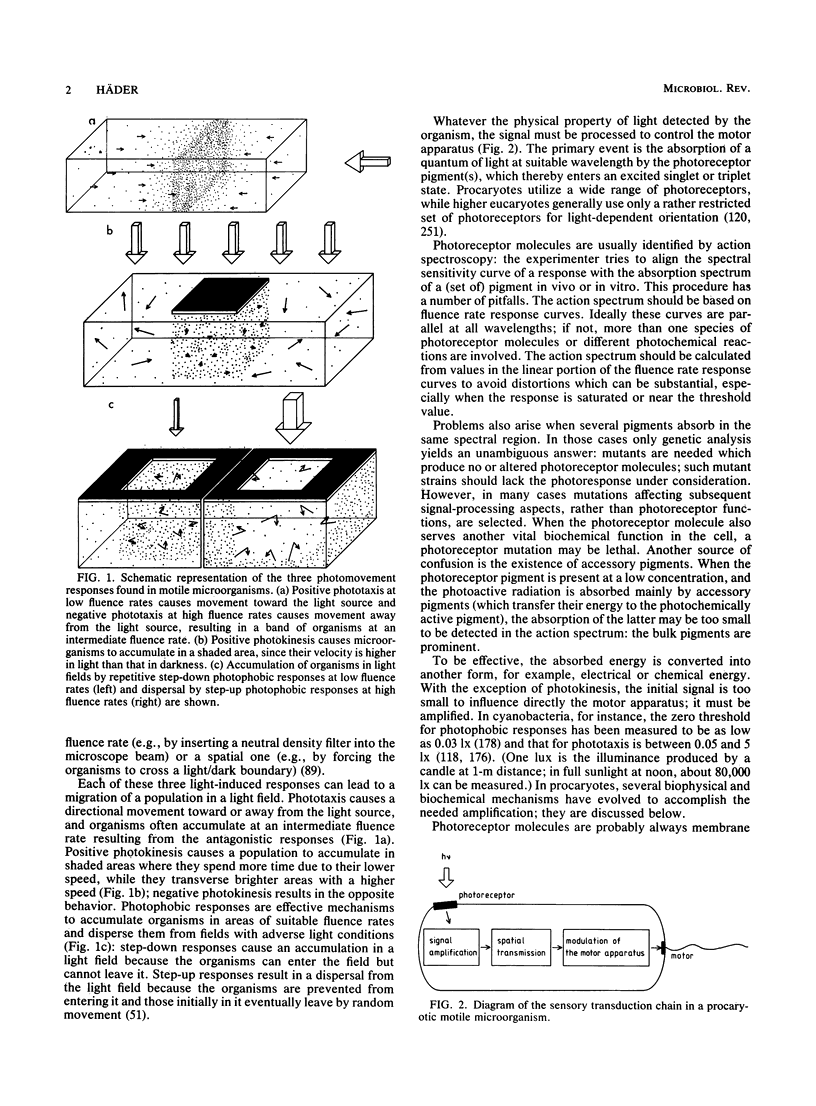
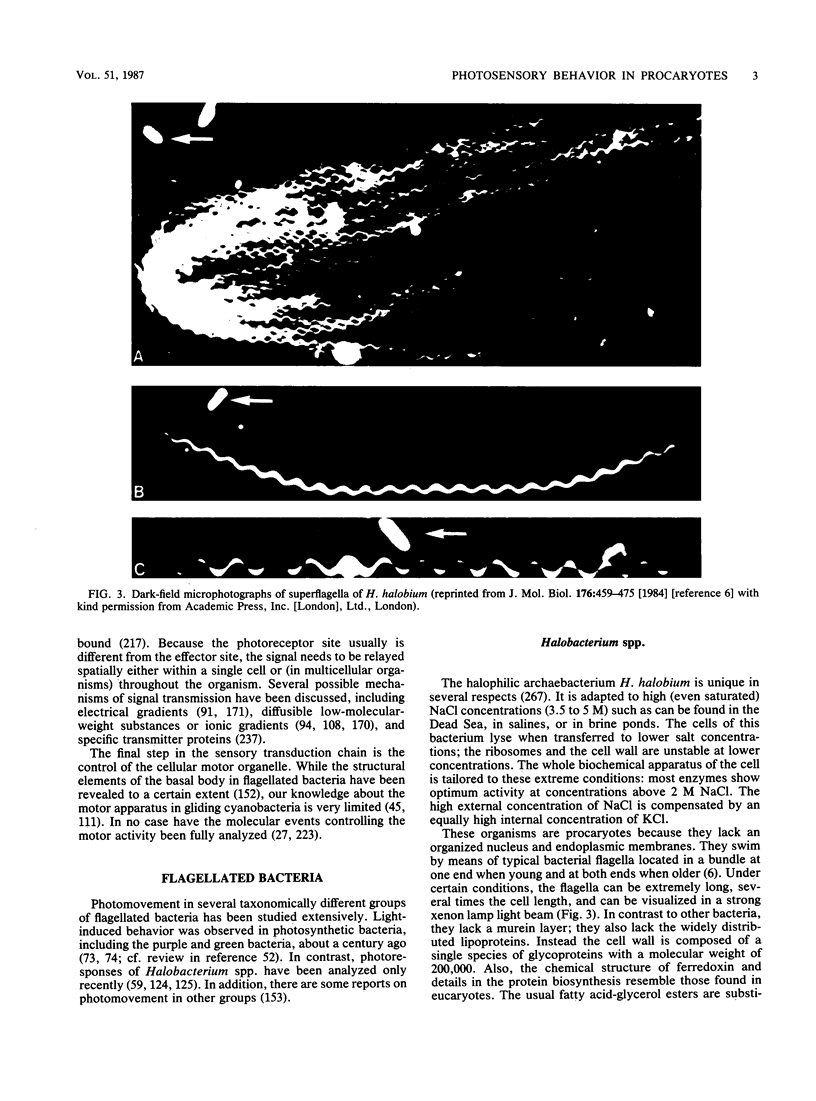
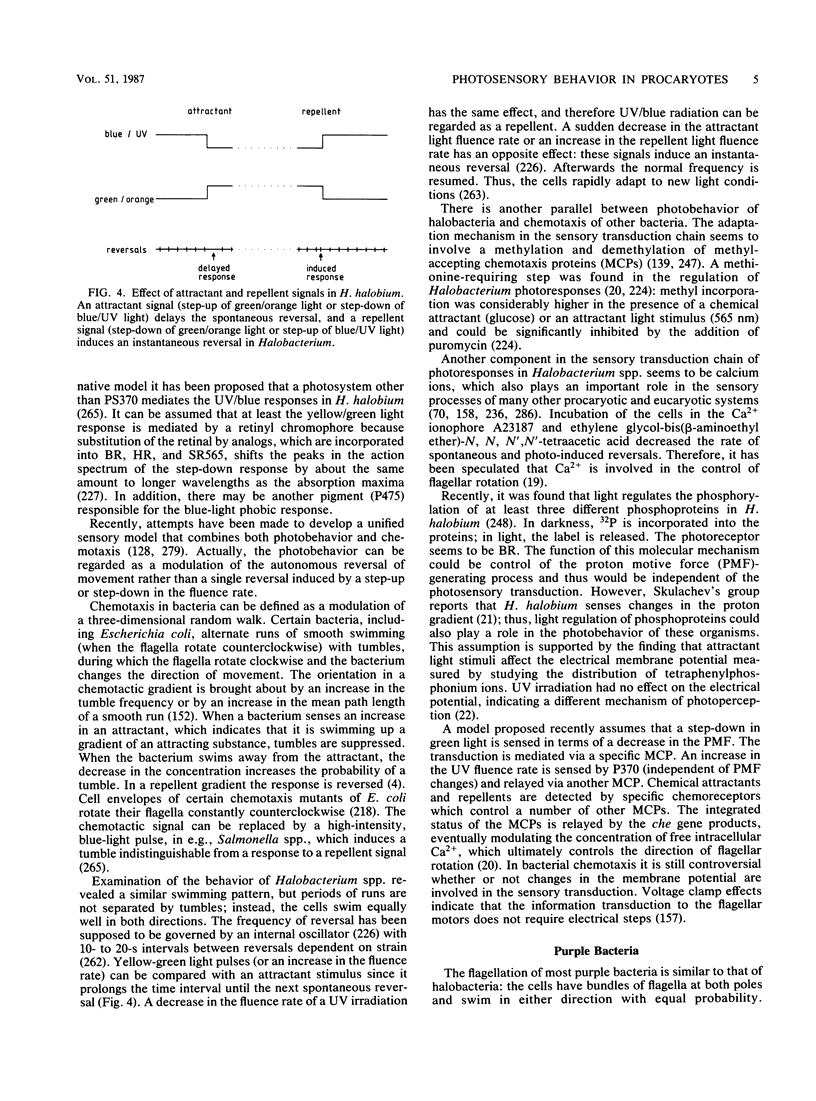
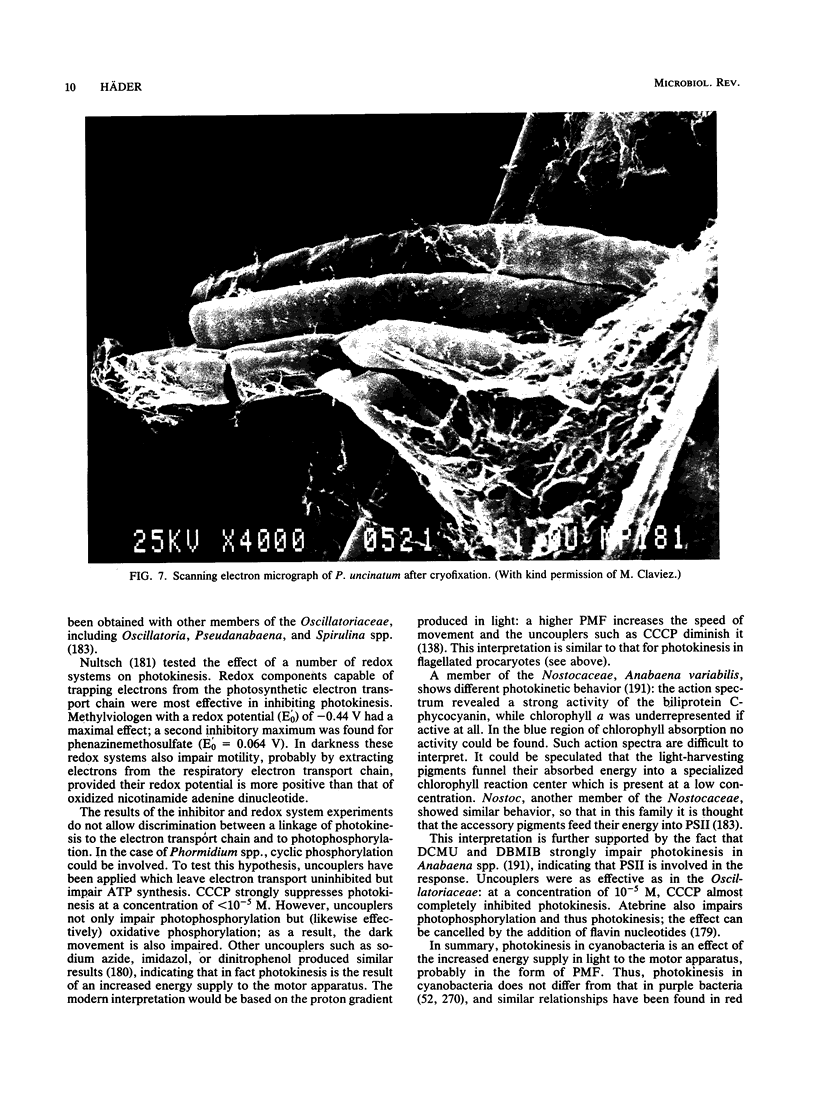
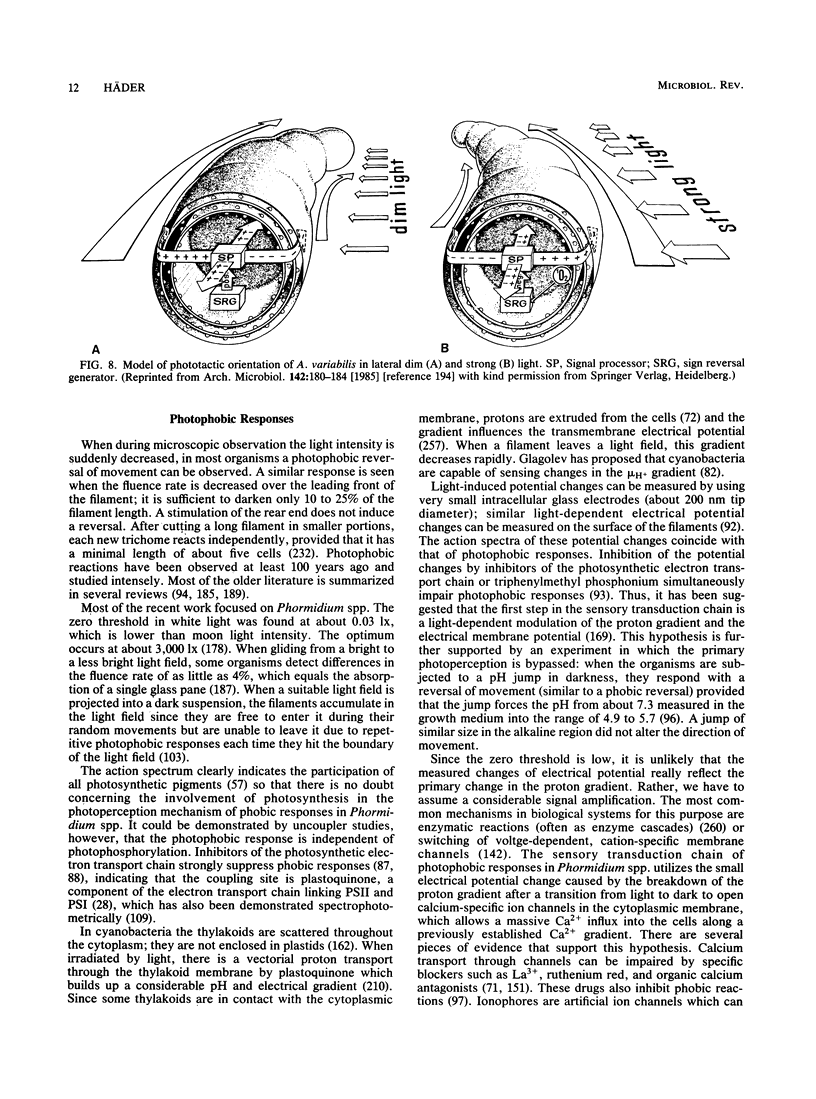
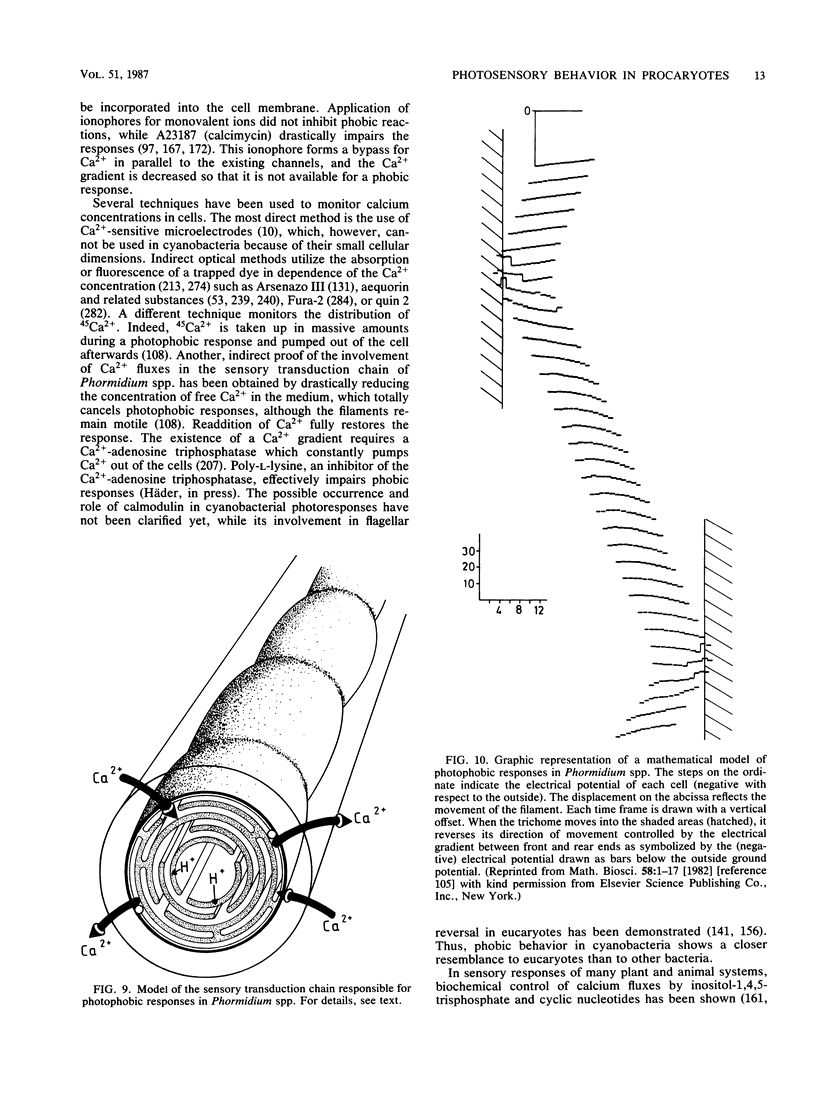
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Bacterial chemotaxis and molecular neurobiology. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):803–804. doi: 10.1101/sqb.1983.048.01.082. [DOI] [PubMed] [Google Scholar]
- Ahl P. L., Cone R. A. Light activates rotations of bacteriorhodopsin in the purple membrane. Biophys J. 1984 Jun;45(6):1039–1049. doi: 10.1016/S0006-3495(84)84251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Oesterhelt D. Morphology, function and isolation of halobacterial flagella. J Mol Biol. 1984 Jul 15;176(4):459–475. doi: 10.1016/0022-2836(84)90172-4. [DOI] [PubMed] [Google Scholar]
- Ammann D. Ca2+-selective microelectrodes. Cell Calcium. 1985 Apr;6(1-2):39–55. doi: 10.1016/0143-4160(85)90033-8. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Evans M. C. Chemotactically induced increase in the membrane potential of spheroplasts of Rhodopseudomonas sphaeroides. FEBS Lett. 1980 Mar 24;112(1):5–9. doi: 10.1016/0014-5793(80)80113-x. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Evans M. C. Comparison of the carotenoid bandshift and oxanol dyes to measure membrane potential changes during chemotactic stimulation of Rhodopseudomonas sphaeroides and Escherichia coli. FEBS Lett. 1981 Apr 6;126(1):98–102. doi: 10.1016/0014-5793(81)81042-3. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Evans M. C. Membrane potential changes during chemotaxis of Rhodopseudomonas sphaeroides. FEBS Lett. 1979 Jun 1;102(1):143–146. doi: 10.1016/0014-5793(79)80946-1. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Evans M. C. The motile and tactic behaviour of Pseudomonas aeruginosa in anaerobic environments. FEBS Lett. 1983 May 30;156(1):113–118. doi: 10.1016/0014-5793(83)80259-2. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Ingham C., Evans M. C. Role of proton motive force in phototactic and aerotactic responses of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Mar;161(3):967–972. doi: 10.1128/jb.161.3.967-972.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassilana M., Damiano E., Leblanc G. Relationships between the Na+-H+ antiport activity and the components of the electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1984 Feb 28;23(5):1015–1022. doi: 10.1021/bi00300a033. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Bacterial flagellar rotation and its chemotactic control. Prog Clin Biol Res. 1984;164:215–219. [PubMed] [Google Scholar]
