Abstract
Advanced analytical modern technology such as coupling a gas chromatography to a mass spectrometric technique provides sufficient information to the environmental and analytical chemists to identify the presence of a variety of components of the specific volatile organic product, determine the degree of the product weathering and in some instances estimate the age of the product as well in the testing sample. In this study, we estimated BTEX in groundwater sample by using gas chromatography–mass spectrometry (GC–MS) after standardization of this technique for advancement towards purification check of water samples in the petro-polluted regions of the soil.
Keywords: BTEX, GC–MS, Organic, Purge, Trap, Volatile
Abbreviations: B, benzene; T, toluene; E, ethylbenzene; X, xylenes; GC, gas chromatography; MS, mass spectrometry
1. Introduction
In this laboratory, first we established research on kinetics of human serum butyrylcholinesterase along with its inhibition by novel experimental Alzheimer therapeutics, bisnorcymserine and dihydrobenzodioxepine cymserine (Kamal et al., 2006, 2008). Moreover, the fatty acids in oils were estimated by Gas Capillary Chromatography (Kamal and Klein, 2007). Currently, we are undertaking assessment of BTEX in groundwater samples by using gas chromatography–mass spectrometry (GC–MS).
Generally, GC–MS has extensive use in various disciplines of medical, analytical and biological sciences such as estimation of critical/essential substances like (i) phenolic microbial fermentation products of flavonoids (in foods and beverages) in urine, plasma, and fecal water. The importance of it reflects through the epidemiological study shown that a flavonoid-rich diet is associated with reduced risk of cardiovascular diseases (Grün et al., 2008); (ii) non-steroidal acidic anti-inflammatory, antipyretic and analgesic drugs in water samples used in human health care. Moreover veterinary applications based on in situ derivatization of analytes to their methyl esters with dimethyl sulfate (Araujo et al., 2008); (iii) monitoring of endocrine-disrupting chemicals, either of animal (estrone and estradiol), pharmaceutical (17α-ethynylestradiol), vegetal (daidzein, genistein, and biochanin A), or industrial in origin (bisphenol A, 4-octylphenol, and 4-nonylphenol) can pollute the area. Therefore, this is possessing potential hazard for water habitat via fish endocrine disruption in ocean/river/pond water (Ribeiro et al., 2009). Hence, for care of water creation particularly the fish population monitoring is required of micro-trace contents in the water using GC–MS technology.
Coupling of gas chromatography to mass spectrometry system is an expensive but a powerful analytical technique for characterization in term of identification as well as quantification of unknown chromatographic components. Some specific volatile aromatics such as benzene, toluene, ethylbenzene and the xylenes are known as BTEX. These are usually found in soils and groundwater samples polluted by leaks from underground fuel tanks. These pollutants pose problems when they leach into groundwater used for drinking purpose and when old petrol stations as well as fuel depots are redeveloped. These samples often composed of many other contaminations, which can interfere with the analysis and also contaminate the gas chromatographic system. Traditional laboratory clean up procedures have poor recoveries due to the lost of these volatile analytes during sample preparation. While static headspace sample preparation leaves a proportion of the analytes in the original matrix. On the other hand, the use of the dynamic headspace (purge and trap) strips all the volatile components from the sample, leaving non-volatile contaminate behind in the matrix, therefore, this system is applied in this study.
2. Materials and methods
2.1. Materials
Glass syringe (10 ml) fitted with a valve for injections of high purity water to expel any air and also for loading standards as well as sample through the purge vessel via its valve was used in the current study. Helium used as carrier gas and set at 70 kPa. 4-Bromofluorobenzene (25 μg/ml in methanol) was used as tuning test standard for tuning verification according to USEPA criteria (Anon., 1992, 1994, 1996). Intermediate standard of BTEX was prepared from combined stock standard of BTEX.
2.2. Optimization of gas chromatograph–mass spectrometer settings
The column (30 m × 0.32 mm ID; Sol–Gel based polyethylene glycol stationary phase 1 μm film thickness) was installed while its initial and final temperature was setup at 40 °C (6 min) and 120 °C, respectively. Injector and interface temperature was at 200 °C and 230 °C, respectively. Ramp rate, inlet pressure, run time and injection mode was adjusted at 15 °C per min, 25.8 kPa, 12 min and 3 min (splitless with purge activation), respectively.
Mass spectrometer setting was as follows: scan range (35–270 m/z at 500 m/z s), interval sampling rate (0.5 s), threshold (1000), ionization (electron impact at 70 eV) detector relative to tuning value (0) and solvent cut time (3 min).
3. Results and discussion
Generally various analytical methods and instrumental setup are used for the forensic characterization of escaped petroleum related substances as presented in the Table 1.
Table 1.
Table of analytical systems for characterization of petroleum related substances.
| Substances | System |
|---|---|
| Gaseous hydrocarbons in the C1 – C5 range | GC (FID) |
| n-Alkanes (C8 – C35)/specific branched-chain alkanes | GC/FID or GC/MS |
| Major volatile hydrocarbons in the gasoline range | HR GC/FID or GC/MS |
| BTEX | PD-GC or GC/MS |
| Alkyl lead speciation and lead scavengers | GC (ECD) |
| Oxygenated blending agents (alcohols & ethers) | T-d-GC/FID or GC/MS |
| Lead, organic lead and trace metals (V / Ni) | ICPS |
| AB, ACH, PNAHC, PCSHC (steranes / terpanes) | GC/MS |
| Stable isotope ratios for carbon, hydrogen, sulfur and nitrogen | DCIRMS |
GC, Gas chromatography; FID, flame ionization detector; HR, high resolution; BTEX, benzene, toluene, ethylbenzene, xylenes (volatile aromatic hydrocarbons); PD, photoionization detector; ECD, electron capture detector; T-d, two-dimensional; TLC, thin layer chromatography; ICPS, inductively coupled plasma spectrometry; V, vanadium; Ni, nickel; AB, Alkylbenzenes; ACH, alkylcyclohexanes; PNAHC, polynuclear aromatic hydrocarbons; PCSHC, polycyclic saturated hydrocarbons; DCIRMS, Dual collecting isotope ratio mass spectrometry.
The profiles for GC–MS chromatogram for 5–25 μl intermediate BTEX standards are presented in Figs. 1–3 while for sample in Fig. 4. Comparative mass/charge ratio (m/z) profiles for various components in the BTEX detected by GC–MS are outline in Fig. 5. The conclusion of sample analysis in this study is as follows:
Figure 1.
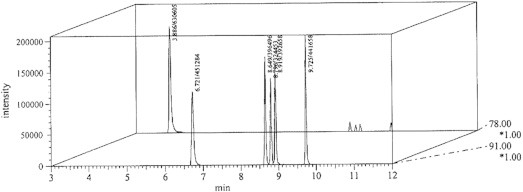
GC–MS chromatogram for 5 μl intermediate BTEX standard.
Figure 2.

