Abstract
Global warming is occurring at an alarming rate and predictions are that air temperature (Ta) will continue to increase during this century. Increases in Ta as a result of unabated production of greenhouse gases in our atmosphere pose a threat to the distribution and abundance of wildlife populations worldwide. Although all the animals worldwide will likely be affected by global warming, diurnal animals in the deserts will be particularly threatened in the future because Tas are already high, and animals have limited access to water. It is expected that Saudi Arabia will experience a 3–5 °C in Ta over the next century. For predicting the consequences of global warming for animals, it is important to understand how individual species will respond to higher air temperatures. We think that populations will not have sufficient time to make evolutionary adjustments to higher Ta, and therefore they will be forced to alter their distribution patterns, or make phenotypic adjustments in their ability to cope with high Ta. This report examines how increases in Ta might affect body temperature (Tb) in the animals of arid regions. We chose three taxonomic groups, mammals, birds, and reptiles (Arabian oryx, Arabian spiny-tailed lizard, vultures, and hoopoe larks) from Saudi Arabia, an area in which Ta often reaches 45 °C during midday in summer. When Ta exceeds Tb, animals must resort to behavioral and physiological methods to control their Tb; failure to do so results in death. The observations of this study show that in many cases Tb is already close to the upper lethal limit of around 47° C in these species and therefore allowing their Tb to increase as Ta increases are not an option. We conclude that global warming will have a detrimental impact on a wide range of desert animals, but in reality we know little about the ability of most animals to cope with change in Ta. The data presented should serve as base-line information on Tb of animals in the Kingdom for future scientists in Saudi Arabia as they explore the impact of global warming on animal species.
Keywords: Climate change, Global warming, Desert animals, Body temperature, Wildlife, Conservation
1. Introduction
The world’s population has reached seven billion people (Tollefson, 2011). The impact that these staggering numbers of people will have on earth is the subject of considerable debate, but it is clear that as requirements for food, electrical power, and transportation increase, we will increase our usage of fossil fuels. Many find it difficult to comprehend that human activity can affect a system as large as earth’s climate, but burning of fossil fuels and production of domestic animals for food result in the addition of large quantities of gases like CO2 and methane into our atmosphere. Currently, we emit over 29 billion metric tons of CO2 in our atmosphere each year (Boden et al., 2009). Most climatologists agree that the increase in greenhouse gases in our atmosphere is causing an increase in air temperature (Ta) and that future increases in Ta pose a clear and present danger to the distribution and abundance of animal and plant populations worldwide (Thompson, 2010). Even though a large number of people doubt the reality of global warming, and others simply choose to ignore it, the increase in the earth’s air temperature over the last 100 years seems incontrovertible, as does the fact that these increases are not a result of natural phenomena (Thompson, 2010; Oerlemans, 2005; Thompson et al., 2009; Briffa et al., 2002; Crowley and Lowery, 2000; Moberg et al., 2005). In the decades to come, if they are to survive, species will need to alter their distribution patterns, change their behavior patterns, and/or make adjustments in their physiology, either by short-term acclimation through phenotypic flexibility or by longer-term evolutionary shifts in physiological phenotype by means of natural selection (Angilletta, 2009; Chown et al., 2010).
For larger animals with long generation times, it is doubtful that evolutionary adaption will be a solution to global warming: there will not be sufficient time for new adaptations to appear that will allow living at such high Ta. If scientists are to predict the consequences of global warming for animals, we will need to understand how individual animals will respond to higher air temperatures through phenotypic flexibility (Pörtner and Farrell, 2008; Somero, 2011).
In this commentary we will briefly examine the evidence for and causes of global warming, comment on how increases in Ta will affect the control of body temperature in animals of Saudi Arabia, an area that already has extremes in Ta during the day, and then speculate on the impact of increases in Ta for animals in Saudi Arabia. We end the paper by pointing out how little we know about the ability of animals to adjust to higher temperatures, and a plea for scientists in Saudi Arabia to study the impact of increasing Ta on animals in the Kingdom.
2. Evidence for global warming
The leading international body for the assessment of climate change, the Intergovernmental Panel on Climate Change (IPCC) produced a synthesis report in 2007 that concluded “warming of the climate system is unequivocal, as is now evident from observations of increases in global average air and ocean temperatures, widespread melting of snow and ice and rising global average sea level” (Fig. 1). To further emphasize how the planet is warming, the IPCC pointed out that eleven of the last twelve years (1995–2006) ranked among the warmest years on record.
Figure 1.
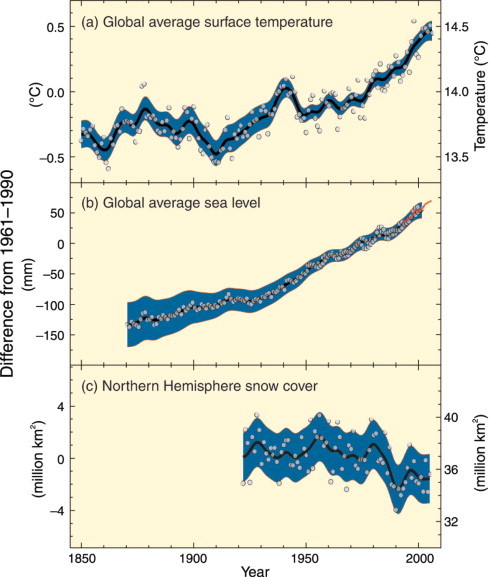
Observed changes in (a) global average surface temperature; (b) global average sea level from tide gauge (blue) and satellite (red) data; and (c) Northern Hemisphere snow cover for March–April. All differences are relative to corresponding averages for the period 1961–1990. Smoothed curves represent decadal averaged values while circles show yearly values. (IPCC Synthesis Report, 2007).
