Abstract
This study was conducted to investigate phytophagous and predatory mites associated with vegetable plants in Riyadh, Saudi Arabia. Eight phytophagous and 10 predacious mites were collected from 14 species of vegetable crops covering five major production localities. Out of these 18 mite species, 13 species are new to the mite fauna of Saudi Arabia. In addition, the two species, Tenuipalpus punicae and Agistemus exsertus, are reported for the first time on vegetable crops in Saudi Arabia. For each mite species found, notes on host plant association and occurrence period are given. An illustrated key for the identification of the 18 mite species reported in this study is provided and this can be used to improve the IPM programs by applying the local natural predatory mites in controlling mite pests in Saudi Arabia.
Keywords: Pest mites, Taxonomy, Mite fauna of Saudi Arabia
1. Introduction
The groundwork for essential taxonomic studies of agricultural mites is extremely rare in Saudi Arabia (SA). Consequently, plant feeding and predatory mites have been poorly investigated in SA resulting in insufficient information about the biology and ecology of these mites (Al-Atawi and Halawa, 2011; Al-Shammery, 2009). Such information is highly required for successful integrated pest management (IPM) programs. Moreover, this information will help to improve the recently introduced programs to SA such as organic farming and biological control programs (Fouly and Al-Rehiani, 2011).
A few numbers of phytophagous mite species have been previously reported on vegetable crops in SA (Martin, 1972). Yet, no predatory mites on vegetable crops have been reported in SA. However, some predatory mites have been found on different plants other than vegetables (Soliman and Al-Yousef, 1979; Dabbour and Abdel-Aziz, 1982). Among these studies that were carried out in SA, only Soliman and Al-Yousef (1979) provided an identification key for 13 phytophagous and predatory prostigmatid mites associated with some important agricultural crops in Riyadh.
However, the previously mentioned studies did not provide enough information about the description and the distribution of the recorded mites. In addition, neither illustrated keys nor deposited specimens were available. Therefore, the main objectives of this study were to (i) investigate phytophagous and both prostigmatid and mesostigmatid predatory mites associated with 30 species of vegetable crops covering different localities in Riyadh, SA, (ii) take notes of occurrence period and distribution of mites in different plants and localities, and (iii) provide an identification key for the previous and new recorded mite species found in this study. The mite identification key presented in this study can serve to lay the groundwork for further taxonomical investigations in the kingdom.
2. Materials and methods
The survey was conducted in five localities surrounding Riyadh city and included 30 species of vegetable plants (Table 1). At each locality, sampling was carried out weekly from June 2008 to May 2009. Samples were collected from both plant foliages and surface soil surrounding the plants, and individually bagged in tightly-closed plastic bags and transported the same day to the Acarology Laboratory, Department of Plant Protection, College of Food and Agriculture Sciences, King Saud University for mites’ extraction.
Table 1.
Distribution of mite species on vegetable crops from five different localities in Riyadh during the period from Jun 2008 to May 2009.
| Host plant | Locality |
No. of samples (soil-foliage) |
No. of mite species phytophagous/predaceous |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Allium cepa | × | ✓ | ✓ | ✓ | × | 0 | 16 | 20 | 8 | 0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 |
| Allium kurrat | ✓ | × | ✓ | × | × | 4 | 0 | 6 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Allium sativum | × | × | ✓ | × | × | 0 | 0 | 16 | 8 | 0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 |
