Abstract
Honey is a rich conventional natural resource of sweetness and energy for human beings. A protocol for the determination of two important monosaccharide sugars (fructose and glucose) in honey was established in the current study by using normal phase partition liquid chromatography and 1–5% combined working standard of glucose, fructose and sucrose.
Abbreviation: LC, Liquid chromatography
Keywords: Beverages, Chromatography, Fructose, Glucose, Honey, Sugars
1. Introduction
Generally, carbohydrates are one of the most important components in many food items and they may be either present as isolated form or associated form to other macromolecules. Sugars are simple carbohydrates and are important for everyday life biological functions such as providing energy for running vital roles of the living body. The majority of the natural sugars contain 6 or 12 carbon atoms in their molecules. Sugars are crystalline, soluble in water and generally have a sweet taste. The commercial sugar is the disaccharide sucrose white sugar. Honey is composed primarily of the simple sugars glucose and fructose – known as monosaccharides and a further 17% to 20% of water.
Honey also contains other types of sugars such as sucrose (which is a disaccharide composed of fructose and glucose linked together through α-1–4 linkage). The chemical structure of the fructose and glucose is presented in various fundamental text books of Chemistry and Biochemistry, therefore, this isn’t critical to mention in this article. Usually, fructose is slightly sweeter than sucrose and glucose is less sweet. The sweetness of mono-floral honey – a honey made from a single flower source – is dependent on the ratio of fructose to glucose that results from the bee’s processing the nectar of the homo mono-specific flower. Most of the honey sold in the markets is a blend of varieties, to create a consistent flavor and sweetness profile. However, most of the honey’s fructose becomes predominating, thus, it achieves creation of a sweet honey taste. Sugars have been separated by ion exchange and normal phase bonded phase methods (Dyson, 1990). In this laboratory, first we established research on kinetics of human serum butyrylcholinesterase along with its inhibition by novel experimental Alzheimer therapeutics, bisnorcymserine and dihydrobenzodioxepine cymserine (Kamal et al., 2006, 2008).
In addition to prior research undertaken in this laboratory, different analytical techniques were considered. This involved the estimation of fatty acids in oils and BTEX in groundwater samples, estimated by Gas Capillary Chromatography and Gas Chromatography–Mass Spectrometry (GC–MS) respectively (Kamal and Klein, 2007, 2010). Currently, we are undertaking a study of the normal phase partition Liquid Chromatographic (LC) system for the separation and quantification of sugars in the honey.
2. Materials and methods
2.1. Materials
Manual syringe (>100 μL) was used for injecting the sample on Rheodyne injector with 20 μL loop on the system. Column was Waters Radial-Pak silica (7 × 100 mm) while Cartridges was RCM 8 × 10 Cartridge Holder. A radial compression column was used in this LC system for sugar analysis, which has several advantageous such as minimize channeling, fluid velocity front due to flexible wall moldable around the packing media and cheaper to replace cartridge then the whole column. Moreover, dynamically close-up voids characteristic of radial compression can be explained due to its decreased void spaces capacity within the bed and by this way eliminates “wall effects”, which cause channeling and loss of efficiency.
3. Results and discussion
LC profiles for 1–5% combined working standard of glucose, fructose and sucrose are represented in Figs. 1–4 while sample profile is obvious in Fig. 5. Differential Refractometer (KNAUER) ICI – Comp (Range 16.3) was used in this analysis. The refractive index (RI) is bulk property and a virtually universal detector based on density. However, this detector does lack its sensitivity. In LC, a normal phase chromatography is a separation mode which runs on unbonded, anhydrous silica (polar) using a nonpolar mobile phase. Generally, in such a case of LC, properties of mobile phase such as pH and concentration have vital roles in controlling the retention time, therefore, a different mode of gradient was applied to allow the strongly retained analyte to be eluted quickly. In this sugar analysis, percentage ratio of acetonitrile and water played this role. Usually, decreasing the particle size of the column packing material and reducing the column length produces better peaks and reduces retention time.
Figure 1.
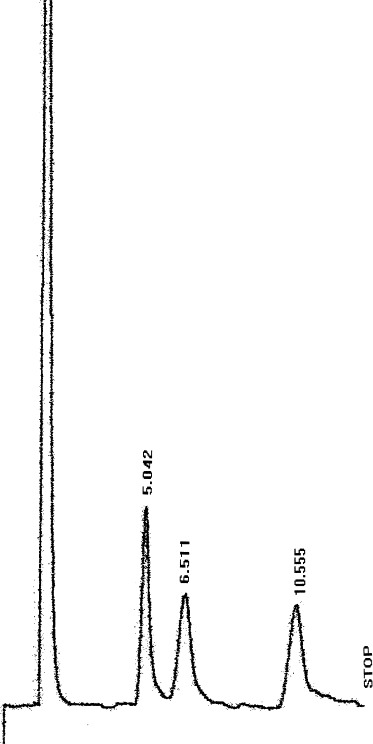
LC profile for 1% combined working standard of glucose, fructose and sucrose.
