Abstract
Hyaluronic acid (HA), an immunoneutral polysaccharide that is ubiquitous in the human body, is crucial for many cellular and tissue functions and has been in clinical use for over thirty years. When chemically modified, HA can be transformed into many physical forms -- viscoelastic solutions, soft or stiff hydrogels, electrospun fibers, non-woven meshes, macroporous and fibrillar sponges, flexible sheets, and nanoparticulate fluids -- for use in a range of preclinical and clinical settings. Many of these forms are derived from the chemical crosslinking of pendant reactive groups by addition/condensation chemistry or by radical polymerization. Clinical products for cell therapy and regenerative medicine require crosslinking chemistry that is compatible with the encapsulation of cells and injection into tissues. Moreover, an injectable clinical biomaterial must meet marketing, regulatory, and financial constraints to provide affordable products that can be approved, deployed to the clinic, and used by physicians. Many HA-derived hydrogels meet these criteria, and can deliver cells and therapeutic agents for tissue repair and regeneration. This progress report covers both basic concepts and recent advances in the development of HA-based hydrogels for biomedical applications.
Keywords: tissue engineering, glycosaminoglycan, cell therapy, regenerative medicine, hyaluronan, biomaterials
1. Introduction
Hyaluronic acid (HA), or hyaluronan, is a linear polysaccharide that consists of alternating units of a repeating disaccharide, β-1,4-D-glucuronic acid - β-1,3-N-acetyl-D-glucosamine. HA is a non-sulfated glycosaminoglycan, and is found throughout the body, from the vitreous of the eye to the extracellular matrix (ECM) of cartilage tissues.[1] HA, a highly hydrated polyanionic macromolecule, exists with molecular weights from 100,000 in serum to 8,000,000 Da in the vitreous. HA is an essential component of the ECM, in which its structural and biological properties mediate its activity in cellular signaling, wound repair, morphogenesis, and matrix organization.[2, 3] Additionally, HA is rapidly turned over in the body by hyaluronidase, with tissue half-lives ranging from hours to days.[4] HA and its derivatives have been clinically used as medical products for over three decades.[5] More recently, HA has become recognized as an important building block for the creation of new biomaterials with utility in tissue engineering and regenerative medicine. [6–9]
HA can be modified through numerous means to alter the properties of resulting materials, including related to hydrophobicity and biological activity.[10] Chemical modifications of HA have been extensively reviewed,[11] and target three functional groups: the glucuronic acid carboxylic acid, the primary and secondary hydroxyl groups, and the N-acetyl group (following deamidation). Most prominently, carboxylates have been modified by carbodiimide-mediated reactions, esterification, and amidation; hydroxyls have been modified by etherification, divinylsulfone crosslinking, esterification, and bis-epoxide crosslinking.
These HA derivatives fall into two primary categories: “monolithic” and “living”.[12] Monolithic HA derivatives are “terminally modified” forms of HA that cannot form new chemical bonds in the presence of cells or tissues, and must be processed and fabricated into different forms. In contrast, living derivatives of HA can form new covalent bonds in the presence of cells, tissues, and therapeutic agents. In most cases, living HA derivatives are required for clinical and preclinical uses in 3-D cell culture and in vivo cell delivery.[13] Nonetheless, caution is required to ensure biological compatibility of the crosslinking chemistry, as well as to establish that the reagents and byproducts are benign in both the short- and long-term. The last decade has seen the development of a growing number of living HA derivatives with clinical potential, which will be the general focus of this progress report.
2. Clinical Biomaterials Derived from HA
Traditionally, tissue biology has inspired chemists, physicians, and engineers to develop innovative technologies that ever more closely approximate the architecture and biological complexity of a given target organ. This focus on elegant technology has not been entirely successful in the marketplace.[14] An alternative approach has been advocated recently,[7, 13] in which the chemical, mechanical, and biological criteria for clinical biomaterials are integrated from the outset with market research. That is, products should be simple for use by physicians under potentially stressful situations. Research in the laboratory should, from the very beginning, be informed by downstream concerns of manufacturing, scalability, economics, regulatory approval pathways, business and reimbursement models, product form and usage, and most importantly, by “market pull” - the unmet needs of physicians and their patients.
For many of these reasons, we have argued that successful clinical biomaterial products should be simple and have defined chemical compositions that can be easily used by physicians.[7] Many commonly used synthetic materials meet these criteria, yet most elicit some degree of inflammatory response, lack an intrinsic biological interaction with delivered cells and host tissues, and are cleared by non-biological degradation mechanisms. In contrast, biomaterials based on chemically-modified biopolymers offer intrinsic biodegradation pathways and recognition by biological systems. Below we describe the progress in creating clinical biomaterials based on living HA derivatives, first by using addition and condensation reactions, second by photochemically-induced radical polymerization, and then by combinations of these methods.
3. Chemical Reactions of HA derivatives
3.1. Chemistry
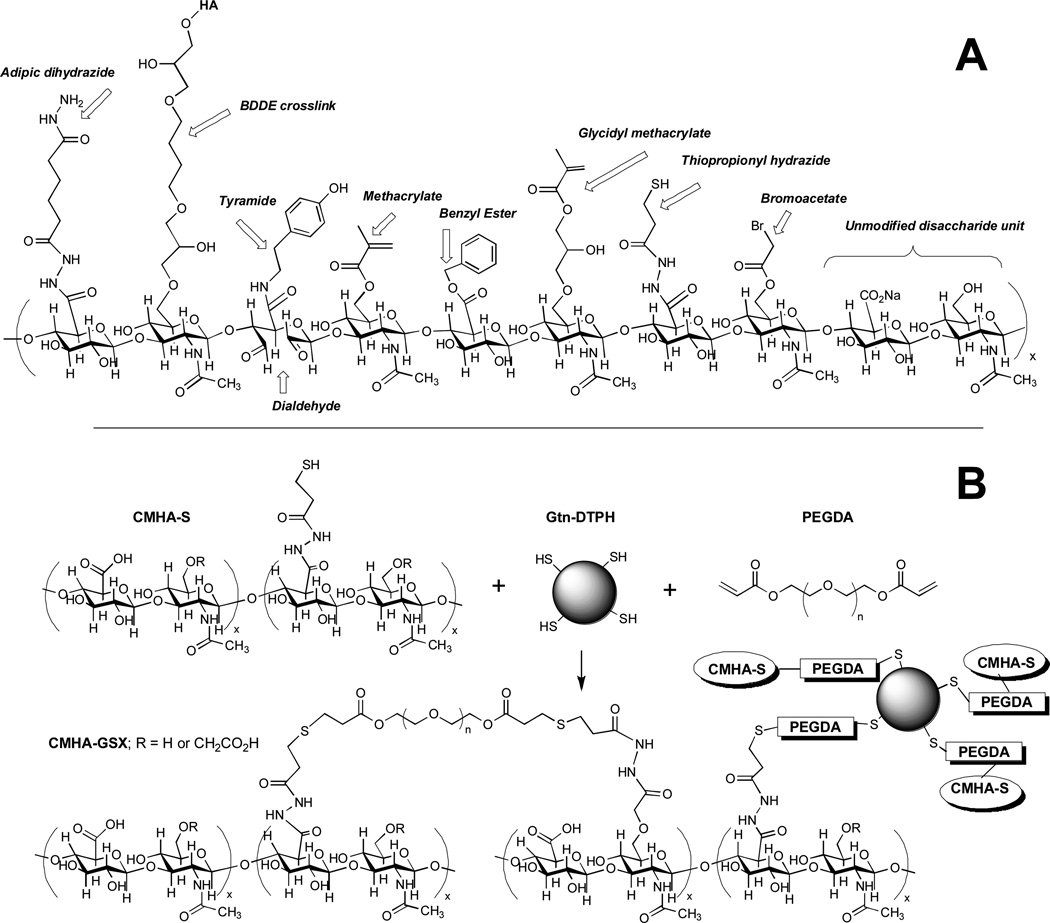
Figure 1 shows a composite structure of an HA decasaccharide containing selected chemical modifications of the carboxylic acid of the glucuronic acid moiety or the C-6 hydroxyl group of the N-acetylglucosamine sugar. The modifications include some monolithic modifications, such as the benzyl ester and BDDE crosslink; the majority of the others represent living HA derivatives that can be further modified or crosslinked in the presence of cells and tissues. At far right is an unmodified disaccharide unit of HA.
Figure 1. Chemical modifications of HA.
(A) A hypothetical composite structure illustrating selected primary modifications discussed herein: adipic dihydrazide for use in further crosslinking via acrylamide or hydrazone linkages; butane-1,4-diol diglycidyl ether, a prototypical monolithic crosslinker for HA; tyramide for peroxidase crosslinking; dialdehyde obtained by periodate oxidation; methacrylate on primary 6-hydroxyl group; benzyl ester; glycidyl methacrylate; thiopropionyl hydrazide from DTPH modification; bromoacetate; an unmodified disaccharide unit for comparison. (B) A thioether crosslinked semi-synthetic ECM formed by crosslinking thiol-modified carboxymethyl HA (CMHA-S) with thiol-modified gelatin using the bifunctional crosslinker, PEGDA.
3.1.1. Thiol-modified HA
To create modular, clinically versatile and readily-manufactured synthetic extracellular matrices (sECMs) for use in drug evaluation and regenerative medicine,[15, 16] we developed a thiol-introduction chemistry based upon the modification of the carboxylate groups of glycosaminoglycans (GAGs) and polypeptides using hydrazide reagents containing a disulfide bond.[17, 18] Thiol-modified macromonomers spontaneously, but slowly, crosslinked in air only to a hydrogel; this gel could be dried to give a thin film or lyophilized to produce a porous sponge.[19] Alternatively, crosslinking with difunctional electrophiles[20] could be accomplished, in the presence or absence of cells, to give injectable and biocompatible hydrogels (Figure 1). The mechanical properties and rates of biodegradation can be altered by several varying parameters:[21] (i) molecular weight of starting HA employed; (ii) percentage of thiol modification; (iii) concentrations of thiolated HA and thiolated gelatin; (iv) molecular weight of the crosslinker poly(ethylene glycol) diacrylate (PEGDA); and (v) ratio of thiols to acrylates.
3.1.2. Haloacetate-modified HA
HA bromoacetate (HABA) with a degree of substitution (SD) of 18% was synthesized in aqueous solution using excess bromoacetic anhydride.[22] This modification is shown in Figure 1. The reaction occurred almost exclusively on the more reactive primary 6-hydroxy groups of the N-acetylglucosamine residues. Using HABA as a polyvalent electrophile, reaction of thiol-modified HA (with or without thiol-modified gelatin) resulted in biocompatible crosslinker-free HA hydrogels. Cells failed to proliferate on hydrogels lacking gelatin, but showed attachment and viability on the gelatin-containing hydrogels similar to the sECM Extracel.
3.1.3. Dihydrazide modified HA
The original hydrazide modification employed adipic dihydrazide (ADH),[23] and later other mono- and polyhydrazides,[24] to create living HA derivatives (Figure 1). HA-ADH has often been employed subsequently, as it is capable of forming hydrazone linkages with ketones and aldehydes, as well as acylhydrazides with acylating agents, thereby allowing crosslinking, addition of hydrophobic groups, and attachment of drugs or polypeptides.
3.1.4. Aldehyde-modified HA
Doubly crosslinked networks composed of HA microgels and crosslinked hydrogels with tunable viscoelasticity in the relevant frequency range have also been proposed for vocal fold healing. These partially monolithic and partially living materials feature divinylsulfone-crosslinked HA particles that have been oxidized with periodate that produce surface aldehyde functionalities (Figure 1). Addition of a solution of HA-ADH effectively formed a double-crosslinked network (DXN), entrapping the stiffer HA-DVS particles in a compliant and stable elastic gel. These DXNs become stiffer at higher frequencies, and the DXNs have a structural hierarchy and mechanical properties suitable for soft tissue repair.[25, 26] A representative micrograph of the DXN gels is shown in Figure 2.
Figure 2. Double-crosslinked HA hydrogels.
Hydrogels formed from the crosslinking of particles in a secondary network leading to hierarchical networks with unique microstructures. Reprinted with permission from [26].