Increases in sea level are consistent with global warming (Fig. 1b). Global average sea level rose at an average rate of 1.8 mm per year over 1961–2003, and at a rate of about 3.1 mm per year from 1993 to 2003. More than half of the increase in sea level can be attributed to the thermal expansion of ocean water whereas about 30% came from melting of glaciers and polar ice sheets. Satellite data since 1978 show that the extent of the annual average Arctic sea ice has shrunk by 2.7% per decade, with larger decreases in summer of 7.4% per decade. The maximum areal extent of seasonally frozen ground has decreased by about 7% in the Northern Hemisphere since 1900 (Fig. 1c), with decreases in spring of up to 15%.
Glacial ice atop mountains the world over is rapidly melting (Thompson, 2010). Since 1912, 85% of the ice on the African mountain Kilimanjaro has melted, visible evidence of warming in Ta (Thompson et al., 2009). In 1910, there were 100 glaciers in Glacier National Park in Northern USA. In 2011 there remain 26 glaciers, and by 2030, it is projected that all glacial ice will have melted in this park. It is difficult to draw any other conclusion than our planet is warming at an alarming rate. The melting of glacial ice embodies the “canary in the coal mine”, and signals a portent of physiological problems for animal populations as they are faced with the consequences of increases in Ta. In the decades to come, species will need to make adjustments in their physiology, especially adjustments that allow maintenance of normal body temperature (Tb) as Ta increases, either by short-term acclimation or by longer-term evolutionary shifts in physiological phenotype (Angilletta, 2009; Chown et al., 2010).
3. Causes of global warming
Global warming is related to the increase in emission greenhouse gases into our atmosphere, such as carbon dioxide, methane, nitrous oxide, and ozone (Fig. 2). Although the evidence that our globe is warming seems incontrovertible, the causes of global warming are less certain and currently hotly debated. Many of the arguments against anthropogenic causes of global warming are rooted in economic or political gain, rather than scientific reasoning. Some argue that our current trend in climate warming is the result of natural variation in Ta, rather than caused by human activity. Close scrutiny of the data contradicts such arguments and points to anthropogenic causes for our current exposure to unusually warm Tas (Thompson, 2010). The IPCC (2007) concluded, “Anthropogenic warming over the last three decades has likely had a discernible influence at the global scale on observed changes in many physical and biological systems”. This report goes on to say that “warming is unlikely to be due solely to natural variability of temperatures”.
Figure 2.
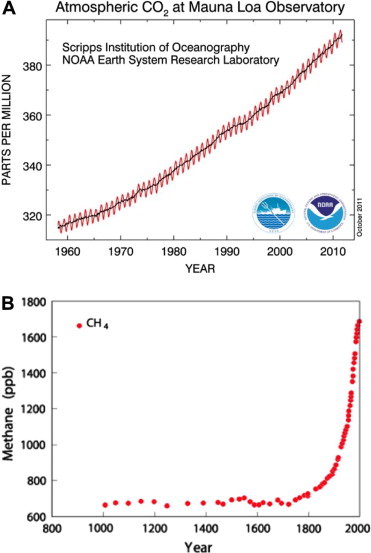
(A) The increase in atmospheric carbon dioxide measured at Mauna Loa, Hawaii. Units are parts per million per volume. From http://www.esrl.noaa.gov/gmd/ccgg/trends/B. The increase in methane gas in the atmosphere. Units are parts per billion. From the European Project for Ice Coring in Antarctica, which has recorded a history of CO2 and methane levels preserved in bubbles in the ice (Loulergue et al., 2008; Lüthi et al., 2008).
4. Global warming in Saudi Arabia
Animals the world over will be impacted by global warming, but because they already face high Tas and intense solar radiation, and do not have access to water, diurnal animals in the deserts of Saudi Arabia are particularly threatened (Louw and Seeley, 1982; Williams and Tieleman, 2005; Williams et al., 2012). Simulations for climate change in Saudi Arabia predict that daily Ta will increase by 3–5° C by the end of the 21st century, but rainfall patterns will remain the same (Al Zawad, 2008). Most desert animals resident in the Kingdom are non-migratory, and thus will be forced to cope with increases in Ta if they are to survive. In Saudi Arabia, Ta often reaches 45° C during midday in summer and soil surface temperatures now regularly exceed 60° C (Tieleman et al., 2003).
5. Normal body temperatures
Predictions of the biological impacts of climate change require information on deviations in environment, such as Ta, solar radiation, and wind, as well as knowledge about body temperature, physiology, and behavior of species (Angilletta, 2009; Kearney et al., 2009). Under normal conditions, body temperature for many mammals and birds is regulated within narrow limits over a broad range of Tas, for mammals around 36–37° C, and for birds near 41–42° C (McNabb, 1966, White et al., 2006; Clarke and Rothery, 2008). Lizards are ectotherms, and therefore mainly regulate their Tb behaviorally and to a lesser extent by physiological means. Tbs typically range from 20 to 40° C in the field for most species (Huey et al., 2009). When ambient temperatures exceed normal Tb, as are predicted to occur in Saudi Arabia, animals must resort to behavioral and physiological methods to control their Tb, such that it does not increase to lethal levels. Failure to control body temperature can result in the loss of muscle coordination and eventually death. Unfortunately we know little about how desert animals control their body temperature below lethal levels.