Figure 2.
LC profile for 2% combined working standard of glucose, fructose and sucrose.
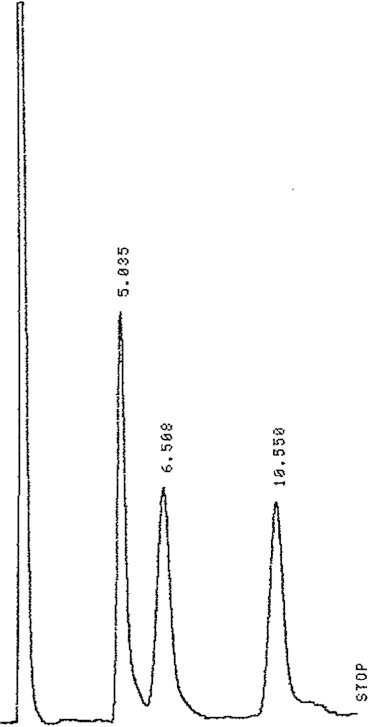
Figure 3.
LC profile for 3% combined working standard of glucose, fructose and sucrose.
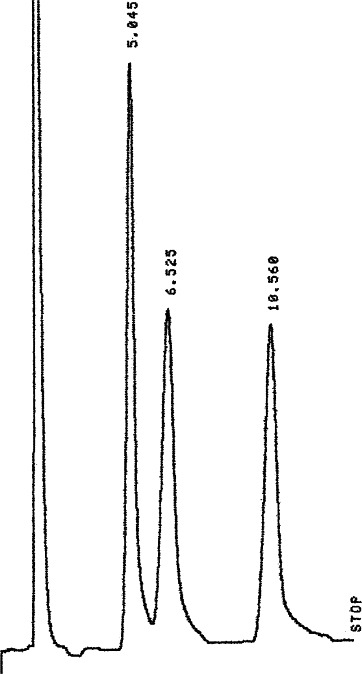
Figure 4.
LC profile for 5% combined working standard of glucose, fructose and sucrose.
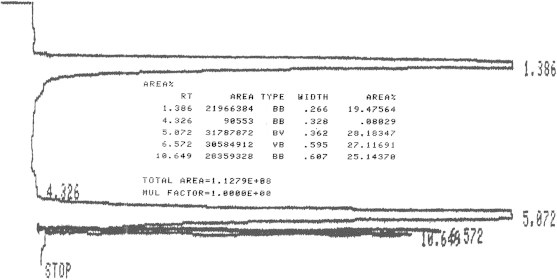
Figure 5.
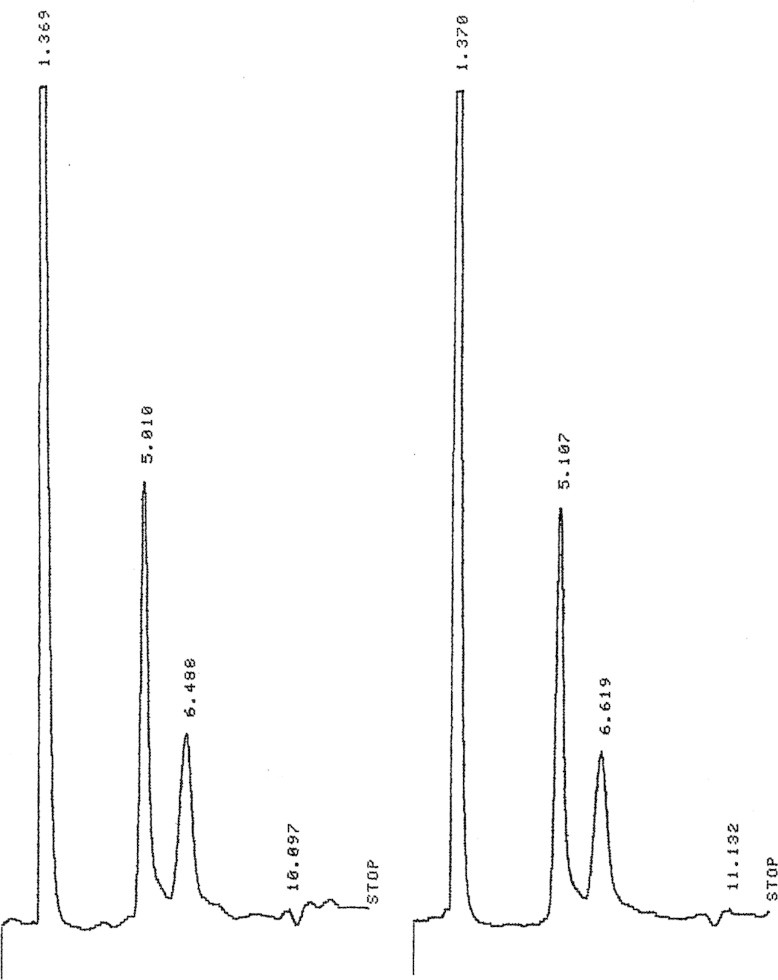
LC profile for honey sample (duplicate experiment).
