Abstract
Administering high levels of inspired oxygen, or hyperoxia, is commonly used as a life-sustaining measure in critically ill patients. However, prolonged exposures can exacerbate respiratory failure. Our previous study showed that toll-like receptor 4 (TLR4) confers protection against hyperoxia-induced lung injury and mortality. Hsp70 has potent cytoprotective properties and has been described as a TLR4 ligand in cell lines. We sought to elucidate the relationship between TLR4 and Hsp70 in hyperoxia-induced lung injury in vitro and in vivo and to define the signaling mechanisms involved. Wild type, TLR4−/− and Trif−/− (a TLR4 adapter protein) murine lung endothelial cells (MLEC) were exposed to hyperoxia. We found markedly elevated levels of intracellular and secreted Hsp70 from mice lung and MLEC after hyperoxia. We confirmed that Hsp70 and TLR4 co-immunoprecipitate in lung tissue and MLEC. Hsp70-mediated NFκB activation appears to depend upon TLR4. In the absence of TLR4, Hsp70 loses its protective effects in endothelial cells. Furthermore, these protective properties of Hsp70 are TLR4 adapter Trif-dependent, MyD88-independent. Hsp70-deficient mice have increased mortality during hyperoxia and lung-targeted adenoviral delivery of Hsp70 effectively rescues both Hsp70-deficient and wild type mice. Our studies are the first to define an Hsp70-TLR4-Trif cytoprotective axis in the lung and endothelial cells. This pathway is a potential therapeutic target against a range of oxidant-induced lung injuries.
Introduction
Acute respiratory failure is a frequent cause of hospital admissions with over 150,000 cases of acute lung injury (ALI), or its severe form, acute respiratory distress syndrome (ARDS), every year in the United States (1). Oxygen therapy is a necessary and life-saving component in the treatment of these critically ill patients, but prolonged oxygen therapy at high concentrations (hyperoxia) has been shown to exacerbate organ injury and potentially lead to increased mortality (2). Animal models have demonstrated that hyperoxia leads to elevated levels of oxidants that damage both pulmonary epithelial and endothelial cells, thereby causing increased pulmonary capillary permeability, inflammation, and eventual respiratory demise (3, 4).
Toll-like receptors (TLRs) have been intensely studied in the context of microbial challenges, inflammation and immune cells, but their role in non-infectious challenges remains an emerging area. We have shown that mammalian TLR4 is required for extended survival during lethal oxidant stress resulting from hyperoxia (5, 6) or bleomycin-induced injury (7). However, the potential ligands and signaling pathways of TLR4 during hyperoxia-induced lung injury are still unknown.
Heat shock proteins (Hsps) are highly conserved proteins found in all prokaryotes and eukaryotes that maintain cellular homeostasis and, when induced, can serve as a “danger” signal by activating both innate and adaptive immunity (8, 9). In most cells the predominant Hsps are approximately 25, 70, 90, and 110 kDa (10, 11). Among these proteins, Hsp70 (also called Hsp72), is the most highly induced and conserved in all organisms from Escherichia coli to humans (12). The mouse is known to express two inducible Hsp70s, Hsp70.1 and Hsp70.3, which are products of two nearly identical genes. Interestingly, a recent gene expression analysis of airway epithelial cells obtained from patients breathing 100% oxygen revealed increased Hsp70, suggesting a clinical relevance (13). In terms of function, overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increased the resistance to ischemic heart injury (14, 15). Adenoviral-mediated Hsp70 overexpression decreased ischemic injury as well as LPS-induced acute lung injury (16, 17). Hsp70−/− mice (targeting both Hsp70.1 and 70.3) are fertile and develop normally but exhibit increased susceptibility to models of cardiac and lung injury (18, 19). Despite descriptions of the protective effects of Hsp70, the signaling mechanisms that underlie these protective responses are poorly defined. Hsp70 has been reported to be an endogenous TLR4 ligand but solely in vitro and predominantly in immune cells (20–22).
Our current studies show for the first time that Hsp70 acts as a TLR4 ligand in the lung and in lung endothelial cells. We detected an interaction between Hsp70 and TLR4. Overexpression or exogenous administration of Hsp70 using viral expression vectors confers significant protection against hyperoxia-induced cell death and oxidant injury. Hsp70 loses its protective effect in the absence of TLR4 or Trif, a TLR4 adapter molecule. These data indicate that Hsp70 is a functionally important TLR4 ligand during lethal oxidant injury that promotes endothelial cell survival.
Materials and Methods
Isolation of primary MLEC and hyperoxia exposures
Isolation of murine lung endothelial cells (MLEC) from wild-type (WT), TLR4−/−, Trif−/−, MyD88−/− and Hsp70−/− mice and hyperoxic conditions have been described previously (23).
Animal hyperoxic exposure
Adult 6- to 8-wk-old C57BL/6 mice were obtained from Jackson Laboratories. TLR4−/−, and Hsp70−/− have been described (18, 23). Trif−/− mice were provided by Dr Masahiro Yamamoto in Osaka University, Japan. All the mice were backcrossed for >10 generations onto a C57BL6 background. WT C57BL/6 mice bred and housed in the same facilities as the knockout mice were used as controls. Mice were exposed to 100% O2 in a Plexiglass exposure chamber and permitted food and water ad libitum. Control mice were exposed to room air. All protocols were reviewed and approved by the Animal Care and Use Committee at Yale University.
Hsp70 constructs
We purchased pEGFP Hsp70 (Addgene plasmid 15215) and pEGFP Hsp70 K71E (Addgene plasmid 15216) from Addgene. The function of these constructs has been previously reported (24).
Protein labeling
Human recombinant Hsp70 protein was purchased from Boston Biochem. We labeled Hsp70 with fluorescent dye using the Alexa Fluor 488 Protein Microscale Labeling kit (Molecular Probes), according to the manufacturer’s recommendations. Briefly, 100 µg Hsp70 was incubated with Alexa Fluor 488 in 0.1 M sodium bicarbonate for 15min at room temperature. Unconjugated dye was removed by the spin filter provided in the kit. The number of dye molecules bound per protein molecule was determined by measuring the optical density at 280 and 494 nm. Calculations predicted that six to nine Alexa Fluor molecules bind to each protein molecule.
Hsp70 detection and uptake
MLEC were incubated with various concentrations of Alexa488-labeled Hsp70 or PBS for 1h on ice for the binding studies. For the competition assays, the MLEC were preincubated with unlabeled Hsp70 protein for 30 min on ice. Then labeled Hsp70 was added and incubated for another 1h on ice. Subsequently, cells were washed with PBS/1% BSA and resuspended in PBS containing 1% paraformaldehyde. Throughout the assay, the temperature was kept at 4°C to minimize nonspecific endocytosis of exogenously added protein. The samples were evaluated using a FACScan flow cytometer (BD Biosciences). Cell surface detection of fluorescent-labeled proteins was calculated using the geometric mean fluorescence value after subtracting the value for autofluorescence of the cells.
Confocal microscopy
MLEC were adjusted to a density of 1 × 105 cells/ml and seeded on Poly-L-Lysine coated cover slips (BD Biosciences) in a volume of 200 µl (2 × 104 cells). After incubation for 24 h (37°C, 5% CO2), cells were treated with Alexa488-labeled Hsp70 or PBS on ice as described above. Then the cells were washed with PBS, followed by fixation with 4% paraformaldehyde for 15 min. Finally, the cells were washed three times with PBS and then mounted with Prolong gold mounting media with DAPI (Invitrogen). For immunofluorescence staining, aspirate liquid, then cover cells to a depth of 2–3 mm with 4% formaldehyde in PBS for 15 min, and then washed the cells with PBS. Fixed cells were incubated with a 1:200 dilution of anti-TLR4 (Santa Cruz Biotechnology) or anti-Hsp70 (Stressgen) at 4°C for overnight. After PBS washes, cells were incubated with secondary antibody (Alexa Fluor® 488 goat anti-rabbit IgG or Alexa Fluor® 594 goat anti-mouse IgG from Invitrogen at 1:300 dilution) at room temperature for 1 hour. Samples were washed three times by immersing in PBS for 5 min and then mounted with Prolong gold mounting media with DAPI (Invitrogen). Labeled cells were visualized using a Nikon Ti-E Eclipse inverted microscope equipped with Perfect Focus (auto focus system), motorized XY stage and Nano Focusing Piezo Stage. Images were prepared by Photoshop (Adobe) for the visualization.
Electromobility Shift Assay (EMSA)
EMSAs of nuclear protein isolated from MLEC were performed as previously described with minor modifications (25). Nuclear extracts were prepared using an NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Thermo Scientific) according to the manufacturer’s protocol. The NFκB site was synthesized as complementary oligodeoxyribonucleotide strands. The sequence of NFκB consensus oligonucleotides was 5’-AGT TGA GGG GAC TTT CCC AGG C-3’ (Santa Cruz Biotechnology). The DNA binding ability of NFκB in the nuclear extracts was assessed by EMSA with biotin-labeled, double-stranded NFκB. EMSA was carried out using the LightShift Chemiluminescent EMSA Kit (Pierce). Specific binding was confirmed using 200-fold excess of unlabeled probe as a specific competitor. Protein–DNA complexes were separated using a 6% non-denaturing acrylamide gel electrophoresis and then transferred to positively charged nylon membranes and cross-linked by UV irradiation. Gel shifts were visualized with streptavidin HRP according to standard protocols.
Measurement of lung injury markers
Mice were removed from the exposure chamber and killed after 72h hyperoxia exposure. Bronchoalveolar lavage (BAL) was performed twice with 0.8 ml PBS (pH 7.4). Cell pellets were pooled, resuspended in PBS and counted. The supernatant was used for BAL protein determination. The protein concentration in each sample was determined by the bicinchoninic acid protein assay (Thermo Scientific) using BSA as the standard.
Microarrays
Total RNA (100 ng per sample) from WT or TLR4−/− mice was subjected to amplification, followed by labeling and hybridization to Affymetrix GeneChip® Mouse Genome 430 2.0 GeneChips (Affymetrix). Sample amplification, labeling, hybridization, and detection were performed by the W.M. Keck Foundation Biotechnology Resource Laboratory in Yale University, West Campus, Orange, CT.