- Bogomolni R. A. Photochemical reactions of halorhodopsin and slow-rhodopsin. Prog Clin Biol Res. 1984;164:5–12. [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolni R. A., Taylor M. E., Stoeckenius W. Reconstitution of purified halorhodopsin. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5408–5411. doi: 10.1073/pnas.81.17.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker T. M., Sherman L. A. Triton X-114 phase fractionation of membrane proteins of the cyanobacterium Anacystis nidulans R2. Arch Biochem Biophys. 1984 Nov 15;235(1):204–211. doi: 10.1016/0003-9861(84)90269-8. [DOI] [PubMed] [Google Scholar]
- Brown I. I., Galperin MYu, Glagolev A. N., Skulachev V. P. Utilization of energy stored in the form of Na+ and K+ ion gradients by bacterial cells. Eur J Biochem. 1983 Aug 1;134(2):345–349. doi: 10.1111/j.1432-1033.1983.tb07573.x. [DOI] [PubMed] [Google Scholar]
- Bräuner T., Hülser D. F., Strasser R. J. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim Biophys Acta. 1984 Apr 11;771(2):208–216. doi: 10.1016/0005-2736(84)90535-2. [DOI] [PubMed] [Google Scholar]
- Burchard R. P. Evidence for contractile flexing of the gliding bacterium Flexibacter FS-1. Nature. 1982 Aug 12;298(5875):663–665. doi: 10.1038/298663a0. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Patterns of accumulation resulting from taxes and changes in motility of micro-organisms. Arch Mikrobiol. 1957;27(3):311–319. doi: 10.1007/BF00409531. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Studies in the phototaxis of Rhodospirillum rubrum. I. Action spectrum, growth in green light, and Weber law adherence. Arch Mikrobiol. 1953;19(2):107–124. doi: 10.1007/BF00446395. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Studies in the phototaxis of Rhodospirillum rubrum. III. Quantitative relations between stimulus and response. Arch Mikrobiol. 1953;19(2):141–165. doi: 10.1007/BF00446397. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Govindjee R., Ebrey T., Bagley K. A., Dollinger G., Eisenstein L., Marque J., Roder H., Vittitow J., Fang J. M. Trans/13-cis isomerization is essential for both the photocycle and proton pumping of bacteriorhodopsin. Biophys J. 1985 Apr;47(4):509–512. doi: 10.1016/S0006-3495(85)83944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. Y., Martynowicz H. Lack of detectable polyamines in an extremely halophilic bacterium. Biochem Biophys Res Commun. 1984 Oct 30;124(2):423–429. doi: 10.1016/0006-291x(84)91570-5. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Draheim J. E., Cassim J. Y. Large Scale Global Structural Changes of the Purple Membrane during the Photocycle. Biophys J. 1985 Apr;47(4):497–507. doi: 10.1016/S0006-3495(85)83943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S. Ca2+ in biological systems. Experientia. 1985 Aug 15;41(8):978–981. doi: 10.1007/BF01952117. [DOI] [PubMed] [Google Scholar]
