolism constraining the primary metabolism, particularly the enzymes of Krebs cycle and photosynthesis (Fig. 1). Furthermore, the nutrients availability for soil-nutrient-plant system relies on several factors. Soil often preconditioned with chemical fertilizers, waste water, manures, liquid fertilizers, sewage sludge etc. adds a voluminous amount of toxic HMs including Cd (Wahid et al., 2009). Cd taken up by plants transferred to particular organs (Kabata-Pendias and Pendias, 2001) induces chlorosis, necrosis, vein reddening, and root and shoot growth retardation, besides reduced nutrient uptake (Sanita di Toppi and Gabbrielli, 1999; Mohamed et al., 2012). Cd competes for plant nutrient status and subsequently alters its physiology. This is particularly important during studying the physiologically effect of P and Ca (Wang, 1987) and other important micronutrients e.g. Zn (Kim et al., 1988). Yang et al. (1996) reported various effects of Cd on the uptake of Fe, Zn, Mn, Cu, P, K, Ca, Mg and S.
Figure 1.
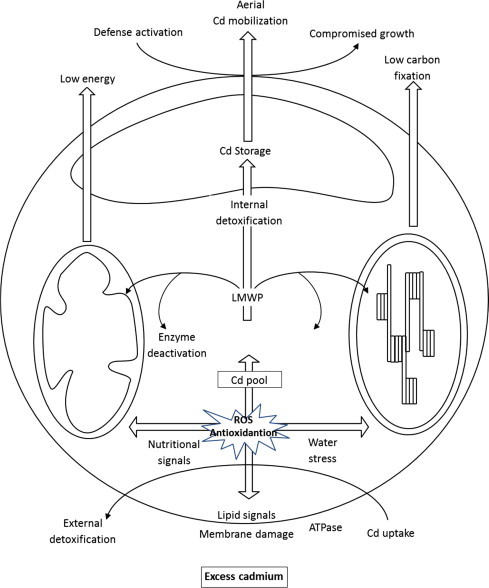
Cadmium uptake and processing in plant.
At the root region Cd competes for absorption of several mineral nutrients generally those sharing similar chemical properties like Ca2+ and Mg2+, therefore, causing mineral deficiency (Barcelo and Poschenrieder, 1990). The reduction of K, Ca and Mg in the tissue due to high concentrations of Cd has been reported in cucumber and tomato plants (Burzynski, 1988). An antagonism between Zn and Cd and their active absorption were also observed in lettuce roots (Costa and Morel, 1994). Moreover, other mineral nutrients such as nitrates, sulfates etc. which do not share chemical characteristics with Cd, has also been restricted by the presence of Cd. Since the plant development responses are quite plastic and opinioned to be controlled by availability of soil nutrient status. The nutritional signal seems to be sensed and coordinated with hormonal signals (Krouk et al., 2011). Soil nitrogen and phosphorus have been evidenced to control the internal hormonal level of plants to interplay with nutritional provisions and efficiency. Molecular switches tightly control the plant growth based upon nutritional cues. Substituting nitrate () to ammonium () supplement triggers a decrease in shoot growth within hours (Walch-Liu et al., 2000). Alternatively, N metabolism of plants plays a central role in HM responses. Plants sensitivity to Cd is affected by both the form supplied (Xie et al., 2009) and availability of N (Finkemeier et al., 2003). Sulfate salts have also been reported to afford protection to Cd toxicity by enhancing sulfate uptake, leading to an increased glutathione synthesis, a precursor of PCs (Van de Mortel et al., 2008).
3. Cadmium induces oxidative signaling
The free state of HMs is never tolerated in the biological system as they have shown to elicit futile reactions generating toxic free radicals (Lindh, 2007) or altering membrane potential and enzyme activities (Schützendübel and Polle, 2002; Fediuc et al., 2005; Janicka-Russak et al., 2008). Apoplastic low pool of thiol buffers and ROS-sensors instantaneously led to outburst of ROS initiating defense or death responses (-apoptosis) depending upon the extent of ROS leaked (Foyer and Noctor, 2005; Keunen et al., 2011). Upsurge of ROS is optimally regulated through sub-cellular pool of antioxidants to protect the tissue from oxidative damage (Milone et al., 2003; Gill and Tuteja, 2010) which is regulated via systemic network of plant hormones (Overmyer et al., 2003). Superoxide dismutase (SOD) is the first enzyme in the ROS detoxification process converting O2− radicals into H2O2 at a very rapid rate (Foyer and Noctor, 2005). Cd was found to result in oxidative stress (Hendy et al., 1992) by either inducing oxygen free radical production (Demirevska-Kepava et al., 2006) or by decreasing concentrations of enzymatic and non-enzymatic antioxidants (Milone et al., 2003). These non-enzymic antioxidant molecules include ascorbate, glutathione, tocopherol, etc., while enzymes include peroxidases (POX), catalases (CAT) and SOD (Milone et al., 2003; Mohamed et al., 2012).
4. Cadmium uptake, accumulation and transport
To cope up with toxic metal reactions and maintaining the level of essential metals within the nontoxic range, plants induce a variety of mechanisms on very first sensing at root hairs (Table 1). Uptake of essential divalent cations is a natural phenomenon to drive normal metabolic processes drawn by the plasma membrane H+-ATPase mediated acidification of rhizospheric soil. AHA2 gene putatively encodes this proton pump (Fox and Guerinot, 1998). In acidic soils, however, HM problem is much more exaggerated. Excess soil HMs including nonessential divalent cations (e.g. Cd) get enriched excessively through non-specificity of transporters and channels that led to uptake and transport the essential mineral HMs. No report is available indicating specific mechanism that is involved for the uptake or transport of Cd in plant kingdom or its involvement in plant metabolism as a moiety of metalloprotein. The only exceptions are the marine diatoms where Cd has shown its involvement in the activity of carbonic anhydrase and photosynthesis (Morant-Manceau et al., 2007). Cd is known to compete with several essential metal ions. It is reported that plants accumulate a variety of cations under iron-deficiency including Cd (Wuana and Okieimen, 2011). Cd can be easily transported within plants (Epstein and Bloom, 2005) in the form of metallo-organic complexes. The mechanisms of uptake, translocation and deposition depend upon the bio-availability of soil, pH, temperature, redox potential and concentration of other elements. Cd can easily penetrate the root system of xylem through the apoplastic and/or symplastic pathway (Salt and Rauser, 1995) and reach tissues of aerial parts of the plants (Yang et al., 1998). Soil HM status or Cd enrichment is sensed at root epidermal cells. When Cd enters first into the roots it primarily damages the root system (Sanita di Toppi and Gabbrielli, 1999). This damage could result from oxidation of membrane proteins/thiols, inhibition of pumps and channels or altered membrane fluidity (Meharg, 1993). Damaged plasma membrane initiates lipid induced signals to control GABA based cytosolic acidification, or intracellular depleted thiol pool induces ROS based signals. Cd stress induced depletion in GSH/GSSG ratio has been observed in different plant species (Romero-Puertas et al., 2007). The distribution and accumulation of Cd within plant tissue are gated at various key points. The regulatory proteins, which open the door for Cd or work as vehicle to Cd transport have been studied governing plant Cd localization. These key points are as follows (Fig. 2).