Overexpression of Hsp70 in MLEC and mice
Adenovirus-CMV-Hsp70 (Ad-Hsp70) was purchased from Vector Biolabs. Ad-null, an adenovirus empty vector, was used as a control (Ad-Ctrl). Ad-Ctrl or Ad-Hsp70 was transfected at 2.5 MOI (multiplicity of infection: the average number of phage particles that infect a single cell) 24 h before experiment and hyperoxia exposure. Intranasal administration of either the Ad-Hsp70 or Ad-Ctrl was performed on mice (8×108 TU/mouse); 2 weeks later hyperoxia exposure was initiated or left in room air. Mice received only a single intranasal treatment for each experiment.
Hsp70 ELISA
Cell culture supernatant or mice bronchoalveolar lavage (BAL) was assessed for Hsp70 protein levels by Hsp70 ELISA (R&D Systems).
Western blot analysis
Lung protein analyses were performed as previously described with minor modifications (23). Whole-lung tissues were homogenized in 62.5 mM Tris buffer, and cell pallets were lysed in 1× RIPA lysis buffer (Upstate Biotechnology). The protein concentrations of the lysates were determined by BCA Protein Assay (Thermo Scientific). Samples were electrophoresed in a 12% ready-made Tris-HCl gel (Bio-Rad Laboratories) and electrophoretically transferred onto a nitrocellulose membrane. The membranes were then incubated overnight with anti–Hsp70 (Stressgen) or β-Actin antibody (Santa Cruz Biotechnology). The membranes were incubated with HRP-conjugated goat anti-rabbit IgG antibody followed by the detection of signal with a chemiluminescence LumiGLO detection kit (Cell Signaling Technology).
Cell membrane isolation
Isolation of total cellular membrane protein of MLEC was performed using Plasma Membrane Protein Extraction Kit (BioVision).
Apoptosis Assays
We used a fluorescence-activated cell sorter (FACS) to detect annexin V-fluorescein isothiocyanate labeling (BD Biosciences) according to the manufacturer’s instruction. Briefly, MLEC were washed with cold phosphate-buffered saline and resuspended with binding buffer (10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, 2.5mM CaCl2); a solution containing 1×105 cells was transferred to a 5-ml tube, and 5 µl each of annexin V and propidium iodide were added. Binding buffer was then added to each tube and analyzed by FACS (BD Biosciences). The annexin V-fluorescein isothiocyanate signal was detected by FL1 (fluorescein isothiocyanate detector) at 518 nm, and the propidium iodide signal was detected by FL2 (phycoerythrin fluorescence detector) at 620 nm. Mouse lung sections were subjected to terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay using the in situ cell death detection kit (Roche Diagnostics) as described previously (26).
Total RNA isolation, RT-polymerase chain reaction (RT-PCR) amplification and Real time RT-PCR amplification
Total RNA from lung tissue was extracted by using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was synthesized using Superscript II Reverse Transcriptase (Invitrogen) with random hexamers; conditions were 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C. RT-PCR was performed using PCR Master Mix (Promega). Primers used for mouse Hsp70 were sense: 5’-CGTGGAGGAGTTCAAGAGGA-3’; antisense: 5’-CTCGTACACCTGGATCAGCA-3’; and for mouse β-actin were sense: 5’-GTGGGCCGCTCTAGGCACCAA-3’; antisense: 5’-CTCTTTGATGTCACGCACGATTTC-3’. Conditions for PCR were 1 cycle at 95°C for 3 min; 30 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s; and 1 cycle at 72°C for 5 min. Each reaction product (10 µl) was then separated on a 1% agarose gel containing 0.5 µg/ml of ethidium bromide. Real-time RT-PCR reactions were carried out in Power SYBR Green PCR Master Mix (Applied Biosystems) and an ABI Prism 7000 Sequence Detection System (Applied Biosystems). GAPDH was amplified as a control. Real-time PCR conditions were 95°C for 10 min, 40 cycles of: 95°C for 15 s, followed by 60°C for 1 min. For real-time PCR, the Hsp70 primers were sense 5'-TGGTGCTGACGAAGATGAAG-3' and antisense 5'-CCGCTGAGAGTCGTTGAAGT-3'. GAPDH primers were sense 5'TGTGTCCGTCGTGGATCTGA-3' and antisense was 5'-CCTGCTTCACCACCTTCTTGAT-3'.
Immunoprecipitation
Immunoprecipitation was performed using the Catch and Release v2.0 Kit (Upstate Biotechnology), per the manufacturer’s protocol. Briefly, lung tissue lysates were incubated with anti-TLR4 (Santa Cruz Biotechnology) or anti-Hsp70 (Stressgen) antibody (Rabbit IgG was used as negative control antibody) and Antibody Capture Affinity Ligand for 30 min on a rotator by using Catch and Release spin columns. We washed the column 3 times with 400 µl of 1× Wash Buffer, spinning at 5000 rpm 15–30 seconds for each wash. Protein bound to the beads was eluted by Denaturing Elution Buffer containing 0.5% β-mercaptoethanol. Proteins were separated by 10% SDS-PAGE and then transferred to a PVDF membrane. The membrane was incubated with either Hsp70 or TLR4 antibody at 1:500 dilutions. Western Blot was done by using the Phototope-HRP Western Blot Detection System kit according to the manufacturer's instructions (Cell Signaling Technology).
Caspase 3 Activity
Caspase 3 activity was measured with colorimetric assays using the CaspACE assay system (Promega). MLEC lysates were centrifuged and the supernatants were incubated with colorimetric substrate, Ac-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA). The release of p-nitroanilide from Ac-DEVD-pNA was measured at 405 nm using a spectrophotometer.
Statistics
Data are expressed as mean ± SE and analyzed by Student’s t test. Significant difference was accepted at p < 0.05.
Results
TLR4 deficiency leads to increased Hsp70 mRNA and protein expression during hyperoxia in vitro
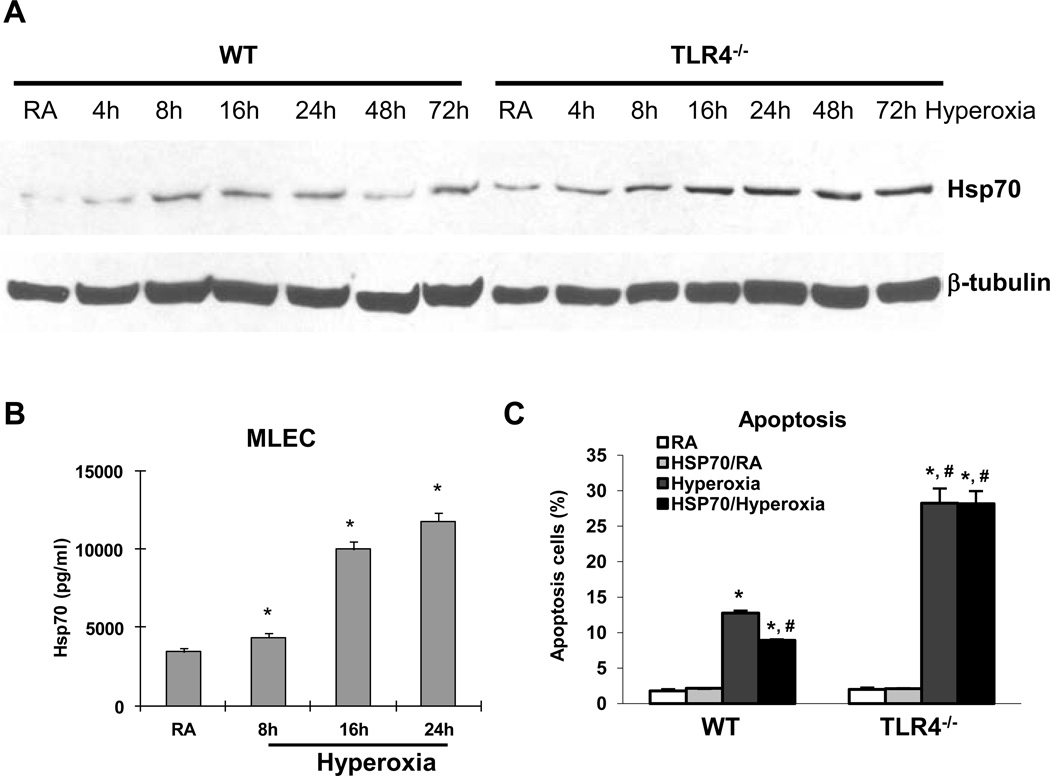
We have previously shown that TLR4 deficiency confers susceptibility to hyperoxia-induced lung and endothelial cell injury (23) and that lung-targeted activation of TLR4 confers resistance to hyperoxia-induced apoptosis (5). We were interested in identifying the relevant ligand for the protective effects of TLR4. Hsp70 has been shown to be protective in various conditions (16, 17) and a potential TLR4 ligand (27). Our microarray analyses of lung lysates from WT and TLR4−/− mice exposed to 72 h of hyperoxia showed markedly elevated levels of Hsp70 in TLR4−/− mice (data not shown). We confirmed our array results with Hsp70 protein level in MLEC. WT MLEC exposed to a time course of hyperoxia showed Hsp70 protein induction by 8h but TLR4−/− MLEC showed consistently higher Hsp70 protein induction (Fig. 1A). We believed that extracellular, or secreted Hsp70, likely serves as a TLR4 ligand and therefore sought to determine levels of secreted Hsp70 protein in MLEC culture media as well as lung lavage fluid. We detected increased Hsp70 protein by ELISA after hyperoxia in MLEC culture medium (Fig. 1B). In order to determine whether exogenous Hsp70 protein serves a cytoprotective role, we performed cell survival studies after treating WT and TLR4−/− MLEC with recombinant human Hsp70 protein and found that Hsp70 decreased cell death in WT cells but not in TLR4−/− cells (Fig. 1C). These data suggested that extracellular Hsp70 has an important physiologic role and that TLR4 is involved.
Figure 1. Hsp70 is inducted and secreted by MLEC during hyperoxia.
A) Hsp70 protein in WT and TLR4−/− MLEC by western blot after a time course of hyperoxia. β-tubulin as loading control. B) Hsp70 protein in WT MLEC supernatant was measured by ELISA after 8h, 16h, and 24h of hyperoxia. RA, room air control. * p <0.05 vs RA. C) WT and TLR4−/− MLEC were treated with recombinant human Hsp70 protein at 8.65µg/ml and were exposed to 72h of hyperoxia. RA, room air control. Graphical quantitation of flow cytometry analysis of apoptosis was determined. The values are expressed as mean ± SD. *p<0.05, vs RA WT; #p<0.05 vs hyperoxia WT (experiments were performed in triplicates).