3.1.5. Tyramine-modified HA
A living hydrogel utilizing enzymatic in situ crosslinking was recently described in which coupling of tyramine to a small percentage of the HA carboxylates produced an HA-tyramide (Figure 1).[27] Crosslinking was induced with the addition of hydrogen peroxide to solutions of HA-tyramide to which horseradish peroxidase (HRP) was added, either in the presence or absence of cells. The resulting peroxidase reaction formed phenolate radicals that isomerized and dimerized to form C-C bonded and fluorescent dityramine adducts as robust hydrogel crosslinks. However, both the use of HRP and peroxide may be problematic from a regulatory point of view for development of a clinical product for cell delivery.
3.1.5. Huisgen cycloaddition (click chemistry)
The Huisgen cycloaddition reaction of azides with alkynes to produce triazoles, or “click chemistry,” was used to produce HA hydrogels and to encapsulate yeast cells during crosslinking.[28] The HA carboxylates were modified using carbodiimide chemistry either as propargyl amides or as 11-azido-triethyleneglycol amides. The hydrogel was formed at room temperature with 0.01% CuCl as a catalyst. This first “clicked” HA hydrogel is clinically impractical because of the complexity of the chemistry and toxicity of preparation. However, direct encapsulation of cells in a clicked PEG-peptide hydrogel was achieved using macromolecular alkyne and azide precursors in combination with a copper-free difluorocyclooctyne click chemistry.[29] This new result suggests a potential alternative approach for preparing clickable, biocompatible, and functionally more complex HA hydrogels.
3.2. Applications
3.2.1. Cell delivery
In the first preclinical use of the sECM formed from PEGDA-crosslinked thiolated HA and gelatin for cell therapy, mesenchymal stem cells were delivered to full-thickness defects in the patellar groove of rabbit femoral articular cartilage. After 12 weeks, defects were completely repaired and the sECM remodeled, showing trabecular bone in the osteal portion of the defect, and integrated, translucent zonated cartilage in the chondral region of the defect.[30] The primary role of the sECM appeared to be cell retention, thereby enhancing the natural biological repair processes mediated by endogenous cells. In a more recent study, chondrogenic cells derived from human embryonic stem cells (H9 hESCs) encapsulated in the commercial sECM Extracel gave functional repair of an osteochondral defect in a rat model. Orderly spatial and temporal remodeling took place over 12 weeks, affording characteristic architecture features including hyaline-like neocartilage integrated with existing cartilage and regenerated subchondral bone.[31]
Design criteria have been established for bioartificial stem cell niches intended to provide microenvironments for expansion of stem cells and maintenance of their undifferentiated phenotype.[9] Embryonic endothelial progenitor cells (eEPC, murine) were encapsulated into HA-hydrogels (HyStem-C/Extracel) to create a bioartifical stem cell niche.[32] Thus, implantation of the eEPC-hydrogel into mice with drug-induced nephropathy or renal ischemia allowed eEPC mobilization to injured kidneys and improved renal function relative to cells delivered in buffer. HA hydrogels protected eEPC against adriamycin cytotoxicity and implantation of eEPC in the sECM supported renal regeneration in ischemic and cytotoxic nephropathy, and promoted neovascularization of an ischemic hindlimb.[32]
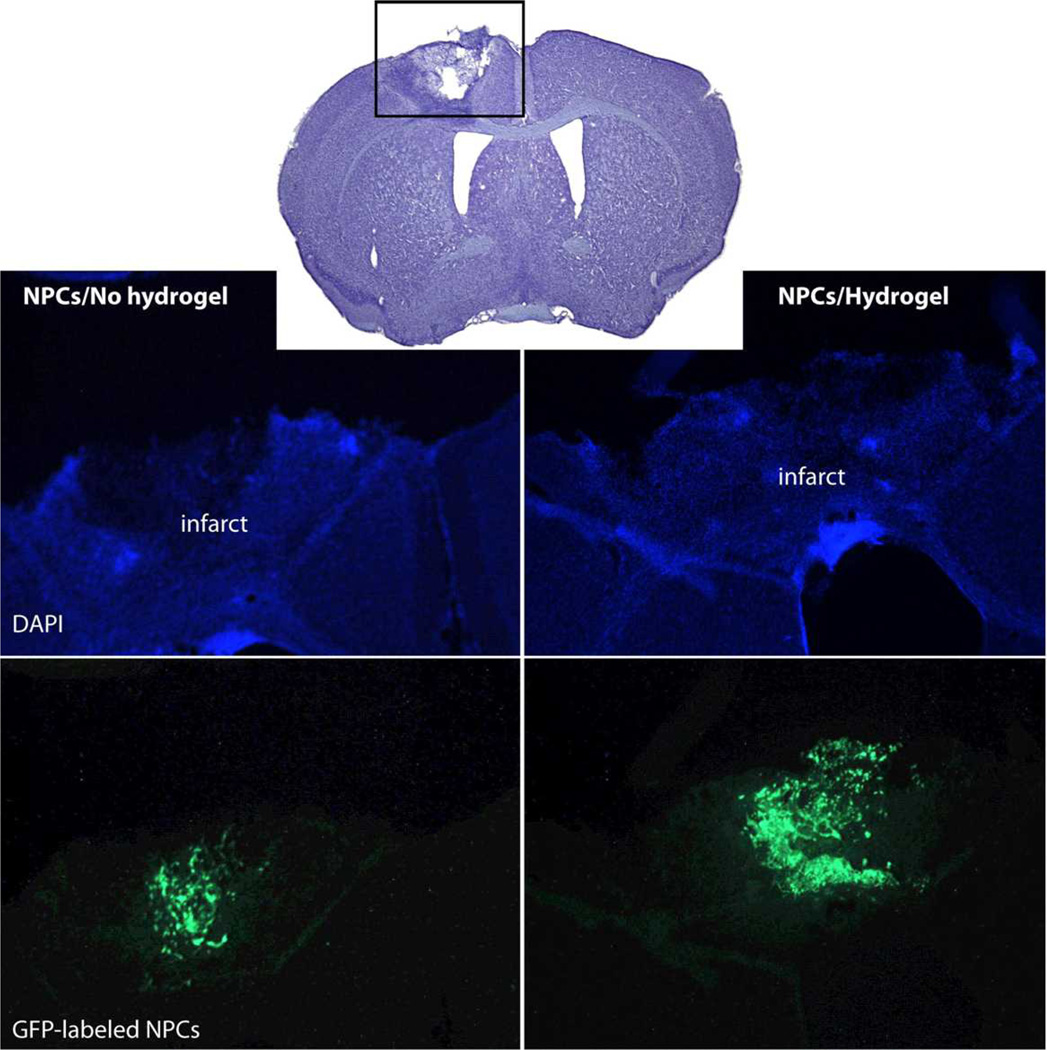
The sECM hydrogel composed of crosslinked thiol-modified heparin, gelatin, and HA (HyStem-HP) significantly promoted the survival of two neural progenitor cell (NPC) lines in vitro under conditions of stress, and in vivo delivery into the cavity of a stroke-infarcted brain (Figure 3).[33] Cell survival was improved, glial scar formation was reduced, and local inflammation was minimized for hydrogel-delivered cells in comparison to NPCs delivered in buffer only. Thus, stem cell transplantation into the infarct cavity within a pro-survival hydrogel matrix may provide a translational therapy for stroke recovery.[33]
Figure 3. Repair of stroke infarct by HA hydrogel-encapsulated NPCs.
HyStem-HP gel significantly increased survival and proliferation of murine GFP-tagged embryonic cortex-derived neural progenitor cells (NPCs) injected into the infarct cavity after a photochemically-induced stroke in mouse brain [33]. Of 100,000 injected NPCs, 4000 survived in buffer while 8000 survived in HyStem-HP (p = 0.035). New figure provided by Drs. J. Zhong and S. T. Carmichael.
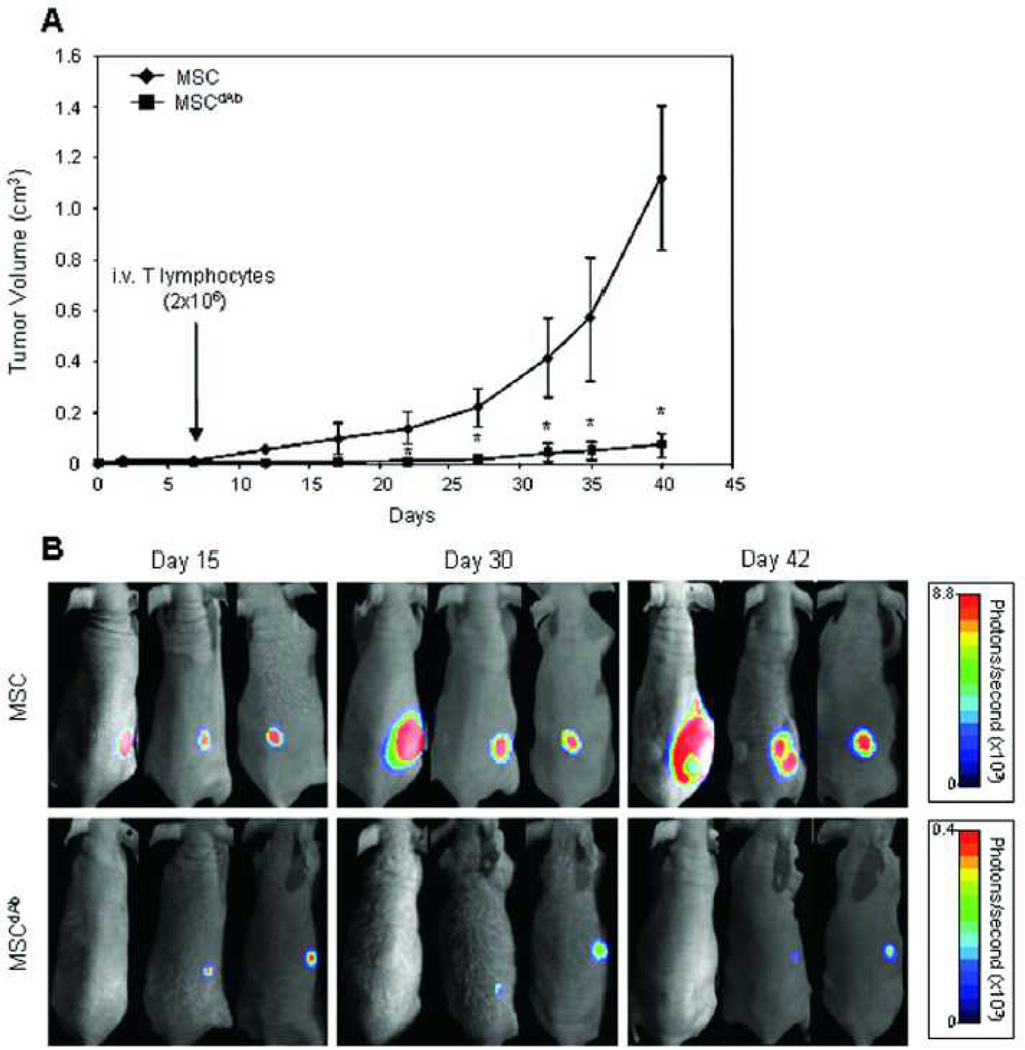
In another example, human MSCs expressing a therapeutic diabody were seeded into the sECM hydrogel Extracel-X and injected subcutaneously into nude mice. The human MSCs had been genetically engineered for the production of a bispecific diabody, and the locally-produced therapeutic antibody showed systemic anti-tumor effects on HCT-116 tumors (Figure 4). In addition, tumors in which wild-type MSCs were co-cultured with HCT-116 colon cancer cells produced 2.5 X larger tumors after 40 days than HCT-116 cells in Extracel-X alone.[34]
Figure 4. Delivery of therapeutic antibody-releasing MSCs reduces tumors.
Co-encapsulation of wild-type MSCs and HCT-116 colon cancer cells in Extracel-X resulted in robust tumor growth (top). In contrast, use of diabody-releasing MSCs with HCT-116 dramatically suppressed tumor growth. Reprinted with permission from [32].