Table 1.
Cadmium in soil environment initiates physiological constraints and subsequent consequences.
| Cd in environment | Consequences |
|---|---|
| Phase I | |
| Enrichment in plant | Accumulation |
| Nutritional competition | Nutritional deficiency symptoms |
| Disturbed osmolality | Altered water potential |
| Binding to proteins (membrane proteins/enzymes) | Altered physiology (stressed state) |
| Phase II | |
| Defense activation | Response |
| Accumulation of osmolytes | Induction of homeostasis restoration |
| Expression of chelates | Detoxification of free Cd |
| Anti-oxidation | ROS management compartmentation |
| Redirection of Cd | |
| Phase III | |
| Phytohormones | Enhanced response |
| Temporal/spatial regulation of defense molecules | Systemic defense activation |
| Up-expression/activation of antioxidant system | Decline of oxidative/stressed state |
| Regulation of hierarchical expression of enzymes | Elicitation of secondary metabolism |
| Co-ordination of primary and sec. metabolism promotion | Maintaining house-state and growth |
Figure 2.
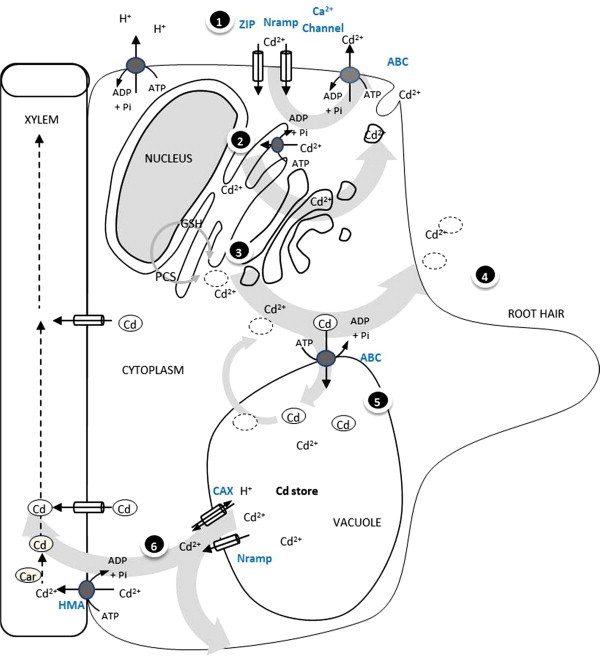
Mechanisms to counter excess cadmium in root epidermal cells.
4.1. Root plasma membrane facing apoplastic space
Absorption of Cd could also occur as inorganic complexes of Cd such as CdCl+, CdCl2, CdSO4, etc. or as organic complexes such as phytometallophore complexes (McLaughlin et al., 1996). Cd, the potent antagonist of Zn and Cu, also competes with Fe and Ca transporters and channels to get pass to the cytoplasm. Cd absorption across the plasma membrane of root cells is controlled by the electrochemical potential difference between the activity of Cd2+ in the cytosol and that in the root apoplasts. The large negative membrane potential alone provides more than enough energy to drive Cd2+ uptake even at low concentrations of Cd2+ (Costa and Morel, 1993).
Cd has been shown to be transported through several Fe and Zn transporters collectively known as ZIP (ZRT, IRT-like Proteins). The cation transporter; IRT1 (iron-regulated transporter) is one of the member of this group up-expressed in the iron deficient roots of Arabidopsis. Several divalent metal ions (e.g. Cd, Co, Mn, and Zn) could be transported via IRT1 (Hall and Williams, 2003). Besides, ZIPs; Nramps, LCT1 and Ca2+ channels facilitate the uptake of Cd2+ within the cytoplasm (Connolly et al., 2002). The plasma membrane-localized enzyme; FRO2 is known for the Fe(III)-chelate reductase activity induced in the roots of Arabidopsis under iron deficient conditions (Robinson et al., 1999). Overexpression of ferritin also induces Cd uptake (Sappin-Didier et al., 2005). Sufficient amount of excess Cd load is actively effluxed through ABC transporters located at the plasma membrane of root epidermis. ABC transporters are suggested to confer HM resistance and even iron homeostasis (Chen et al., 2007). Kim et al. (2007) have implicated the role of PDR8 in Cd2+ and Pb2+ resistance. PDR8 over-expresser plants have comparatively reduced Cd levels in their roots and shoots and were resistant to Cd2+. Also ATPase ZntA overexpression conferred reduced Cd2+ accumulation and enhanced tolerance in Arabidopsis (Lee et al., 2003).
