Abstract
This study compared the response of common bean (Phaseolus vulgaris L.) to arbuscular mycorrhizal fungi (AMF) and rhizobia strain inoculation. Two common bean genotypes i.e. CocoT and Flamingo varying in their effectiveness for nitrogen fixation were inoculated with Glomus intraradices and Rhizobium tropici CIAT899, and grown for 50 days in soil–sand substrate in glasshouse conditions. Inoculation of common bean plants with the AM fungi resulted in a significant increase in nodulation compared to plants without inoculation. The combined inoculation of AM fungi and rhizobia significantly increased various plant growth parameters compared to simple inoculated plants. In addition, the combined inoculation of AM fungi and rhizobia resulted in significantly higher nitrogen and phosphorus accumulation in the shoots of common bean plants and improved phosphorus use efficiency compared with their controls, which were not dually inoculated. It is concluded that inoculation with rhizobia and arbuscular mycorrhizal fungi could improve the efficiency in phosphorus use for symbiotic nitrogen fixation especially under phosphorus deficiency.
Keywords: Arbuscular mycorrhizal fungi, Glomus intraradices, Nitrogen fixation, Phaseolus vulgaris, Phosphorus, Rhizobia, Symbiosis
1. Introduction
Phosphorus and nitrogen constitute the most limited nutriment for vegetative growth. In order to assess the capacity of plant to acquire nutrients, arbuscular mycorrhizal fungi and rhizobia are two of the most important plant symbionts. They play a key role in natural ecosystems and influence plant productivity, plant nutrition and improved inhibition of fungal plant pathogens (Demir and Akkopru, 2007). Mycorrhiza benefits the host through mobilization of phosphorus from non-labile sources, whereas rhizobia fixes N2 (Scheublin and Vander Heijden, 2006). Previous works on the tripartite symbiosis legume–mycorrhiza–rhizobia have shown stimulatory (Edwards et al., 1998; Xiao et al., 2010) or inhibitory (Söderberg et al., 2002; Scheublin and Vander Heijden, 2006; Franzini et al., 2010) effects on each other or on the growth of plants.
A few studies have shown that some bacterial species respond to the presence of certain AM fungi (Andrade et al., 1997; Artursson et al., 2006), suggesting a high degree of specificity between bacteria associated with AM fungi. Thus, the specific bacteria together with AM fungi may create more indirect synergism for plant growth (Barea, 1997), including nutrient acquisition (Barea et al., 2002) and enhancement of root branching (Gamalero et al., 2004). In addition, the AM fungi themselves have also been shown to have an impact on the composition of bacterial communities in their mycelium environment (Artursson et al., 2006).
On the other hand Aysan and Demir (2009) reported that the information on the mechanisms controlling interactions of bacteria with AM fungi and plant roots in the mycorrhizosphere and their activities are very difficult to generalize because the interactions involving arbuscular mycorrhiza, root rot fungi and Rhizobium vary with the microbial species and plant cultivars.
In this paper, we focus on interactions between rhizobia and AM fungi with common bean in their influence on plant growth and investigate whether sensitivity of symbiotic nitrogen fixation to phosphorus deficiency was restored by symbiosis with arbuscular mycorrhizal fungi in soil condition.
2. Material and methods
Two common bean (Phaseolus vulgaris L.) genotypes (CocoT and Flamingo) were used in this study grown in sand–soil culture. The common bean genotypes were inoculated or not (controls) with AMF and in both cases received similar rhizobial inoculation (see below).
2.1. Biological material
The common bean genotypes were CocoT and Flamingo. The first one was selected as a pure line from the local cultivar Coco whereas Flamingo was selected on the basis of its tolerance to salinity (Jebara et al., 2001) among a collection initially supplied by B. Voyesset from CIAT (Colombia). Seeds were surface-sterilized with 1.3% calcium hypochloride for 15 min with constant stirring, and subsequently washed with sterile distilled water. They were germinated on 0.8% sterile agar plates for 3 days at 28 °C in the dark, with a germination rate of 80%. Rhizobial inoculation was performed by soaking the seedlings of common bean for 45 min within a freshly prepared suspension of Rhizobium tropici CIAT899 containing 108 bacteria ml−1.
Thereafter the seedlings were grown in 1000 ml pots filled with autoclaved sand–soil mixture (9:1 v:v) recolonized with soil bacteria according to Jansa et al. (2002). This potting mixture was inoculated with AMF or a non-mycorrhizal inoculum (Control was not inoculated with Glomus intraradices). By placing the bean seedlings in the potting mixture, they became inoculated with G. intraradices BEG157 (Schenck & Smith) or with the non-mycorrhizal mixture (as a control). The inoculum used for the pots consisted of chopped roots of pot cultures planted with leek (Allium porrum) and grown for 18 months in a glasshouse. Fifty grams of AMF inoculum was thoroughly mixed into each pot that received approximately 1000 spores of the AMF species contained at least 20 infective propagules of AMF per gram of chopped root.