- Elferink J. G., Deierkauf M. The effect of verapamil and other calcium antagonists on chemotaxis of polymorphonuclear leukocytes. Biochem Pharmacol. 1984 Jan 1;33(1):35–39. doi: 10.1016/0006-2952(84)90367-8. [DOI] [PubMed] [Google Scholar]
- Fattom A., Shilo M. Hydrophobicity as an adhesion mechanism of benthic cyanobacteria. Appl Environ Microbiol. 1984 Jan;47(1):135–143. doi: 10.1128/aem.47.1.135-143.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. W., Saranak J., Patel N., Zarilli G., Okabe M., Kline T., Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984 Oct 25;311(5988):756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Groma G. I., Helgerson S. L., Wolber P. K., Beece D., Dancsházy Z., Keszthelyi L., Stoeckenius W. Coupling between the bacteriorhodopsin photocycle and the protonmotive force in Halobacterium halobium cell envelope vesicles. II. Quantitation and preliminary modeling of the M----bR reactions. Biophys J. 1984 May;45(5):985–992. doi: 10.1016/S0006-3495(84)84243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfen L. N., Castenholz R. W. Gliding in a blue-green alga: a possible mechanism. Nature. 1970 Mar 21;225(5238):1163–1165. doi: 10.1038/2251163a0. [DOI] [PubMed] [Google Scholar]
- Harayama S., Iino T. Phototactic response of aerobically cultivated Rhodospirillum rubrum. J Gen Microbiol. 1976 May;94(1):173–179. doi: 10.1099/00221287-94-1-173. [DOI] [PubMed] [Google Scholar]
- Harayama S., Iino T. Phototaxis and membrane potential in the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1977 Jul;131(1):34–41. doi: 10.1128/jb.131.1.34-41.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt W. Phototaxis in plants. Int Rev Cytol. 1966;19:267–299. [PubMed] [Google Scholar]
- Hazemoto N., Kamo N., Terayama Y., Kobatake Y., Tsuda M. Photochemistry of two rhodopsinlike pigments in bacteriorhodopsin-free mutant of Halobacterium halobium. Biophys J. 1983 Oct;44(1):59–64. doi: 10.1016/S0006-3495(83)84277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand E., Dencher N. Two photosystems controlling behavioural responses of Halobacterium halobium. Nature. 1975 Sep 4;257(5521):46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- Hildebrand E. Halobacteria: the role of retinal-protein complexes. Symp Soc Exp Biol. 1983;36:207–222. [PubMed] [Google Scholar]
- Häder D. P. Influence of electric fields on photophobic reactions in blue-green algae. Arch Microbiol. 1977 Jul 26;114(1):83–86. doi: 10.1007/BF00429635. [DOI] [PubMed] [Google Scholar]