3.2.2. Molecule delivery
The most common use of sECM hydrogels is for spatiotemporal control over growth factor release. Growth factors are expensive, diffuse away from sites of administration, and are very rapidly degraded by proteolysis in vitro and in vivo. Moreover, a suite of growth factors is often required to recapitulate a desired biologic outcome. To this end, sECMs were developed by co-crosslinking thiolated HA with thiol-modified heparin, creating an immobilized heparin that acted as a mimic of a heparan sulfate proteoglycan.[35] Cell growth and rates of neovascularization were increased in this sECM, which had a half-life for basic fibroblast growth factor (bFGF) release of over one month in vitro.[35] By varying the thiolated GAG composition, and by adding thiolated gelatin, different release rates were realized for a variety of growth factors.[36] VEGF, bFGF, angiopoietin-1, and keratinocyte growth factor (KGF) each increased microvessel density and maturity and in many cases exhibited synergistic effects when incorporated in Extracel-HP, a product that combines covalently modified heparin into the HA-gelatin sECM. [37, 38] Optimal vascularization and vascular maturation using films implanted in mouse ear pinnae in vivo was accomplished by dual release of VEGF and KGF.[37, 38]
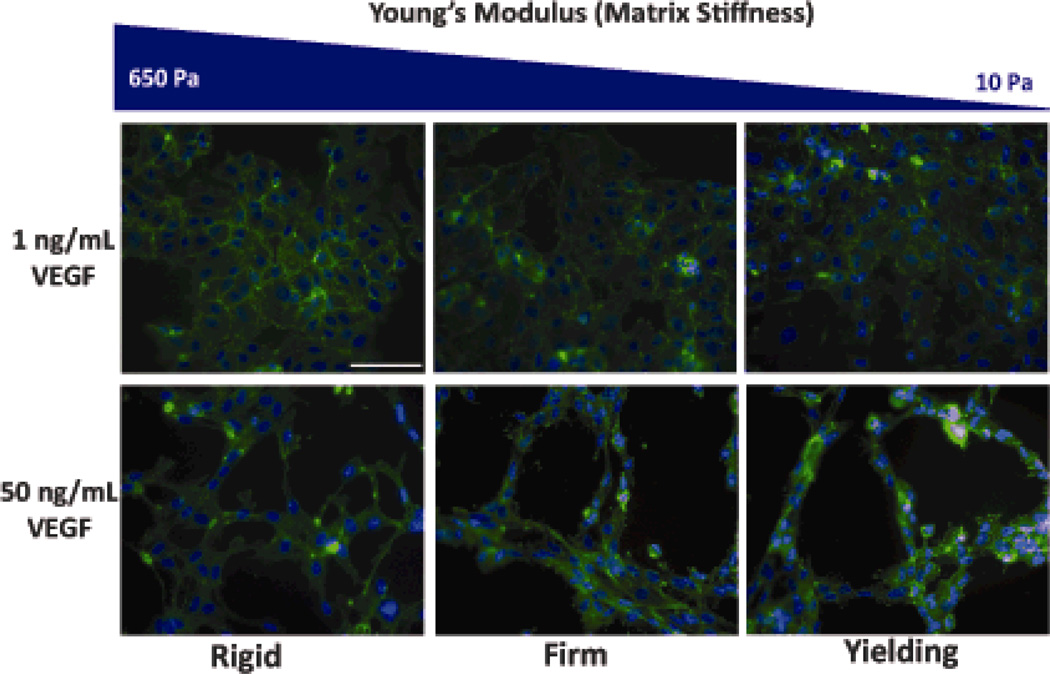
In addition to growth factors, the mechanical environment of the ECM is crucial for vasculogenesis. For example, VEGF and substrate mechanics co-regulated tubulogenesis by endothelial progenitor cells (EPCs) encapsulated in Extracel-HP (Figure 5). Higher VEGF and softer gels promoted EPC migration, increased cellular elongation, and increased lumenization by EPCs in vitro.[39]
Figure 5. Mechanical sensitivity of cells to HA hydrogels.
Endothelial progenitor cells cultured on HA gels at a range of mechanical properties and two concentrations of VEGF. Capillary-like structures only formed on gels at the higher VEGF concentration and the morphology (e.g., tube length, area, and thickness) was dependent on the gel mechanical properties. Reprinted with permission from [39].
An alternative to cell delivery per se is to attract endogenous stem cells and precursor cells to the defect site for de novo tissue regeneration. Hepatocyte growth factor (HGF) induces migration of MSCs in vitro but is rapidly degraded in vivo. Extended, localized delivery of HGF was achieved using sECM hydrogels containing Heprasil, and an sECM composition for controlled release of HGF resulted in recruitment of human bone marrow MSCs into a scaffold in vitro.[40]
Hydrogels alone lack the robustness required for many applications in tissue engineering. Recently, a hybrid biomaterial was created by electrospinning of poly (ε-caprolactone)-collagen (PCL/Col) microfibers with concomitant electrospraying of the thiolated HA-heparin product Heprasil™.[41] VEGF165 and PDGF-BB were released in biologically active form over a period of five weeks in vitro. These hybrid meshes allowed co-cultured human umbilical vein endothelial cells and lung fibroblasts to attach and infiltrate into the mesh, thereby recapitulating a primitive vascular network within the architecture of the scaffold. [41]
Nanoporous HA-hydrogel microparticles (10 mm) prepared by crosslinking of HA-aldehyde with HA-ADH, were grafted with a perlecan domain with HS chains.[42] The perlecan domain-conjugated HA hydrogel particles provided a release system for bone morphogenetic protein 2 (BMP-2), and stimulated robust cartilage-specific ECM production.
3.2.3. Cell expansion and recovery
It is frequently desirable to recover cells for analysis or subsequent culture following encapsulation and expansion. With the sECMs based on thiolated HA, we enabled rapid recovery of cells expanded in 3-D by incorporating disulfide groups within the PEGDA crosslinkers. [43] The triblock PEGSSDA contained a single disuflide-containing block, and cells were released from PEGSSDA crosslinked sECMs using a one hour incubation with 25 mM N-acetyl-cysteine (NAcCys) or glutathione to induce a thiol-disulfide reaction. The sECM simply dissolves, permitting cell recovery under non-enzymatic conditions. NIH 3T3 fibroblasts, HepG2 C3A hepatocytes, bone marrow-derived mesenchymal stem cells (MSCs), and human umbilical vein endothelial cells (HUVECs) all showed excellent viability and growth during expansion in 3-D and following cell recovery by gentle centrifugation.
An alternative to 3-D encapsulation is the use of sECMs in a microparticulate 3-D on top modality. Porous microcarrier beads were infused with a solution of thiolated sECM components, which then crosslinked by disulfide bond formation. Following cell proliferation in 3-D in a rotating wall vessel (RWV) bioreactor designed to mimic the low fluid shear stress environments in the body, human intestinal epithelial cells (Int 407) formed multilayered cell aggregates on the sECM beads. Cell clusters were harvested using N-acetyl cysteine to dissolve the gel, and were further expanded in a scaffold-free state in the RWV bioreactor to produce spheroidal microtissues that have utility for studying host-pathogen interactions, evaluating new therapeutic agents, and creating clusters for bioprinting and cell therapy.[44]
Human hepatoblasts (hHBs) and human hepatic stem cells (hHpSCs) were maintained on plastic versus thiol-modified HA hydrogels mixed with specific combinations of extracellular matrix components (e.g., type I collagen and laminin). NMR spectroscopy was used to define metabolomic profiles for each substratum tested. Both hHpSCs and hHBs survived and expanded in all soft disulfide-bonded Glycosil (thiolated HA) hydrogel-matrix combinations tested for more than 4 weeks. The metabolomic profiles indicated that hHpSCs on plastic remained as stem cells, while those in hydrogels were primarily hHBs, expressing AFP, albumin, and urea. Variations in hyaluronan-matrix chemistry resulted in distinct profiles correlating with growth or with differentiative responses.[45] In a related study, human fetal liver cells were embedded in the same HA-based sECM, disulfide-crosslinked Glycosil, with the hydrogel contained within the capillary system of a three-dimensional perfusion bioreactor. The culture model incorporating three-dimensionality, constant perfusion, and integral oxygenation in combination with a thiolated HA-based hydrogel provided the best conditions for liver cell survival and differentiation.[46] Earlier we had found that primary rat hepatocytes cultured in thiol-modified HA and gelatin retained cytochrome P-450 activity, a key metabolic function for drug testing models.[15]
Finally, hESCs grown on a soft hydrogel (Extracel-HP) substrate showed reduced vimentin levels than hESCs cultured on Matrigel or on murine embryonic fibroblast layers. The soft HA-rich hydrogels also maintained other proteomic and morphological indicators characteristic of 3D culture and superior to that of feeder layers. The expression of vimentin exemplifies a stress-induced response by hESCs to growth on stiff substrata.[47] Combining these observations with the techniques for cell expansion and recovery suggests that soft HA-rich matrices have the potential for clinical-grade stem cell expansion, differentiation, and implantation in regenerative medicine. This potential also extends to photochemically crosslinked HA hydrogels, as will be demonstrated below.
3.2.4. Drug evaluation and tumor models
Using in situ crosslinkable sECM hydrogels, cancer cells were encapsulated and injected in vivo, introducing a “tumor engineering” strategy for creation of orthotopic xenografts.[48] These orthotopic tumor models in immune compromised mice have utility for drug development, cancer research, and potential applications in personalized medicine. Engineered tumors showed improved „take“ for various cell lines, more consistent tumor size, better tissue integration and vascularization (with reduced necrosis), better control of tumor location, and generally improved animal health compared with cell injection in serum free medium.[48] Tumor growth and metastasis were also enhanced in a pancreatic adenocarcinoma model.[49] To date, human cancer lines have been injected in CMHA-S with gelatin: colon (HCT-116, Caco-2), breast (MCF-7, Sk-Br-3, MDA-MB-231, MDA-MB-468), ovarian (OVCAR-3, SK-OV-3, pancreatic (MiaPaCa-2), and lung (A-549). In addition, mice implanted with tumors in which wild-type human MSCs were co-injected with HCT-116 colon cancer cells in Extracel-X produced 2.5 X larger tumors after 40 days, when compared to mice injected with HCT-116 cells in Extracel-X alone (see Figure 4).[34]
Novel cell types and cell aggregates have been generated using HA-rich hydrogel matrices. In one study, tumor-like stem cells derived from human keloid (keloid precursor cells, KPCs) were suspended in Extracel-HP containing IL-6 or IL-17 and implanted subcutaneously in immune compromised mice. This inflammatory niche contributed to a benign tumor-like stem cell phenotype of the KPCs characterized by uncontrolled self-renewal and increased proliferation. Modification of this pathological stem cell niche with anti-cytokine antibodies had an anti-tumor effect.[50] In another study, Extracel was used to create a Matrigel-free 3-D environment for the production of tumor spheroids in a microgravity environment in a high aspect ratio vessel bioreactor. The co-culture of keratinocytes and melanoma cells in an HA-rich sECM demonstrated the potential for reducing the use of laboratory animals in anti-tumor drug evaluation.[51]
Finally, engineered tumors can be used to examine responses to newly-developed signal transduction modifiers that modulate the lysolipid signaling pathway.[52] First, we showed that BrP-LPA, a novel dual function LPA antagonist/ATX inhibitor (LPAa/ATXi), inhibited growth and angiogenesis in MB-231 breast tumors grown in Extracel.[53] Second, we showed that the engineering of tumors from non-small cell lung cancer (NSCLC) cells, required re-formulation to use Extracel-HP with added growth factors. Reproducibly-sized subcutaneous lung tumors were formed, and growth and vascularization were inhibited by the LPAa/ATXi. We also used Extracel to deliver HCT-116 colon cancer cells directly in the liver of a nude mouse, mimicking a colon cancer metastasis site. The LPAa/ATXi agent also significantly reduced tumor growth and angiognesis in this model. Taken together, these improved, more realistic xenografts show considerable utility for evaluating the potential of novel anti-metastatic, anti-proliferative, and anti-angiogenic compounds that modify signal transduction through the LPA signaling pathway.