6. Upper lethal body temperature
The upper lethal body temperature of complex multicellular animals is thought to be around 47° C (Pörtner, 2002; Pörtner and Farrell, 2008). The ‘Pompeii worm’, thought to be the ‘most thermotolerant eukaryote on earth’ (Desbruyères et al., 1998), because it can be exposed to temperatures as low as 2° C and as high as 105° C in hydrothermal deep sea vents for short periods, will likely not live long-term beyond 31° oC (Dahlhoff and Somero, 1991). The upper thermal limit of nematodes is around 45–47° C, which is considered the upper temperature limit of continuous inhabitation by these animals (Nicholas, 1984). A desert ant can tolerate a Tb of 53.6° C, but only for brief periods (Wehner et al., 1992). The average upper thermal limit for insects was evaluated as 47.4 ± 0.4° C (Addo-Bediako et al., 2000). In general, body temperatures beyond 47° C are tolerated only temporarily by metazoans (Schmidt-Nielsen, 1997). For some birds and mammals, this upper limit for Tb may even be lower, around 43–44° C (Bligh, 1985).
Increases in Ta and in soil surface temperature associated with climate change in Saudi Arabia will undoubtedly challenge populations of animals in the future because they must maintain their Tb below the upper lethal limit. Desert animals control their Tb below their upper lethal limit by evaporative cooling when Ta is above Tb, which may challenge their fragile water economy, or behaviorally such as by burrowing underground or seeking deep shade. Avoidance of high Ta behaviorally often restricts foraging time, and consequently food intake, which impacts survival (Sinervo et al., 2010). Given that control of Tb will be a significant component of an animal’s response to global warming, we explore what we know of Tb for free-living animals of Saudi Arabia.
7. Arabian Oryx
The Arabian oryx (Oryx leucoryx), a desert antelope (body mass, 80–100 kg) that once ranged throughout most of the Arabian Peninsula, was extirpated from the wild by 1972 (Henderson, 1974). In 1990, Arabian oryx were reintroduced into Mahazat as-Sayd, a large protected area 160·km north-east of Taif, Saudi Arabia. The population has increased significantly and now numbers >600 individuals (Ostrowski et al., 1998; Treydte et al., 2001). Arabian oryx can live without access to drinking water in deserts (Williams et al., 2001), including the Rub al-Khali, one of the driest regions in the world (Meigs, 1953). Survival of oryx in such harsh areas is noteworthy when one considers its large size, its inability to shelter in burrows and that herbivory is typically associated with high rates of water turnover (Nagy and Peterson, 1988). Arabian oryx have one of the lowest mass-specific water-influx rates among ungulates living in hot environments: 76.9% below allometric prediction in summer (Nagy and Peterson, 1988; Williams et al., 2001; Ostrowski et al., 2002a,b). During the hot Arabian summer, Oryx on average consumed 1310 mL H2O per day in their food, whereas in spring when the vegetation contained more water, they consumed 3438 mL H2O per day (Ostrowski et al., 2003).
Ostrowski et al. (2003) measured core Tb of six free-ranging, adult Arabian Oryx during 2 years in the arid desert of West-Central Saudi Arabia. They found that oryx varied their Tb by 4.1 ± 1.7 °C per day during summer (June to September), a phenomenon called heterothermy (Fig. 3). Hence, Oryx stored heat in their body during the summer day, which could be dissipated during the night without using water to evaporatively cool themselves. Over the entire year, mean Tb was 38.4 ± 1.3 °C. In this study, daily variation in Tb appeared to reflect thermal load (Ta,max–Ta,min) rather than an endogenous rhythm. Oryx used behavioral thermoregulation to cope with thermal stress during summer: animals lay down in shade in the morning shortly before Ta exceeded Tb and remained there until evening when Tb–Ta became positive. Oryx saved a minimum of 280 mL H2O per day and 110 mL H2O per day in summer and winter, respectively, by allowing their body to store heat during the day and dissipate it at night by non-evaporative means. Without heat storage in summer, Ostrowski et al. (2003) estimated that Oryx would have to increase their water intake by 19%, a requirement that would be difficult to meet in their desert environment.
Figure 3.
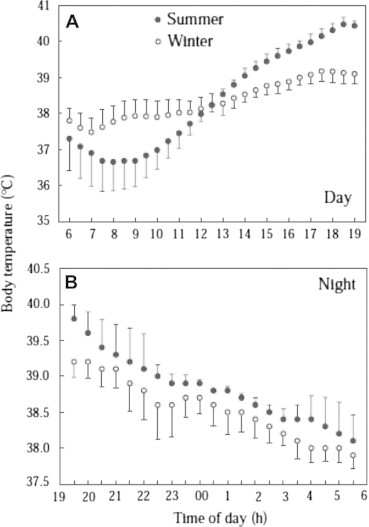
Mean body temperature of six free-ranging Arabian Oryx as a function of time of day. Means ± S.D. for 59 days and 12 nights between May 1998 and September 2001 (Ostrowski et al., 2002b).
How will Oryx respond to increases in Ta during the next century? If increases in Ta are 3–5 °C as the models predict, then daytime and nighttime temperatures that Oryx experience will be higher. Hence, they will not be able to reduce their Tb as low at night as they do now, with the consequence that they would not be able to store as much heat during the day. With higher Tas, Oryx will likely be forced to use more water to thermoregulate as their environment warms.