Hsp70 is a TLR4 ligand during hyperoxia in vitro and in vivo
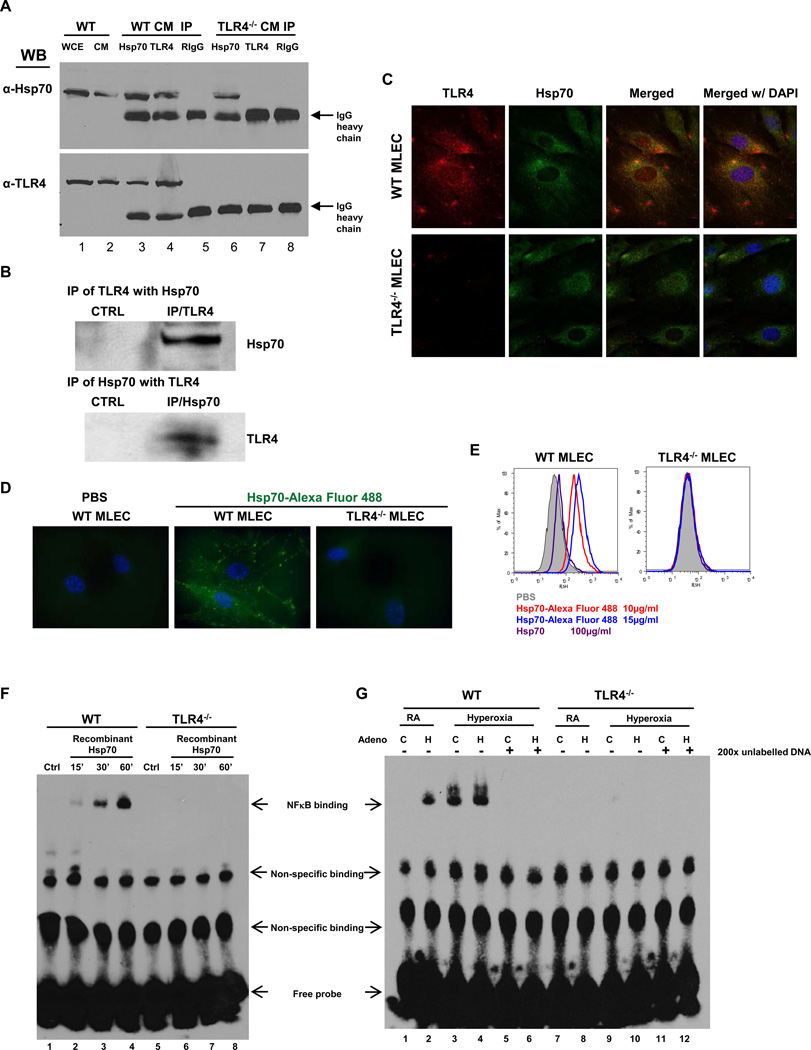
Reports of Hsp70 as an endogenous TLR4 ligand have been predominantly in immune cells and in vitro (e.g. monocyte, macrophage and dendritic cells) (20–22). We sought to determine whether there were Hsp70-TLR4 interactions in MLEC cell membranes and detected Hsp70 and TLR4 binding (Fig. 2A). Therefore, we performed co-immunoprecipitation studies determine whether there was a physical interaction between TLR4 and Hsp70 in WT mice exposed to 72 h of hyperoxia. We were able to detect Hsp70 protein using TLR4 antibody and vice a versa in lung lysates (Fig. 2B). These data indicated that there was an interaction between Hsp70 and TLR4 in lung during hyperoxia in MLEC and in vivo. We determined co-localization of endogenous TLR4 and Hsp70 on the surface of WT MLEC using immunofluoresence detection of TLR4 and Hsp70 on non-permeabilized MLEC (Fig. 2C). Of note, Hsp70 and TLR4 co-localization was not detected in TLR4−/− MLEC. We also confirmed an interaction between Hsp70 and cell surface TLR4 using exogenous, recombinant Hsp70 protein labeled with Alexa488 fluorescence dye and confocal microscopic visualization. WT and TLR4−/− MLEC were incubated either with Hsp70-Alexa488 or PBS, as a negative control (Fig. 2D). In WT MLEC, we were able to detect labeled Hsp70 but not in TLR4−/− MLEC. We quantitated fluorescent Hsp70 detection on the surface of WT and TLR4−/− MLEC using flow cytometry (Fig. 2E). Cells were incubated with varying doses of Hsp70-Alexa488 (10µg/ml or 15µg/ml) before cytometric detection. We also performed competition assays in which cells were preincubated with unlabeled Hsp70 protein (100µg/ml), followed by Hsp70-Alexa488 and then subjected to flow cytometry. Mean fluorescence values were calculated. We found a dose-response with increased Hsp70 detection with a higher dose (15µg/ml) and the ability of unlabeled Hsp70 to decrease labeled Hsp70 detection in WT MLEC. We did not detect any exogenously added labeled Hsp70 on the surface of TLR4−/− MLEC, indicating that exogenous Hsp70 requires TLR4 expression for cell surface detection.
Figure 2. Hsp70 interacts with TLR4 in MLEC and lungs.
A) Immunoprecipitation (IP) of Hsp70 and TLR4 in WT and TLR4−/− MLEC cell membrane (CM). WCE, whole cell extract. RIgG: Rabbit normal IgG. B) IP of TLR4 and Hsp70 with lung lysates. CTRL, control. C) Co-localization of TLR4 and Hsp70 in MLEC using immunofluorescence. WT or TLR4−/− MLEC were immunostained for TLR4 (red) and Hsp70 (green). Nuclei were stained with DAPI (blue) and imaged by confocal microscopy. The left panels show single immunostained images and the rights panels show merged images. Original magnification of all photomicrographs, ×600. D) Recombinant human Hsp70 protein was labeled with Alexa Fluor 488, incubated with WT or TLR4−/− MLEC (10µg/ml) and imaged with laser microscopy. Original magnification of all photomicrographs: ×1000. E) WT or TLR4−/− MLEC were incubated with two concentrations of Alexa 488-labeled recombinant human Hsp70 (10µg/ml and 15µg/ml). Unlabelled Hsp70 protein (100µg/ml) was used to “compete off” labeled Hsp70. Fluorescence intensity was measured by flow cytometry. F) Nuclear extracts prepared from WT and TLR4−/− MLEC incubated with recombinant Hsp70 (10µg/ml) for the indicated time or no treatment (Ctrl) were mixed with biotin-labeled oligonucleotide containing NFκB motif. Bound complexes were analyzed by electrophoresis. The results are representative of at least three independent experiments. G) WT and TLR4−/− MLEC were treated with Ad-Ctrl (C) or Ad-Hsp70 (H) and were exposed to 72h of hyperoxia. RA, room air control. Nuclear extracts were mixed with biotin-labeled oligonucleotide containing NFκB motif. Competitive inhibition of NFκB binding with nonlabeled probe (200×). Bound complexes were analyzed by electrophoresis. The results are representative of at least 3 independent experiments.
To confirm the extracellular Hsp70 is not only interacting with TLR4 at the cell membrane but is also signaling, we performed EMSAs using recombinant Hsp70. Hsp70 activated NFκB in a time-dependent manner, which was abolished in TLR4−/− MLEC (Fig. 2F). Given previously reported concerns of recombinant Hsp70 protein and LPS contamination (28), we used virally-driven Hsp70 expression constructs for subsequent studies. Similar to recombinant Hsp70, Ad-Hsp70 and hyperoxia induced NFκB activation in MLEC (Fig. 2G). Next, we determined whether Hsp70 had protective effects during hyperoxia.
Hsp70 is protective in hyperoxia
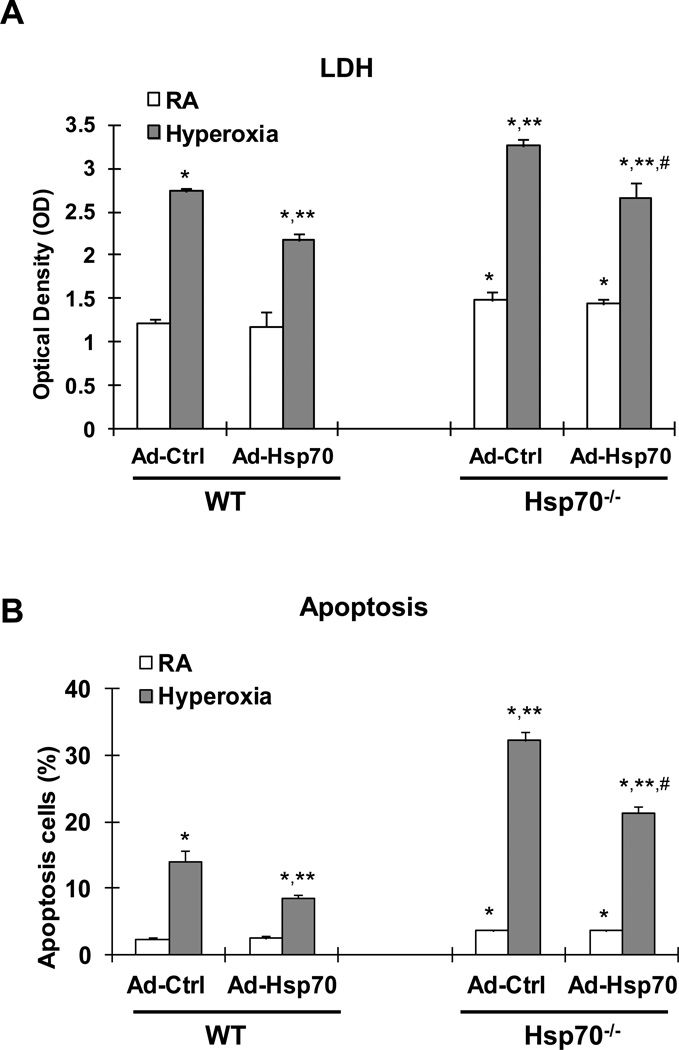
We first confirmed the efficacy of our Ad-Hsp70 vector in total and secreted Hsp70 protein in WT MLEC at baseline, compared to cells treated with empty vector control (Ad-Ctrl) by Western blot and ELISA (Supplemental Figure 1A & 1B). We also tested for hyperoxia-induced Hsp70 with and without Ad-Hsp70 treatment. Our results show that hyperoxia induces Hsp70 secretion and mRNA in MLEC and that Ad-Hsp70 treatment augments Hsp70 expression both in WT and Hsp70−/− MLEC (Supplemental Figure 2A & 2B). Ad-Hsp70 decreased hyperoxia-induced MLEC injury, as assessed by lactate dehydrogenase (LDH) release (Fig 3A), as well as apoptosis in WT MLEC (Fig. 3B).