4.2. Vacuolar sequestration
Plant vacuoles serve various purposes including the accumulation of toxic secondarily produced metabolites or those eventually taken up through soil mineral solutions. Cd appeared to be complexed to the cell wall surrounding root apoplast, effluxed or checked at very first entrance while majority of Cd entered is channelized to the root vacuoles or of older leaves. AtMTP1 and ShMTP encode Zn and Mn transporters, putatively involved in sequestering metals to the root vacuole to confer higher tolerance (Van der Zaal et al., 1999). Cytosolic low molecular weight proteins (LMWPs) transport Cd-complexes to vacuoles. LMWPs are recycled subsequently. Cation/H+ antiporters located at tonoplast constitute the system for Cd2+ sequestered from the cytoplasm to the vacuole (Vogelilange and Wagner, 1990). CAX (CAtion eXchangers) transporters are vacuolar Ca2+/H+ antiporters. Salt and Wagner (1993) and Korenkov and co-workers (2007) demonstrated vacuolar Cd2+/H+ antiport activity in plants while Shigaki and Hirschi (2000) have shown the catalysis of Cd2+ and with protons at vacuolar membrane via CAX transporters. Comparative and overexpression studies with CAX4 and CAX2 genes in Nicotiana tobaccum have shown a high transport activity of Cd2+ through root tonoplast vesicles therefore, affecting root to shoot distribution and level of uptake of Cd responsible for this distribution (Korenkov et al., 2007). NRAMP (Natural Resistance Associated Macrophage Protein) transporters are distributed ubiquitously in plants. Fe transport NRAMP genes also confer Cd2+ uptake activity when expressed in Saccharomyces cerevisiae. NRAMP4 as well as NRAMP3 are localized in the vacuolar membrane (Thomine et al., 2003; Lanquar et al., 2005).
4.3. Cellular detoxification
Plants’ provision for detoxification mechanisms employs chelating compounds such as glutathiones (GSH), PCs, MTs and other cystein-rich membrane proteins (Clemens et al., 2001; Cobbett and Goldsbrough, 2002). These either detoxify the cellular pool of free toxic metals to sequester in vacuole or transport to sink. Another group of Cys-rich chelater is MTs, the low molecular peptides induced against HMs (Cobbett and Goldsbrough, 2002). MTs also reduce cytosolic toxicity load of free HMs. MT overexpressing plants for instance; tobacco, Arabidopsis thaliana, Vicia faba also favored metal detoxification via MTs (Lee et al., 2004). MTs increased tolerance to Cd and other HMs in different transgenic plants overexpressing PCs supported the role of PCs in stress resistance (Mohamed et al., 2012). Metal complexation to the PCs in the cytosol also lowers its toxicity (Cobbett, 2000). Cd is chelated with glutathione and PCs (Mohamed et al., 2012; Cobbett, 2000) which complex with Cd to sequesters it in vacuole or expel in apoplasts through facilitated membrane pumps. The PCs are expressed constitutively and their expression is enhanced under HM stress (Cobbett and Goldsbrough, 2002) for instance under excess Cd (Wang et al., 2009). The high level of PCs determines the root to shoot transport of Cd, therefore, maintain low pool of Cd in the shoot as compared to the root (Mohamed et al., 2012).
4.4. Extracellular soil detoxification
Exudation of organic chelates and LWMPs also detoxifies HMs extra-cellularly. Different amino acids and carboxylic acid viz. citric, malic and histidine are exuded from the roots to bind and detoxify HMs in soil, therefore, playing an important role in tolerance (Rauser, 1999; Clemens et al., 2001). Kramer et al. (1996) reported a 36-fold increase of HMs content in xylem sap when Alyssam lesbiacum, a Ni hyper-accumulator species was exposed to Ni. Phytosiderophores (PSPs) in monocots are commonly exuded for Fe acquisition. YSL, the member of OPT family transporting oligopeptide is another important plasma membrane localized protein transporting Fe(III)-PSPs (Curie et al., 2001).
4.5. Golgi–Endoplasmic reticulum secretory pathway
The evidence of Golgi–Endoplasmic reticulum secretory network to exocytose waste vesicular cargo of excess metals to confer tolerance is studied (Peiter et al., 2007) but not fully investigated yet as a plant mechanism to get rid of excess metal. Cytosolic excess HMs are taken up into the labyrinth of endoplasmic reticulum and Golgi vesicles through MTPs (metal transporter proteins). In yeast ATX1 delivers Cu into the Golgi vesicle. Ubiquitous metallo-chaperone ATX1 has its homologs in plants, microbes and animals transferring Cu through P-type ATPase across membrane (Huffman and O’Halloran, 2000). This ATPase (Ccc2) in yeast is involved in transferring Cu into lumen of Golgi vesicles (Culotta et al., 1997). The Arabidopsis Homolog of Ccc2, RAN1 pumps Cu into Golgi vesicles (Hirayama et al., 1999). Possibly vesicular transport of excess HMs is effluxed through the plasma membrane.
4.6. Xylem loading to sinks
The regulation of long distance Cd transport to the aerial part is also an important determinant of resistant approach. Several transporter families aid to this necessity to cope with Cd toxicity. NRAMP (natural resistance-associated macrophage protein) family is another important family of metal transporters to mobilize Cd. NRAMP and CAX export vacuolar Cd to cytosol from where Heavy Metal transporting ATPases (HMAs) and unknown other transporters load Cd to xylem. Eight HMA genes identified in rice and A. thalinana (Baxter et al., 2003) suggested the role of this class in HMs xylem loading in plants. The class of HMAs transporting divalent cations also transports Cd along with Zn, Co and Pb hydrolyzing ATP (Verret et al., 2005). HMA4 overexpressed in both the root and shoot of Cd/Zn hyperaccumulators (Courbot et al., 2007) contributes the tolerance to genotypes. The OPT members e.g. OPT6, are also able to transport Cd–Glutathione (Cd–GSH) complexes and GSH derivatives (Cagnac et al., 2004). Ligands and carriers (Car) Cd-complexes are carried to sink tissues.
5. Cadmium toxicity induced metabolic alterations and manifestations
5.1. Hormonal signaling and cross talk
Cd accumulation triggers the production of ROS altering activity of antioxidant enzymes (Romero-Puertas et al., 2007). Plant defense system counters HM stress through altering hormonal regulation (Hsu and Kao, 2008; Krouk et al., 2011). This regulation includes the detoxification and sequestration mechanisms, regulation of redox status and replenishing basal nutrient supply (Mohamed et al., 2012). The change in hormone profile is often evident from the key enzymes of biosynthetic pathway (Fediuc et al., 2005) or mutant study. The signals within the cell join through phosphorylation of an array of kinases and phosphatases (e.g. MAPK). This stress, in part, could also be contributed through Cd competed reduced availability of P for phosphorylation-relay-signals (Jonak et al., 2004). Heavy metal (HM) stress signals besides phosphorylation cascades transduced through Ca–calamudulin system, or ROS signaling to converge at transcriptional regulation (Romero-Puertas et al., 2007; Tamas et al., 2008). Several transcriptional factors have been identified induced in response to Cd applications (Van de Mortel et al., 2008) which share the culminating pathways of other related abiotic stresses (Singh et al., 2002).