In addition, during summer Oryx feed at night when the relative humidity is higher than during the day. Foraging at night allows them to avoid the extremes of daytime solar radiation and Ta, and to obtain more water in the grasses that they eat. At night, when Ta is lower and relative humidity higher, the grasses that they eat contain more water (Fig. 4). To demonstrate the hygroscopic nature of desert plants, Taylor (1968) dried the leaves of Diasperma sp., a desert plant, and then placed the leaves at 17° C-85% RH, 23° C-39% RH, and 40° C-17% RH. He noted that the leaves at the highest relative humidity and lowest temperature gained the most moisture. This demonstrated that desert plants are hygroscopic, and that the best time for desert ungulates to feed would be in the early morning hours before dawn when plants contain the most water. As the global temperature warms, Ta will increase at night, and humidity will decrease. This will result in Oryx obtaining less water in the grasses that they eat. With less water intake, but a higher water requirement to thermoregulate, we doubt that Oryx will survive in Mahazat short of intervention by man. Providing food and water to Oryx may be necessary, but also it removes their “wild animal” status, and reduces them to captive zoo animals. It then will become an economic and/or political issue of whether government officials will be willing to supply food and water to them.
Figure 4.
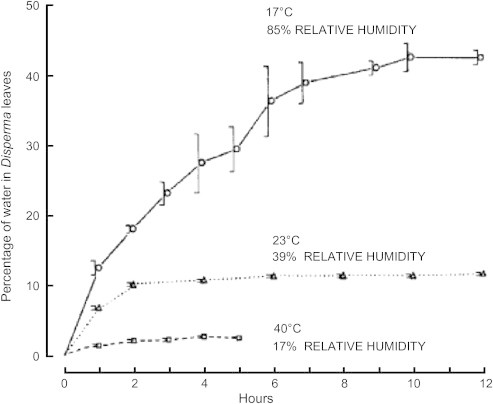
Leaves of Disperma sp. were dried to constant mass at 110 °C and then placed at 19 °C at 85% RH, or 23 °C and 39% RH, or 40 °C and 17% RH. The figure demonstrates the hygroscopic nature of leaves and the time required for equilibration. (From Taylor, 1968).
8. Arabian spiny-tailed lizard
Reptiles are common in deserts and semi-deserts across the Arabian Peninsula. Some of them (like members of the Agamidae, Lacertidae and Varanidae) are typically heliotherms that bask in direct sun to attain physiologically active Tbs, whereas others, like most members of the Gekkonidae and Trogonophidae, are nocturnal and exhibit Tbs not significantly different from the nighttime environment. Activity at high Tas can elevate Tb above the critical maximum leading to death (Porter, 1989). As ectotherms, reptiles control their Tb mainly by means of behavioral adjustments, for example, by changes in daily activity rhythms, shuttling in and out of direct sun, darkening their skin coloration to absorb more solar radiation when its cooler, or panting to evaporatively cool themselves, or retreating into burrows when environmental heat load becomes extreme. However, spending time in underground thermal refuges limits foraging, constraining functions like growth and reproduction, and thereby may undermine population growth rates and raising extinction risk (Sinervo et al., 2010). Relative to birds and mammals, ectotherms, like lizards, are thought to be especially sensitive to changes in environmental temperature (Porter and Gates, 1969; Deutsch et al., 2008; Huey et al., 2010), and as such, they are considered to be good indicator organisms for ecosystem monitoring (Sinervo et al., 2010).
A large (2000–2500 g), diurnal, primarily herbivorous lizard, the Arabian spiny-tailed lizard (Uromastyx aegyptia microlepis) or Dabb lizard, occurs throughout much of the Arabian Peninsula. In spring these lizards are active between 8:00–10:00 h and 15:00–18:00 h), but less active during the hottest part of the day (Wilms et al., 2011). However, during midday large adults are often seen outside of their burrows in full sun suggesting that high Tas force juveniles and subadults underground, where temperatures are cooler, whereas larger individuals can withstand higher Ta, at least up to a point (Wilms et al., 2009).
The Tb of adult Uromastyx was studied in Mahazat as-Sayd during 2006 and 2007 using small data loggers implanted into the peritoneal cavity of free ranging lizards (Wilms et al., 2011) (Fig. 5). During July to September, 2006, maximum Tb achieved by field-active Uromastyx often reached 46° C during midday, and for one lizard was 47.2 °C, near the presumed upper lethal limit for vertebrates. For an animal that does not control its body temperature by metabolism, as does an endotherm, average Tb was remarkably constant for Uromastyx during summer, around 37–38° C. Some other desert lizards are capable of withstanding body temperatures near 50 °C under laboratory conditions for short periods of time (Ulmasov et al., 1999), but it is not known if this also applies to Uromastyx.
Figure 5.
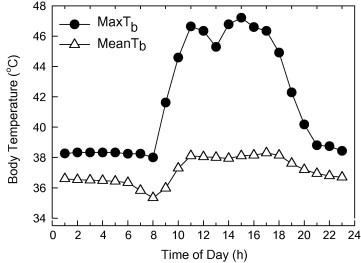
Maximum and mean body temperature of adult Uromastyx lizards in Mahazat protected area during July–September, 2006. N = 6 individuals.
Operative temperature (Te) is a measurement of the actual temperature experienced by an animal, and includes properties of Ta, solar radiation, and wind, as well as properties of the animal such as surface area and skin color (Bakken et al., 1985). It is typically measured using physical models of the animal such as a copper cast or taxidermic mount placed throughout the environment. This information provides a picture of the possible thermal niche available to the animal. When Te was measured for models of Uromastyx, it ranged between 14.3–61.8 °C (spring) and 19.5–63.4 °C (summer). Within burrows, Te ranged between 28.6–34.1 °C in spring and 35.5–37.8 °C in summer. Hence when Te soars above 60 °C on the soil surface in Mahazat, Uromastyx can find a thermal refuge below ground (Wilms et al., 2011). During winter Uromastyx are active from about 11:00–15:00 h, only about 20% of summer activity levels. During winter, Te available to the animals ranged from 1.2 to 44.4 °C.