- Häder D. P., Lebert M. Real time computer-controlled tracking of motile microorganisms. Photochem Photobiol. 1985 Nov;42(5):509–514. doi: 10.1111/j.1751-1097.1985.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Häder D. P. Participation of two photosystems in the photo-phobotaxis of Phormidium uncinatum. Arch Microbiol. 1974 Mar 7;96(3):255–266. doi: 10.1007/BF00590181. [DOI] [PubMed] [Google Scholar]
- Jubb J. S., Worcester D. L., Crespi H. L., Zaccaï G. Retinal location in purple membrane of Halobacterium halobium: a neutron diffraction study of membranes labelled in vivo with deuterated retinal. EMBO J. 1984 Jul;3(7):1455–1461. doi: 10.1002/j.1460-2075.1984.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N., Hazemoto N., Kobatake Y., Mukohata Y. Light and dark adaptation of halorhodopsin. Arch Biochem Biophys. 1985 Apr;238(1):90–96. doi: 10.1016/0003-9861(85)90144-4. [DOI] [PubMed] [Google Scholar]
- Kasianowicz J., Benz R., McLaughlin S. The kinetic mechanism by which CCCP (carbonyl cyanide m-chlorophenylhydrazone) transports protons across membranes. J Membr Biol. 1984;82(2):179–190. doi: 10.1007/BF01868942. [DOI] [PubMed] [Google Scholar]
- Kehry M. R., Doak T. G., Dahlquist F. W. Sensory adaptation in bacterial chemotaxis: regulation of demethylation. J Bacteriol. 1985 Sep;163(3):983–990. doi: 10.1128/jb.163.3.983-990.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller K. H., Grady M., Dworkin M. Surface tension gradients: feasible model for gliding motility of Myxococcus xanthus. J Bacteriol. 1983 Sep;155(3):1358–1366. doi: 10.1128/jb.155.3.1358-1366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont H. C. Shear-oriented microfibrils in the mucilaginous investments of two motile oscillatoriacean blue-green algae. J Bacteriol. 1969 Jan;97(1):350–361. doi: 10.1128/jb.97.1.350-361.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Light-dependent trans to cis isomerization of the retinal in halorhodopsin. FEBS Lett. 1984 Oct 1;175(2):337–342. doi: 10.1016/0014-5793(84)80764-4. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Nature of the principal photointermediate of halorhodopsin. Biochem Biophys Res Commun. 1984 Jul 18;122(1):91–96. doi: 10.1016/0006-291x(84)90443-1. [DOI] [PubMed] [Google Scholar]
- Lapidus I. R., Berg H. C. Gliding motility of Cytophaga sp. strain U67. J Bacteriol. 1982 Jul;151(1):384–398. doi: 10.1128/jb.151.1.384-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Govindjee R., Ebrey T. G. A correlation between proton pumping and the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7079–7082. doi: 10.1073/pnas.81.22.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukács G. L., Fonyó A. Ba2+ ions inhibit the release of Ca2+ ions from rat liver mitochondria. Biochim Biophys Acta. 1985 Sep 19;809(2):160–166. doi: 10.1016/0005-2728(85)90058-1. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Margolin Y., Eisenbach M. Voltage clamp effects on bacterial chemotaxis. J Bacteriol. 1984 Aug;159(2):605–610. doi: 10.1128/jb.159.2.605-610.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno-Yagi A., Mukohata Y. Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977 Sep 9;78(1):237–243. doi: 10.1016/0006-291x(77)91245-1. [DOI] [PubMed] [Google Scholar]
- Maugh T. H., 2nd What is the risk from chlorofluorocarbons? Science. 1984 Mar 9;223(4640):1051–1052. doi: 10.1126/science.6695193. [DOI] [PubMed] [Google Scholar]
- Miller K. R. The photosynthetic membrane: prokaryotic and eukaryotic cells. Endeavour. 1985;9(4):175–182. doi: 10.1016/0160-9327(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Molecular mechanics of protonmotive F0F1 ATPases. Rolling well and turnstile hypothesis. FEBS Lett. 1985 Mar 11;182(1):1–7. doi: 10.1016/0014-5793(85)81142-x. [DOI] [PubMed] [Google Scholar]
- Murakami N., Konishi T. DCCD-sensitive, Na+-dependent H+-influx process coupled to membrane potential formation in membrane vesicles of Halobacterium halobium. J Biochem. 1985 Oct;98(4):897–907. doi: 10.1093/oxfordjournals.jbchem.a135369. [DOI] [PubMed] [Google Scholar]
- Murvanidze G. V., Glagolev A. N. Electrical nature of the taxis signal in cyanobacteria. J Bacteriol. 1982 Apr;150(1):239–244. doi: 10.1128/jb.150.1.239-244.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer D. C., Zingsheim H. P., Oesterhelt D. Biogenesis of purple membrane in halobacteria. Methods Enzymol. 1983;97:218–226. doi: 10.1016/0076-6879(83)97134-3. [DOI] [PubMed] [Google Scholar]
- Nultsch W. Effect of desaspidin and DCMU on photokinesis of blue-green algae. Photochem Photobiol. 1969 Aug;10(2):119–123. doi: 10.1111/j.1751-1097.1969.tb07228.x. [DOI] [PubMed] [Google Scholar]
- Nultsch W., Schuchart H., Koenig F. Effects of sodium azide on phototaxis of the blue-green alga Anabaena variabilis and consequences to the two-photoreceptor systems-hypothesis. Arch Microbiol. 1983 Jan;134(1):33–37. doi: 10.1007/BF00429403. [DOI] [PubMed] [Google Scholar]
- Nultsch W., Throm G. Equivalence of light and ATP in photokinesis of Rhodospirillum rubrum. Nature. 1968 May 18;218(5142):697–699. doi: 10.1038/218697a0. [DOI] [PubMed] [Google Scholar]
- Nultsch W. Uber den Antagonismus von Atebrin und Flavinnucleotiden im Bewegungs- und Lichtreaktionsverhalten von Phormidium uncinatum. Arch Mikrobiol. 1966 Nov 11;55(2):187–199. [PubMed] [Google Scholar]