Figure 3. Hsp70−/− MLEC have increased injury and apoptosis, which improved with Hsp70 reconstitution.
WT and Hsp70−/− MLEC were treated with Ad-Ctrl or Ad-Hsp70 and were exposed to 72h of hyperoxia. RA, room air control. A) LDH activity assay from MLEC supernatant. B) Graphical quantitation of flow cytometry analysis of apoptosis. The values are expressed as mean ± SD. *p <0.05 vs RA Ad-Ctrl WT; **p <0.05 vs hyperoxia Ad-Ctrl WT; #p<0.05 vs corresponding Ad-Ctrl (experiments were performed in triplicates).
The protective effect of Hsp70 depends upon a TLR4-Trif pathway
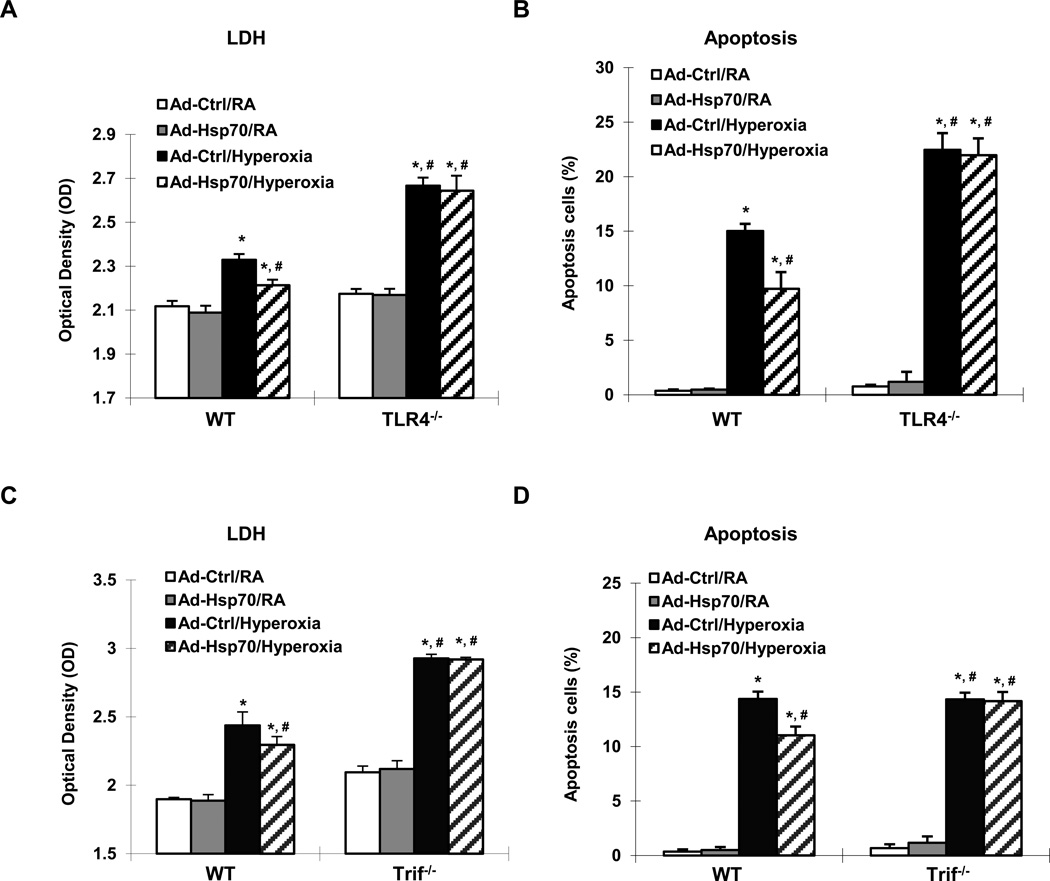
Ad-Hsp70 decreased hyperoxia-induced MLEC injury, as assessed by LDH release (Fig 4A), as well as decreased cell death in WT MLEC (Fig. 4B). Next we wanted to determine whether Hsp70 was dependent upon TLR4 and found that Ad-Hsp70 lost its protective effects in TLR4−/− MLEC, indicating that Hsp70 was dependent upon TLR4 in order to decrease hyperoxia-induced cell injury and death. In order to test whether secreted Hsp70 was responsible for the observed effects of Ad-Hsp70, we pretreated MLEC with methyl β-cyclodextrin (MBC) (29) before administering Ad-Hsp70. We found that MBC completely blocked the ability of Ad-Hsp70 to decrease hyperoxia-induced LDH release and apoptosis in WT MLEC, leading to levels of injury and death equivalent to that of TLR4−/− MLEC (Supplemental Figure 3A & 3B). As further evidence that secreted (or extracellular) Hsp70 conferred anti-apoptotic effects, we were also able to block the effects of Ad-Hsp70 with anti-Hsp70 neutralizing antibody (Supplemental Figure 3C).
Figure 4. Hsp70 is protective and depends upon a TLR4-Trif pathway in MLEC.
WT, TLR4−/− and Trif−/− MLEC were treated with Ad-Ctrl or Ad-Hsp70 and were exposed to 72h of hyperoxia. RA, room air control. LDH activity of WT and TLR4−/− MLEC in (A), and WT and Trif−/− MLEC in (C) was determined. Graphical quantitation of flow cytometry analysis of apoptosis in WT and TLR4−/− MLEC in (B), and WT and Trif−/− MLEC in (D) was determined. The values are expressed as mean ± SD. *p<0.05, vs Ad-Ctrl/RA WT; #p<0.05 vs Ad-Ctrl/hyperoxia WT (experiments were performed in triplicates).
Myd88 and Trif are the major adapter proteins for TLR4 signaling. In order to determine the involvement of each, we isolated MLEC from Trif−/− and MyD88−/− mouse lungs. Of note, Trif−/− MLEC exhibited increased levels of injury and cell death compared to WT MLEC (Fig 4C & 4D) whereas MyD88−/− MLEC were not significantly different from WT MLEC (Supplemental Figure 4A & 4B). Importantly, Ad-Hsp70 lost its protective effects in Trif−/−MLEC (Fig 4C & 4D) but not in MyD88−/− MLEC (Supplemental Figure 4A & 4B). These data indicated that Hsp70 was dependent upon Trif for its protective effects.
Hsp70-ATPase mutant still had a protective effect in MLEC
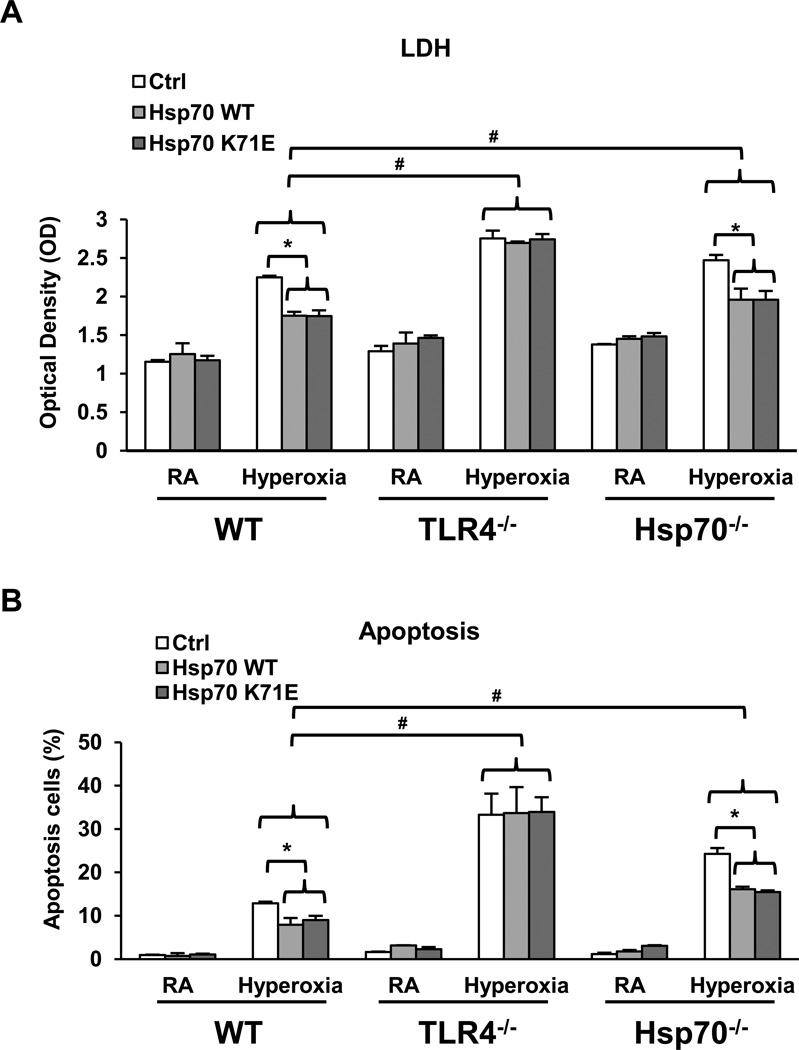
There is the possibility that Hsp70 may be acting as an extracellular chaperone for TLR4. To investigate if the protective actions of Ad-Hsp70 are dependent on the chaperone functions of Hsp70, we transfected cells with a chaperone-mutant form of Hsp70 before hyperoxia exposure. ATPase-deficient mutant of Hsp70, Hsp70 (K71E), has reduced mobility relative to the cytoplasm, whereas it is almost completely immobilized both in the nucleoplasm and the cytoplasm (24). WT, TLR4−/− and Hsp70−/− MLEC were transfected with GFP-Hsp70 WT or GFP-Hsp70 K71E constructs. EGFP mRNA expression was used as a measure of the efficiency of construct transduction. The data are presented as the relative proportion of cells expressing EGFP compared to the Sham group (Ctrl). MLEC showed increased Hsp70 and EGFP mRNA expression whereas the Sham-treated MLEC did not (Supplemental Figure 1C &1D). WE also detected GFP-Hsp70 fusion proteins in cell lysates, indicating that Hsp70 is secreted out of cells (Supplemental Figure 1E). Consistent with the high efficiency of expression of both WT and mutant K71E Hsp70 in MLEC, we also detected increased levels of Hsp70 secretion the supernatant of WT, TLR4−/− and Hsp70−/− MLEC after transfection, especially after hyperoxia (Supplemental Figure 1F). As expected, Hsp70−/− MLEC had lower levels of hyperoxia-induced Hsp70 because they lack endogenous Hsp70. These data indicated that Hsp70 expression and secretion were not affected by the loss TLR4 or by the loss of ATPase function. Importantly, the Hsp70 K71E construct had the same effect on hyperoxia-induced LDH release (Fig. 5A) and apoptosis (Fig. 5B) as that of WT Hsp70 in WT and Hsp70−/− MLEC. These data suggest that the protective effect of Hsp70 transfection was independent of its chaperone activities. Neither the WT nor mutant K71E Hsp70 constructs had any protective effects on TLR4−/− MLEC, confirming that Hsp70 utilizes TLR4 for its cytoprotective actions.