From these studies, it is clear that summer is the time of the year when lizards experience the highest level of potential heat stress. In summer, when Te approached 60 °C, and plants were becoming dry, lizards drastically lowered their above ground activity not only to escape the heat outside their burrows, but also to conserve body water. It has been shown, that the lizards selected areas with significantly higher moisture content in burrows during the night in summer, indicating some level of water stress for Uromastyx at this time (Wilms et al., 2010).
The effect of climate warming on Uromastyx has been assessed by Wilms et al. (2011) who developed a climate model to predict the impact of global warming on populations of Uromastyx. The temperature increase of 3–5° C predicted by climate models for Saudi Arabia will likely affect the activity patterns and thermoregulation of these lizards, and will also interfere with the general ecology of the species. Lizards will be confined below ground for longer periods of time, thus affecting their growth and reproduction. The model suggested that Uromastyx will still be found on most of the Arabian Peninsula at the end of this century, but the model also predicted a remarkable decrease of environmental suitability for these lizards of up to 70–80% (Wilms et al., 2011). We suspect that Uromastyx will disappear from many regions on the Arabian Peninsula as global temperatures increase.
9. Vultures
Weighing nearly 9 kg and with a wing-span of 2.75 meters, the lappet-faced vulture (Torgos tracheliotus) is the largest of the old world vultures found in Africa and the Arabian Peninsula (Mundy et al., 1992; Newton and Shobrak, 1993). These vultures were first recorded in Saudi Arabia in 1947, and now number around 600 pairs, an increase coincident with the establishment of protected reserves in the Kingdom (Newton and Shobrak, 1993; Newton and Newton, 1996). Lappet-faced vultures in Saudi Arabia are listed on the IUCN Red list as Vulnerable to extinction (Shobrak, 1996; BirdLife International, 2000). Because they eat and recycle nutrients from decaying animal material, these birds play a major role in the desert ecosystem. Houston (1974) estimated that in the Serengeti, 85% of the diet of lappet-faced vultures came from the carcasses of animals which had died from disease or starvation, but Shobrak (1996) thought that their diet was mostly dead domestic animals in Saudi Arabia. Like all vultures, lappet-faced vultures require several years to reach sexual maturity and, because they generally lay one egg per year, have a low reproductive rate (Houston, 1980; Mundy et al., 1992). Hence this species is particularly vulnerable to any changes in their environment that might impact their reproductive success.
Lappet-faced vultures build their nests atop Maerua and Acacia trees typically in winter (December) when Tas are relatively low in the desert, and incubate their eggs for about 56 days, with eggs hatching in March–April, when Ta is beginning to increase (Brown, 1986; Bridgeford et al., 1995; Ferguson-Lees and Christie, 2001). Chicks remain in the nest for ca. 125 days exposed to the sun and higher Tas during late spring and summer and therefore often need to be shaded by parents during the midday. One or the other parent stays at the nest continuously until the chicks fledge, to ward of potential predators, and to protect the chick from the sun (Shobrak, 1996). Given their full exposure to the desert sun, and their threatened status, a study of the Tb of lappet-faced vultures should produce important baseline information to address how these birds might respond to global warming.
Shobrak et al. (unpubl.) implanted temperature-sensitive data loggers into the peritoneal cavity of young vulture chicks in Mahazat in 2004–2008, and monitored their Tb as they grew. They found that the mean core Tb of the nestlings implanted with data loggers was 39.1 ± 0.68 °C, and the minimum and maximum Tb recorded were 37.6 ± 0.6° C and 41.1 ± 0.8° C, respectively. Hence the body temperature of chicks varied only a few degrees, from 38–41° C, despite chicks being located on the tops of trees in the desert (Shobrak et al., unpubl., Fig. 6). Nestlings maintained their Tb by adjusting their orientation to the direct sun or they were shaded by the parent (Shobrak, 2001; Shobrak unpublished data). Shading of young nestlings by adults was critically important for their survival: one 10-day old nestling died after the adult left the nest and chick Tb reached 42.6° C. Older chicks were able to survive body temperatures of at least 44° C.
Figure 6.
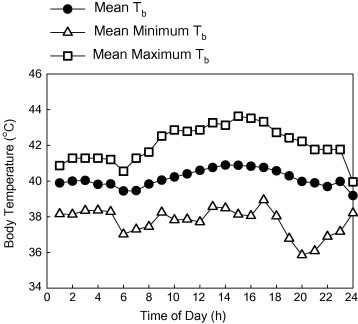
Mean, minimum, and maximum average Tb of vulture chicks during the entire nesting cycle (Shobrak unpubl.).
Gauging the response of vultures to global warming is difficult because we know so little about upper lethal limits of Tb for chicks or adults. We also know nothing of how vultures can evaporatively cool themselves during the heat of the day. For young chicks, the upper lethal Tb seems to be around 43° C, but for older chicks somewhat higher. We suspect that as Ta increases in Saudi Arabia, adults will be forced to spend more time away from the chicks, and hence chick mortality may increase, especially during the first few days of life. With a reproductive rate that is already low, we doubt that the lappet faced vulture will be able to survive an increase in Ta of 3–5° C in Saudi Arabia.
10. Hoopoe larks
Hoopoe larks (Alaemon alaudipes) are small (45 g) ground-foraging birds of the Arabian Desert with a diet that consists mainly of insects and spiders. Drinking water is not available to them except for short periods after rains, so they obtain all of their preformed water from their food. Hoopoe larks breed in Mahazat from February to June in years when adequate rainfall has occurred. If rains do not fall in spring, Hoopoe Larks will forego breeding until the following year. Hoopoe larks in Mahazat defended territories with a mean size of 0.41 ± 0.18 km2 (N = 9) (Tieleman and Williams, 2002). Pairs remain together throughout most of the day, mutually calling while foraging or resting in shade.