| Anethum graveolens | ✓ | ✓ | ✓ | ✓ | ✓ | 20 | 20 | 20 | 20 | 20 | 1/0 | 1/0 | 1/0 | 1/0 | 1/0 |
| Brassica. campestris var rapa | × | ✓ | × | × | ✓ | 0 | 6 | 0 | 0 | 2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Brassica chinensis | × | ✓ | × | × | × | 0 | 4 | 0 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Brassica oleracea var capitata | × | × | ✓ | ✓ | × | 0 | 0 | 4 | 8 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Capsicum annuum | × | × | × | ✓ | × | 0 | 0 | 0 | 8 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Capsicum frutescense | × | ✓ | × | × | × | 0 | 9 | 0 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Corchorus olitorius | ✓ | × | ✓ | × | × | 4 | 0 | 3 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Cucumis sativus | ✓ | ✓ | ✓ | ✓ | ✓ | 20 | 20 | 20 | 20 | 20 | 1/0 | 1/0 | 1/0 | 1/0 | 1/0 |
| Cucurbita mixta | ✓ | ✓ | ✓ | ✓ | ✓ | 20 | 20 | 20 | 20 | 20 | 1/0 | 1/0 | 1/0 | 1/0 | 1/0 |
| Cucurpita moshata | ✓ | × | ✓ | × | × | 20 | 0 | 12 | 0 | 0 | 2/0 | 0/0 | 2/0 | 0/0 | 0/0 |
| Cucurpita pepo | ✓ | ✓ | ✓ | ✓ | ✓ | 15 | 8 | 20 | 16 | 25 | 2/0 | 2/0 | 2/0 | 2/0 | 2/0 |
| Cynara scolymus | × | × | ✓ | × | × | 0 | 0 | 12 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Eruca sativa | × | × | ✓ | × | × | 0 | 0 | 16 | 0 | 0 | 0/0 | 0/0 | 16 | 0/0 | 0/0 |
| Foeniculum vulgare var dules | ✓ | × | × | ✓ | × | 4 | 0 | 0 | 6 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Hibiscus esculentus | ✓ | ✓ | ✓ | ✓ | ✓ | 20 | 20 | 20 | 20 | 20 | 1/0 | 1/0 | 1/0 | 1/0 | 1/0 |
| Ipomoea batatas | × | × | ✓ | × | × | 0 | 0 | 12 | 0 | 0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 |
| Lactuca sativa | × | ✓ | ✓ | ✓ | ✓ | 0 | 12 | 12 | 12 | 12 | 0/0 | 0/1 | 0/2 | 0/1 | 0/1 |
| Lactuca sativa var capitata | × | × | ✓ | × | × | 0 | 0 | 4 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Lycopersicon esculentum | × | × | ✓ | × | × | 0 | 0 | 5 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Malva parviflora | × | × | × | × | ✓ | 0 | 0 | 0 | 0 | 6 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Petroselinum crispum | × | × | ✓ | × | × | 0 | 0 | 8 | 0 | 0 | 0/0 | 0/0 | 0/1 | 0/0 | 0/0 |
| Phaseoulus vulgaris | × | × | ✓ | × | × | 0 | 0 | 6 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Phaseoulus lunatus | × | × | ✓ | × | × | 0 | 0 | 6 | 0 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Raphenus sativus | ✓ | ✓ | ✓ | ✓ | ✓ | 20 | 15 | 20 | 20 | 8 | 1/0 | 1/1 | 1/1 | 1/1 | 1/1 |
| Solanum melongena | ✓ | ✓ | ✓ | ✓ | ✓ | 36 | 36 | 36 | 36 | 36 | 3/1 | 3/2 | 3/3 | 3/3 | 3/2 |
| Vicia faba | × | × | × | ✓ | × | 0 | 0 | 0 | 4 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Vigna sinensis | × | × | × | ✓ | × | 0 | 0 | 0 | 8 | 0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
1 – Al-Deraiya; 2 – Al-Oiayna; 3 – Al-Hayer; 4 – Al-Waseel; 5 – Al-Ammaria.
Mites were extracted from leaves and soil samples by using a fine hair brush under a high quality Olympus Stereo-microscope (SZX-10) at magnifications 100–200× and/or a modified Berlese funnel apparatus for 24 h, then preserved in 70% ethanol. Selected mites were cleared in “Nesbitt’s” solution for 10–12 h. Subsequently, mites were mounted onto micro-slides with Hoyer’s medium, and later dried in a 40 °C-oven for one week. In case of eriophyid mites, the individuals were directly mounted on micro-slides with Keifer medium (Zhang, 2003).
An Olympus compound microscope (BX-51) with an attached drawing tube was used for examination and initial pencil drawing of mite diagnostic features at magnifications of 400–1200×. The line drawings of mites were scanned and imported into Adobe Photoshop and used as templates for final illustrations in Adobe illustrator. The figure measurement lines were fixed at 25 μm.
The terminology used in the key presented in this study was according to Krantz (1978) and Amrine et al. (2003).
All specimens are deposited in the King Saud University Museum of Arthropod (KSMA), College of Food and Agriculture Sciences, King Saud University.
