Abstract
The insecticide chlorpyrifos (CPF) is widely used in the Kingdom of Saudi Arabia (KSA) to control agricultural pests. The present work is a preliminary investigation of the effect of CPF on healing of cutaneous leishmaniasis (CL) lesions, caused by Leishmania major in farmers exposed to this insecticide, after treatment with Pentostam®. Lesion diameters were measured and CPF concentrations in the blood plasma of farmer and non-farmer CL patients in Al-Ahsa were detected by gas chromatography/mass spectrometry/mass spectrometry before and 6 weeks after treatment with Pentostam®. CPF concentrations in the blood of farmer patients ranged between 4.570 and 7.096 ng/μl (mean = 6.19 ± 0.881 ng/μl) before and after treatment with Pentostam®. The mean lesion diameter in these patients decreased by a factor of 2.21 after treatment with Pentostam®; they measured 1.85–11.75 mm, (mean = 6.165 ± 3.500 mm) before treatment and 0.22–6.10 mm (mean = 2.796 ± 2.102 mm) after treatment. Lesion diameter increased exponentially with the increase of CPF concentration in the patients’ blood. CPF was not detected in the non-farmer patients before or after treatment. Their mean lesion diameter decreased by a factor of 6.86 after treatment with Pentostam®; they measured 1.33–7.10 mm (mean = 2.882 ± 1.764 mm) before treatment and 0.11–0.92 mm (mean = 0.425 ± 0.277 mm) after treatment. The mean lesion diameter in farmer patients was much greater than that of non-farmer patients both before (2.14×) and after (6.657×) treatment with Pentostam®. Chronic exposure to low levels of the pesticide aggravates the development and delays the healing of CL lesions due to immunotoxicity and/or peripheral neurotoxicity caused by CPF. Further detailed studies would assess CPF effect on the severity of infection with CL in agricultural workers continuously exposed to this insecticide in different areas of KSA in conformity of their finding.
Keywords: Chlorpyrifos, Organophosphates, Leishmania major, Cutaneous leishmaniasis, Kingdom of Saudi Arabia, GC/MS/MS, Pentostam®, Pentavalent antimonials
1. Introduction
Pentostam® is considered as an effective treatment for CL caused by Leishmania major and has been used extensively for the treatment of this disease in the Kingdom of Saudi Arabia (KSA) (Peters and Al-Zahrani, 1987; Al Tawfiq and AbuKhamsin, 2004; Al-Jaser, 2005). Al-Jaser (1995) observed some patients do not respond properly to treatment and attributed this unresponsiveness due to exposure of patients to chemical pollutants such as pesticides (Breckenridge et al., 1987). Chlorpyrifos (CPF) (O,O′-diethyl 3,5,6-trichloro-2-pyridinyl-phosphorothioate), a broad-spectrum organo-phosphorous (OP) insecticide, has been widely used in KSA for the control of agricultural pests. OP compounds are highly toxic to vertebrates (Sultatos and Murphy, 1983; Spies et al., 1988; Kousba et al., 2004; Zhao et al., 2006), and CPF is particularly considered to be neurotoxic (Albers et al., 2004), cardiotoxic, hepatotoxic (Meyer et al., 2004) and immunotoxic (Galloway and Handy, 2003).
Lately, we observed that the development of CL lesions in BALB/c mice infected with L. major was aggravated by exposure to CPF. The aim of this paper is to investigate the effect of chronic exposure of humans to CPF on the healing of CL lesions.
2. Materials and methods
2.1. Patients’ treatment
Forty-three farmer and non-farmer CL patients attending the Dermatology Clinic at Al-Ahsa Clinical Center in KSA were the subject of this study. However, only 16 farmers and 15 non-farmers reported after treatment. After obtaining an informed consent from all patients, they were examined for skin lesions. Lesions, mainly on the forearms and lower parts of the legs of these patients, were clinically diagnosed and confirmed to be caused by L. major by the polymerase chain reaction (unpublished data). Each patient was given a single course of daily intramuscular injections of 600 mg Pentostam® (pentavalent antimony sodium stibogluconate, the Wellcome Foundation Ltd., Berkhamstead, England) for 21 days. The lesion diameters were measured using a micrometer (Pocotest® dial caliper gauge, Carbonze, UK) and blood samples were collected from the patients in vacutainer tubes (Becton Dickenson, USA) containing disodium ethane diamine tetraacetic acid dihydrate (EDTA) (10 mg/ml) from Serva Feinbiochemica (Heidelberg, Germany) before and 6 weeks after treatment with Pentostam®.