Crucial role of certain plant hormones has been suggested in plants in response to different environmental stresses including HM stress (Hsu and Kao, 2008). The treatment with Cd, Cu, Fe, and Zn led to the increase of ethylene. Increased activity of ACC synthase and up-regulation of its transcription in particular treating with Cd and Cu were also reported (Maksymiec et al., 2007). The jasmonic acid content in Arabidopsis, Oryza and bean get increased when treated with Cd or Cu. Cd has been shown to increase abscisic acid (ABA) contents in plants (Poschenrieder et al., 1989; Fediuc et al., 2005). Cd-induced ABA accumulation was observed in rice leaves (Hsu and Kao, 2008) and roots of Typha and Phragmites plants (Fediuc et al., 2005). Cd has also been shown to stimulate SA accumulation in the roots. Cd-induced increases in ABA and salicylic acid (SA) contents were observed in citrus leaves but not in the roots (López-Climent et al., 2011). Cd treatment following the application of SA partially protected the barley seedlings against HM toxicity (Maksymiec et al., 2007). Salicylic acid mediates the H2O2 accumulation in rice leaves and protects against Cd toxicity (Chao et al., 2009). In cell cultures exposed to Cd an increase in NO production was observed in pea roots (Bartha et al., 2005) and soybean (Kopyra et al., 2006). However, long exposure led to reduction in NO production (Rodríguez-Serrano et al., 2009) and lateral root induction (Lombardo et al., 2006). Exogenous application of NO has shown to augment HM stress (Wang and Yang, 2005; Laspina et al., 2005) and is assumed through activation of antioxidant system (Wang and Yang, 2005; Rodríguez-Serrano et al., 2009; Singh et al., 2008).
5.2. Biochemical changes
It has been shown that the excess HMs cause cell death in plants by inactivating enzymes, through metal sensitive groups, and rendering them to be catalytically inactive (Fediuc et al., 2005). The presence of Cd decreased nodulation and nitrogenase activity in Trifolium repens (McGrath et al., 1988), Phaseolus vulgaris (Dewdy and Ham, 1997), Pisum sativum (Dhingra and Priefer, 2006), mungbean (Wahid et al., 2007), and chickpea (Hasan et al., 2008). Nitrogen assimilation in pea plants was severely affected on exposure to Cd (Dhingra and Priefer, 2006). A positive correlation was observed between leghemoglobin content and nitrogenase activity (Dakora, 1995) and both these parameters exhibited a parallel decrease in the presence of Cd (Fernandez-Pascual et al., 1996).
Nitrate reductase (NR), the primary enzyme in the nitrate assimilation pathway, is the limiting factor in plant growth and development (Solomonson and Barber, 1990) and its level is influenced by a variety of environmental factors (Murphy et al., 1997). The presence of Cd in the soil retarded the assimilation of NO3 in Silene vulgaris (Mathys, 1975), pea (Burzynski, 1988), tomato (Quariti et al., 1997), bean (Gouia et al., 2003) and in Cicer arietinum (Hasan et al., 2008).
The decreased activity of carbonic anhydrase in plants (Siedlecka et al., 1997) could be coupled with Cd induced stomatal closure (Poschenrieder et al., 1989). The plants exposed to HMs seem to induce accumulation of free proline. Among the four tested HMs that induce proline accumulation Cd was the strongest inducer (Saradhi and Saradhi, 1981). An increase in constitutive proline levels has been observed in a copper-tolerant ecotype of Armeria meritima exposed to Cd (Farago, 1981). Cd induced proline in rice (Roy et al., 1992), Armeria moritima (Farago, 1981), and sunflower (Kastori et al., 1992) and Hordeum vulgare (Tamas et al., 2008) and in Cicer arietinum (Hasan et al., 2008). In addition, proline could be involved in metal chelation in the cytoplasm (Farago and Mullen, 1979).
5.3. Photosynthesis and carbon fixation efficiency
Cd is an effective inhibitor of photosynthesis (Chugh and Sawhney, 1999; Vassilev et al., 2005; Mohamed et al., 2012). A linear relationship between photosynthesis and inhibition of transpiration was observed in clover, lucerne, and soybean that suggest Cd inhibited stomatal opening (Barcelo and Poschenrieder, 1990). Cd damages the photosynthetic apparatus, in particular the light harvesting complex II and photosystems I and II (Krupa et al., 1993; Siedlecka et al., 1997). The inhibition of root Fe(III) reductase induced by Cd leads to Fe(II) deficiency which seriously affects photosynthesis (Alkantara et al., 1994). Cd also causes alteration in leaf gas exchange (Costa and Morel, 1994; López-Climent et al., 2011) stomatal closure in higher plants (Poschenrieder et al., 1989) and an overall inhibition of photosynthesis (Ekmekci et al., 2008; Mohamed et al., 2012). In A. thaliana Cd altered the activity of photosynthetic apparatus (Mohamed et al., 2012) while decreased the potential quantum yield of PSII (Maksymiec et al., 2007). Similarly, the synthesis and level of pigments are decreased in other plant species under the influence of Cd (Ekmekci et al., 2008; Mohamed et al., 2012).
5.4. Vegetative growth and potential yield
The presence of Cd in the soil retards the growth of many crop plants; to name some are soybean (Dewdy and Ham, 1997), Corchorus olitorius (Mazen, 2004), Medicago sativa (Drazic et al., 2006), maize (Krantev et al., 2008) and chickpea (Hasan et al., 2008). High concentrations of Cd decreased the cell growth as well as the plant yield (Prasad, 1995; Yang et al., 1998). The interaction of Rhizobium in the nodules of chickpea was found to be very sensitive to HMs resulting in a decrease in dry mass of chickpea and green gram (Woolhouse, 1983; Rana and Ahmad, 2002). An increase in Cd concentration decreased the fresh mass in mung bean (Wahid and Ghani, 2007), Medicago sativa (Drazic et al., 2006), and maize (Ekmekci et al., 2008). Moreover, a marked decrease in root and shoot mass was observed when treated with low concentrations of Cd in Brassica juncea (Mohamed et al., 2012), Vigna ambacensis (Al-Yemeni, 2001) and wheat (Milone et al., 2003). Phytotoxicity of the metal in other crop plants has been observed in the form of a loss in protein levels (Krantev et al., 2008). Moreover, the grains developed on the plants grown under Cd stress had lower protein content (Salgare and Acharekar, 1992). Hasan et al. (2008) reported lower seed protein content in chickpea plants grown under Cd stress.