3. Results and discussion
Eighteen phytophagous and predatory mite species belonging to 14 genera and nine families were recorded on 14 vegetable species (Tables 1–3). Among these, 13 are recorded for the first time in SA. Out of these 13 newly recorded mite species, four are phytophagous (Aceria lycopersici Wolffenstein, Eotetranychus fallugiae Tuttle and Baker, Eutetranychus palmatus Attiah, Pyllotetranychus aegyptiacus Sayed) whereas nine are predaceous; (Agistemus vulgaris Soliman and Gomma, Cheylostigmaeus californicus Summer and Ehara, Cheylostigmaeus torulus Summer, Eustigmaeus kermesinus (Koch), Ereynetes sp., Exothorhis sp., Lasioseius lindquisti Nasr and Abou-Awad, Macrocheles glaber Muller and Neoseiulus cucumeris Oudemans). Moreover, the phytophagous mite Tenuipalpus punicae Pritchard and Baker, and the predatory mite A. vulgaris Gonzalis are reported for the first time on vegetable crops.
Table 2.
List of phytophagous mites on vegetable crops from different localities, Riyadh, SA.
| Family | Species | Locality | Host plant | Sample period |
|---|---|---|---|---|
| Tetranychidae | Tetranychusurticae | 1, 2, 3, 4, 5 | Cucurbitamixta, Cucumissativus, Hibiscusesculentus | June–May |
| Raphenus sativus, Solanummelongena | ||||
| T. cinnabarinus | 1, 2, 3, 4, 5 | Allium sp. | June–May | |
| Anethumgraveolens | ||||
| Cucurbita sp. | ||||
| S.melongena | ||||
| Eutetranychusorientalis | 1 | Cucurbitamoschata | March–May | |
| E. palmatus | 1, 3 | C.moschata | March–May | |
| Eotetranychus fallugiae | 3 | Ipomoea batats | May | |
| Tenuipalpidae | Phyllotetranychusaegyptiacus | 1, 3, 4 | I.batatas, S. melongena | November |
| Tenuipalpuspunicae | 1, 3 | Capsicum sp. | July, September, and November | |
| Cucurbita sp. | ||||
| Eriophyidae | Aceria lycopersici | 1, 2, 3, 4, 5 | S. melongena | November–May |
1 – Al-Deraiya; 2 – Al-Oiayna; 3 – Al-Hayer; 4 – Al-Waseel; 5 – Al-Ammaria.
Table 3.
List of predacious mites on vegetable crops from different localities, Riyadh, SA.
| Family | Species | Locality | Host plant | Sample period |
|---|---|---|---|---|
| Stigmaidae | Agistemus vulgaris | 2, 3, 4, 5 | Capsicum sp., Lactucasativa soil, S. melongena | November and January |
| A. exsertus | 2, 3, 4, 5 | S. melongena, R. sativus | July, November and December | |
| Eustigmaeus kermesinus | 1, 5 | S. melongena | July and November | |
| Cheylostigmaeus californicus | 4 | S. melongena | July and November | |
| C. torulus | 4 | Capsicum sp soil | July and November | |
| Phytoseiidae | Neoseiulus cucumeris | 3, 4 | Alliumsativum, Capsicum sp. | December, February and April |
| Ascidae | Lasioseius lindquisti | 3 | Capsicum sp. | November, February and April |
| Macrochelidae | Macrocheles glaber | 3 | Alliumcepa, soil, Capsicum sp., L. sativa | November and March |
| Eupalopsellidae | Exothoris sp. | 3, 4 | Erucasativa, S. melongena | December |
| Ereynetidae | Ereynetes sp. | 3 | Petroselinum crispum soil | November |
1 – Al-Deraiya; 2 – Al-Oiayna; 3 – Al-Hayer; 4 – Al-Waseel; 5 – Al-Ammaria.
Sixteen plant species observed were free from phytophagous and predacious mites (Table 1). Compared to the other 14 plant species populated with different mites, these plants were neither spread over more than two localities nor found during most of the time of this study. However, six of them (Brassica oleracea var capitata, Capsicum annuum, Corchorus olitorius, Lycopersicon esculentum, Phaseoulus vulgaris and Vicia faba) were previously inhabited with Tetranychus cinnabarinus in different regions of SA (Martin, 1972). Also, Abd Elsalam et al. (1980) found some phytophagous and predaceous mites on all these sixteen plant species in Egypt.