GC–MS chromatogram for 10 μl intermediate BTEX standard.
Figure 3.

GC–MS chromatogram for 25 μl intermediate BTEX standard.
Figure 4.

GC–MS chromatogram for sample.
Figure 5.

Comparative mass/charge ratio (m/z) profiles for various components in the BTEX detected by GC–MS (R, retention; T, time; 78 for benzene; 91 for toluene).
(1) RT 3.9 m/z 78 (0.728 μg); (2) RT 6.7 m/z 91 (0.722 μg); (3) RT 8.65 m/z 91 (0.038 μg); (4) RT 8.75 m/z 91 (0.027 μg); (5) RT 8.86 m/z 91 (0.072 μg) and (6) RT 9.54 m/z 91 (0.065 μg).
References
- Anon., 1992. Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS): Capillary Column Technique. USEPA, Method 8260.
- Anon., 1994. Purge and Trap for Aqueous Samples. USEPA, Method 5030.
- Anon., 1996. Purge and Trap for Aqueous Samples. USEPA, Method 5035B.
- Araujo L., Wild J., Villa N., Camargo N., Cubillan D., Prieto A. Determination of anti-inflammatory drugs in water samples, by in situ derivatization, solid phase microextraction and gas chromatography–mass spectrometry. Talanta. 2008;75(1):111–115. doi: 10.1016/j.talanta.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Grün C.H., van Dorsten F.A., Jacobs D.M., Le Belleguic M., van Velzen E.J., Bingham M.O., Janssen H.G., van Duynhoven J.P. GC–MS methods for metabolic profiling of microbial fermentation products of dietary polyphenols in human and in vitro intervention studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008;871(2):212–219. doi: 10.1016/j.jchromb.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Kamal M.A., Klein P. Estimation of fatty acids in oils by gas capillary chromatography. Saudi J. Biol. Sci. 2007;14(1):17–20. [Google Scholar]
- Kamal M.A., Yu Q.-S., Holloway H.W., Tweedie D., Klein P., Greig N.H. Kinetics of human serum butyrylcholinesterase and its inhibition by a novel experimental Alzheimer therapeutic, bisnorcymserine. J. Alzheimers Dis. 2006;10(1):43–51. doi: 10.3233/jad-2006-10108. [DOI] [PubMed] [Google Scholar]
- Kamal M.A., Klein P., Luo W., Li Y., Holloway H.W., Tweedie D., Greig N.H. Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental Alzheimer therapeutic, dihydrobenzodioxepine cymserine. Neurochem. Res. 2008;33(5):745–753. doi: 10.1007/s11064-007-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C., Tiritan M.E., Rocha E., Rocha M.J. Seasonal and spatial distribution of several endocrine-disrupting compounds in the Douro River Estuary, Portugal. Arch. Environ. Contam. Toxicol. 2009;56(1):1–11. doi: 10.1007/s00244-008-9158-x. [DOI] [PubMed] [Google Scholar]