6. Conclusion
Cd, the most abundant and a highly toxic nonessential HM is well known for its negative influence on the enzymatic systems of cells, oxidative stress and inducing nutritional deficiency in plants. Nutritional deficiency and breakdown of photosynthetic efficiency have been perceived as major checkpoints of growth. Nonspecific enrichment of least or non-essential HMs (e.g. Cd) prove futile for the plant system, channelized into plant body through various mechanisms of their entry. Their pooling and mobilization into resistant and sensitive genotypes elucidate the key events of physiological and biochemical perturbations. Cd interruptions at electron transport chain of major membranous organelles and cytosolic depletion of LMWP pool induce the defense metabolism at the cost of primary metabolism. Plant hormones’ cross talk orchestrates the plant basal defense detoxifying, sequestering or expelling these toxic molecules to manifest optimal vegetative growth.
Acknowledgements
The first author is thankful to the University Grant Commission, New Delhi, India for non-NET-JRF fellowship and the Chairman of the Department of Botany, Aligarh Muslim University, India for providing necessary facilities.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alkantara E., Romera F.J., Cannete M., De La Guardia M.D. Effects of heavy metals on both induction and function of root Fe(III) reductase in Fe-deficient cucumber (Cucumis sativus L.) plants. J. Exp. Bot. 1994;45:1893–1898. [Google Scholar]
- Al-Yemeni M.N. Effect of cadmium, mercury and lead on seed germination and early seedling growth of Vigna ambacensis. Indian J. Plant Physiol. 2001;6:147–151. [Google Scholar]
- Arora M., Kiran B., Rani S., Rani A., Kaur B., Mittal N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008;111:811–815. [Google Scholar]
- Barcelo J., Poschenrieder C. Plant water relations as affected by heavy metal stress: a review. J. Plant Nut. 1990;13:1–37. [Google Scholar]
- Bartha B., Kolbert Z., Erdei L. Nitric oxide production induced by heavy metals in Brassica juncea L. Czern. and Pisum sativum L. Acta Biol. Szeged. 2005;49:9–12. [Google Scholar]
- Baxter I., Tchieu J., Sussman M.R., Boutry M., Palmgren M.G., Gribskov M., Harper J.F., Axelsen K.B. Genomic comparison of P-Type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 2003;132:618–628. doi: 10.1104/pp.103.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynski M. The uptake and accumulation of phosphorus and nitrates and the activity of nitrate reductase in cucumber seedlings treated with Pb and Cd. Acta Soc. Bot. 1988;57:349–359. [Google Scholar]
- Cagnac O., Bourbouloux A., Chakrabarty D., Zhang M.Y., Delrot S. AtOPT6 transports glutathione derivatives and is induced by primisulfuron. Plant Physiol. 2004;135:1378–1387. doi: 10.1104/pp.104.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y.Y., Chen C.-Y., Huang W.-D., Kao C.H. Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant and Soil. 2009;329:327–337. [Google Scholar]
- Chen J., Zhu C., Li L.P., Sun Z.Y., Pan X.B. Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. J. Environ. Sci. 2007;19:44–49. doi: 10.1016/s1001-0742(07)60007-2. [DOI] [PubMed] [Google Scholar]
- Chugh L.K., Sawhney S.K. Photosynthetic activities of Pisum sativum seedlings grown in presence of cadmium. Plant Physiol. Biochem. 1999;37:297–303. [Google Scholar]
- Clemens S., Schroeder J.I., Degenkolb T. Caenorhabdites elegans expresses a functional phytochelatin synthase. Eur. J. Biochem. 2001;268:3640–3643. doi: 10.1046/j.1432-1327.2001.02293.x. [DOI] [PubMed] [Google Scholar]
- Cobbett C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett C.S., Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- Connolly E.L., Fett J.P., Guerinot M.L. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 2002;14:1347–1357. doi: 10.1105/tpc.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G., Morel I.L. Cadmium uptake by Lypins albus (L.) cadmium excretion, a possible mechanism of cadmium tolerance. J. Plant Nutr. 1993;16:1921–1929. [Google Scholar]
- Costa G., Morel J.L. Water relations gas exchange and amino acid content in Cd treated lettuca. Plant Physiol. Biochem. 1994;32:561–570. [Google Scholar]
- Courbot M., Willems G., Motte P., Arvidsson S., Roosens N., Saumitou-Laprade P., Verbruggen N. A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri co-localizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiol. 2007;144:1052–1065. doi: 10.1104/pp.106.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta V.C., Klomp L.W.J., Strain J., Casareno R.L.B., Krems B., Gitlin J.D. The copper chaperone for superoxide dismutase. J. Biol. Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- Curie C., Panaviene Z., Loulergue C., Dellaporta S.L., Briat J.F., Walker E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Dakora F.D. A functional relationship between legheamoglobin and nitrogenase based on novel measurement of the two proteins in legume root nodules. Ann. Bot. 1995;75:4–54. doi: 10.1016/S0305-7364(05)80008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirevska-Kepava K., Simova-Stoilova L., Stoyanova Z., Feller U. Cadmium stress in barley: growth, leaf pigment and protein composition and detoxification of reactive oxygen species. J. Plant Nutr. 2006;29:451–468. [Google Scholar]
- Dewdy R.H., Ham G.E. Soybean growth and elemental content as influenced by soil amendments of sewage sludge and heavy metals: seedling studies. Agron. 1997;69:300–303. [Google Scholar]
- Dhingra H.R., Priefer U.R. Impact of cadmium on structural and functional aspect of pea (Pisum sativum L.) root nodules. J. Plant Biol. 2006;33:207. [Google Scholar]
- Drazic G., Mihailovic N., Lojic M. Cadmium accumulation in Medicago sativa seedlings treated with salicylic acid. Biol. Plant. 2006;50:239–244. [Google Scholar]