- Oesterhelt D., Hegemann P., Tittor J. The photocycle of the chloride pump halorhodopsin. II: Quantum yields and a kinetic model. EMBO J. 1985 Sep;4(9):2351–2356. doi: 10.1002/j.1460-2075.1985.tb03938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofer S., Nowik I., Bauminger E. R., Papaefthymiou G. C., Frankel R. B., Blakemore R. P. Magnetosome dynamics in magnetotactic bacteria. Biophys J. 1984 Jul;46(1):57–64. doi: 10.1016/S0006-3495(84)83998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurusu T., Maeda A., Yoshizawa T. Absorption spectral properties of purified halorhodopsin. J Biochem. 1984 Apr;95(4):1073–1082. doi: 10.1093/oxfordjournals.jbchem.a134695. [DOI] [PubMed] [Google Scholar]
- Peschek G. A., Czerny T., Schmetterer G., Nitschmann W. H. Transmembrane Proton Electrochemical Gradients in Dark Aerobic and Anaerobic Cells of the Cyanobacterium (Blue-Green Alga) Anacystis nidulans: Evidence for Respiratory Energy Transduction in the Plasma Membrane. Plant Physiol. 1985 Sep;79(1):278–284. doi: 10.1104/pp.79.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson B. K., Giovannoni S. J., Castenholz R. W. Physiological ecology of a gliding bacterium containing bacteriochlorophyll a. Appl Environ Microbiol. 1984 Mar;47(3):576–584. doi: 10.1128/aem.47.3.576-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro G., Cleemann L., Morad M. Optical measurement of voltage-dependent Ca2+ influx in frog heart. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1864–1868. doi: 10.1073/pnas.82.6.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polland H. J., Franz M. A., Zinth W., Kaiser W., Hegemann P., Oesterhelt D. Picosecond events in the photochemical cycle of the light-driven chloride-pump halorhodopsin. Biophys J. 1985 Jan;47(1):55–59. doi: 10.1016/S0006-3495(85)83876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid S., Eisenbach M. Direction of flagellar rotation in bacterial cell envelopes. J Bacteriol. 1984 Apr;158(1):222–230. doi: 10.1128/jb.158.1.222-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie R. J. A critical assessment of the use of lipophilic cations as membrane potential probes. Prog Biophys Mol Biol. 1984;43(1):1–32. doi: 10.1016/0079-6107(84)90002-6. [DOI] [PubMed] [Google Scholar]
- SCHULZ G. Bewegungsstudien sowie elektronenmikroskopische Membranuntersuchungen an Cyanophyceen. Arch Mikrobiol. 1955;21(4):335–370. [PubMed] [Google Scholar]
- Satir P. Meeting report: mechanisms and controls of prokaryotic and eukaryotic flagellar motility. Cell Biol Int Rep. 1979 Nov;3(8):641–650. doi: 10.1016/0309-1651(79)90094-8. [DOI] [PubMed] [Google Scholar]
- Schimz A. Methylation of membrane proteins is involved in chemosensory and photosensory behavior of Halobacterium halobium. FEBS Lett. 1981 Mar 23;125(2):205–207. doi: 10.1016/0014-5793(81)80719-3. [DOI] [PubMed] [Google Scholar]