2.2. GC/MS/MS
CPF analysis was performed as described by Tarbah et al. (2001) using a Hewlett Packard GC/MS/MS system: GC 5890 MSD 5970 equipped with a Hewlett Packard automatic liquid sampler HP 7673. Column was HP 5 MS, 30 m length, i.d. 0.25 mm (0.25 μm film thickness), carrier gas was helium (pressure 70 kPa) and split/purge off time was 2 min. The injector temperature was 270 °C, transfer line temperature was 280 °C, temperature program included an initial temperature 60 °C for 2 min, 40 °C/min to 90 °C, 15 °C/min to 300 °C and maintained for 3 min (total run time: 20 min). The mass spectrometer was in the electron impact mode at 70 eV scanning from m/z 50–450 mass units. Fig. 1 shows the CPF chromatogram obtained (Retention time, tR = 13.133 min). A standard curve was formed by measuring serial 4-fold dilutions (2, 4, 6 and 8 × 10−5 mg/ml) of CPF (94.5% purity purchased from Avonchem, UK) using GC/MS/MS analysis described above.
Figure 1.

Chlorpyrifos chromatogram.
2.3. Preparation of blood plasma samples and CPF extraction procedure
In Eppendorf tubes, aliquots each of 0.7 ml plasma sample were vortexed with 1 ml toluene for 2 min and then centrifuged at 14,000 rpm for 5 min at 4 °C (to avoid emulsion formation). An amount of 1 μl of the supernatant was analyzed directly for the presence CPF by GC/MS/MS as described above (Tarbah et al., 2001).
2.4. Statistical analysis
The means and standard deviations (SD) of the data were obtained using GraphPad Prizm software version 3.0 and were compared using paired Students’ t-test.
3. Results
3.1. Lesion diameter before and 6 weeks after treatment
The mean lesion diameter in farmer patients before treatment (range 1.85–11.75 mm, mean 6.165 ± 3.500 mm) was 2.21× larger (P < 0.0001) than that after treatment (range 0.22–6.10 mm, mean 2.796 ± 2.102 mm) (Table 1). In non-farmer patients, the mean lesion diameter before treatment (range 1.33–7.10 mm, mean 2.882 ± 1.764 mm) was 6.86× larger (P < 0001) than that after treatment (range 11–0.92 mm, mean 0.425 ± 0.277 mm) (Table 2). The mean lesion diameter of farmer patients was 2.14× larger (P < 0.0005) than that of non-farmer patients before treatment and 6.657× after treatment.
Table 1.
Results of GC/MS/MSa assay for chlorpyrifos concentration in the blood plasma of farmer patients and their lesion diameters before and 6 weeks after treatment with Pentostam®.
| Patients’ no. | CPF concentration before and 6 weeks after treatment (ng/μl) |
Lesion diameter before and 6 weeks after treatment (mm) |
||
|---|---|---|---|---|
| Before | After | Before | After | |
| 1 | 6.116 | 6.116 | 3.95 | 0.91 |
| 2 | 7.096 | 7.096 | 14.75 | 3.60 |
| 3 | 6.043 | 6.043 | 3.85 | 1.22 |
| 4 | 6.043 | 6.043 | 3.55 | 2.11 |
| 5 | 6.956 | 6.956 | 7.65 | 4.80 |
| 6 | 6.546 | 6.546 | 7.05 | 5.10 |
| 7 | 4.570 | 4.570 | 1.85 | 0.50 |
| 8 | 6.421 | 6.421 | 4.65 | 2.33 |
| 9 | 7.096 | 7.096 | 11.75 | 6.10 |
| 10 | 6.721 | 6.721 | 7.10 | 3.11 |
| 11 | 4.570 | 4.570 | 2.10 | 1.10 |
| 12 | 6.974 | 6.974 | 9.10 | 2.32 |
| 13 | 4.570 | 4.570 | 2.60 | 0.22 |
| 14 | 5.610 | 5.610 | 3.50 | 0.99 |
| 15 | 6.721 | 6.721 | 7.35 | 4.10 |
| 16 | 6.956 | 6.956 | 7.85 | 3.22 |
| Mean ± SD | 6.188 ± 0.881 | 6.188 ± 0.881 | 6.165 ± 3.500 | 2.796 ± 2.102 |
Gas chromatography/mass spectrometry/mass spectrometry.
Table 2.
Lesion diameters in non-farmer patients before and 6 weeks after treatment with Pentostam®.a
| Patients’ no. | Lesion diameter before and 6 weeks after treatment (mm) |
|
|---|---|---|
| Before | After | |
| 1 | 1.86 | 0.41 |
| 2 | 2.20 | 0.11 |
| 3 | 3.10 | 0.12 |
| 4 | 1.55 | 0.44 |
| 5 | 4.65 | 0.21 |
| 6 | 2.70 | 0.71 |
| 7 | 2.00 | 0.11 |
| 8 | 3.40 | 0.84 |
| 9 | 6.11 | 0.92 |
| 10 | 7.10 | 0.61 |
| 11 | 1.78 | 0.22 |
| 12 | 2.22 | 0.55 |
| 13 | 1.13 | 0.15 |
| 14 | 1.05 | 0.75 |
| 15 | 1.33 | 0.22 |
| Mean ± SD | 2.882 ± 1.764 | 0.425 ± 0.277 |
No chlorpyrifos was detected in the blood of these patients.