3.2.5. Effects of matrix elasticity
Using a physiologically relevant ECM mimic composed of crosslinked thiolated HA and fibronectin domains, adult human dermal fibroblasts modified their mechanical response in order to match substrate stiffness. That is, cells on stiffer substrates had higher modulus and a more stretched and organized actin cytoskeleton, which translated into larger traction forces exerted on the substrate.[54] Similarly, migration of human dermal fibroblasts (HDFs) was examined with the same HA-fibronectin hydrogels. Traction stresses were observed to be a sensitive indicator of the modulus of the hydrogel substrate, as determined by crosslinking density within the hydrogel. Moreover, the traction stresses caused by cell migration led to nuclear distortion.[55]
Experimental control of both composition and gel stiffness is possible with thiolated HA and gelatin crosslinked with PEGDA, and mechanical properties of the sECM largely determine the resulting cell phenotype. The rheology of Extracel sECM hydrogels spanning three orders of magnitude of storage shear modulus, from 11 Pa to 3.5 kPa, was examined, since this is the critical range for engineering of soft tissues. The concentration of the chemically modified HA and the cross-linking density were the main determinants of gel stiffness. Increasing the ratio of thiol-modified gelatin reduced gel stiffness by diluting the effective concentration of the HA component.[21]
Finally, human bone-marrow-derived multipotent MSCs were cultured on crosslinked sECM hydrogels (Extracel) with compliance values that matched muscle or brain tissue elasticity. Over 90 MSC-secreted cytokines and growth factors were measured, and many exhibited elasticity-dependent expression. For example, IL-8 was 90-fold up-regulated on hard surfaces relative to soft surfaces.[56]
3.3. Processing
3.3.1. Centrifugal casting
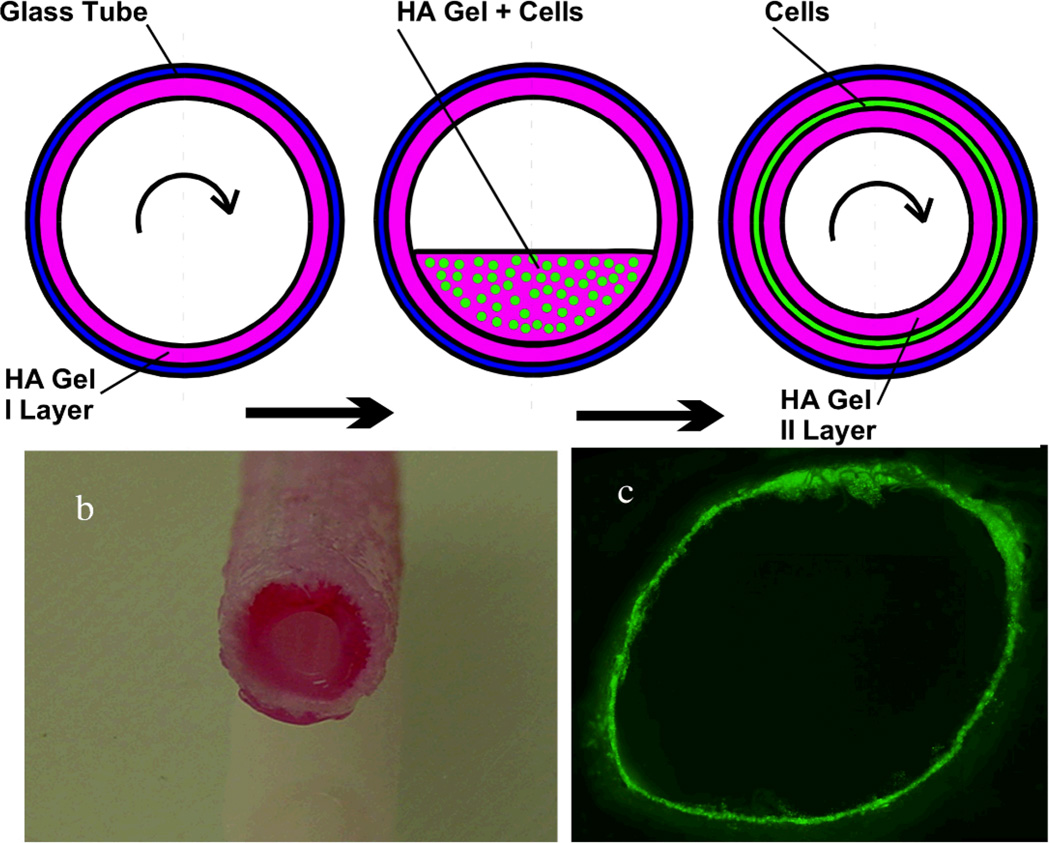
Centrifugal casting of sECM encapsulated endothelial cells was first employed create a variety of tubular constructs, allowing crosslinking of the living thiolated macromolecules to occur during axial spinning of a tube containing a suspension of endothelial cells (Figure 6).[57] Subsequently, example 5 mm vessels with highly viable cell densities were created from small intestine submucosa tubular scaffolds with laser-machined micropores.[58] Indeed, the abundance of tubular tissues in the human body – from capillaries to bones, GI tract, kidney tubules, genitourinary structures – suggests that centrifugal casting could have an important impact on tissue engineering with living sECMs.[59]
Figure 6. Centrifugal casting of cells in hollow hydrogel cylinders.
(Top) The inner walls of a capillary tube or dacron vascular prosthesis are precoated with the Extracel sECM by axial rotation at 2000 rpm (11.2 × g) for 10 min to effect uniform coating during crosslinking and gelation. Then, cells are entrapped between two sECM layers by repeating the process with a cell suspension, giving a concentric sandwich construct. Panels b shows a gel-coated dacron vascular graft, and panel c shows GFP-labeled QCE-6 quail vascular progenitor cells. Partially reprinted with permission from [57].
3.3.2. Electrospinning and electrospraying
Thiolated HA has been electrospun into three-dimensional nanofibrous scaffolds with PEO as a leachable diluent, and the fibers were crosslinked with PEDGA prior to removal of PEO. The fibrous scaffold was coated with fibronectin and seeded with fibroblasts, which attached and spread to a dendritic morphology. [60]
A common drawback of electrospun scaffolds is the poor cellular infiltration into the structure. To address this, micron-sized fibers electrospun fibers were combined with co-deposition of the thiolated HA-heparin product Heprasil. The resulting µPCL/Col fibers co-electrosprayed with Heprasil showed optimal penetration of fetal osteoblasts.[61] Additional examples of electrospun photoactivatable HA-derived materials are described below.
3.3.3. Bioprinting
Bioprinting is an approach to tissue engineering that employs layer-by-layer robotic biofabrication of three-dimensional (3-D) constructs to create functional living macrotissues,[62] depositing “bio-ink” (cell aggregates or spheroids) and “bio-paper” (scaffold materials) into predesigned 3-D organizations.[59, 63, 64] The success of bioprinting so far has been limited by a paucity of biomaterials that are compatible with printing devices, having the optimal balance of robustness, extrudability, cytocompatibility, and biodegradability.
A four-armed polyethylene glycol 3400 tetracrylate, TetraPAc13, was recently used to co-crosslink thiolated HA and gelatin derivatives into biocompatible, extrudable sECM hydrogels.[65] A high-density suspension of NIH3T3 cells in a 2% (w/v) TetraPAc13-crosslinked sECM hydrogel (9% cell mass/hydrogel volume, 25 million cells/mL) afforded an extrudable hydrogel that could be printed from microcapillaries into macrofilaments that held their shape during and after bioprinting. Cellularized tubular constructs akin to simplified blood vessel-like structures were fabricated with a rapid prototyping device, and these structures maintained viability in culture for up to 4 weeks.[65] Functional blood vessel structures and branched vascular networks should be accessible using this bioprinting strategy.
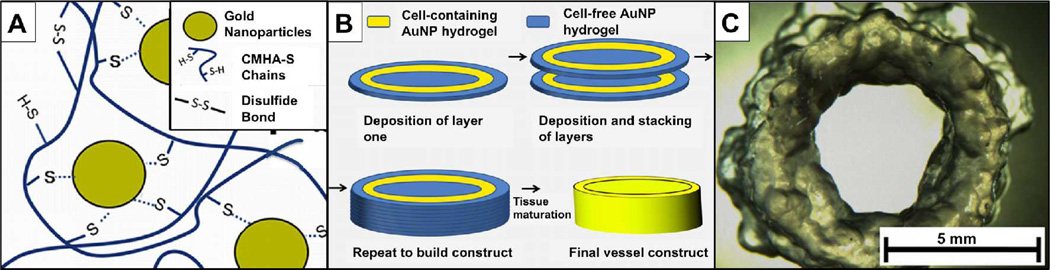
Most recently, gold nanoparticles (AuNP) were employed as multifunctional crosslinkers for the thiol-modified macromonomers comprising the sECM hydrogels described above (Figure 7). These AuNP-crosslinked HA-gelatin sECM hydrogels exhibited a new and unusual property that was called “dynamic crosslinking.”[66] The initially-formed hydrogel macrofilaments extruded from a syringe were held together by intra-gel crosslinks. Within hours, the extruded gel filaments formed inter-gel crosslinks, leading to fusion of the macrofilaments. For bioprinting, when cellularized AuNP-crosslinked sECM hydrogels were bioprinted, the dynamic crosslinking facilitated cell growth and maturation within the printed constructs. After maturation of the construct, the addition of NAcCys effectively dissolved the hydrogel and only cell-secreted ECM remained. Additional bioprintable photoactivatable HA-gelatin based hydrogels are described below.
Figure 7. Dynamic crosslinking of macromolecular thiols with gold nanoparticles.
(A) Au-NPs act as multivalent crosslinkers for thiol-modified HA. (B) Bioprinting consists of deposition of acellular AuNP-CMHA-S gels (blue) and cell-containing AuNP-CMHA-S/Gelatin-DTPH gels to produce a cylindrical structure. (C) A tubular cellularized construct printed without the central core outer annulus of the acellular gel. Partially reprinted with permission from [66].
4. Photopolymerization and Electropolymerization Reactions to Form HA Hydrogels
Another area that has found widespread use is the application of radical polymerization for the formation of HA-based hydrogels. Radical polymerizations are used currently in clinical settings for the in situ formation of biomaterials, such as bone cements and for the filling of caries in dental applications.[67] Radical polymerizations involve the formation of a radical through some initiation source (e.g., light, temperature, redox reaction) that reacts with a reactive group on the HA macromer to form kinetic chains. As long as there is greater than one reactive group on the HA macromer, a gel forms. As reviewed elsewhere, [68] there are numerous advantages to radical polymerizations, including the controllability of the reactions and the ability to react in the presence of aqueous solutions.
Photoinitiated polymerizations are the most common example of radical polymerizations being applied to the formation of HA hydrogels. Photopolymerization is advantages due to the temporal and spatial control that is afforded by using light as the initiation trigger.[68] For example, control of light with lasers and masks can be used to spatially control crosslinking of hydrogels, leading to advanced hydrogel systems and complex biomaterials. Direct cellular encapsulation is also possible as long as the initiation conditions are mild enough, so that radical concentrations or light intensities are not detrimental to the viability of cells.[69, 70]
4.1. Chemistry
4.1.1. Reaction with methacrylic anhydride
Acrylates and methacrylates are the most common reactive groups for use in radical polymerizations since they react rapidly with radicals. Fortunately, the HA backbone presents several groups for modifications, including carboxyls and acids. One of the simplest and most widely used reactions for HA modification is the simple reaction of HA with methacrylic anhydride under basic conditions to form a methacrylated HA (MeHA) [71, 72]. The structure of MeHA is illustrated in Figure 1, and additional derivatives are shown in Figure 8. This reaction was first used by Grinstaff and coworkers [71, 72] to modify both HA and alginate and although the reaction is not efficient, it is simple and effective. One application that was initially pursued with this group of materials was the sealing of corneal lacerations, which was very successful [73]. As will be discussed later, this MeHA system has been applied to many other applications from scaffolds for tissue regeneration to microdevices.