- Ekmekci Y., Tanyolac D., Ayhan B. Effect of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 2008;165:600–611. doi: 10.1016/j.jplph.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Epstein E., Bloom J.A. second ed. Sinauer; Sunderland, MA: 2005. Mineral Nutrition of Plants: Principles and Perspective. [Google Scholar]
- Farago M.E. Metal tolerant plant. Coord. Chem. Rev. 1981;36:155–182. [Google Scholar]
- Farago M.E., Mullen W.A. Plants which accumulate metals. IV. A possible copper–proline complex from the roots of Armeria maritime. Inorg. Chem. Acta. 1979;32:L93–L94. [Google Scholar]
- Fediuc E., Lips S.H., Erdei L. O-Acetylserine (thiol) lyase activity in Phragmities and Typha plants under cadmium and NaCl stress conditions and the involvement of ABA in the stress response. J. Plant Physiol. 2005;162:865–872. doi: 10.1016/j.jplph.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pascual M., de Lorenozo C., de Felipe M.R., Rajalakshmi S., Gordon A.J., Thomas B.J., Minchin F.R. Possible reasons for relative salt stress tolerance in nodules of white lupine cv. Multolypa. J. Expt. Bot. 1996;47:1709–1716. [Google Scholar]
- Finkemeier I., Kluge C., Metwally A., Georgi M., Grotjohann N., Dietz K.J. Alterations in Cd-induced gene expression under nitrogen deficiency in Hordeum vulgare. Plant Cell Environ. 2003;26:821–833. doi: 10.1046/j.1365-3040.2003.01014.x. [DOI] [PubMed] [Google Scholar]
- Fox T.C., Guerinot M.L. Molecular biology of cation transport in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:669–696. doi: 10.1146/annurev.arplant.49.1.669. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Noctor G. Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005;29:1056–1071. [Google Scholar]
- Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gouia H., Suzuki A., Brulfert J., Gharbal M.H. Effect of cadmium on the co-ordination of nitrogen and carbon metabolism in bean seedlings. Plant Physiol. 2003;160:367–376. doi: 10.1078/0176-1617-00785. [DOI] [PubMed] [Google Scholar]
- Hall J.L., Williams L.E. Transition metal transporters in plants. J. Exp. Bot. 2003;54:2601–2613. doi: 10.1093/jxb/erg303. [DOI] [PubMed] [Google Scholar]
- Hasan S.A., Hayat S., Ali B., Ahmad A. 28-homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidant. Environ. Poll. 2008;151:60–66. doi: 10.1016/j.envpol.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hendy G.A.F., Baker A.J.M., Evart C.F. Cadmium tolerance and toxicity, oxygen radical processes and molecular damage in cadmium tolerant and cadmium-sensitive clones of Holcus lanatus. Acta Bot. Neerl. 1992;41:271–281. [Google Scholar]
- Hirayama T., Kieber J.J., Hirayama N., Kogan M., Guzman P., Nourizadeh S., Alonso J.M., Dailey W.P., Dancis A., Ecker J.R. Responsive-to-antagonist1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell. 1999;97:383–393. doi: 10.1016/s0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Hsu Y.T., Kao C.H. Distinct roles of abscisic acid in rice seedlings during cadmium stress at high temperature. Bot. Stud. 2008;49:335–342. [Google Scholar]
- Huffman D.L., O’Halloran T.V. Energetics of copper trafficking between the Atx1 metallo-chaperone and the intracellular copper transporter, Ccc2. J. Biol. Chem. 2000;275:18611–18614. doi: 10.1074/jbc.C000172200. [DOI] [PubMed] [Google Scholar]
- Janicka-Russak M., Kabala K., Burzynski M., Klobus G. Response of plasma membrane H+-ATPase to heavy metal stress in Cucumis sativus roots. J. Exp. Bot. 2008;59:3721–3728. doi: 10.1093/jxb/ern219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C., Nakagami H., Hirt H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004;136:3276–3283. doi: 10.1104/pp.104.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabata-Pendias A., Pendias H. third ed. CRC Press; Boca Raton, FL: 2001. Trace Elements in Soils and Plants. [Google Scholar]
- Kastori R., Petrovic M., Petrovic N. Effect of excess lead, cadmium, copper and zinc on water relations in sunflower. J. Plant Nutr. 1992;15:2427–2439. [Google Scholar]
- Keunen E., Remans T., Bohler S., Vangronsveld J., Cuypers A. Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci. 2011;12:6894–6918. doi: 10.3390/ijms12106894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.Y., Bovet L., Maeshima M., Martinoia E., Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007;50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Chang A.C., Page A.L., Warpake J.E. Relative concentrations of cadmium and zinc in tissue of selected food plants grown on sludge-treated soils. J. Environ. Qual. 1988;17:568–573. [Google Scholar]
- Kopyra M., Stachín-Wilk M., Gwozdz E.A. Effect of exogenous nitric oxide on the antioxidant capacity of cadmium-treated soybean cell suspension. Acta Physiol. Plant. 2006;28:525–536. [Google Scholar]
- Korenkov V., Hirschi K., Crutchfield J.D., Wagner G.J. Enhancing tonoplast Cd/H antiport activity increases Cd, Zn, and Mn tolerance, and impacts root/shoot Cd partitioning in Nicotiana tabacum L. Planta. 2007;226:1379–1387. doi: 10.1007/s00425-007-0577-0. [DOI] [PubMed] [Google Scholar]
- Kramer U., Cotter-Howells J.D., Charnock J.M., Baker A.J.M., Smith J.A.C. Free histidine as a metal chelator in plants that accumulate nickel. Nature. 1996;379:635–638. [Google Scholar]
- Krantev A., Yordanova R., Janda T., Szalas G., Papova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008;165:920–931. doi: 10.1016/j.jplph.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Krouk G., Ruffel S., Gutiérrez R.A., Gojon A., Crawford N.M., Coruzzil G.M., Lacombe B. A framework integrating plant growth with hormones and nutrients. Trend. Plant Sci. 2011;16:178–182. doi: 10.1016/j.tplants.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Krupa Z., Quist G., Hurner N.P.A. The effect of cadmium on photosynthesis of Phaseolus vulgaris – a fluorescence analysis. Physiol. Plant. 1993;88:626–630. doi: 10.1111/j.1399-3054.1993.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Lanquar V., Lelièvre F., Bolte S., Hames C., Alcon C., Neumann D., Vansuyt G., Curie C., Schroder A., Kramer U., Barbier-Brygoo H., Thomine S. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005;24:4041–4051. doi: 10.1038/sj.emboj.7600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laspina N.V., Groppa M.D., Tomaro M.L., Benavides M.P. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005;169:323–330. [Google Scholar]