Figure 5. Hsp70-ATPase mutant still had a protective effect in MLEC.
WT, TLR4−/− or Hsp70−/− MLEC were transfected with human wild type Hsp70 WT, Hsp70 K71E mutant (which has a mutated ATPase binding domain) or SHAM control and exposed to 72h of hyperoxia or RA (room air) as control. A) LDH activity of MLEC. B) Graphical quantitation of flow cytometry analysis of apoptosis. The values are expressed as mean ± SD. *p<0.05, vs Ctrl; #p<0.05, vs corresponding WT hyperoxia (experiments were performed in triplicates).
Hsp70 protects hyperoxia-induced MLEC injury and Caspase 3 activation through TLR4
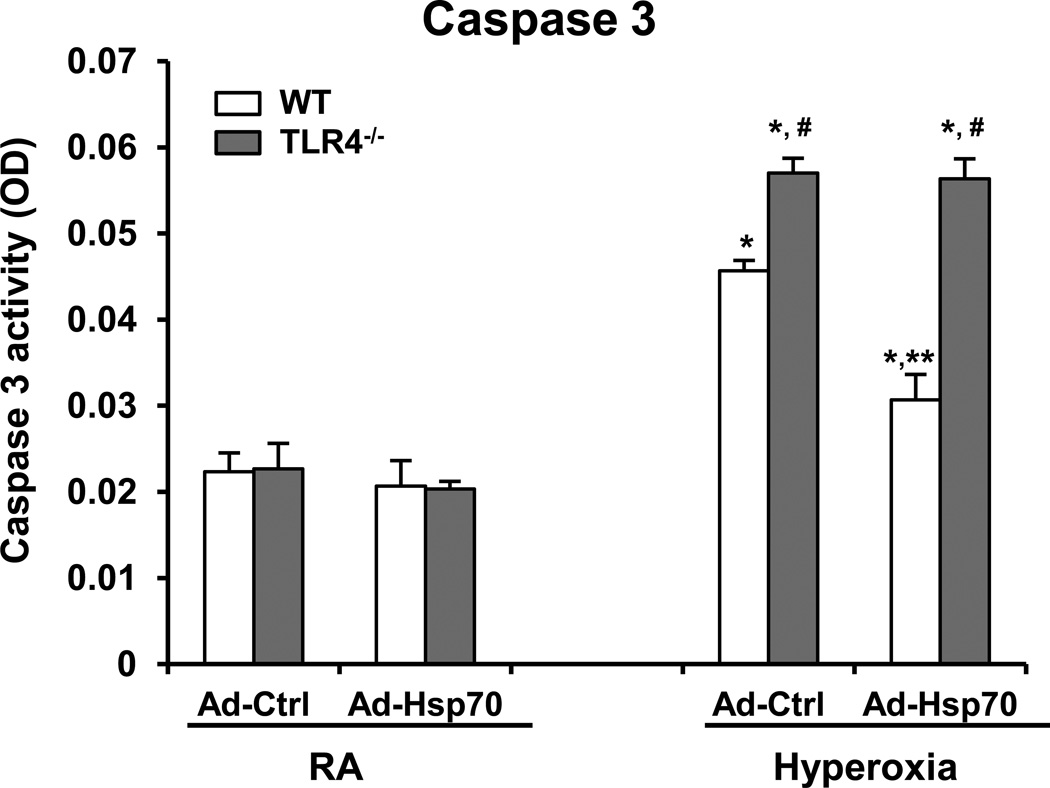
Our previous study showed that activation of Caspase 3 plays important role in hyperoxia-induced cell death (30). We sought to determine if Hsp70 has the ability to inhibit Caspase 3 activation and the role TLR4, if any. We overexpressed Hsp70 using Ad-Hsp70, in WT and TLR4−/− MLEC and found that Ad-Hsp70 significantly inhibited MLEC from hyperoxia-induced Caspase 3 activation in WT MLEC but not in TLR4−/− MLEC, suggesting that Hsp70-mediated anti-apoptotic effects are mediated by TLR4 (Fig. 6).
Figure 6. Hsp70-mediated protection requires TLR4 in MLEC.
Caspase 3 activity was measured in WT and TLR4−/− MLEC transfected with Ad-Ctrl or Ad-Hsp70 and were exposed to 72h of hyperoxia. The values are expressed as mean ± SD. *p<0.01 vs RA WT Ad-Ctrl; **p<0.01 vs corresponding Ad-Ctrl; #p<0.01 vs corresponding WT (experiments were performed in triplicates).
TLR4 deficiency leads to increased Hsp70 mRNA and protein expression during hyperoxia in vivo
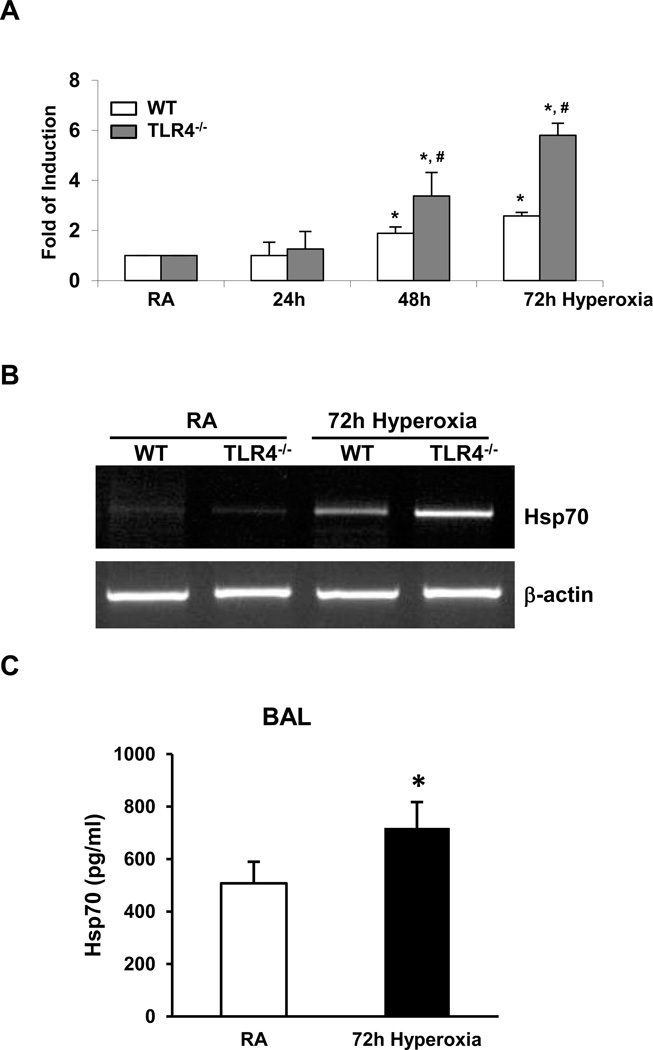
We also confirmed the increased Hsp70 expression by using RT-PCR and quantitative (real-time) RT-PCR analyses in lung tissue. Lung lysates from WT mice exhibited Hsp70 mRNA induction during hyperoxia but this induction was markedly exaggerated in TLR4−/− mice (Fig. 7A & 7B). We detected increased Hsp70 protein by ELISA after hyperoxia in lung lavage fluid (Fig. 7C).
Figure 7. Hsp70 is induced and secreted in lungs during hyperoxia.
A) Hsp70 mRNA expression in WT and TLR4−/− mice lung lysates was measured by real time RT-PCR after 24h, 48h, and 72h of hyperoxia. B) Hsp70 mRNA expression in lung lysates was measured by RT-PCR after 72h of hyperoxia. C) Hsp70 ELISA assay in BAL fluid of WT mice. WT mice were exposed to 72h of hyperoxia. RA, room air control. The values are expressed as mean ± SD. *p<0.05, vs RA; #p<0.05, vs corresponding WT hyperoxia (n=5 for each group).