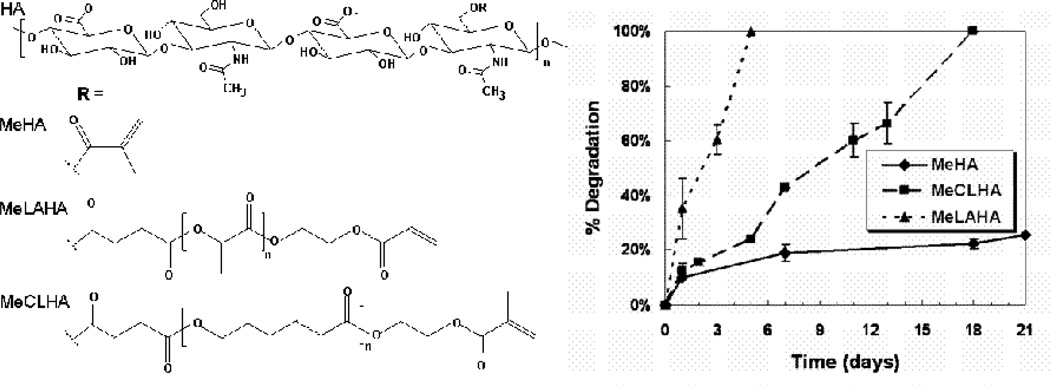
Figure 8. Structures of a range of photopolymerizable HA macromers.
HA macromers can be synthesized to include reactive methacrylate groups either directly (MeHA) or with a hydrolytically degradable spacer of lactic acid (MeLAHA) or caprolactone (MeCLHA). When photocrosslinked, these macromers form hydrogels with varied degradation behavior (measured with release of uronic acid), with degradation rates of MeLAHA > MeCLHA > MeHA.
One benefit to hydrogels formed from the MeHA macromer is that the properties of the formed networks can be tailored by modification of the HA molecular weight, the number of reactive groups, and the concentration of the macromer [74]. For instance, HA hydrogels that range in volumetric swelling ratios from ~42 to 8, compressive moduli from ~2 to over 100 kPa, and degradation times from less than one day up to almost 38 days in the presence of hyaluronidase can be fabricated using these modifications [74]. Generally, these hydrogels are fairly stable and degrade in short periods only with the presence of hyaluronidases [74, 75]. Interpenetrating networks (IPNs), where the HA network is polymerized around an alternate network, can also be obtained using this MeHA system. In one example, IPNs of collagen within an HA hydrogel were produced that permitted advantages of both the HA networks with respect to mechanical stability and the collagen with respect to cellular adhesion [76]. This approach opens up many possibilities for tailoring HA hydrogels for a range of applications. There are other examples where this MeHA macromer has been copolymerized with poly(amino acids) [77] and processed into hydrogel beads [78] to further their tunable properties and application potential.
4.1.2. Reaction with glycidyl methacrylate
An alternate method for the functionalization of HA is the reaction of glycidyl methacrylate with HA to form GMHA conjugates [79]. Schmidt and coworkers crosslinked the GMHA macromers into hydrogels and illustrated a range of degradation rates, as well as material properties [79]. A wide range of GMA modifications have been explored, with methacrylation up to 90% occurring with long reaction times at room temperature [80]. Photocrosslinking of highly modified GMA-HA affords densely crosslinked, robust gels. Moreover, a range of complex fluids can be fabricated with GMA-HA. Lightly crosslinked near-gels and emulsion-crosslinked-microspheres are strongly viscoelastic, while centrifuged microspheres formed elastic microgels [81]. The implanted hydrogels also showed good biocompatibility with minimal inflammation. These macromers have also been investigated for cytocompatibility and showed a relatively favorable response when directly exposed to cells [82].
Hydrogels were also formed from the GMHA macromer in combination with acrylated versions of poly(ethylene glycol) (PEG) and PEG-peptide macromers [83]. In this case, stable hydrogels were formed from these macromer combinations at high peptide-conjugations. Likewise, GMHA was combined with hydroxyethyl acrylate in a range of combinations to produce hydrogels with variable properties [84]. IPNs of the GMHA and N-dimethylacrylamide have also been fabricated to produce networks with high compressive moduli [85]. These gels were not cytotoxic to cells cultured on their surface, but encapsulation in these high modulus gels has not been performed.
HA has also been modified with other derivatives of glycidyl methacrylate, including oxidizing the HA prior to coupling and grafting HA with poly(2-hydroxyethyl methacrylate) and then coupling to HA [25]. These alterations in the methacrylate conjugation led to photopolymerized hydrogels with a wide range of properties, depending on the chemistry that was used. These gels showed minimal cytotoxicity in indirect and direct toxicity assays. Photocrosslinkable HA has also been synthesized by coupling cinnamic acid through the carboxyl groups of HA using a 3-aminopropanol spacer [86]. Hydrogels form from this HA through a direct photodimerization, rather than the typical chain polymerization and generation of kinetic chains. Unfortunately, the photochemical energy required for photodimerization precludes cell encapsulation during irradiation, and this would not be considered a living HA derivative. Protein adsorption on unmodified HA hydrogels is minimal, leading to relatively low cell adhesion; however, this can be overcome with sulfate derivatives [87] or through peptide modification [88].
4.1.3. Hydrolytically degradable HA
Although these previously described HA modifications permit the fabrication of stable and enzymatically degradable hydrogels, there are instances where further control over the degradation behavior of the HA gels is desirable. Specifically, non-degrading or slowly degrading hydrogels may limit cellular migration and cell-cell contacts or be inhibitory where enzymes are not present, whereas a system with controlled degradation could be used for the delivery of biological molecules or for tailored temporal properties. To meet these criteria, HA macromers were synthesized that form hydrogels that are both hydrolytically (via ester group hydrolysis) and enzymatically degradable [75]. This was accomplished by introducing hydrolytically degrading esters (e.g., lactic acid or caprolactone) between the HA backbone and the photoreactive groups and the structures are shown in Figure 8. The kinetics of hydrogel degradation and molecule release was controlled through the hydrogel crosslinking density (i.e., macromer concentration), type of degradable unit (i.e., caprolactone versus lactic acid) and copolymerization with purely enzymatically degradable macromers. The distribution of produced matrix by encapsulated mesenchymal stem cells (MSCs) was controlled by the copolymer concentration (i.e., degradation behavior). Specifically, the distribution of released extracellular matrix molecules (e.g., chondroitin sulfate (CS)) was improved with increasing amounts of the hydrolytically degradable component. Overall, these macromers allow for enhanced control over the structural evolution of the HA hydrogels towards applications as biomaterials.
4.1.4. Electropolymerizable pyrrole-HA
Reaction of N-(1-aminoprop-3-yl) pyrrole with HA using carbodiimide-NHS chemistry afforded a 5–15% PyHA conjugate. PyHA was then electrochemically polymerized to give a stable, biocompatible 20–40 nm HA coating on conducting polymer substrates such as platinum, indium-tin oxide, and polystyrene sulfonate-doped polypyrrole surfaces.[89] The poly(PyHA) coated electrode surfaces were hydrophilic and resistant to fibroblast and astrocyte adhesion, and the immobilized HA films were stable under physiological conditions for 3 months. Importantly, the poly(PyHA) surfaces retained the electrical properties of the underlying electrodes.
4.2. Applications
4.2.1. Cartilage tissue engineering
As with PEGDA crosslinked thiol-HA hydrogels mentioned above, the encapsulation of cells for cartilage regeneration has been extensively investigated in photopolymerizable HA hydrogels. Elisseeff and coworkers first pioneered the use of a photopolymerization process for the encapsulation of chondrocytes in hydrogel networks for treating damaged cartilage tissue, mainly due to the benefits of this approach for injectable constructs and for the filling of irregular defects [90–92]. Auricular (ear) chondrocytes have been directly encapsulated in MeHA hydrogels with a range of variations in molecular weight (50 to 1100 kDa) and macromer concentration (2 to 20 wt%) to investigate the influence of gel properties on neocartilage formation [93]. After 12 weeks of subcutaneous implantation, neocartilage production varied depending on the gel formulation, including being 81 to 93% water, containing between 0.1 × 106 and 0.6 × 106 chondrocytes per sample, and consisting of 0 to 0.049 µg CS/ µg wet weight (GAG content) and 0.002 to 0.060 µg collagen/ µg wet weight. Hydrogels fabricated from 2 wt% of the 50 kDa HA macromer most resembled the properties of native cartilage and showed the greatest promise for continued development for cartilage regeneration.
The crosslinking of HA hydrogels also influences the diffusion through hydrogels and the overall tissue production by encapsulated MSCs [94]. Furthermore, the expansion of chondrocytes prior to encapsulation played a role in the extent of neocartilage formation in these HA hydrogels, with extended passaging leading to inferior neocartilage properties [93]. Likewise, the type of chondrocyte (auricular versus articular) influenced neocartilage tissue properties, with auricular chondrocytes forming better tissue, potentially due to their increased ability to remodel the hydrogels [95]. However, some enhanced gene expression was observed in articular chondrocyte constructs when they were mechanically loaded in compression, mimicking features of the native tissue environment. Chondrocytes have also been photoencapsulated in HA hydrogels and investigated in an in vivo model of cartilage repair in swine [96]. The constructs appeared to integrate well with the native tissue, and showed enhanced matrix synthesis and cellularity after implantation.
The above mentioned hydrolytically degradable HA macromers have also been used to influence tissue formation by encapsulated MSCs.[97] MSCs were photoencapsulated in combinations of hydrolytically and enzymatically degradable HA hydrogels to investigate the tunability of these hydrogels and the influence of network evolution on neocartilage formation. Specifically, the compressive mechanical properties increased when degradation complemented extracellular matrix deposition and decreased when degradation was too rapid. Evolving hydrogels also showed an increase in tissue distribution and an increase in GAG content over static hydrogels. The influence of hydrogel type has also been investigated towards their ability to support cartilage formation, with HA hydrogels being compared to those of Puramatrix and agarose.[98] Interestingly, the performance of chondrocytes in agarose wa superior that that in either the HA hydrogels or the Puramatrix, yet MSCs performed similarly throughout the different hydrogels. This may be related to specific MSC interactions with HA hydrogels that will be reviewed below.[99]
4.2.2. Cardiac repair
A recent study investigated the utility of redox-initiated HA hydrogels in cardiac repair. [100] After myocardial infarction, the increase in inflammatory molecules and alterations in local enzymatic activity, in combination with the pumping of fluid through the heart, leads to an expansion of the left ventricle and thinning of the heart wall. This process can have detrimental effects on cardiac function and ultimately lead to the onset of congestive heart failure. One approach to overcome this and to reduce stresses in the heart wall is the injection of an acellular biomaterial. HA hydrogels that had uniform gelation and degradation behavior, but different mechanical properties were injected into the heart and it was found that the gel with a higher modulus enhanced functional outcomes better than the gel with a lower modulus.[100] This gel system provided a means to probe the optimal design criteria for such as system and this information will be useful in future design considerations of hydrogels to effect cardiac outcomes.
4.2.3. Molecule delivery
Due to the excellent biocompatibility, non-toxic nature of HA hydrogels, and tunability in properties and degradation, they are potentially useful for molecule delivery applications. Additionally, photopolymerization provides a simple technique for the encapsulation of molecules for delivery applications. [92, 101] The delivery of proteins from GMHA derivatives of HA alone and combined with poly(ethylene glycol) was investigated with a range of hydrogel formulations [102]. Bovine serum albumin was used as a model protein and release could either be very rapid (<6 hours) or very slow (several weeks), depending on the extent of crosslinking and the incorporation of microspheres. These hydrogel and composite systems can be used for a wide range of applications, including in regenerative medicine where the timing of protein delivery is crucial in tissue formation. Others have characterized the degradation of degradable microsphere and HA hydrogel composites using techniques such as optical coherence tomography [103].
Modified HA macromers have been combined with other reactive systems as encapsulation and release systems for DNA delivery [104]. Gene therapy approaches are becoming useful in regenerative medicine to alter the gene expression of cells towards directed tissue repair, thus the local and controlled delivery of DNA is important. In this work, the release profiles were dependent on the extent of crosslinking and amount of HA incorporated, and the activity of the released DNA was dependent on the encapsulation conditions and material formulations. Likewise, Shea and coworkers investigated vector delivery from hydrogels with acrylated HA and 4-arm poly(ethylene glycol) precursors and assessed delivery profiles based on material compositions [105]. This provides a non-viral approach for the local delivery of DNA and illustrates the importance of release on hydrogel properties and the specific vector used.