Because Hoopoe larks forage for terrestrial insects during the day, they are particularly exposed to the climate near the ground. Tieleman and Williams (2002) measured soil surface temperature, operative temperature of copper casts covered with the feathers and skin of a Hoopoe Lark, and Ta during the summer in Mahazat (Fig. 7). By 9 am, Te and soil surface temperature had exceeded the normal body temperature of larks meaning that as they foraged, they were likely gaining heat from the environment. Hence, their body temperature must increase, or they need to dissipate heat in other ways. During midday, Hoopoe Larks were forced into the shade of acacia trees where they often pressed their body to the ground (soil temperature = 37° C) to conduct heat away from their body.
Figure 7.
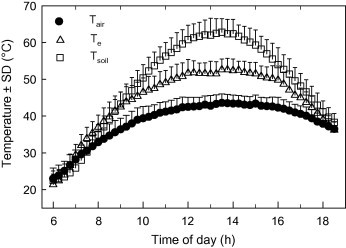
Average soil surface temperature (open squares), operative temperature of hoopoe lark copper models (open triangles), and air temperature (closed circles) in Mahazat during summer (Tieleman and Williams, 2002).
To explore how Hoopoe Larks managed their Tb in the deserts of Saudi Arabia, Tieleman, Williams, and Shobrak (unpubl.) measured the Tb of Hoopoe Larks in Mahazat during the summer using temperature sensitive radio transmitters implanted in the bird’s peritoneal cavity. Body temperature of Hoopoe Larks reached 45° C during midday, near their upper lethal limit. Given that these birds already employ various behaviors to regulate their Tb, such as going into the burrow of Uromastyx lizards during midday (Williams et al., 1999), it is hard to imagine them developing new behavioral repertoires for this purpose. To control Tb below lethal limits, during periods of extreme heat, one might imagine that desert birds will increase their evaporative water losses to control their Tb, but this will impose severe constraints on their water budget. Over the long term desert birds will likely be forced to further reduce their evaporative water losses, especially water loss through their skin (Williams et al., 2012). How desert birds will resolve these conflicting demands of water shortage and thermoregulation remains unknown. This could be a subject of profitable research by scientists in Saudi Arabia.
11. Conclusions
The earth’s temperature is warming. It is predicted that animals in Saudi Arabia will experience a 3–5° in Ta over the next century. We have explored the body temperature of four species of desert animals, Oryx, Uromastyx, lappet faced vultures, and hoopoe larks, and have shown that in many cases Tb is already close to the upper lethal limit in these species. Allowing their Tb to increase as Ta increases in Saudi Arabia is likely not an option. Hence we argue that global warming will have a detrimental impact on all four of these species. These data should serve as base-line information on body temperature of animals in the Kingdom for future scientists in Saudi Arabia as they explore the impact of global warming on animals. Finally we end the paper by pointing out how little we know about the ability of animals to adjust to higher Ta or Tb, and a plea for scientists in Saudi Arabia to study the impact of increasing Ta on animals in the Kingdom.
Acknowledgments
This paper resulted from an invited seminar presentation of the Saudi Biological Society Meeting, 2011. Financial support for our research has come from the National Plan for Science and Technology (NPST) Program by King Saud University, Project Number BIO1116-02-10, and from the National Science Foundation (IBN-0212092 to J.B.W).
References
- Addo-Bediako A., Chown S.L., Gaston K.J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Zawad, F.M., 2008. Impacts of climate change on water resources in Saudi Arabia. The 3rd International Conference on Water Resources and Arid Environments and the 1st Arab Water Forum.
- Angilletta M.J., Jr. Looking for answers to questions about heat stress: researchers are getting warmer. Funct. Ecol. 2009;23:231–232. [Google Scholar]
- Bakken G.S., Santee W.R., Erskine D.J. Operative and standard operative temperature: tools for thermal energetics studies. Amer. Zool. 1985;25:933–943. [Google Scholar]
- BirdLife International, 2000. Threatened birds of the World. Barcelona, Spain and Cambridge, UK: Lynx Edicions and BirdLife International.
- Bligh . Temperature regulation. In: Yousef M.K., editor. Stress Physiology in Livestock. vol. 1. CRC Press, Inc.; Boca Raton: 1985. pp. 75–96. (Basic Principles). [Google Scholar]
- Boden, T.A., Marland, G., Andres, R.J., 2009. Global, regional, and national fossil fuel CO2 emissions. Oak Ridge, TN: Carbondioxide Information Analysis Center, Oak.