- Schleicher A., Hofmann K. P. Proton uptake by light induced interaction between rhodopsin and G-protein. Z Naturforsch C. 1985 May-Jun;40(5-6):400–405. doi: 10.1515/znc-1985-5-619. [DOI] [PubMed] [Google Scholar]
- Schobert B., Lanyi J. K. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982 Sep 10;257(17):10306–10313. [PubMed] [Google Scholar]
- Schutt C. Protein structure. Hands on the calcium switch. Nature. 1985 May 2;315(6014):15–15. doi: 10.1038/315015a0. [DOI] [PubMed] [Google Scholar]
- Segall J. E., Ishihara A., Berg H. C. Chemotactic signaling in filamentous cells of Escherichia coli. J Bacteriol. 1985 Jan;161(1):51–59. doi: 10.1128/jb.161.1.51-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Shimomura A. Effect of calcium chelators on the Ca2+-dependent luminescence of aequorin. Biochem J. 1984 Aug 1;221(3):907–910. doi: 10.1042/bj2210907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Shimomura A. Halistaurin, phialidin and modified forms of aequorin as Ca2+ indicator in biological systems. Biochem J. 1985 Jun 15;228(3):745–749. doi: 10.1042/bj2280745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. D. Gliding motility in Aphanothece halophytica: analysis of wall proteins in mot mutants. J Bacteriol. 1981 Oct;148(1):315–321. doi: 10.1128/jb.148.1.315-321.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangle L., Armstrong P. B. Gliding motility of algae in unaffected by cytochalasin B. Exp Cell Res. 1973 Aug;80(2):490–493. doi: 10.1016/0014-4827(73)90331-5. [DOI] [PubMed] [Google Scholar]
- Sperling W., Schimz A. Photosensory retinal pigments in Halobacterium halobium. Biophys Struct Mech. 1980;6(2):165–169. doi: 10.1007/BF00535752. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Zanolari B. Sensory transduction in Escherichia coli: regulation of the demethylation rate by the CheA protein. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5061–5065. doi: 10.1073/pnas.81.16.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Biochemical characterization of halorhodopsin in native membranes. J Biol Chem. 1985 Jan 25;260(2):1208–1212. [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Spectroscopic discrimination of the three rhodopsinlike pigments in Halobacterium halobium membranes. Biophys J. 1983 Aug;43(2):243–246. doi: 10.1016/S0006-3495(83)84345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L. Genetic demonstration of a sensory rhodopsin in bacteria. Prog Clin Biol Res. 1984;164:221–229. [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M. G. Origin and magnitude of transmembrane resting potential in living cells. Philos Trans R Soc Lond B Biol Sci. 1983 May 24;301(1104):85–141. doi: 10.1098/rstb.1983.0023. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W. The purple membrane of salt-loving bacteria. Sci Am. 1976 Jun;234(6):38–46. doi: 10.1038/scientificamerican0676-38. [DOI] [PubMed] [Google Scholar]