4.2.4. Valvular engineering
The engineering of heart valves is important due to the disease and damage that inflict natural heart valves and tissue engineered approaches are particularly interesting as a biological substitute for damaged valves. Photocrosslinked HA hydrogels are being explored for this application due to the presence of HA within the structure of the native valve [106]. Interestingly, valvular interstitial cells (VICs) have been difficult to culture in a range of common culture environments, including with peptide and protein modified surfaces; however, these cells adhered to and proliferated on HA-based hydrogels [106]. Additionally, the degradation products of these HA gels increased VIC proliferation in culture, with a dependence on molecular weight [107]. The VICs were able to internalize the HA and activate signaling to influence the biological responses. When encapsulated, the VICs remained viable and produced important matrix molecules found in the native tissue. When the HA gels were combined with poly(ethylene glycol) –based crosslinkers, further tunability of the gel was possible to better organize the timing and structure of produced matrix [108].
4.2.5. Control of stem cell behavior
HA hydrogels have been extensively used to control the differentiation of entrapped stem cells, as described in detail for a variety of crosslinked thiolated HA gels. Similarly, photocrosslinked HA hydrogels have featured prominently for use in 3-D stem cell encapsulation. In one study,[99] MSC differentiation towards chondrocytes was investigated in photopolymerized HA hydrogels using the MeHA system, particularly since HA is a native component of cartilage and MSCs may interact with HA via surface receptors. MSCs are multipotent progenitor cells whose plasticity and self-renewal capacity have generated significant interest for applications in tissue engineering [109, 110]. Notably, both in vitro and in vivo cultures permitted chondrogenic differentiation, measured by the early gene expression (up-regulation of type II collagen, aggrecan, sox9) and production of cartilage specific matrix proteins (type II collagen and CS).[99] To assess the importance of hydrogel chemistry on MSC chondrogenesis, HA hydrogels were compared to relatively inert poly(ethylene glycol) (PEG) hydrogels in the presence of chondrogenic factors (e.g., TGF-β3). MSCs in HA hydrogels showed greatly enhanced expression of cartilage specific markers when compared to the PEG hydrogels in vitro and in vivo. This work indicates that hydrogel chemistry alone can play a role in MSC differentiation and particularly that HA can enhance chondrogenesis.
However, this effect on stem cell differentiation was specific to the type of stem cell. HA hydrogels were also investigated as a 3-dimensional environment for controlling the self-renewal and differentiation of human embryonic stem cells (hESCs) [111]. It is known that levels of HA are very high during embryogenesis and that only when these levels decrease is differentiation observed [2, 3]. When encapsulated in three-dimensional HA hydrogels (but not within other hydrogels such as those based on Dextran, or in monolayer cultures on HA), hESCs increased in number, maintained their undifferentiated state, and maintained their full differentiation capacity. Additionally, differentiation could be induced within the gel by simply altering soluble factors. Thus, this system provides a culture system for hESC expansion that does not involve culture on mouse or human cell feeder layers. This work provides evidence that the developmental relevance of HA can be utilized in material design for advanced culture systems.
4.2.6. Microdevices
Beyond direct tissue engineering and cell culture, photopolymerized HA hydrogels have also been used in the development of microdevice systems.[112] Micropatterning of hydrogels is potentially useful for a variety of applications, including tissue engineering, fundamental biological studies, diagnostics, and high-throughput screening.[113] This process takes advantage of the spatial control of the photopolymerization of the MeHA macromers to form microwells or microgels that cells can either be cultured within or encapsulated in, respectively [112]. Cells within the microwells remained viable, could generate spheroids, and could be retrieved using mechanical disruption. When encapsulated, arrays of viable embryonic stem (ES) cells or fibroblasts were obtained and could later be recovered using enzymatic digestion of the microstructures. Using a similar approach, the liquid MeHA macromer can be micromolded using a hydrophilic polydimethylsiloxane stamp and crosslinked with light to fabricate cell containing microscale hydrogels out of HA [114]. These microgels can potentially be assembled into tissue structures or used in cell-based assays.
HA hydrogels have also been processed into microbioreactor systems that allow for the 3-dimensional cultivation of hESCs in hydrogels with controlled perfusion of culture media [115]. The device used common lithography techniques and was fabricated in polydimethylsiloxane and glass, and consisted of a microfluidic layer placed over an array of wells (which contain the hESCs encapsulated in the HA hydrogels) adhered to a standard microscope slide. Dynamic flow conditions improved hESC viability in the microreactor and enhanced the vascular differentiation of hESCs after the administration of growth factors.
4.3. Processing
4.3.1. Macroporous hydrogels
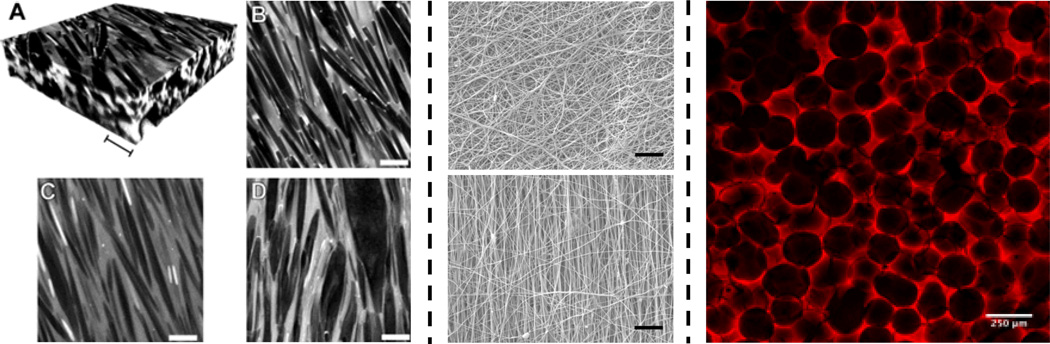
Radically polymerized materials can be fabricated into functional materials using a number of routes. Due to the nature of the polymerization, hydrogels can be injected directly into tissues or into void spaces (e.g., dual barrel syringe for redox systems, macromer injection and light exposure for photoinitiated systems) [116]. Using these same techniques, injection into molds is also possible to fabricate hydrogels with endless variations in shape and size. For example, a microsphere templating process can be used to fabricate a macroporous scaffold, where the macromer is crosslinked around beads (either sintered or packed together) and then the beads can be dissolved away in a solvent [117, 118]. A representative example of a scaffold fabricated with this procedure with HA hydrogels is shown in Figure 9. These macroporous HA scaffolds possess many of the advantages of a hydrated HA system, yet provide porosity for cell and tissue invasion.
Figure 9. Examples of photocrosslinked HA scaffold structures.
Photopolymerizable HA macromers can be processed into a range of structures based on: crystal templating (left, scale bars = 10 µm), electrospinning fibrous structures (middle, non-aligned on top, aligned on bottom, scale bar = 10 µm) and macroporous scaffolds from sphere templating (right, scale bar = 250 µm). For crystal templating, confocal images (A: reconstruction, B–C: scan) of hydrogels containing urea crystals before (A, B) and after (C: swollen, D: dry) crystal removal are shown. Partially reprinted with permission from [128].
4.3.2. Electrospinning
As discussed above, electrospinning is finding increased utility in creating scaffolds for tissue engineering from chemically and photochemically crosslinkable HA derivatives. A charge is applied to a polymer solution for fiber formation, and collection can occur either in a random or aligned (e.g., via a rotating mandrel) orientation [119, 120]. These structures are important in that they can mimic the size-scale of the natural extracellular matrix for enhanced biological interactions and that the alignment can be used to direct cell orientation and tissue formation for the engineering of anisotropic tissues (e.g., meniscus and cardiac tissues) [121, 122]. There are numerous examples of where HA has been electrospun into scaffolds, including as a photopolymerizable version [60, 123, 124]. The HA macromer is electrospun into a scaffold and then exposed to light for crosslinking within and between fibers. Electron microscopy images of HA fibers in both aligned and non-aligned configurations are shown in Figure 9. An added advantage to this process is that the light exposure can be performed with spatial control to pattern porosity into the fibrous scaffolds, which can be used to enhance cellular infiltration and vascularization [125].
4.3.3. Patterning HA hydrogels
The three-dimensional patterning of HA hydrogels is also possible based on spatially controlled light exposure. In one example, Schmidt and coworkers developed IPN and semi-IPNs of collagen and photocrosslinked HA [126]. The HA influences the properties of these gels, compared to collagen alone, and HA IPNs can be patterned throughout the collagen gels to spatially manipulate the properties. Protein microstructures can also be patterned within three-dimensional HA hydrogels using multiphoton excitation, even with sub-micron resolution [127]. This patterning method enhances the complexity of scaffolds for use in regenerative medicine, including for neural applications.
A completely different form of patterning was employed to create dendritic pore networks. In a clever and simple approach, dendritic crystals of urea were grown within solution-cast films of GMHA that were supersatured with urea.[128] Following induction of crystal growth, the GMHA was photocrosslinked to preserve the dendritic network, and the urea was dissolved, leaving a resulting porous, fibrillar HA network. Representative confocal images of the gels before and after crystal removal are shown in Figure 9. The technique is general for natural and synthetic polymers capable of forming viscous concentrated solutions.
4.3.4. Bioprinting
In a new application, a methacrylated ethanolamide derivative of gelatin (GE-MA) was combined with methacrylated HA (HA-MA) and partially crosslinked to give an extrudable gel-like fluid. [44] Gels were printed through a syringe needle into robust structures, followed by a second photocrosslinking step to create a bioprinted tubular construct. The viscoelasticity of methacrylated HA gels can be varied by adjusting the degree of methacrylation and by post-processing. The new HA-MA:GE-MA hydrogels were biocompatible, supporting cell attachment and proliferation of HepG2 3CA, Int-407, and NIH3T3 cells. Moreover, a computer-driven prototyping device was used to print a cellularized tubular construct with an acellular core and acellular support halo. Cells in the printed construct were viable in culture, and gradually remodeling the synthetic matrix to create an endogenous ECM.[129]
5. Combining Addition and Photoinitiated Polymerizations
Beyond the approaches discussed in the previous sections related to the formation of HA hydrogels with either addition or radical polymerizations alone, it may also be advantageous to combine these techniques to utilize the advantages of both systems. For instance, the crosslinking of a hydrogel can be used to control cellular interactions, and particularly their spreading and migration behavior. Generally, cells remain rounded in a directly photopolymerized system since the cells are unable to remodel the kinetic chains that are formed during the polymerization reaction. However, this may be overcome with very low macromer concentrations [130], by using modified natural polymers (e.g., gelatin or collagen), or through the incorporation of matrix metalloprotease (MMP) cleavable peptides into the crosslinks [88, 131]. Likewise, the behavior of cells in addition crosslinked HA hydrogels depends on the related crosslinker chemistry and systems are again available that cells can remodel with the use of enzymes, such as MMPs [88, 131–133].
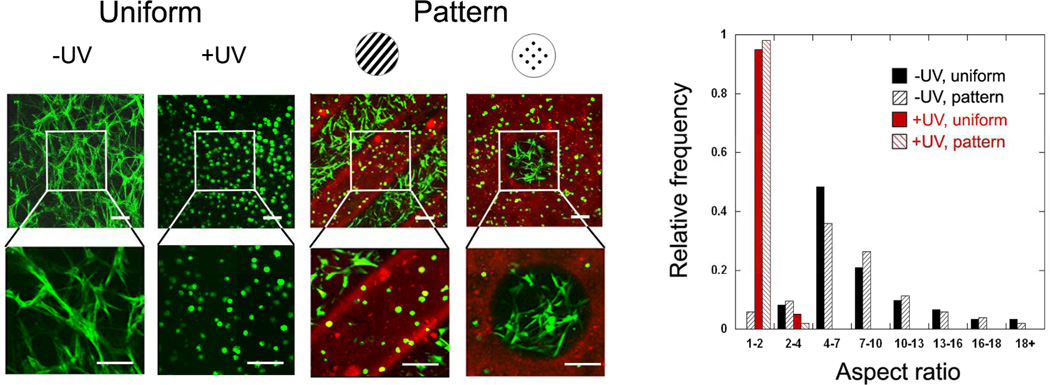
In one study, addition and radical polymerizations were utilized in series, in a mechanism termed sequential polymerization [88]. First, acrylated HA was crosslinked with thiol terminated peptide crosslinkers that were also MMP cleavable to form a network. However, only a fraction of the acrylates was consumed during this reaction, leaving remaining acrylates that are accessible to undergo a radical polymerization. With the addition of light and a photoinitiator, the remaining acrylates reacted into non-degradable kinetic chains. As long as an adhesive peptide (e.g., RGD) was incorporated on the HA, cells were able to remodel the hydrogels after the first crosslinking step; however, the radical polymerization prevented hydrogel remodelling and cellular spreading after the second step. Importantly, the second step can be implemented with spatial control due to the light, giving rise to spatially controlled hydrogel remodeling by cells. This was completed in a follow-up study [134] and precise control over cellular spreading was observed in patterned gels, as illustrated in Figure 10. Interesting, the spread behavior of the cells led to differences in stem cell differentiation in both uniform and patterned gels, where spread cells underwent a primarily osteogenic differentiation and rounded cells underwent a primarily adipogenic differentiation, likely dependent on tension generated within the cells due to differences in spreading.[134] This may be interesting for applications in multicellular tissues or for vascularization of tissue engineered constructs.