- Briffa K.R., Jones P.D., Schweingruber F.H., Shiyatov S.G., Cook E.R. Unusual twentieth-century summer warmth in a 1000-year temperature record from Siberia. Nature. 2002;376:156–159. [Google Scholar]
- Bridgeford P., Bridgeford M., Erasmus J. First record of two Lappet-faced Vulture chicks reared in one nest Ostrich. 1995;66:35–37. [Google Scholar]
- Brown C.J. Biology and conservation of the Lappet faced Vulture in SWA/Namibia. Vulture News. 1986;14:4–15. [Google Scholar]
- Chown S.L., Hoffmann A.A., Kristensen T.N., Angilletta M.J., Jr., Stenseth N.C., Pertoldi C. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 2010;43:3–15. [Google Scholar]
- Clarke A., Rothery P. Scaling of body temperature in mammals and birds. Funct. Ecol. 2008;22:58–67. [Google Scholar]
- Crowley T.J., Lowery T.S. How warm was the medieval warm period? AMBIO. J. Hum. Environm. 2000;29:51–54. [Google Scholar]
- Dahlhoff E., Somero G. Pressure and temperature adaptation of cytosolic malate dehydrogenases of shallow and deep-living marine invertebrates: evidence for high body temperatures in hydrothermal vent animals. J. Exp. Biol. 1991;159:473–487. [Google Scholar]
- Desbruyères D., Chevaldonné P., Alayse A.M., Jollivet D., Lallier F., Jouin-Toulmond C., Zal F., Sarradin P.M., Cosson R., Caprais J.C., Arndt C., O’Brien J., Guezennec J., Hourdez S., Riso R., Gaill F., Laubier L., Toulmond A. Biology and ecology of the “Pompeii worm” (Alvinella pompejana Desbruyères and Laubier), a normal dweller of an extreme deep-sea environment: a synthesis of current knowledge and recent developments. Deep-Sea Res. 1998;45:383–422. [Google Scholar]
- Deutsch C.A., Tewksbury J.J., Huey R.B., Sheldon K.S., Ghalambor C.K., Haak D.C., Martin P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Lees, J., Christie, D.A., 2001. Raptors of the world. Houghton Mifflin Harcourt.
- Henderson D.S. Were they the last Arabian oryx? Oryx. 1974;12:347–350. [Google Scholar]
- Houston D.C. Mortality of the Cape Vulture. Ostrich. 1974;45:57–62. [Google Scholar]
- Houston D.C. A possible function of sunning behavior by Griffon Vultures Gyps sp. and other large soaring birds. Ibis. 1980;122:366–369. [Google Scholar]
- Huey R.B., Losos J.B., Moritz C. Ecology. Are lizards toast? Science. 2010;328:832–833. doi: 10.1126/science.1190374. [DOI] [PubMed] [Google Scholar]
- Huey R.B., Deutsch C.A., Tewksbury J.J., Vitt L.J., Hertz P.E., Álvarez Pérez H.J., Garland T., Jr Why tropical forest lizards are vulnerable to climate warming. Proc. R Soc. Lond. B. 2009;276:1939–1948. doi: 10.1098/rspb.2008.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC, 2007. Summary for policymakers. In: Solomon S, Qin D, Manning M, Chen Z and others (eds.), Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
- Kearney M.R., Porter W., Shine R. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl. Acad. Sci. USA. 2009;106:3835–3840. doi: 10.1073/pnas.0808913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulergue L., Schilt A., Spahni R., Masson-Delmotte V., Blunier T., Lemieux B., Barnola J.M., Raynaud D., Stocker T.F., Chappellaz J. Orbital and millennial-scale features of atmospheric CH4 over the past 800,000 years. Nature. 2008;453:383–386. doi: 10.1038/nature06950. [DOI] [PubMed] [Google Scholar]
- Louw G.N., Seeley M.K. Longman, 1982; New York: 1982. Ecology of Desert Organisms. [Google Scholar]
- Lüthi D., Le Floch M., Bereiter B., Blunier T., Barnola J.M., Siegenthaler U., Raynaud D., Jouzel J., Fischer H., Kawamura K., Stocker T.F. High-resolution carbon dioxide concentration record 650, 000–800,000 years before present. Nature. 2008;453:379–382. doi: 10.1038/nature06949. [DOI] [PubMed] [Google Scholar]
- McNabb B.K. An analysis of body temperature of birds. Condor. 1996;68:47–55. [Google Scholar]
- Meigs P. World distribution of arid and semi-arid homoclimates. Arid Zone Res. 1953;1:203–210. [Google Scholar]
- Moberg A., Sonechkin D.M., Holmgren K., Datsenko N.M., Karlen W. Highly variable Northern Hemisphere temperatures reconstructed from low- and high-resolution proxy data. Nature. 2005;433:613–617. doi: 10.1038/nature03265. [DOI] [PubMed] [Google Scholar]
- Mundy P., Butchart D., Ledger J., Piper S. Russel Friedman and Acorn Books; Johannesburg: 1992. The Vultures of Africa. [Google Scholar]
- Newton S., Newton A. Breeding biology and seasonal abundance of lappet-faced vultures Torgos tracheliotus in Western Saudi Arabia. Ibis. 1996;138:675–683. [Google Scholar]
- Newton S.F., Shobrak M. The lappet-faced vulture Torgos tracheliotus in Saudi Arabia. Proc. VIII Pan-African orn. Congr. 1993:111–117. [Google Scholar]
- Nagy K.A., Peterson C.C. Scaling of water flux rate in animals. Univ. California Public. Zool. 1988;120:1–172. [Google Scholar]
- Nicholas W.L. Clarendon Press; Oxford: 1984. The Biology of Free-Living Nematodes. [Google Scholar]
- Oerlemans J. Extracting a climate signal from 169 glacier records. Science. 2005;675:677. doi: 10.1126/science.1107046. [DOI] [PubMed] [Google Scholar]
- Ostrowski S., Bedin E., Lenain D.M., Abuzinada A.H. Ten years of Arabian oryx conservation breeding in Saudi Arabia – achievements and regional perspectives. Oryx. 1998;32:209–222. [Google Scholar]
- Ostrowski S., Williams J.B., Bedin E., Ismail K. Water flux and food consumption of free-leaving oryx (Oryx leucoryx) in the Arabian desert during summer. J. Mammal. 2002;83:665–673. [Google Scholar]
- Ostrowski S., Williams J.B., Ismail K. Heterothermy and the water economy of free-living Arabian oryx (Oryx leucoryx) J. Exp. Biol. 2002;206:1471–1478. doi: 10.1242/jeb.00275. [DOI] [PubMed] [Google Scholar]
- Porter W.P. New animal models and experiments for calculating growth potential at different elevations. Physiol. Zool. 1989;62:286–313. [Google Scholar]
- Porter W.P., Gates D.M. Thermodynamic equilibria of animals with environment. Ecol. Monogr. 1969;39:227–244. [Google Scholar]
- Pörtner H.O. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. Part A. 2002;132:739–761. doi: 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O., Farrell A.P. Physiology and climate change. Science. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; Cambridge: 1997. Animal Physiology: Adaptation and Environment. [Google Scholar]
- Shobrak, M., 1996. Ecology of the lappet-faced vulture Torgos tracheliotus in Saudi Arabia. Ph. D. diss. Glasgow University. Glasgow.