Hsp70 is protective in hyperoxia in vivo
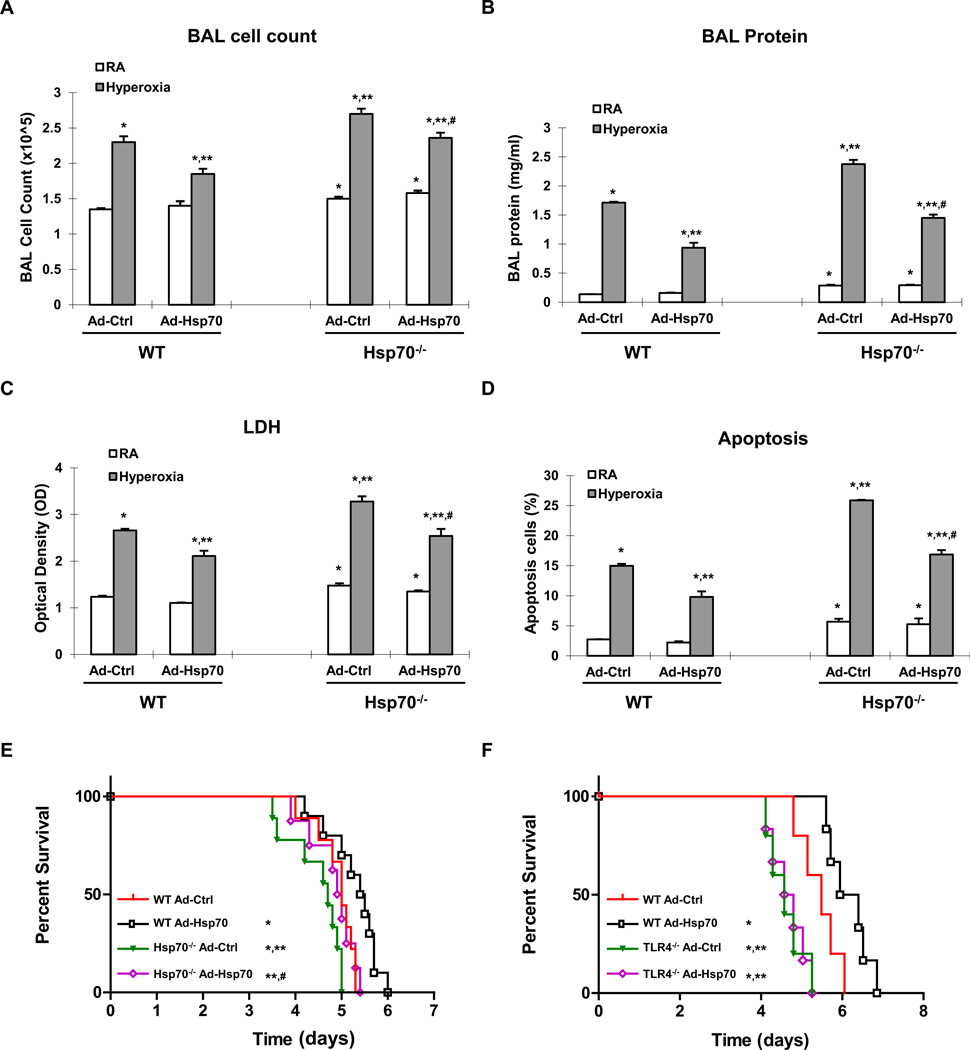
To demonstrate the impact of Ad-Hsp70 in vivo, we treated WT and Hsp70−/− mice with intranasal, and thus lung-targeted, Ad-Ctrl or Ad-Hsp70 prior to 72h hyperoxia, a time point of maximal lung injury. We confirmed that hyperoxia induces Hsp70 secretion in lung lavage fluid and Hsp70 mRNA in lung lysates (Supplemental Figure 2C & 2D, respectively). Intranasal Ad-Hsp70 administration markedly induced Hsp70 expression in lung lavage fluid and lung lysates both in WT and Hsp70−/− mice. Hyperoxia increased bronchoalveolar lavage (BAL) leukocytes and protein (an indication of increased lung permeability), which improved with Ad-Hsp70 treatment in WT and Hsp70−/− mice compared to Ad-Ctrl treatment (Fig. 8A & 8B, respectively). Mice treated with Ad-Hsp70 showed markedly less lung lavage LDH activity (Fig. 8C) and lung apoptosis (Fig. 8D) than controls did after 72 h hyperoxia. Hyperoxia is a highly lethal model and strategies that improve survival by even several hours are significant. We tested the impact of Ad-Hsp70 on survival by treating WT and Hsp70−/− mice (Fig. 8E) with Ad-Ctrl or Ad-Hsp70 prior to hyperoxia. Hsp70−/− mice were significantly more susceptible to hyperoxia than WT mice but were successfully rescued with Ad-Hsp70 treatment. WT mice given Ad-Hsp70 vector achieved the highest level of protection against lethal hyperoxia, likely due to augmented Hsp70 expression above that induced by hyperoxia alone. Taken together, these studies show that Hsp70 administration in vitro and in vivo has significant protective properties against lethal hyperoxic injury.
Figure 8. Hsp70 protects against lethal hyperoxia and is TLR4-dependent.
WT and Hsp70−/− mice were treated with Ad-Ctrl or Ad-Hsp70 and were exposed to 72h of hyperoxia. RA, room air control. A) Lung inflammation was detected by bronchoalveolar lavage (BAL) cell counts. B) Lung permeability was assessed by BAL protein content. C) LDH activity assay from BAL fluid. D) TUNEL staining was performed on lung sections and the number of TUNEL-positive cells were quantitated and expressed as a percentage of the total number of lung cells counted on each section. The values are expressed as mean ± SD. *p <0.05 vs RA Ad-Ctrl WT mice; **p <0.05 vs hyperoxia Ad-Ctrl WT mice; #p<0.05 vs corresponding Ad-Ctrl mice (n=5 for each group). WT and Hsp70−/− mice (E) or WT and TLR4−/− mice (F) were treated with intranasal Ad-Ctrl or Ad-Hsp70 and were exposed to hyperoxia. Survival proportions were compared among four groups. *p <0.05 vs WT Ad-Ctrl mice; **p <0.05 vs WT Ad-Hsp70 mice; #p<0.05 vs Hsp70−/− Ad-Ctrl mice (n=10 for each group in (E)n=5~6 for each group in (F)).
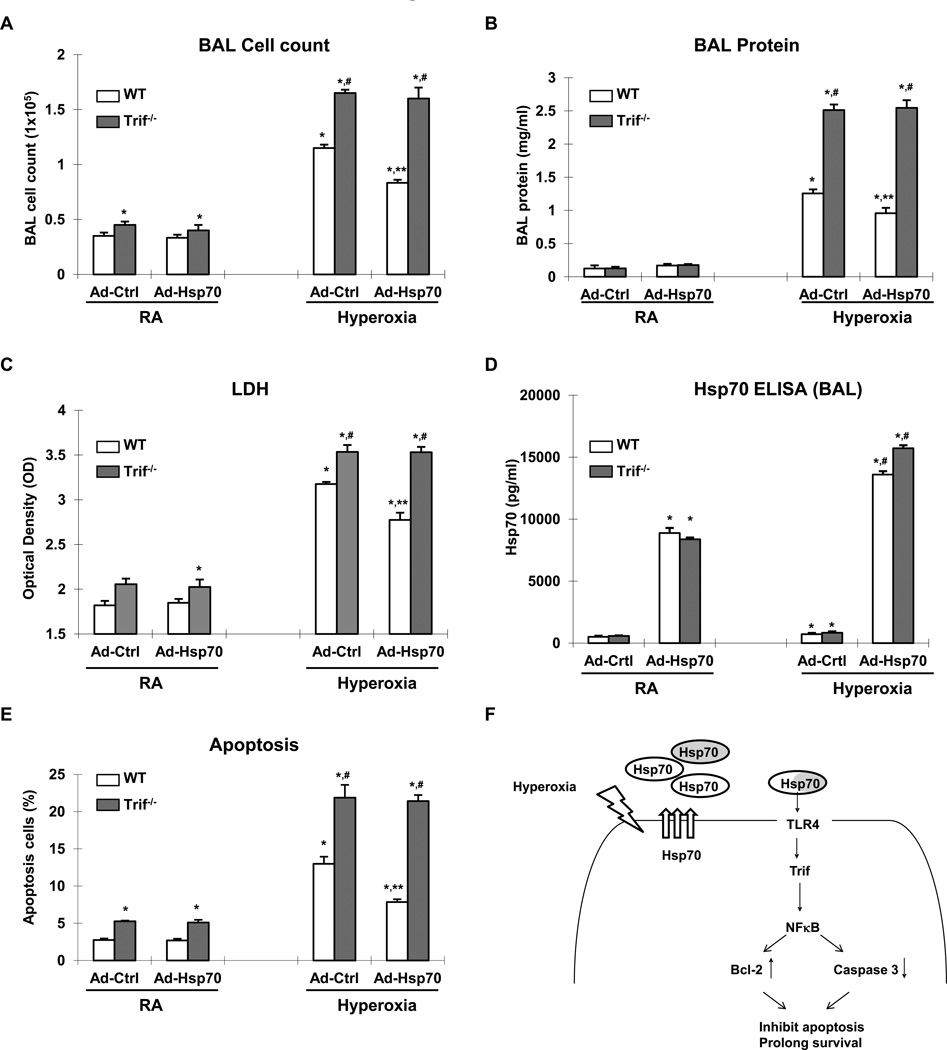
The protective effect of Hsp70 depends upon a TLR4-Trif pathway in vivo
We previously reported that TLR4−/− mice are more susceptible to hyperoxia-induced death and successfully rescued by adenoviral delivery of another known cytoprotective heat shock protein, heme oxygenase 1 (HO1) (23). In contrast, Ad-Hsp70 did not improve the survival of TLR4−/− mice exposed to hyperoxia (Fig. 8F). These data suggested that Hsp70 had specific TLR4-dependent therapeutic effects that are distinct from that of other protective heat shock proteins. To confirm that Trif is involved in vivo, we administered Ad-Hsp70 or Ad-Ctrl to WT and Trif−/− mice before 72h hyperoxia, a time point of maximal lung injury. We first confirmed that hyperoxia and Ad-Hsp70 induced Hsp70 in both WT and Trif−/− lungs (data not shown). We found that not only do hyperoxia-exposed Trif−/− mice have increased BAL cell influx, protein leak and LDH release compared to WT, but also Ad-Hsp70 completely lost its ability to decrease lung injury in the absence of Trif (Fig. 9). Taken together, these studies indicate that Hsp70 ameliorates hyperoxic injury via Trif in vitro and in vivo.
Figure 9. Hsp70-mediated protection is Trif-dependent in mice.
WT or Trif−/− mice treated with Ad-Ctrl or Ad-Hsp70 and were exposed to 72h of hyperoxia. RA, room air control. A) Lung inflammation was detected by BAL cell counts. B) Lung permeability was assessed by BAL protein content. C) LDH activity assay as assessed in BAL fluid. D) Hsp70 ELISA assay in BAL fluid. E) TUNEL staining was performed on lung sections and the number of TUNEL-positive cells were quantitated and expressed as a percentage of the total number of lung cells counted on each section. The values are expressed as mean ± SD. *p<0.01 vs RA WT Ad-Ctrl mice; **p<0.01 vs corresponding Ad-Ctrl mice; #p<0.01 vs corresponding WT mice (n=5 for each group). F) Summary of Hsp70-TLR4 signaling under hyperoxia. We postulate that endothelial TLR4 is required for an adaptive mechanism that delays hyperoxic apoptosis, and prolongs survival. Hyperoxia-induced Hsp70 secretion from MLEC (white circle labeled Hsp70) or other cell type (gray circle labeled Hsp70). Extracellular Hsp70 interacted with TLR4 and transduced signal through Trif-NFκB patyway. Induced expression of Bcl-2 and decreased activation of Caspase 3 inhibit the cell apoptosis and prolong survival.