- Lee J., Bae H., Jeong J., Lee J.Y., Yang Y.Y., Hwang I., Martinoia E., Lee Y. Functional expression of a bacterial heavy metal transporter in Arabidopsis enhances resistance to and decreases uptake of heavy metals. Plant Physiol. 2003;133:589–596. doi: 10.1104/pp.103.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Shim D., Song W.Y., Hwang I., Lee Y. Arabidopsis metallothioneins 2a and 3 enhance resistance to cadmium when expressed in Vicia faba guard cells. Plant Mol. Biol. 2004;54:805–815. doi: 10.1007/s11103-004-0190-6. [DOI] [PubMed] [Google Scholar]
- Lindh U. Metal biology: aspects of beneficial effects. AMBIO. 2007;36:107–110. doi: 10.1579/0044-7447(2007)36[107:mbaobe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lombardo M.C., Graziano M., Polacco J.C., Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal. Behav. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Climent M.F., Arbona V., Pérez-Clemente R.M., Gómez-Cadenas A. Effects of cadmium on gas exchange and phytohormone contents in citrus. Biol. Plant. 2011;55:187–190. [Google Scholar]
- Maksymiec W., Malgorzata W., Krupa Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere. 2007;66:421–427. doi: 10.1016/j.chemosphere.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Mathys W. Enzymes of heavy metals resistant and non-resistant populations of Silene cucubatus and their interactions with some heavy metal in vitro and in vivo. Physiol. Plant. 1975;33:161–165. [Google Scholar]
- Mazen A.M.A. Accumulation of four metals in tissues of Corchorus olitorius and possible mechanism of their tolerance. Biol. Plant. 2004;48:267–272. [Google Scholar]
- McGrath S.P., Brooks P.C., Giller K.E. Effects of potentially toxic metals in soils derived from part applications of sewage sludge on nitrogen fixation by Trifolium repens. Soil Biol. Biochem. 1988;2:415–425. [Google Scholar]
- McLaughlin M.J., Tiller K.G., Naidu R., Stevens D.P. Review: the behaviour and impact of contaminants in fertilizers. Aust. J. Soil Res. 1996;34:1–54. [Google Scholar]
- Meharg A.A. Integrated tolerance mechanisms constitutive and adaptive plant response to elevated metal concentrations in the environment. Plant Cell Environ. 1993;17:989–993. [Google Scholar]
- Milone M.T., Sgherri C., Clijsters H., Navari-Izzo F. Antioxidative responses of wheat treated with realistic concentration of cadmium. Environ. Exp. Bot. 2003;50:265–276. [Google Scholar]
- Mohamed A.A., Castagna A., Ranieri A., Sanita di Toppi L. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012;57:15–22. doi: 10.1016/j.plaphy.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Morant-Manceau A., Nguyen T.L.N., Pradier E., Tremblin G. Carbonic anhydrase activity and photosynthesis in marine diatoms. Eur. J. Phycol. 2007;42:263–270. [Google Scholar]
- Murphy A., Zhao J., Goldbrough P.B., Taiz L. Purification and immunological identification of metallothioneins 1 and 2 from Arabidopsis thaliana. Plant Physiol. 1997;113:1293–1301. doi: 10.1104/pp.113.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K., Brosché M., Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- Peiter E., Montanini B., Gobert A., Pedas P., Husted S., Maathuis F.J.M., Blaudez D., Chalot M., Sanders D. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. PNAS. 2007;104:8532–8537. doi: 10.1073/pnas.0609507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschenrieder C., Gunsé B., Barceló J. Influence of cadmium on water relations, stomatal resistance and abscisic acid content in expanding bean leaves. Plant Physiol. 1989;90:1365–1371. doi: 10.1104/pp.90.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M.N.V. Cadmium toxicity and tolerance in vascular plants. Environ. Exp. Bot. 1995;35:525–545. [Google Scholar]
- Quariti O., Govia H., Ghorbal M.H. Response of bean and tomato plants to cadmium, growth mineral nutrition and nitrate reduction. Plant Physiol. Biochem. 1997;35:347–354. [Google Scholar]
- Rana A., Ahmad M. Heavy metal toxicity in legume microsymbiont system. J. Plant Nutr. 2002;25:369–386. [Google Scholar]
- Rauser W.E. Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin and metallothioneins. Cell Biochem. Biophys. 1999;31:19–48. doi: 10.1007/BF02738153. [DOI] [PubMed] [Google Scholar]
- Robinson N.J., Procter C.M., Connolly E.L., Guerinot M.L. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Flores M., Rodriguez-Castellon E. Lead and cadmium levels in soil and plants near highways and their correlation with traffic density. Environ. Pollut. Ser. B. 1982;4:281–290. [Google Scholar]
- Rodríguez-Serrano M., Romero-Puertas M.C., Pazmiño D.M., Testillano P.S., Risueño M.C., del Río L.A., Sandalio L.M. Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009;150:229–243. doi: 10.1104/pp.108.131524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas M.C., Corpas F.J., Rodriguez-Serrano M., Gomez M., del Rio L.A., Sandalio L.M. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J. Plant Physiol. 2007;164:1346–1357. doi: 10.1016/j.jplph.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Roy D., Bhumnia A., Basu N., Banerjee S.K. Effect of NaCl salinity on metabolism of proline in salt sensitive and salt-resistant cultivars of rice. Biol. Plant. 1992;34:159–162. [Google Scholar]
- Salgare S.A., Acharekar C. Effect of industrial pollutant on growth and content of certain weeds. J. Nat. Conserv. 1992;4:1–6. [Google Scholar]