Figure 10. Spatially controlled behavior of stem cells in 3D hydrogels.
Human mesenchymal stem cells (confocal images on left, quantification of aspect ratios on right) were encapsulated in HA hydrogels using MMP-cleavable crosslinkers using a sequential crosslinking process. The introduction of light introduces kinetic chains in a spatially controlled manner (illustrated in red) that alters the ability of a cell to remodel the hydrogel, leading to spatially controlled cell spreading. Reprinted with permission from [134].
HA hydrogels have also been developed in a similar fashion to spatially control mechanical properties in the gels [135]. In this case, significant differences in the extent of crosslinking during the primary and secondary steps were used to produce gels with moduli of ~3 kPa after the addition reaction and ~80 kPa after the radical polymerization. These mechanics are within the regime of mechanosensitivity by a range of cells and can influence cellular behaviour such as spreading and differentiation [136]. In this case, the spatial control led to MSCs that were round and did not proliferate on the softer regions and spread and proliferated on the stiffer regions [135].
In another study, spatial patterning of photocrosslinks in a chemically crosslinked HA hydrogel was used to produce hydrogels with anisotropic swelling behavior, based on differences in crosslink density within the hydrogel [137]. This controlled swelling provides excellent control over hydrogel shape changes, leading to more advanced hydrogel systems.
6. Conclusion and Outlook
This progress report illustrates the wide range of materials based on HA that have been developed in recent years. The versatility in HA macromer synthesis and processing of the materials has transitioned into materials with a range of properties useful in applications such as tissue engineering and drug delivery. Thus, unique design criteria can be met using tunable material development. Additionally, HA-based hydrogels may impart biological activity to cells, as evident by changes in cellular behaviour, including stem cell differentiation, when interaction with biomaterials based on HA compared to other polymers. One area that is clear is the potential utility of HA-based materials in translational applications, particularly due to the processing capabilities, biocompatibility, and efficacy of these materials. It is likely that the upcoming years will see an expansion in this area through new material development with unique and interesting properties.
Acknowledgements
The authors gratefully acknowledge funding from a David and Lucile Packard Fellowship in Science and Engineering (JAB), the Utah Centers of Excellence (GDP), the National Science Foundation (EF-0526854, GDP) and the National Institutes of Health (EB008722, JAB; DC004336, GDP; SBIRs to Sentrx Surgical and Glycosan BioSystems, GDP). We also thank our many co-workers and colleagues for their assistance in developing the figures.
Biographies

Glenn D. Prestwich is Presidential Professor of Medicinal Chemistry and Presidential Special Assistant for Faculty Entrepreneurism. He co-founded several life science start-ups, including Echelon Biosciences, Sentrx Animal Care, Glycosan BioSystems, and GlycoMira, and he is a scientific advisor for Novozymes, Elastin Specialities, ContraDyn, Carbylan BioSurgery, Frontier Scientific, Organonovo, and Brickell Biotech. He received the Governor’s Medal for Science and Technology for 2006, and was awarded the 1998 Paul Dawson Biotechnology Award and the 2008 Volwiler Research Award of the American Association of Colleges of Pharmacy. In 2010, he received the University of Utah Distinguished Scholarly and Creative Research Award, as well as the “Rooster Prize” of the International Society for Hyaluronan Science. During his 34 years as a faculty member, he has published over 600 technical papers, patents, and book chapters, and has trained over 125 postgraduate scientists. He is a pianist, commercially-rated pilot, a first tenor in the Utah Symphony Chorus, and chairs the board of the National Center for Voice and Speech.

Jason A. Burdick is an Associate Professor in the Department of Bioengineering at the University of Pennsylvania, USA. The focus of work in his laboratory is the development of biodegradable polymers for applications in tissue engineering and drug delivery. One specific area is the development of hydrogels based on hyaluronic acid for controlled stem cell behavior, altering cardiac function after infarction, and tissue regeneration. He has received several research awards, including a K22 Scholar Development and Career Transition Award from the National Institutes of Health, a Fellowship in Science and Engineering from the David and Lucile Packard Foundation, an Early Career Award from the Wallace H. Coulter Foundation, and a CAREER Award from the National Science Foundation. He has published over 90 peer-reviewed papers and book chapters and is on the editorial boards of the Journal of Biomedical Materials Research A, Biomacromolecules, and Tissue Engineering
References
- 1.Fraser JR, Laurent TC, Laurent UB. J Intern Med. 1997;242:27. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 2.Toole BP. Semin Cell Dev Biol. 2001;12:79. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 3.Toole BP. Nat Rev Cancer. 2004;4:528. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 4.Laurent TC, Fraser JR. Ciba Found Symp. 1986;124:9. doi: 10.1002/9780470513385.ch2. [DOI] [PubMed] [Google Scholar]
- 5.Kuo JW. Practical aspects of hyaluronan based medical products. Boca Raton: CRC/Taylor & Francis; 2006. [Google Scholar]
- 6.Allison DD, Grande-Allen KJ. Tissue Eng. 2006;12:2131. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 7.Prestwich GD. Organogenesis. 2008;4:42. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condie RC, Prestwich GD. In: Injectable Biomaterials: Science and Application. Vernon B, editor. London: Woodhead Publishing; 2010. [Google Scholar]
- 9.Prestwich GD, Ghaly T, Brudnicki P, Ratliff B, Goligorsky MS. In: Regenerative Nephrology. Goligorsky MS, editor. Elsevier; 2010. [Google Scholar]
- 10.Prestwich GD. Glycoforum. 2001 http://glycoforum.gr.jp/science/hyaluronan/HA18/HA18E.html.
- 11.Kuo JW, Prestwich GD. In: Materials of biological origin- Materials analysis and implant uses, Comprehensive biomaterials. Ducheyne P, Healy K, Hutmacher D, Kirkpatrick J, editors. Elsevier; 2010. [Google Scholar]
- 12.Prestwich GD, Kuo JW. Curr Pharm Biotechnol. 2008;9:242. doi: 10.2174/138920108785161523. [DOI] [PubMed] [Google Scholar]
- 13.Prestwich GD. J Cell Biochem. 2007;101:1370. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 14.Pangarkar N, Pharaoah M, Nigam A, Hutmacher D, Champ S. Regen. Med. 2010;5 doi: 10.2217/rme.10.66. in press. [DOI] [PubMed] [Google Scholar]
- 15.Prestwich GD. Acc Chem Res. 2008;41:139. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 16.Serban MA, Prestwich GD. Methods. 2008;45:93. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Biomacromolecules. 2002;3:1304. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 18.Shu XZ, Ahmad S, Liu Y, Prestwich GD. J. Biomed. Mater. Res. 2006;79A:902. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 19.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Biomaterials. 2003;24:3825. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 20.Vanderhooft JL, Mann BK, Prestwich GD. Biomacromolecules. 2007;8:2883. doi: 10.1021/bm0703564. [DOI] [PubMed] [Google Scholar]
- 21.Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Macromol Biosci. 2009;9:20. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serban MA, Prestwich GD. Biomacromolecules. 2007;8:2821. doi: 10.1021/bm700595s. [DOI] [PubMed] [Google Scholar]
- 23.Pouyani T, Prestwich GD. Bioconjugate Chem. 1994;5:339. doi: 10.1021/bc00028a010. [DOI] [PubMed] [Google Scholar]
- 24.Vercruysse KP, Marecak DM, Marecek JF, Prestwich GD. Bioconjugate Chem. 1997;8:686. doi: 10.1021/bc9701095. [DOI] [PubMed] [Google Scholar]
- 25.Jia XQ, Burdick JA, Kobler J, Clifton RJ, Rosowski JJ, Zeitels SM, Langer R. Macromolecules. 2004;37:3239. [Google Scholar]
- 26.Jha AK, Hule RA, Jiao T, Teller SS, Clifton RJ, Duncan RL, Pochan DJ, Jia X. Macromolecules. 2009;42:537. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darr A, Calabro A. J Mater Sci Mater Med. 2009;20:33. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 28.Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Biomacromolecules. 2007;8:1844. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- 29.DeForest C, Polizzotti B, Anseth K. Nature Materials. 2009;8:659. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Shu XZ, Prestwich GD. Tissue Eng. 2006;12:3405. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 31.Toh W, Lee E, Guo X, Chan J, Yeow C, Choo A, Cao T. Biomaterials. 2010;31:6968. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 32.Ratliff B, Ghaly T, Brudnicki P, Yasuda K, Rajdev M, Bank M, Mares J, Hatzopoulous A, Goligorsky MS. Am. J. Physiol. Renal Physiol. 2010;299:F178. doi: 10.1152/ajprenal.00102.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Neurorehabil. Neural Repair. 2010;24:636. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Compte M, Cuesta AM, Sanchez-Martin D, Alonso-Camino V, Vicario JL, Sanz L, Alvarez-Vallina L. Stem Cells. 2009;27:753. doi: 10.1634/stemcells.2008-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai S, Liu Y, Shu XZ, Prestwich GD. Biomaterials. 2005;26:6054. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Peattie RA, Pike DB, Yu B, Cai S, Shu XZ, Prestwich GD, Firpo MA, Fisher RJ. Drug Deliv. 2008;15:389. doi: 10.1080/10717540802035442. [DOI] [PubMed] [Google Scholar]
- 37.Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Biomaterials. 2010;31:4630. doi: 10.1016/j.biomaterials.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosack L, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Biomaterials. 2008;29:2336. doi: 10.1016/j.biomaterials.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanjaya-Putra D, Yee J, Ceci D, Truitt R, Yee D, Gerecht S. J. Cell. Molec. Med. 2009 doi: 10.1111/j.1582-4934.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J, Zhang N, Prestwich GD, Wen X. Macromol Biosci. 2008;8:836. doi: 10.1002/mabi.200700334. [DOI] [PubMed] [Google Scholar]
- 41.Ekaputra A, Prestwich G, Cool S, Hutmacher D. J. Controlled Release. 2010 submitted. [Google Scholar]
- 42.Jha AK, Yang W, Kirn-Safran C, Farach-Carson M, Jia X. Biomaterials. 2009;30:6964. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Skardal A, Prestwich GD. Biomaterials. 2008;29:4521. doi: 10.1016/j.biomaterials.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skardal A, Sarker S, Crabbé A, Nickerson CA, Prestwich GD. Biomaterials. 2010;31:8426. doi: 10.1016/j.biomaterials.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 45.Turner WS, Seagle C, Galanko JA, Favorov O, Prestwich GD, Macdonald JM, Reid LM. Stem Cells. 2008;26:1547. doi: 10.1634/stemcells.2007-0863. [DOI] [PubMed] [Google Scholar]
- 46.Schmelzer E, Triolo F, Turner ME, Thompson RL, Zeilinger K, Reid LM, Gridelli B, Gerlach JC. Tissue Eng Part A. 2010;16:2007. doi: 10.1089/ten.TEA.2009.0569. [DOI] [PubMed] [Google Scholar]
- 47.Van Hoof D, Braam SR, Dormeyer W, Ward-van Oostwaard D, Heck AJ, Krijgsveld J, Mummery CL. Stem Cells. 2008;26:2777. doi: 10.1634/stemcells.2008-0365. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Shu XZ, Prestwich GD. Tissue Eng. 2007;13:1091. doi: 10.1089/ten.2006.0297. [DOI] [PubMed] [Google Scholar]