Discussion
A protective role for Hsp70 has been found in models of sepsis-related lung injury, as found in cecal ligation and puncture (CLP)-induced. Rats administered vector-driven porcine Hsp70 prior to CLP had significantly decreased lung inflammation and edema as well as reduced mortality (17). Singleton, K.D. et al. subjected Hsp70 knockout mice to CLP and found prolonged NFκB activation, increased inflammatory cytokine release and injury in lung tissue that was associated with increased mortality (19). Hsp70 has also been implicated in glutamine-mediated attenuation of lung injury during sepsis and in hypoxia-induced preconditioning to combat high altitude-associated lung injury in rats (31, 32). Our studies show for the first time that Hsp70 is a critical protective response in sterile, oxidant-induced lung injury incurred during hyperoxia exposure.
Our current studies also build upon previous reports of Hsp70-associated cytoprotection by elucidating the signaling mechanisms. Specifically, we identified Hsp70 as a functionally important TLR4 ligand in vitro and in vivo. We previously reported that TLR4 signaling is necessary to prevent hyperoxia-induced lung structural cell injury and mortality (23). Prior to our report, TLR4 deficiency was thought to be generally protective against non-infectious challenges such as endotoxin, ischemia-reperfusion and ozone (33–35). We confirmed the central role of TLR4 in maintaining lung structural integrity in a chronic model when TLR4 knockout mice were allowed to age. We detected increased levels of lung as well as systemic oxidants in TLR4 knockout lungs and circulation, eventually resulting in age-related, spontaneous emphysema (36). However, the relevant TLR4 ligand remained elusive. We invoked the role of endogenous and damage-associated TLR ligands.
Multiple endogenous TLR2 or TLR4 ligands have been described. These ligands range damage-associated molecular pattern (DAMP) molecules and matrix turn over to inflammatory mediators and lipids. Of the endogenous TLR ligands described, five are intracellular, six are matrix components, four are modified lipids or lipoproteins, and eight fall into other categories (37). DAMPs generated from injured cells and damaged matrix can activate TLR4 signaling in the absence of microbial-derived molecules (38). Less is known about endogenous TLR4 ligands in the lung. In pre-metastatic lungs, S100A8 and serum amyloid A have been described as endogenous TLR4 ligands (32). In hemorrhagic shock-resuscitation injury, oxidant-induced neutrophil activation serves an important signaling function in mediating alveolar macrophage priming and lung inflammation. The endogenous TLR4 ligand, HMGB1 was thought to mediate neutrophil oxidant activation in the lung, leading to inflammation and tissue injury (39).
Heat shock proteins are endogenous DAMPS that are released by cells during stress and injury. For example, Hsp60 and Hsp70 are thought to regulate of bacteria-induced inflammation when released (40). Extracellular Hsp70 has been shown to be released by virally infected lung airway epithelial cells and activates neutrophils via TLR4 (41). Hsp70 was shown to be released and biologically active in human and mouse lung lavage fluid and regulate airway epithelial cell cytokine expression in a TLR4 and NF-κB-dependent manner (42). In the heart, cardiomyoctes secrete Hsp70 and mediate the expression of cardiodepressant cytokines via a TLR4-dependent mechanism (43). Ischemia-reperfusion injury of the liver led to increased Hsp70 in the circulation which signaled via TLR2 and TLR4 on hepatocytes with subsequent NF-κB activation and MIP-2 induction (44). To the best of our knowledge, Hsp70-TLR4 interactions in the lung or in vivo have not been explored. We show for the first time a TLR4-Hsp70 protective pathway in lungs and endothelial cells.
Given reports that Hsp70 is a stress-response gene that can act as a “marker” for the degree of injury (45), we theorized that the exaggerated levels of Hsp70 induction during hyperoxia in TLR4−/− mice were due to the increased oxidant stress found in the setting of TLR4 deficiency, as we have previously described (36). In addition, we hypothesized that despite elevated levels of Hsp70, a putative TLR4 ligand, in the absence of TLR4 there is inadequate Hsp70 protective signaling. We confirmed our contention by showing that exogenous Hsp70 protein or adeno-Hsp70 can delay apoptosis and prolong survival of wild type as well as Hsp70-deficient cells and mice. Furthermore, the dependence of Hsp70 on TLR4 seems specific to Hsp70. Adenoviral overexpression of a well-known heat shock protein, HO1, had no rescue effect on TLR4−/− mice (23). In general, we have found that the impact of silencing heat shock proteins may be more dramatic than overexpression, depending on the specific protein. This may be a function of protein distribution, timing or level of induction achieved by intranasal adenoviral administration, the fact that hyperoxia is so toxic (100% lethal) or because there is a threshold effect of specific heat shock overexpression in lungs.
We have delineated an Hsp70-TLR4 signaling pathway that serves important cytoprotective functions using both in vitro and in vivo models of oxidant injury. Cultured lung endothelial cells and mice exposed to hyperoxia exhibit significant injury and, ultimately, death. Lung endothelial cell injury and death are critical features of hyperoxia-induced lung failure and eventual demise. We found that Hsp70 is not only induced during hyperoxia exposure but also necessary to protect against excessive oxidant generation and Caspase 3-mediated cell death in endothelial cells and lungs. Hsp70-deficient mice or endothelial cells exhibited increased injury, oxidant generation, as measured by lipid peroxidation, and death. Using adenoviral Hsp70 overexpression constructs, we were able to rescue Hsp70-deficient mice and cells from hyperoxia-induced mortality and cell death. Even wild type mice, which retain the ability to induce endogenous Hsp70 during hyperoxia challenge, demonstrated prolonged survival when Hsp70 is overexpressed compared to mice given control vector. Binding to the coxsackievirus and adenovirus receptor (CAR) mediates adenovirus endocytosis (46). Unlike epithelial cells, MLEC lack CAR. Lung epithelial cells have higher efficiency of infection with adenovirus (47) and likely also play a major role in Ad-Hsp70-mediated protection. We chose to focus on MLEC based on our previous hyperoxia studies in which MLEC signaling is important but recognize that epithelium, as a source or target of Hsp70, are likely involved as well.
We describe for the first time increased baseline levels of Hsp70 mRNA and protein expression in the lungs and endothelial cells of TLR4 knockout mice. This indicated that TLR4 is not necessary for basal Hsp70 expression and that the increased Hsp70 expression observed in TLR4-deficient cells and animals is likely a stress-response. As we have previously shown, TLR4-deficient lungs and mice have increased levels of oxidant stress, even in an unchallenged state, due to increased NADPH oxidase activity leading to systemic stress and ultimately lung destruction (36). This is distinct from that found in the colon, where TLR4 is known to be required for Hsp70 induction (48). We employed loss-of-function and gain-of-function approaches to confirm the specific roles of Hsp70 in hyperoxic lung injury and TLR4-dependent mechanisms. Hsp70 expression and induction are robust in both wild type and TLR4 knockout lung tissue and endothelial cells during hyperoxia but there is a complete lack of Hsp70-mediated cytoprotection in the absence of TLR4.
In the present study, we found recombinant Hsp70 itself has protective effects and was dependent on TLR4. Hsp70 appears to be an extracellular ligand of TLR4, although the chaperone function of Hsp70 is also an important consideration. Hyperoxia induced Hsp70 secretion and interaction with TLR4. Given that Hsp70 is released by a non classical protein transport pathway and that intact surface membrane lipid rafts are required for efficient stress-induced Hsp70 release, we used methyl-β-cyclodextrin (MBC) to test the role of secreted Hsp70 (49). MBC and soluble Hsp70 antibody abolished the protective effects of Hsp70, which implicated extracellular Hsp70 rather than intracellular Hsp70 in our model. Extracellular Hsp70, known as a “chaperokine,” has been reported to be a danger signal produced and released when cells are under stress and as activators of the immune system (49). Our data show an alternative, protective role of extracellular Hsp70.
Hsp70’s best-known function is as a molecular chaperone, by selectively binding to denatured or partially unfolded domains in polypeptides. Hsp70s are weak adenosine triphosphatases (ATPases) and cycle through low- and high-affinity substrate-binding states driven by nucleotide hydrolysis: adenosine triphosphate–(ATP)-bound Hsp70 binds substrates with low affinity and adenosine 5-diphosphate–(ADP) bound and nucleotide-free Hsp70 exhibits tight binding to substrate (50). The Hsp70 K71 mutation abolishes the ATPase function and loses affinity to substrate (24, 51). We used Hsp70 WT and Hsp70 K71E constructs to show that the adaptive functions of Hsp70 were independent of its chaperone activities.
Although we demonstrate that Hsp70 binding to MLECs requires the expression of TLR4, we have not specifically addressed the role of co-receptors or binding proteins. The mechanism of TLR4 activation is quite complex and (unlike other TLRs) involves several auxiliary proteins (LPS-binding protein, CD14) as well as a coreceptor (MD-2) (52). TLR4 ligands, such as LPS, high-mobility group box 1 (HMGB1), hyaluronan, and biglycan, have been shown to require CD14 and LPS-binding proteins (31, 53). Hsp70 likely has similar requirements. Both Hsp70 and Hsp90 have been reported to associate with TLR4 on the cell surface after bacterial stimulation and thought to serve as endogenous regulators of the innate immune response (53, 54). Our co-immunoprecipitation and co-immunofluorescence studies demonstrate binding and proximity between TLR4 and Hsp70 but additional studies, such as fluorescence resonance energy transfer, chromatography, and specific targeting of co-receptors, would be necessary to confirm the role of TLR4-associated complexes.
Moreover, we found that Hsp70-dependent TLR4 signaling requires the TLR4 adapter Trif but not the well-characterized MyD88 adapter pathway. TLR4 is the toll-like receptor known to activate two distinct signaling pathways in the production of proinflammatory cytokines or type I interferons (IFNs). MyD88 and TIRAP/Mal are essential for the former but not for the latter pathway whereas Trif/TICAM-1 and TRAM/TICAM-2 are essential for both (55). Little is known about Trif-mediated TLR4 signal transduction in response to ligands other than LPS. Imai et al. reported that avian flu-induced lung injury is triggered by the signaling of oxidized phospholipids through TLR4 and Trif in lung macrophages (56). Similarly, oxidized phospholipid, OxPAPC, promoted avian flu-induced lung injury and inflammation by activating lung macrophages via TLR4-Trif (57). Notably, both these reports showing that TLR4-Trif activation promotes lung injury, unlike our findings that Hsp70-TLR4-Trif is lung-protective, are viral infection models whereas hyperoxia is a non-microbial lung challenge. As we have shown previously, TLR4 has a distinct role in microbial and non-microbial challenges of the lungs. In addition, the type of infection also determines the role TLR4-Trif activation plays. For example, rapid Trif-dependent TLR4 activation serves important host immunity against Gram-negative bacteria (58). There appears to be tissue specificity as well. In liver Kuppfer cells, MyD88-independent, TLR4-Trif signaling promoted alcoholic steatohepatitis, whereas in liver stellate cells, TLR4 activation promoted fibrosis (59).