The phytophagous mites included eight species represented by three families (Tetranychidae, Tenuipalpidae, and Eriophyidae) found on nine plant species (Table 2). Three species (Tetranychus urticae Koch; T. cinnabarinus, and Eutetranychus orientalis (Klein)) were previously recorded on vegetable crops in different regions of the Kingdom (Martin, 1972). However, the eriophyid mite Aculus lycopersici (Massee) recorded on vegetables by Martin (1972) was not found in this study. Unsurprisingly, this study showed that T. urticae; and T. cinnabarinus were sampled from a wide range of plants from the five localities surveyed (Table 2). These two spider mite species are major pests on fruit trees and crops in both greenhouses and fields. Moreover, both have the ability of developing resistance to the pesticides used intensively (Dittrich, 1975). Noticeably, the eriophyid mite A. lycopersici (Wolffenstein) was found on Solanum melongina which was grown in all five localities (Table 2).
The predaceous mites included 10 species belonging to six families (Stigmaeidae, Phytoseiidae, Ascidae, Macochelidae, Eupalopsellidae, and Ereyenetidae) and associated with eight plant species (Table 3). All these mites are recorded for the first time on vegetables in SA. While Agistemus exsertus Gonzalez and A. vulgaris Soliman occurred on a wide range of host plants and soils, most of the predaceous mites were recorded only in one or two locations (Table 3). This could be related to the intensive use of pesticides that have been applied (Martin, 1972).
However, predatory mites play an important role in suppressing pest population occupying different habitats and used in biological control programs (Amitai, 1992). Members of Phytoseiidae and Stigmaeidae mites are very essential biological control agents of plant and soil-inhabiting pest mites, e.g., tetranychids, tenuipalpids and eriophyids (Helle and Sabelis, 1985; Santos and Laing 1985; Villanueva and Harmsen, 1998; Kheradmand et al., 2007).
Members of Stigmaeidae family recorded in this work (A. vulgaris; A. exsertus; E. kermesinus; C. torulus; C. californicus) are known to prey on Tetranychidae, Tenuipalpidae, and Eriophyidae (Ehara and Wongsiri, 1984; Arruda Filho and Moraes, 2003). In greenhouses, N. cucumeris provides an effective biological control of T. urticae at lower cost than chemical control (Zhang, 2003). The ascid mite L. lindquisti and Macrochelid mite M. glaber are considered biocontrol agents to suppress arthropods and dung beetles populations in soil and organic debris (Halliday, 1986; Zaher, 1986).
In conclusion, the identification key reported in this study should help other investigators in identifying both phytophagous and predaceous mites in SA. Also, these results can be used to improve IPM programs by using the local natural enemies in controlling local or invasive pests in SA.
4. Key to identify mites associated with some vegetable crops in Riyadh
-
1
Chelicerae chelate type with digits usually developed and dentate; single seta and pilus dentilis usually present on the base of fixed digit; a pair of stigmata occurs at the level of coxae III–IV ………… Mesostigmata … 2
-
-
Chelicerae with movable digit stylet or with two long movable recurved stylets; stigmata present or absent, if present located on the anterior margin of propodosoma or between chelicerae bases ………… Prostigmata ……4
-
2
Leg I without ambulacrum (Fig. 1A); arthordial membrane at base of movable digit of the chelicera reduced into long filamentous or brush-like processes; genu I with 2 or 3 ventral setae; palp apotele 3 tined……………………………………………… Macrochelidae Vitzthum lateral and marginal setae simple; setae J5 shorter than Z5 (Fig. 1B) …………………………………………Macrocheles glaber Muller
-
-
Leg I typically with ambulacrum (Fig. 1C); arthordial membrane at base of movable digit of the chelicera with or without setiform processes forming a cluster or acoronet; genu I with 3 ventral setae; palp apotele 2 or 3 tined …………………………………3