3.2. CPF concentration in patients’ plasma before and 6 weeks after treatment
The GC/MS/MS analysis to detect CPF concentration in the plasma samples of farmer patients showed that the CPF level was the same before and 6 weeks after treatment and ranged between 4.570 and 7.096 ng/μl (mean 6.188 ± 0.881 ng/μl) (Table 1). On the other hand, no CPF was detected by this assay in the plasma of non-farmer patients before or after treatment.
3.3. Relation between lesion diameter and CPF concentration
When the CPF concentrations in the farmer patients’ plasma were plotted against their lesion diameters (Figs. 2 and 3), a significant positive correlation was observed before (r = 0.819) and after (r = 0.675) treatment; lesion diameter increased exponentially with the increase in the CPF concentration (in ng/μl) both before and after treatment with Pentostam®.
Figure 2.
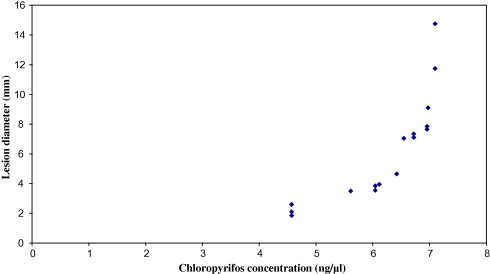
Relationship between lesion diameters and chloropyrifios concentrations in farmer patients’ plasma before treatment with Pentostam®.
Figure 3.
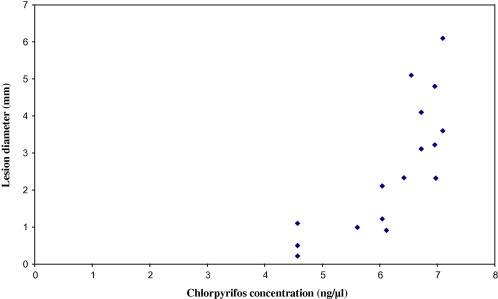
Relationship between lesion diameters and chlorpyrifos concentrations in farmer patients’ plasma 6 weeks after treatment with Pentostam®.
4. Discussion
CL is a common health problem among agricultural workers in KSA (Al-Gindan et al., 1984; Peters and Al-Zahrani, 1987; Peters, 1988; Dye et al., 1989). Although the Saudi Agriculture Ministry forbids the use of the highly toxic CPF since 20/4/2004, it is still in use in KSA to control agricultural pests (http://www.agrwat.gov.sa/public/portal). Thus, farmers are exposed continuously to this pesticide and therefore, their blood was found to contain a steady concentration of CPF during the present study. Although CPF was present in very low concentrations in their blood, it seems that these quantities were sufficient to affect their response to treatment with Pentostam®. In support of this assumption were the larger CL lesions in the farmers than in the non-farmers, the slower healing of the farmers’ CL lesions when compared to those of the non-farmer workers, and the exponential increase in lesion diameter with the increase in CPF concentration in the farmers’ blood.
In a previous study, treatment with CPF for 2 weeks was found to aggravate CL lesion development in BALB/c mice (Al-Dawood et al., 2008). Such aggravation has probably occurred in the infected farmers and might be attributed to alteration of the their immune system since CPF has been reported to be immunotoxic (Galloway and Handy, 2003); alteration of the immune system was reported to interfere with treatment of CL in AID’s patients (Altes et al., 1991; Gradoni and Gramiccia, 1994). CPF also affected neurotransmission by acetylcholine esterase (AChE) in animals and humans (reviewed by Zhao et al., 2006). Sufficient inhibition of AChE by CPF caused excessive accumulation of acetylcholine, which in turn might cause a neurotoxic effect on the peripheral nerves supplying the cutaneous area of the lesion and might have contributed to the aggravation of the lesions.
Acknowledgements
Thanks are due to the authorities in the Central Laboratory of Princess Al-Gowhara at the College of Education for their support and interest in this work, especially Dr. Zakia Tulbah and Ms. Abir Abu-Ali of the Chemistry Department. Authors are also grateful to Dr. Mohamed H. El-Saeid of Plant Protection Department, College of Food and Agriculture at King Saud University for providing valuable information and consultation on pesticide residue analysis.