- Salt D.E., Rauser W.E. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt D.E., Wagner G.J. Cadmium transport across tonoplast of vesicles from oat roots. Evidence for a Cd2+/H+ antiport activity. J. Biol. Chem. 1993;268:2297–12302. [PubMed] [Google Scholar]
- Sanita di Toppi L., Gabbrielli R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999;41:105–130. [Google Scholar]
- Sappin-Didier V., Vansuyts G., Mench M., Briat J.F. Cadmium availability at different soil pH to transgenic tobacco overexpressing ferritin. Plant Soil. 2005;270:189–197. [Google Scholar]
- Saradhi A., Saradhi P.P. Proline accumulation under heavy metal stress. J. Plant Physiol. 1981;138:554–558. [Google Scholar]
- Schützendübel A., Polle A. Plant responses to abiotic stresses: heavy metal induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002;53:1351–1365. [PubMed] [Google Scholar]
- Shigaki T., Hirschi K. Characterization of CAX-like genes in plants: implications for functional diversity. Gene. 2000;257:291–298. doi: 10.1016/s0378-1119(00)00390-5. [DOI] [PubMed] [Google Scholar]
- Siedlecka A., Krupa Z., Samuelsson G., Öoquist G., Gardeström P. Primary carbon metabolism in Phaseolus vulgaris under Cd/Fe interaction. Plant Physiol. Biochem. 1997;35:951–957. [Google Scholar]
- Singh B.K., Foley R.C., Onate-Sanchez L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002;5:430–436. doi: 10.1016/s1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- Singh H.P., Batish D.R., Kaur G., Arora K., Kohli R.K. Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ. Exp. Bot. 2008;63:158–167. [Google Scholar]
- Solomonson L.P., Barber M.J. Assimilatory nitrate reductase: functional properties and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990;4:225–253. [Google Scholar]
- Tamas L., Dudıkova J., Durcekova K., Haluskova L., Huttova J., Mistrık I., Olle M. Alterations of the gene expression, lipid peroxidation, proline and thiol content along the barley root exposed to cadmium. J. Plant Physiol. 2008;165:1193–1203. doi: 10.1016/j.jplph.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Thomine S., Lelievre F., Debarbieux E., Schroeder J., Barbier-Brygoo H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003;34:685–695. doi: 10.1046/j.1365-313x.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- Van de Mortel J.E., Schat H., Moerland P.D., Ver Loren Van Themaat E., van Der Ent S., Blankestijn H., Ghandilyan A., Tsiatsiani S., Aarts M.G.M. Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2008;31:301–324. doi: 10.1111/j.1365-3040.2007.01764.x. [DOI] [PubMed] [Google Scholar]
- Van der Zaal B.J., Neuteboom L.W., Pinas J.E., Chardonnens A.N., Schat H., Verkleij J.A.C., Hooykaas P.J.J. Over-expression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. 1999;119:1047–1055. doi: 10.1104/pp.119.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev A., Perez-Sanz A., Semane B., Carteer R., Vangronsveld J. Cadmium accumulation and tolerance of two salix genotypes hydroponically grown in presence of cadmium. J. Plant Nutr. 2005;28:2159–2177. [Google Scholar]
- Verret F., Gravot A., Auroy P., Preveral S., Forestier C., Vavasseur A., Richaud P. Heavy metal transport by AtHMA4 involves the N-terminal degenerated metal binding domain and the C-terminal His11 stretch. FEBS Lett. 2005;579:1515–1522. doi: 10.1016/j.febslet.2005.01.065. [DOI] [PubMed] [Google Scholar]
- Vogelilange R., Wagner G.J. Subcellular-localization of cadmium and cadmium-binding peptides in tobacco-leaves – implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990;92:1086–1093. doi: 10.1104/pp.92.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid A., Arshad M., Farooq M. Cadmium phytotoxicity: responses, mechanisms and mitigation strategies: a review. Sus. Agric. Rev. 2009;1:371–403. [Google Scholar]
- Wahid A., Ghani A. Varietal differences in mungbean (Vigna radiata) for growth, yield, toxicity symptoms and cadmium accumulation. Ann. App. Biol. 2007;152:59–69. [Google Scholar]
- Wahid A., Ghani A., Ali I., Ashraf M.Y. Effect of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J. Agron. Crop Sci. 2007;193:357–365. [Google Scholar]
- Walch-Liu P., Neumann G., Bangerth F., Engels G. Rapid effects of nitrogen form on leaf morphogenesis. J. Exp. Bot. 2000;51:227–237. doi: 10.1093/jexbot/51.343.227. [DOI] [PubMed] [Google Scholar]
- Wang H.C., Wu J.S., Chia J.C., Yang C.C., Wu Y.J., Juang R.H. Phytochelatin synthase is regulated by protein phosphorylation at a threonine residue near its catalytic site. J. Agric. Food Chem. 2009;57:7348–7355. doi: 10.1021/jf9020152. [DOI] [PubMed] [Google Scholar]
- Wang W. Root elongation method for toxicity testing of organ and inorganic pollutants. Environ. Toxicol. Chem. 1987;6:409–414. [Google Scholar]
- Wang Y.S., Yang Z.M. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol. 2005;46:1915–1923. doi: 10.1093/pcp/pci202. [DOI] [PubMed] [Google Scholar]
- WoolHouse H.W. Encyclopedia of Plant Physiology. In: Lange O.L., Nobel P.S., Osmond C.B., Ziiegler H., editors. vol. v.12C. Springer-Verlag; Berlin: 1983. pp. 245–300. (Toxicity and Tolerance in the Responses of Plants of Metals). [Google Scholar]
- Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risk and best available strategies for remediation. ISRN. 2011 [Google Scholar]
- Xie H.L., Jiang R.F., Zhang F.S., McGrath S.P., Zhao F.J. Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator. Thlaspi caerulescens. Plant Soil. 2009;318:205–215. [Google Scholar]
- Yang M.G., Lin X.Y., Yang X.E. Impact of Cd on growth and nutrient accumulation of different plant species. Chin. J. Appl. Ecol. 1998;19:89–94. [Google Scholar]
- Yang X., Baliger V.C., Martens D.C., Clark R.B. Cadmium effects on influx and transport of mineral nutrients in plant species. J. Plant Nutr. 1996;19:643–656. [Google Scholar]