- Shobrak, M., 2001. Posturing behaviour of Lappet-faced Vulture Torgos tracheliotus chicks on the nest plays a role in protecting them form high ambient temperatures. Asain Raptor Bulliten No. 2.
- Sinervo B., Méndez-de-la-Cruz F., Miles D.B. Erosion of lizard diversity by climate change and altered thermal niches. Science. 2010;328:894–899. doi: 10.1126/science.1184695. [DOI] [PubMed] [Google Scholar]
- Somero G.N. Comparative physiology: a “crystal ball” for predicting consequences of global change. Am. J. Physiol. -Regula. Integ. Compar. Physiol. 2011;301:R1–R14. doi: 10.1152/ajpregu.00719.2010. [DOI] [PubMed] [Google Scholar]
- Taylor C.R. Hygroscopic food: a source of water for desert antelopes. Nature. 1968;219:181–182. doi: 10.1038/219181a0. [DOI] [PubMed] [Google Scholar]
- Thompson L.G. Climate change: the evidence and our options. Behav. Anal. 2010;33:153–170. doi: 10.1007/BF03392211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L.G., Brecher H.H., Mosley-Thompson E., Hardy D.R., Mark B.G. Glacier loss on Kilimanjaro continues unabated. PNAS. 2009;106:19770–19775. doi: 10.1073/pnas.0906029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman B.I., Williams J.B. Cutaneous and respiratory water loss in larks from arid and mesic environments. Physiol. Biochem. Zool. 2002;75:590–599. doi: 10.1086/344491. [DOI] [PubMed] [Google Scholar]
- Tieleman B.I., Williams J.B., Buschur M.E., Brown C.R. Phenotypic variation among and within larks along an aridity gradient: are desert birds more flexible? Ecology. 2003;84:1800–1815. [Google Scholar]
- Tollefson J. Seven billion and counting. Nature. 2011;478:300. doi: 10.1038/478300a. [DOI] [PubMed] [Google Scholar]
- Treydte A.C., Williams J.B., Bedin E., Ostrowski S., Seddon P.J., Marshall E.A., Waite T.A., Ismail K. In search of the optimal management strategy for Arabian oryx. Anim. Conserv. 2001;4:239–249. [Google Scholar]
- Ulmasov K., Zatsepina O., Molodtsov V., Evgenev M. Natural body temperature and kinetics of heat-shock protein synthesis in the toad headed agamid lizard Phrynocephalus interscapularis. Amphib.–Rept. 1999;20:1–9. [Google Scholar]
- Wehner R., Marsh A.C., Wehner S. Desert ants on a thermal tightrope. Nature. 1992;357:586–587. [Google Scholar]
- White C.R., Phillips N.F., Seymour R.S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2006;2:125–127. doi: 10.1098/rsbl.2005.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.B., Tieleman B.I. Physiological adaptation in desert birds. Bioscience. 2005;55:416–425. [Google Scholar]
- Williams J., Tieleman I., Shobrak M. Lizard burrows provide thermal refugia for larks in the Arabian desert. Condor. 1999;101:714–717. [Google Scholar]
- Williams J.B., Ostrowski S., Bedin E., Ismail K. Seasonal variation in energy expenditure, water flux and food consumption of Arabian oryx Oryx leucoryx. J. Exp. Biol. 2001;204:2301–2311. doi: 10.1242/jeb.204.13.2301. [DOI] [PubMed] [Google Scholar]
- Williams, J.B., Agustí Muñoz-Garcia, and Alex Champagne, 2012. Climate change and cutaneous water loss in birds. Invited Commentary. Journal of Experimental Biology. (in press). [DOI] [PubMed]
- Wilms T.M., Wagner P., Shobrak M., Böhme W. Activity profiles, habitat selection and seasonality of body weight in a population of Arabian Spiny-tailed Lizards (Uromastyx aegyptia microlepis Blanford, 1875; Sauria: Agamidae) in Saudi Arabia. Bonner zoologische Beiträge. 2009;56(4):259–272. [Google Scholar]
- Wilms T.M., Wagner P., Shobak M., Lutzmann N., Böhme W. Aspect of the ecology of the Arabian Spinytailed lizard (Uromastyx aeyptia microlepis Blandford 1875) at Mahazat as-Sayd protected Area. Salamandra. 2010;46(3):131–140. [Google Scholar]
- Wilms T.M., Wagner P., Shobrak M., Rodder D., Bohme W. Living on the edge? – On the thermobiology and activity pattern of the large herbivorous desert lizard Uromastyx aegyptia microlepis Blanford, 1875 at Mahazat as-Sayd Protected Area, Saudi Arabia. J. Arid Environ. 2011;75(7):636–647. [Google Scholar]