Despite these reports of a generally injurious, pro-inflammatory TLR4-Trif pathway, emerging evidence suggests the presence of a protective role as well. LPS preconditioning provides neuroprotection against subsequent cerebral ischemic injury through activation of TLR4 and investigators invoked a neuroprotective capacity of Trif/IRF3 signaling (60). Trif was found to contribute to otitis media pathogenesis as well as recovery (61). Similarly, targeting a Trif/IRF-3 pathway ameliorated liver dysfunction associated with chronic EtOH (62). We had previously performed survival studies of Trif−/− and found that Trif−/− mice are more susceptible to hyperoxia compared to WT (data not shown). We summarized our proposed Hsp70–TLR4–Trif signaling in MLEC and lungs during hyperoxia (Fig. 9F). We show for the first time that Hsp70 in lung endothelial cells activates a Trif-dependent pathway to promote anti-apoptotic and anti-inflammatory responses. The distinct results in TLR4-Trif activation likely are a function of the type and degree of injury as well as the specific cells and tissues involved.
In conclusion, we delineated a novel Hsp70-TLR4-Trif therapeutic axis against lethal oxidant lung injury. Both in vivo and in vitro studies using gain-of-function and loss-of-function approaches demonstrate the ability of Hsp70 to ameliorate injury and death via TLR4 and Trif. Hsp70 activates NFκB via TLR4 and significantly modulates not only cell death but also inflammatory cell recruitment and oxidative damage, which are critical to the pathogenesis of oxidant-induced respiratory failure. By demonstrating the pivotal roles of Hsp70 and TLR4 signaling during non-microbial challenges, we expanded our understanding of the critical, non-infectious roles these innate immune molecules play in the lung and provide potentially new therapeutic targets against a range of lung diseases.
Supplementary Material
Acknowledgements
We thank Dr. Evan Eisenberg (Laboratory of Cell Biology, NHLBI, NIH) for the Hsp70 K71E mutant construct.
Footnotes
P.J.L. was supported by NIH Grant HL 071595, HL 090660, AHA 0755917T.
X.Z. was supported by American Heart Heritage Affiliate Grant 0635596T.
T.K.P. was supported by R01CA129537 and R01CA154320.
References
- 1.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 2.Fisher AB. Oxygen therapy. Side effects and toxicity. Am Rev Respir Dis. 1980;122:61–69. doi: 10.1164/arrd.1980.122.5P2.61. [DOI] [PubMed] [Google Scholar]
- 3.Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposure to lethal and adaptive doses of oxygen. Am. Rev. Respir. Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 4.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 5.Qureshi ST, Zhang X, Aberg E, Bousette N, Giaid A, Shan P, Medzhitov RM, Lee PJ. Inducible Activation of TLR4 Confers Resistance to Hyperoxia-Induced Pulmonary Apoptosis. J Immunol. 2006;176:4950–4958. doi: 10.4049/jimmunol.176.8.4950. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 7.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 8.Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001;81:1461–1497. doi: 10.1152/physrev.2001.81.4.1461. [DOI] [PubMed] [Google Scholar]
- 9.Tsan MF, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004;1:274–279. [PubMed] [Google Scholar]
- 10.Ang D, Liberek K, Skowyra D, Zylicz M, Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991;266:24233–24236. [PubMed] [Google Scholar]
- 11.Huang L, Mivechi NF, Moskophidis D. Insights into regulation and function of the major stress-induced hsp70 molecular chaperone in vivo: analysis of mice with targeted gene disruption of the hsp70.1 or hsp70.3 gene. Mol Cell Biol. 2001;21:8575–8591. doi: 10.1128/MCB.21.24.8575-8591.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambellan A, Cruickshank PJ, McKenzie P, Cannady SB, Szabo K, Comhair SA, Erzurum SC. Gene expression profile of human airway epithelium induced by hyperoxia in vivo. Am J Respir Cell Mol Biol. 2006;35:424–435. doi: 10.1165/rcmb.2005-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mestril R, Chi SH, Sayen MR, O'Reilly K, Dillmann WH. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Invest. 1994;93:759–767. doi: 10.1172/JCI117030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestril R, Giordano FJ, Conde AG, Dillmann WH. Adenovirus-mediated gene transfer of a heat shock protein 70 (hsp 70i) protects against simulated ischemia. J Mol Cell Cardiol. 1996;28:2351–2358. doi: 10.1006/jmcc.1996.0228. [DOI] [PubMed] [Google Scholar]
- 17.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–806. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singleton KD, Wischmeyer PE. Effects of HSP70.1/3 gene knockout on acute respiratory distress syndrome and the inflammatory response following sepsis. Am J Physiol Lung Cell Mol Physiol. 2006;290:L956–L961. doi: 10.1152/ajplung.00466.2005. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, Xing PX, Calderwood SK, Gong J. Enhanced immunogenicity of heat shock protein 70 Peptide complexes from dendritic cell-tumor fusion cells. J Immunol. 2006;177:5946–5955. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- 21.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 22.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175:4834–4838. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- 24.Zeng XC, Bhasin S, Wu X, Lee JG, Maffi S, Nichols CJ, Lee KJ, Taylor JP, Greene LE, Eisenberg E. Hsp70 dynamics in vivo: effect of heat shock and protein aggregation. J Cell Sci. 2004;117:4991–5000. doi: 10.1242/jcs.01373. [DOI] [PubMed] [Google Scholar]
- 25.Mannam P, Zhang X, Shan P, Zhang Y, Shinn AS, Lee PJ. Endothelial MKK3 is a critical mediator of lethal murine endotoxemia and acute lung injury. J Immunol. 190:1264–1275. doi: 10.4049/jimmunol.1202012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 27.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 28.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 29.Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Shan P, Sasidhar M, Choi AMK, Lee PJ. Reactive oxygen species and ERK-mitogen activated protein kinase mediate hyperoxia-induced apoptosis in lung epithelium. Am. J. Resp. Cell Mol. Biol. 2003;28:305–315. doi: 10.1165/rcmb.2002-0156OC. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomita T, Sakurai Y, Ishibashi S, Maru Y. Imbalance of Clara cell-mediated homeostatic inflammation is involved in lung metastasis. Oncogene. 30:3429–3439. doi: 10.1038/onc.2011.53. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 34.Ke B, Shen XD, Gao F, Busuttil RW, Kupiec-Weglinski JW. Interleukin 13 gene transfer in liver ischemia and reperfusion injury: role of Stat6 and TLR4 pathways in cytoprotection. Hum Gene Ther. 2004;15:691–698. doi: 10.1089/1043034041361244. [DOI] [PubMed] [Google Scholar]
- 35.Kleeberger SR, Reddy SP, Zhang LY, Cho HY, Jedlicka AE. Toll-like receptor 4 mediates ozone-induced murine lung hyperpermeability via inducible nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L326–L333. doi: 10.1152/ajplung.2001.280.2.L326. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 38.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 3:21. doi: 10.1186/1755-1536-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 40.Bangen JM, Schade FU, Flohe SB. Diverse regulatory activity of human heat shock proteins 60 and 70 on endotoxin-induced inflammation. Biochem Biophys Res Commun. 2007;359:709–715. doi: 10.1016/j.bbrc.2007.05.167. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir Res. 2009;10:31. doi: 10.1186/1465-9921-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NF-kappaB-dependent mechanism. J Immunol. 2007;179:6318–6324. doi: 10.4049/jimmunol.179.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ao L, Zou N, Cleveland JC, Jr, Fullerton DA, Meng X. Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70. Am J Physiol Heart Circ Physiol. 2009;297:H21–H28. doi: 10.1152/ajpheart.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galloway E, Shin T, Huber N, Eismann T, Kuboki S, Schuster R, Blanchard J, Wong HR, Lentsch AB. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008;295:C514–C520. doi: 10.1152/ajpcell.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Wang W, Qian L. Hsp70 may protect cardiomyocytes from stress-induced injury by inhibiting Fas-mediated apoptosis. Cell Stress Chaperones. 2007;12:83–95. doi: 10.1379/CSC-231R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krasnykh VN, Douglas JT, van Beusechem VW. Genetic targeting of adenoviral vectors. Mol Ther. 2000;1:391–405. doi: 10.1006/mthe.2000.0062. [DOI] [PubMed] [Google Scholar]
- 47.Excoffon KJ, Gansemer ND, Mobily ME, Karp PH, Parekh KR, Zabner J. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS One. 2010;5:e9909. doi: 10.1371/journal.pone.0009909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuo K, Zhang X, Ono Y, Nagatomi R. Acute stress-induced colonic tissue HSP70 expression requires commensal bacterial components and intrinsic glucocorticoid. Brain Behav Immun. 2009;23:108–115. doi: 10.1016/j.bbi.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- 50.Shaner L, Morano KA. All in the family: atypical Hsp70 chaperones are conserved modulators of Hsp70 activity. Cell Stress Chaperones. 2007;12:1–8. doi: 10.1379/CSC-245R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajapandi T, Wu C, Eisenberg E, Greene L. Characterization of D10S and K71E mutants of human cytosolic hsp70. Biochemistry. 1998;37:7244–7250. doi: 10.1021/bi972252r. [DOI] [PubMed] [Google Scholar]
- 52.Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609–579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Triantafilou M, Sawyer D, Nor A, Vakakis E, Triantafilou K. Cell surface molecular chaperones as endogenous modulators of the innate immune response. Novartis Found Symp. 2008;291:74–79. doi: 10.1002/9780470754030.ch6. discussion 79–85, 137–140. [DOI] [PubMed] [Google Scholar]
- 54.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 55.Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun. 2008;368:94–99. doi: 10.1016/j.bbrc.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 56.Martin TR, Wurfel MM. A TRIFfic perspective on acute lung injury. Cell. 2008;133:208–210. doi: 10.1016/j.cell.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 59.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 300:G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leichtle A, Hernandez M, Pak K, Webster NJ, Wasserman SI, Ryan AF. The toll-Like receptor adaptor TRIF contributes to otitis media pathogenesis and recovery. BMC Immunol. 2009;10:45. doi: 10.1186/1471-2172-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao XJ, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, Kolls JK. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–3056. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.