-
3
Holodorsal shield with not more than 20 pairs of setae, setae J1 absent …………………………… Phytoseiidae Oudemans most dorsal setae short; dorsal shield consistently reticulate throughout (Fig. 2A) …………………Neoseiulus cucumeris Oudemans
-
-
Holodorsal or schizodorsal shield with more than 20 pairs of setae, setae J1 present ……………………Ascidae Voigts and Oudemans dorsal shield with 22 pairs of setae; lateral membrane of dorsum with four pairs of setae (Fig. 2B) …… Lasioseius lindquisti Nasr and Abou-Awad
-
4
Mites with two or four pairs of legs; chelicerae with movable digit whip-like ………… 5
-
-
Mites with four pairs of legs; chelicerae with movable digit stylet-like ……………… 12
-
5
Body fusiform or wormlike; stigmata and peritremes absent; chelicerae not recurved basally and not arising from stylophore; eyes usually absent; only two pairs of legs, without a thumb-claw process …………………… Eriophyoidae Nalepa ……… Eriophyidae Nalepa body wormlike; shield subtriangular; median and admedian line almost complete; featherclaw 4-rayed; coverflap of female genetalia with 10–12 longitudinal ribs (Fig. 3A–D) ………Aceria lycopersici (Wolffenstein)
-
-
Body shape oval, triangular or flattened, with post cheliceral stigmata and peritremes; chelicerae recurved posteriorly and arising from a stylophore; eyes usually present (Fig. 4A); four pairs of legs; palpus with or without a thumb-claw process ……… Tetranychoidea Baker and Pritchard …… 6
-
6
Palpus with five segments and a thumb-claw process (Fig. 4B) …………Tetranychidae Donnadieu ………… 7
-
-
Palpus with 1–5 segments, simple, lacking claw on penultimate segment (Fig. 5A) …………… Tenuipalpidae Berlese …… 11
-
7
Empodium claw-like or split distally (Fig. 6A and B) ………… 8
-
-
Empodium rudimentary or absent (Fig. 6C) ……Eutetranychus Banks …… 10
-
8
With two pairs of para-anal setae (Fig. 7) ……… Eotetranychus Oudemans male aedeagus tapering distally (Fig. 8A) …… E. fallugiae Tuttle and Baker
-
-
With one pair of para-anal setae … Tetranychus Dufour …… 9
-
9
Male aedeagus with both anterior and posterior angulation’s, anterior angulation of head acute, axes of aedeagus head parallel with axis of shaft (Fig. 8B) ……… T. urticae Koch
-
-
Male aedeagus with both anterior and posterior angulation’s, anterior angulation of head round, axis of head forming a small angle with axis of shaft (Fig. 8C) ………… T. cinnabarinus (Boisd.)
-
10
Idiosoma with most dorsal setae set on small tubercles (Fig. 9A); lobes of propodosomal stria somewhat rounded …… E. orientalis (Klein)
-
-
Idiosoma with none of dorsal body setae on tubercles (Fig. 9B); prodorsum with mediodorsal steria slightly twisted and with obvious lobes ………… E. palmatus Attiah
-
11
Podosoma very broad and narrow opisthosoma; palpus with three segments; first and second dorsal propodosomal setae minute and simple, third longest and narrowly lanceolate (Fig. 5A and B) …………… Tenuipalpus punicae Pritchard and Baker
-
-
Podosoma not strongly differentiated from opisthosoma; palpus with two segments; first pair of dorsal propodosoma setae broadly lanceolate and longer than the distance between bases, second and third propodosomal setae short and fanlike with veined pattern (Fig. 10A and B) ……… Phyllotetranychus aegyptiacus Sayed
-
12
Palpi simple, chelate, produced distidorsally ………… 13
-
-
With a palp thump-claw process in all stages ………Stigmaeidae Oudemans …… 14
-
13
Prodorsal setae paired, similar in size; femora of legs generally undivided (Fig. 11B) ………… Eupalopsellidae Willmann dorsal setae robust, coarsely denticulate, set on tubercles; palpal tibia with one or two setae (Fig. 11A and C) ………Exothorhis sp.
-
-
Prodorsal setae unpaired but differing in form; femora of legs generally subdivided (Fig. 12B) ………… Ereynetidae Oudemans palp with five segments; dorsal plate and eyes present or absent (Fig. 12A and C) ……… Ereynetes sp.