References
- Albers J.W., Garabrant D.H., Schweitzer S.J., Garrison R.P., Richardson R.J., Berent S. The effects of occupational exposure to chlorpyrifos on the peripheral nervous system: a prospective cohort study. Occup. Environ. Med. 2004;61:201–211. doi: 10.1136/oem.2003.008847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dawood A.N., Khalil G.M., Al-Jaser M.H. The effect of the pesticides chlorpyrifos and alphacypermethrin on the development of cutaneous leishmaniasis lesion in BALB/C mice. Saudi J. Biol. Sci. 2008;15:59–68. [Google Scholar]
- Al-Gindan Y., Abdul-Aziz O., Kubba R. Cutaneous leishmaniasis in Al-Hassa, Saudi Arabia. Int. J. Dermatol. 1984;23:194–196. doi: 10.1111/j.1365-4362.1984.tb04510.x. [DOI] [PubMed] [Google Scholar]
- Al-Jaser, M.H., 1995. Drug Sensitivity of Cutaneous Leishmaniasis in Al-Kharj, Saudia Arabia. Ph.D. Thesis. London School of Hygiene and Tropical Medicine, University of London, London, England.
- Al-Jaser M.H. Treatment trends of cutaneous leishmaniasis in Saudi Arabia. Saudi Med. J. 2005;26:1220–1224. [PubMed] [Google Scholar]
- Al Tawfiq J.A., AbuKhamsin A. Cutaneous leishmaniasis: a 46-year study of the epidemiology and clinical features in Saudi Arabia (1956–2002) Int. J. Infect. Dis. 2004;8:244–250. doi: 10.1016/j.ijid.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Altes J., Salas A., Riera M., Udina M., Galmas A., Balazat J., Ballesteros A., Buades J., Salva F., Villalonga C. Visceral leishmaniasis: another HIV-associated opportunistic infection? Report of eight cases and review of the literature. AIDS. 1991;5:201–207. [PubMed] [Google Scholar]
- Breckenridge A., Orme M., Edwards G. Clinical pharmacology looks at tropical medicine. Trans. R. Soc. Trop. Med. Hyg. 1987;81:529–533. doi: 10.1016/0035-9203(87)90394-4. [DOI] [PubMed] [Google Scholar]
- Dye C., Killick-Kendrick R., Ismael R.B., Al-Gindan Y. Zoonotic cutaneous leishmaniasis in Saudi Arabia: results of a preliminary epidemiological survey in Al-Ahsa oasis. Trans. R. Soc. Trop. Med. Hyg. 1989;83:493–498. doi: 10.1016/0035-9203(89)90265-4. [DOI] [PubMed] [Google Scholar]
- Galloway T., Handy R. Immunotoxicity of organo-phosphorous pesticides. Ecotoxicology. 2003;12:345–363. doi: 10.1023/a:1022579416322. [DOI] [PubMed] [Google Scholar]
- Gradoni L., Gramiccia M. Leishmania infantum tropism: stain genotype or host immune status. Parasitol. Today. 1994;10:264–267. doi: 10.1016/0169-4758(94)90142-2. <http://www.agrwat.gov.sa/public/portal> [DOI] [PubMed] [Google Scholar]
- Kousba A.A., Sultatos L.G., Poet T.S., Timchalk C. Comparison of chlorpyrifos-oxon and paraxon acetylcholinestrase inhibition dynamics: potential role of peripheral binding site. Toxicol. Sci. 2004;80:239–248. doi: 10.1093/toxsci/kfh163. [DOI] [PubMed] [Google Scholar]
- Meyer A., Seidler F.J., Slotkin T.A. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ. Health Perspect. 2004;11:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. “The little sister” – a tale of Arabia. Trans. R. Soc. Trop. Med. Hyg. 1988;82:179–184. doi: 10.1016/0035-9203(88)90400-2. [DOI] [PubMed] [Google Scholar]
- Peters W., Al-Zahrani M.A. The leishmaniasis – a public health problem in Saudi Arabia. Saudi Med. J. 1987;8:333–343. [Google Scholar]
- Spies R.B., Rice D.W., Jr., Felton J. Effects of organic contaminants on reproduction of the starry flounder Phatichthys stellatus in San Fransico Bay. Hepatic contamination and mixed-function oxidase (MFO) activity during the reproductive season. Mar. Biol. 1988;98:181–189. [Google Scholar]
- Sultatos L.G., Murphy S.D. Hepatic microsomal detoxification of the organophosphates paraxon and chlorpyrifos oxons in the mouse. Drug Metab. Dispos. 1983;11:232–238. [PubMed] [Google Scholar]
- Tarbah F.A., Mahler H., Temme O., Daldrup T. An analytical method for the rapid screening of organophosphate pesticides in human biological samples and foodstuffs. Forensic Sci. Int. 2001;121:126–133. doi: 10.1016/s0379-0738(01)00462-5. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Doursan M., Gadagbui B. A review of the reference dose for chlorpyrifos. Regul. Toxicol. Pharmacol. 2006;44:111–124. doi: 10.1016/j.yrtph.2005.10.003. [DOI] [PubMed] [Google Scholar]