According to slope values obtained after linear regression analysis of the data (Fig. 6), it was found that fructose and glucose was found as 1.605 ± 0.021 and 1.08 ± 0.085 (SD)% in the sample profile of honey while after multiplication with the dilution factor it becomes 40% and 27% respectively. A setup of integration parameters such as threshold and peak width in this current study was important. This is critical because it allows the process to seek the required peaks within the chart size. Furthermore, it allows the ability to achieve effectively the study of comparable obvious heights. If this setup is inappropriate, it can lead to number of consequences. These are creation of unnecessary peaks or very short peaks or abnormal high height of the peaks, to be observed, that goes out of the range. Therefore, appropriate set up was necessary before the running of the sample or standard on LC.
Figure 6.
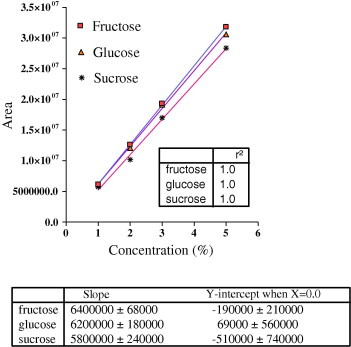
Plot for area of working standard sugars versus concentration.
According to the literature, honey has always been regarded as a food which is advantageous for one’s health and as a product that has healing qualities. For this reason, it is necessary to protect consumers from the fraudulent mislabeling of inferior honeys, therefore, Feás et al. (2010) characterized artisanal honey is produced on the Northwest of Portugal by melissopalynological and physico–chemical analysis. A method has been also developed and validated for the simultaneous analysis of different veterinary drug residues (macrolides, tetracyclines, quinolones, and sulfonamides) in honey by Vidal et al. (2009). Overall this study is composed of a simple protocol for the analysis of monosaccharide in the honey, which can be a guideline for students and junior researchers to demonstrate normal phase partition LC, operation and application of the differential refractometer detector.
References
- Dyson, N., 1990. Chromatographic integration methods. Royal Chemical Society.
- Feás, X., Pires, J., Iglesias, A., Estevinho, M.L., 2010. Characterization of artisanal honey produced on the Northwest of Portugal by melissopalynological and physico-chemical data. Food Chem. Toxicol. [DOI] [PubMed]
- Kamal M.A., Klein P. Estimation of fatty acids in oils by gas capillary. Chromatography. Saudi J. Biol. Sci. 2007;14(1):17–20. [Google Scholar]
- Kamal M.A., Klein P., Luo W., Li Y., Holloway H.W., Tweedie D., Greig N.H. Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental Alzheimer therapeutic, dihydrobenzodioxepine cymserine. Neurochem. Res. 2008;33(5):745–753. doi: 10.1007/s11064-007-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M.A., Yu Q.-S., Holloway H.W., Tweedie D., Klein P., Greig N.H. Kinetics of human serum butyrylcholinesterase and its inhibition by a novel experimental Alzheimer therapeutic, bisnorcymserine. J. Alz. Disease. 2006;10(1):43–51. doi: 10.3233/jad-2006-10108. [DOI] [PubMed] [Google Scholar]
- Kamal M.A., Klein P. Estimation of BTEX in groundwater by using gas chromatography–mass Spectrometry. Saudi J. Biol. Sci. 2010;17(3):205–208. doi: 10.1016/j.sjbs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J.L., Aguilera-Luiz Mdel M., Romero-González R., Frenich A.G. Multiclass analysis of antibiotic residues in honey by ultraperformance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2009;57(5):1760–1767. doi: 10.1021/jf8034572. [DOI] [PubMed] [Google Scholar]