-
14
Setae e1 and f1 situated on different shield or platelets in female; with at least one pair of genital setae in female ………… Agistemus Summer …… 15
-
-
Setae e1 and f1 situated on same shield in female; without genital setae ………… 16
-
15
Suranal plate with one pair of dorsal setae; metapodosomal plate very large; leg I very long; dorsal setae 11 pairs (Fig. 13A) …………A. vulgaris Soliman and Gomma
-
-
Suranal plate with two pairs of dorsal setae; metapodosomal plat partially covering metapodosoma; leg I shorter than idiosoma; dorsal setae 12 pairs (Fig. 13B) …………… A. exsertus Gonzales
-
16
Chelicerae separate (Fig. 14B) ……… Eustigmaeus kermesinus (Koch)
-
-
Chelicerae basally conjunct (Fig. 14A) ……… Cheylostigmaeus Summers and Ehara ……… 17
-
17
Dorsal plates with heavy shallow circular pores (Fig. 15A) ………… C. californicus Summer and Ehara
-
-
Dorsal plates with few shallow circular pores (Fig. 15B) ……… C. torulus Summer
Figure 1.

Macrocheles glaber: (A) tarsus I; (B) dorsal view; (C) tarsus I of Lasioseius.
Figure 2.
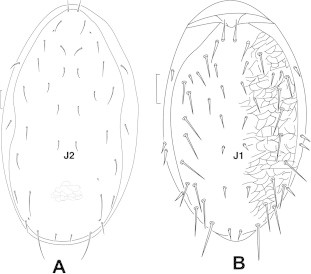
Dorsal view: (A) Neoseiulus cucumeris; (B) Lasioseius lidquisti.
Figure 3.

Aceria lycopersici: (A) legs; (B) male genital coverflap; (C) dorsal shield; (D) female genital coverflap.
Figure 4.

(A) Eyes of Tenuipalpus punicae, (B) palptarsus of Tetranychus urticae.
Figure 5.

Tenuipalpus punicae: (A) palp, (B) dorsal view.
Figure 6.

Empodium: (A) Tetranychus sp.; (B) Eotetranychus fallugiae; (C) Eutetranychus sp.
Figure 7.

Opisthosoma: showing the two pairs of para-anal setae of Eotetranchus fallugiae.
Figure 8.
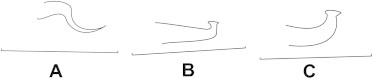
Male aedeagus: (A) E. fallugiae; (B) T. urticae; (C) T. cinnabarinus.
Figure 9.

Dorsal view: (A) Eutetranychus orientalis; (B) Eutetranychus palmatus.
Figure 10.

Phyllotetranychus aegyptiacus: (A) dorsal view; (B) palpus.
Figure 11.

Exothorhis sp.: (A) dorsal view; (B) leg I; (C) palp.
Figure 12.

Ereynetes sp.: (A) dorsal view; (B) leg I; (C) palp.
Figure 13.

Dorsal view: (A) Agistemusvulgaris; (B) Agistemus exsertus.
Figure 14.

Chelicerae: (A) Cheylostigmaeus sp.; (B) Eustigmaeus kermesinus.
Figure 15.
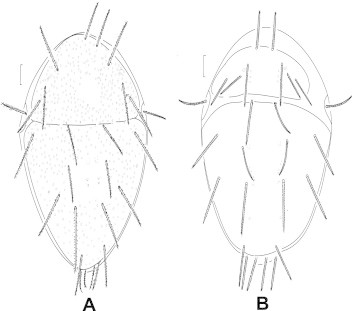
Dorsal view: (A) Cheylostigmaeus californicus; (B) Cheylostigmaeus torulus.
Acknowledgments
The author wishes to express his deep gratitude to Dr. Azzam al-Ahmad for his contribution in providing some laboratory equipments. Deepest thanks also to Dr. Amgad Saleh for revising the manuscript. Many thanks are also extended to the members of Acarology lab for their help, support, and cooperation. This research was conducted with partial financial support from the Research Center of the College of Food and Agriculture Sciences (PLPT-32). All thanks to King Saud University for support and encouragement.