- 49.Scaife CL, Shea JE, Dai Q, Firpo MA, Prestwich GD, Mulvihill SJ. J Gastrointest Surg. 2008;12:1074. doi: 10.1007/s11605-007-0425-3. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Yamaza T, Kelly AP, Shi S, Wang S, Brown J, Wang L, French SW, Le AD. PLoS One. 2009;4:e7798. doi: 10.1371/journal.pone.0007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marrero B, Messina JL, Heller R. In Vitro Cell Dev Biol Anim. 2009;45:523. doi: 10.1007/s11626-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, Yang G, Zhang H, Prestwich GD. Prostaglandins Other Lipid Mediat. 2009;89:140. doi: 10.1016/j.prostaglandins.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, Fells JI, Perygin D, Parrill AL, Tigyi G, Prestwich GD. Cancer Res. 2009;69:5441. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA. Biomaterials. 2007;28:671. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan Z, Ghosh K, Liu Y, Clark RA, Rafailovich M. Biophys. J. 2009;96:4286. doi: 10.1016/j.bpj.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seib FP, Prewitz M, Werner C, Bornhauser M. Biochem Biophys Res Commun. 2009;389:663. doi: 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 57.Mironov V, Kasyanov V, Shu XZ, Eisenberg C, Eisenberg L, Gonda S, Trusk T, Markwald RR, Prestwich GD. Biomaterials. 2005;26:7628. doi: 10.1016/j.biomaterials.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 58.Kasyanov VA, Hodde J, Hiles MC, Eisenberg C, Eisenberg L, Der Castro LEF, Ozolanta I, Murovska M, Fraughn RA, Prestwich GD, Markwald RR, Mironov V. J. Mater. Sci: Mater Med. 2008;20:329. doi: 10.1007/s10856-008-3590-3. [DOI] [PubMed] [Google Scholar]
- 59.Mironov V, Kasyanov V, Markwald RR, Prestwich GD. Expert Opin Biol Ther. 2008;8:143. doi: 10.1517/14712598.8.2.143. [DOI] [PubMed] [Google Scholar]
- 60.Ji Y, Ghosh K, Shu XZ, Li BQ, Sokolov JC, Prestwich GD, Clark RAF, Rafailovich MH. Biomaterials. 2006;27:3782. doi: 10.1016/j.biomaterials.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 61.Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW. Biomacromolecules. 2008;9:2097. doi: 10.1021/bm800565u. [DOI] [PubMed] [Google Scholar]
- 62.Mironov V, Prestwich GD, Forgacs G. J. Mater. Chem. 2007;17:2054. [Google Scholar]
- 63.Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJA. Tissue Eng. 2007;13:1905. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- 64.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Biomaterials. 2009;30:2164. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skardal A, Zhang J, Prestwich G. Biomaterials. 2010;31:6173. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 66.Skardal A, Zhang J, McCoard L, Oottamasathien S, Prestwich G. Advanced Materials. 2010 doi: 10.1002/adma.201001436. [DOI] [PubMed] [Google Scholar]
- 67.Anseth KS, Burdick JA. MRS Bulletin. 2002;27:130. [Google Scholar]
- 68.Ifkovits JL, Burdick JA. Tissue Eng. 2007;13:2369. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 69.Bryant SJ, Nuttelman CR, Anseth KS. J Biomater Sci Polym Ed. 2000;11:439. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 70.Burdick JA, Anseth KS. Biomaterials. 2002;23:4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 71.Smeds KA, Grinstaff MW. Journal of Biomedical Materials Research. 2001;54:115. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 72.Smeds KA, Pfister-Serres A, Hatchell DL, Grinstaff MW. Journal of Macromolecular Science-Pure and Applied Chemistry. 1999;A36:981. [Google Scholar]
- 73.Miki D, Dastgheib K, Kim T, Pfister-Serres A, Smeds KA, Inoue M, Hatchell DL, Grinstaff MW. Cornea. 2002;21:393. doi: 10.1097/00003226-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 74.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Biomacromolecules. 2005;6:386. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sahoo S, Chung C, Khetan S, Burdick JA. Biomacromolecules. 2008;9:1088. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A. Tissue Engineering Part A. 2009;15:1645. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pitarresi G, Pierro P, Palumbo FS, Tripodo G, Giammona G. Biomacromolecules. 2006;7:1302. doi: 10.1021/bm050697m. [DOI] [PubMed] [Google Scholar]
- 78.Bae KH, Yoon JJ, Park TG. Biotechnology Progress. 2006;22:297. doi: 10.1021/bp050312b. [DOI] [PubMed] [Google Scholar]
- 79.Leach JB, Bivens KA, Patrick CW, Schmidt CE. Biotechnology and Bioengineering. 2003;82:578. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 80.Bencherif S, Srinivasan A, Horkay F, Hollinger J, Matyjaszewski K, Washburn N. Biomaterials. 2008;29:1739. doi: 10.1016/j.biomaterials.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 81.Prata J, Barth T, Bencherif S, Washburn N. Biomacromolecules. 2010;11:769. doi: 10.1021/bm901373x. [DOI] [PubMed] [Google Scholar]
- 82.Trudel J, Massia SP. Biomaterials. 2002;23:3299. doi: 10.1016/s0142-9612(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 83.Leach JB, Bivens KA, Collins CN, Schmidt CE. Journal of Biomedical Materials Research Part A. 2004;70A:74. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- 84.Jin Y, Yamanaka J, Sato S, Miyata I, Yomota C, Yonese M. Journal of Controlled Release. 2001;73:173. doi: 10.1016/s0168-3659(01)00234-6. [DOI] [PubMed] [Google Scholar]
- 85.Weng LH, Gouldstone A, Wu YH, Chen WL. Biomaterials. 2008;29:2153. doi: 10.1016/j.biomaterials.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyamoto K, Sasaki M, Minamisawa Y, Kurahashi Y, Kano H, Ishikawa S. Journal of Biomedical Materials Research Part A. 2004;70A:550. doi: 10.1002/jbm.a.30112. [DOI] [PubMed] [Google Scholar]
- 87.Lord MS, Pasqui D, Barbucci R, Milthorpel BK. Journal of Biomedical Materials Research Part A. 2009;91A:635. doi: 10.1002/jbm.a.32219. [DOI] [PubMed] [Google Scholar]
- 88.Khetan S, Katz JS, Burdick JA. Soft Matter. 2009;5:1601. [Google Scholar]
- 89.Lee J, Schmidt CE. Acta Biomater. 2010;2010 doi: 10.1016/j.actbio.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elisseeff J. Expert Opin Biol Ther. 2004;4:1849. doi: 10.1517/14712598.4.12.1849. [DOI] [PubMed] [Google Scholar]
- 91.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Proc Natl Acad Sci U S A. 1999;96:3104. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. Journal of Controlled Release. 2002;78:199. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- 93.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Journal of Biomedical Materials Research Part A. 2006;77A:518. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Osteoarthritis and Cartilage. 2009;17:1639. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung C, Erickson IE, Mauck RL, Burdick JA. Tissue Eng Part A. 2008;14:1121. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Annals of Biomedical Engineering. 2004;32:391. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 97.Chung C, Beecham M, Mauck RL, Burdick JA. Biomaterials. 2009;30:4287. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Tissue Engineering Part A. 2009;15:1041. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chung C, Burdick JA. Tissue Engineering Part A. 2009;15:243. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, Gorman RC, Burdick JA. P Natl Acad Sci USA. 2010;107:11507. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burdick JA, Ward M, Liang E, Young MJ, Langer R. Biomaterials. 2006;27:452. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 102.Leach JB, Schmidt CE. Biomaterials. 2005;26:125. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 103.Patterson J, Stayton PS, Li XD. IEEE Transactions on Medical Imaging. 2009;28:74. doi: 10.1109/TMI.2008.927356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chun KW, Lee JB, Kim SH, Park TG. Biomaterials. 2005;26:3319. doi: 10.1016/j.biomaterials.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 105.Wieland JA, Houchin-Ray TL, Shea LD. Journal of Controlled Release. 2007;120:233. doi: 10.1016/j.jconrel.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Masters KS, Shah DN, Walker G, Leinwand LA, Anseth KS. Journal of Biomedical Materials Research Part A. 2004;71A172 doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 107.Masters KS, Shah DN, Leinwand LA, Anseth KS. Biomaterials. 2005;26:2517. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 108.Shah DN, Recktenwall-Work SM, Anseth KS. Biomaterials. 2008;29:2060. doi: 10.1016/j.biomaterials.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baksh D, Song L, Tuan RS. J Cell Mol Med. 2004;8:301. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Mol Biotechnol. 2002;20:245. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 111.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Proc Natl Acad Sci U S A. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, Langer R, Burdick JA. Journal of Biomedical Materials Research Part A. 2006;79A:522. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 113.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. P Natl Acad Sci USA. 2006;103:2480. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yeh J, Ling YB, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, Langer R, Khademhosseini A. Biomaterials. 2006;27:5391. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Figallo E, Cannizzaro C, Gerecht S, Burdick JA, Langer R, Elvassore N, Vunjak-Novakovic G. Lab on a Chip. 2007;7:710. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- 116.Piantino J, Goldberg D, Burdick JA, Fischer D, Langer R, Benowitz LI. Journal of Neurotrauma. 2005;22:1243. [Google Scholar]
- 117.Brey DM, Ifkovits JL, Mozia RI, Katz JS, Burdick JA. Acta Biomaterialia. 2008;4:207. doi: 10.1016/j.actbio.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stachowiak AN, Bershteyn A, Tzatzalos E, Irvine DJ. Advanced Materials. 2005;17:399. [Google Scholar]
- 119.Tan AR, Ifkovits JL, Baker BM, Brey DM, Mauck RL, Burdick JA. J Biomed Mater Res A. 2008;87:1034. doi: 10.1002/jbm.a.31853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baker BM, Nerurkar NL, Burdick JA, Elliott DM, Mauck RL. Journal of Biomechanical Engineering-Transactions of the Asme. 2009;131:101012. doi: 10.1115/1.3192140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mauck RL, Baker BM, Nerurkar NL, Burdick JA, Li WJ, Tuan RS, Elliott DM. Tissue Engineering Part B-Reviews. 2009;15:171. doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nisbet DR, Forsythe JS, Shen W, Finkelstein DI, Horne MK. J Biomater Appl. 2009;24:7. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 123.Lee KY, Jeong L, Kang YO, Lee SJ, Park WH. Advanced Drug Delivery Reviews. 2009;61:1020. doi: 10.1016/j.addr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 124.Li JX, He AH, Han CC, Fang DF, Hsiao BS, Chu B. Macromolecular Rapid Communications. 2006;27:114. [Google Scholar]
- 125.Sundararaghavan HG, Metter RB, Burdick JA. Macromol Biosci. 2009 doi: 10.1002/mabi.200900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suri S, Schmidt CE. Acta Biomaterialia. 2009;5:2385. doi: 10.1016/j.actbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 127.Seidlits SK, Schmidt CE, Shear JB. Advanced Functional Materials. 2009;19:3543. [Google Scholar]
- 128.Zawko S, Schmidt CE. Acta Biomater. 2010;6:2415. doi: 10.1016/j.actbio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 129.Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Tissue Eng. Part A. 2010;16:2675. doi: 10.1089/ten.tea.2009.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mahoney MJ, Anseth KS. Biomaterials. 2006;27:2265. doi: 10.1016/j.biomaterials.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 131.Gobin AS, West JL. Faseb Journal. 2002:16. doi: 10.1096/fj.01-0759fje. [DOI] [PubMed] [Google Scholar]
- 132.Lutolf MP, Hubbell JA. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 133.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Proc Natl Acad Sci U S A. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khetan S, Burdick JA. Biomaterials. 2010;31:8228. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 135.Marklein RA, Burdick JA. Soft Matter. 2010;6:136. [Google Scholar]
- 136.Burdick JA, Vunjak-Novakovic G. Tissue Eng Part A. 2008;15:205. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zawko SA, Suri S, Truong Q, Schmidt CE. Acta Biomaterialia. 2009;5:14. doi: 10.1016/j.actbio.2008.09.012. [DOI] [PubMed] [Google Scholar]