- Stryer L. Molecular design of an amplification cascade in vision. Biopolymers. 1985 Jan;24(1):29–47. doi: 10.1002/bip.360240105. [DOI] [PubMed] [Google Scholar]
- Stryer L. Sensory and energy transduction. Light-activated retinal proteins. Nature. 1984 Dec 6;312(5994):498–499. doi: 10.1038/312498a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Mukohata Y. Isolation and characterization of halorhodopsin from Halobacterium halobium. J Biochem. 1984 Aug;96(2):413–420. doi: 10.1093/oxfordjournals.jbchem.a134852. [DOI] [PubMed] [Google Scholar]
- THOMAS J. B. On the role of the carotenoids in photosynthesis in Rhodospirillum rubrum. Biochim Biophys Acta. 1950 Apr;5(2):186–196. [PubMed] [Google Scholar]
- Takahashi T., Mochizuki Y., Kamo N., Kobatake Y. Evidence that the long-lifetime photointermediate of s-rhodopsin is a receptor for negative phototaxis in Halobacterium halobium. Biochem Biophys Res Commun. 1985 Feb 28;127(1):99–105. doi: 10.1016/s0006-291x(85)80131-5. [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Miller J. B., Warrick H. M., Koshland D. E., Jr Electron acceptor taxis and blue light effect on bacterial chemotaxis. J Bacteriol. 1979 Nov;140(2):567–573. doi: 10.1128/jb.140.2.567-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Kamo N., Takahashi T., Kobatake Y. Photochemical intermediate of third rhodopsin-like pigment in Halobacterium halobium by simultaneous illumination with red and blue light. Biochem Biophys Res Commun. 1984 Sep 28;123(3):989–994. doi: 10.1016/s0006-291x(84)80231-4. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Measuring and manipulating cytosolic Ca2+ with trapped indicators. Kroc Found Ser. 1984;17:147–155. [PubMed] [Google Scholar]
- Waterbury J. B., Willey J. M., Franks D. G., Valois F. W., Watson S. W. A cyanobacterium capable of swimming motility. Science. 1985 Oct 4;230(4721):74–76. doi: 10.1126/science.230.4721.74. [DOI] [PubMed] [Google Scholar]
- White J. R., Ishizaka T., Ishizaka K., Sha'afi R. Direct demonstration of increased intracellular concentration of free calcium as measured by quin-2 in stimulated rat peritoneal mast cell. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3978–3982. doi: 10.1073/pnas.81.13.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox L. W., Wedemayer G. J. Dinoflagellate with blue-green chloroplasts derived from an endosymbiotic eukaryote. Science. 1985 Jan 11;227(4683):192–194. doi: 10.1126/science.227.4683.192. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]