References
- Abd Elsalam A.L., Metwally A.M., Yousef A.A., El- Bghdady H.A., Hegab M.F.A.H. A mites associated with vegetable plants in Egypt. Proc. 1st Conf. P1. Port. Res. Inst. 1980;1:65–71. [Google Scholar]
- Al-atawi Fahad J., Halawa Alaa M. New records of Eriophyoid mites (Acari: Prostigmata: Eriophyoidae) from Saudi Arabia. Pakistan J. Biol. Sci. 2011;14(2):112–117. doi: 10.3923/pjbs.2011.112.117. [DOI] [PubMed] [Google Scholar]
- Al-Shammery K.A. Different biological aspects of the predaceous mite Euseius scutalis (Athias-Henriot) and the effects due to feeding on three tetranychid mites in Saudi Arabia. Asian J. Biol. Sci. 2009;3:77–84. [Google Scholar]
- Amitai S. New records of Phytoseiid mites (Acarina: Phytoseedae) from Cyprus. Entomolgia Hellenica. 1992;10:19–20. [Google Scholar]
- Amrine J.W., Jr., Stasny T.A.H., Flechtmann C.H.W. Indira Publishing House; West Bloomfield: 2003. Revised Key to World Genera of Eriophyoidea (Acari: Prostigmata) IV+244 p. [Google Scholar]
- Arruda Filho G.P. de, Moraes G.J. Stigmatidae mites (Acari: Raphignathoidea) from Arecaceae of Atlantic forest in SaoPaoulo State. Neotrop. Entomol. 2003;32(1) Londrina Jan. [Google Scholar]
- Dabbour A.E., Abdel-Aziz M.I. Scientific note on Acarina in Saudi Arabia. J. Coll. Agric. King Saud Univ. 1982;4:113–116. [Google Scholar]
- Dittrich V. Acaricide resistance in mites. Z. Angew. Ent. 1975;78:28–45. [Google Scholar]
- Ehara S., Wongsiri T. Stigmaeid mites associated with plants in Thailand (Acarina, Stigmaeidae) Kntya, Tokyo. 1984;52(1):110–118. [Google Scholar]
- Fouly A.H., Al-Deghairi M.A., Abdel Baky N.F. Biological aspects and life tables of Typhlodromips swirskii (Acari: Phytoseiidae) fed on Bemisia tabaci (Hemiptera: Aleyroidae) J. Entomol. 2011 ISSN 1812-56701. [Google Scholar]
- Halliday R.B. Mites of the Macrocheles-Glaber Group in Australia (Acarina, Macrochelidae) Aus. J. Zool. 1986;34(5):733–752. [Google Scholar]
- Helle W., Sabelis M.W. vol. 1B. Elsevier; Amsterdam: 1985. (Spider Mites their Biology, Natural Enemies and Control). [Google Scholar]
- Kheradmand K., Ueckermann E.A., Fathipour Y. Mites of the genera Zetzellia and Eustigmaeus from Iran (Acari: Stigmaeidae) Acarina. 2007;15:143–147. [Google Scholar]
- Krantz G.W. A Manual of Acarology. Oregon State Univ. Stores, Inc.; Corvallis: 1978. (second ed.). p. 509. [Google Scholar]
- Martin, H., 1972. Report to the government of Saudi Arabia on research in plant protection. p. 38.
- Santos M.A., Laing J.E. Stigmaeid predators. In: Hell W., Sabelis M.W., editors. vol. 1B. Elsevier, Amsterdam; The Netherlands: 1985. pp. 197–203. (Spider Mites: Their Biology, Natural Enemies and Control). [Google Scholar]
- Soliman Z.R., Al-Yousef M.S. Prostigmatid mites of Saudi Arabia (Acari: Acariformes: Prostigmata) Bull. Zool. Egypt. 1979;29:82–86. [Google Scholar]
- Villanueva R.T., Harmsen R. Studies on the role of the stigmaeid predator Zetzellia mali in the acarine system of apple foliage. Proc. Entomolog. Soc. Ontario. 1998;129:149–155. [Google Scholar]
- Zaher, M.A., 1986. Predaceous & Non-phytophagous Mites in Egypt. PL.480 Programme U.S.A. Project No. EG-ARS-30. Grant No. FG-EG-139.
- Zhang Z.-Q. School of Life Sciences, Fudan University; Shanghi, China: 2003. Mites of Greenhouse. p. 243. [Google Scholar]