The amount of mycorrhizae substrate was characterized by low available N (0.007%) and P (0.001%). In non mycorrhizal treatments, each pot filled with same amount of mycorrhizae free substrate.
2.2. Growth conditions
Trials were performed in a temperature-controlled glasshouse with night/day temperatures of 25/35 °C, and a 16 h photoperiod with complementary illumination of 400 μmol photons m−2 s−1. Seedlings inoculated with R. tropici CIAT899 were grown in soil–sand substrate with or without G. intraradices.
Pots were watered with distilled water every 2 days until harvest, and received once a week the Vadez et al. (1996) nutrient solution: macroelements: K2SO4 (1.25 mM), MgSO4·7H2O (2.05 mM), CaCl2 (3.3 mM); microelements: Fe EDDHA (8.5 μM Fe as sequestrene), H3BO3 (4.0 μM), MnSO4 (6.0 μM), ZnSO4 (0.9 μM), CuSO4 (1.0 μM), NaMoO4 (0.1 μM). The nutrient solution was supplemented with 2 mmol urea plant−1 during first two weeks, 1 mmol urea plant−1 during the next two weeks and no more urea during the last two weeks.
The pots were distributed in a complete randomized block design with 6 replications and one plant only per pot.
2.3. Assessment of AMF colonization
The plants were harvested after 50 days of growth. Half of the root system was used for estimation of the extent of root colonization by the AMF as follows: roots were cleared in KOH 10% (w:v) at 80 °C for 30 min followed by rinsing with water and two rinses with 1% HCl during 1 h. Thereafter, the roots were immersed at 80 °C for 1.5 h in the staining solution consisting of lactic acid:glycerol:water (1:1:1 v:v:v) and 0.1% of each Trypan Blue and Methylene Blue. After washing away the staining solution the roots were de-stained in tap water for 30 min at room temperature.
The roots were examined under a compound microscope for quantitative colonization assessment by magnified-intersection method according to McGonigle et al. (1990) and colonization parameters (percentages of root length colonized by AMF hyphae, arbuscules, and vesicles) were recorded.
2.4. Biomass and percentages of P and N at harvest
At harvest, shoot, nodules and roots were separated and dried at 70 °C for 2 days. Dry weight of each fraction was measured. The root to shoot ratio from each plant was determined.
The concentration of P was measured in samples of ground tissues following wet digestion with nitric–perchloric acids (6:1, v:v) at 250 °C for 6 h, using the phosphovanado-molybdate method (Taussky and Shorr, 1953). The P use efficiency (PUE) was calculated as the ratio of plant dry mass (g) and plant P content (mg).
Total nitrogen percentage (TN) was measured by Kjeldahl procedure on plants harvested for biomass determinations.
2.5. Statistical analysis
The SAS software (1997) was used to perform the ANOVA of results and the comparison of means was achieved by the Duncan’s multiple range test (p ⩽ 0.05). The regressions were performed using the general linear model procedure of the 2-D graphing Analysis System package (File Version: 1.27).
3. Results
3.1. AMF root colonization and nodulation
Fig. 1(a) shows that in both genotypes, the colonization with arbuscular mycorrhizal fungi was higher for hyphae and vesicles (rates of about 80%) than for arbuscules (rates below 10%). There was no significant difference between both genotypes whatever the structure of colonization.
Figure 1.
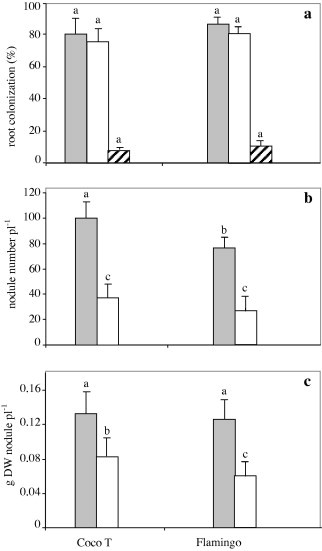
Effect of G. intraradices on root colonization (a) by hyphae(grey bars), vesicles (open bars) and arbuscules (hatched bars) and number (b) and dry weight (c) of nodules of common bean genotypes CocoT and Flamingo, grown in sand–soil culture after inoculation with R. tropici CIAT899, and with G. intraradices (grey bars) or not as control (open bars). Data are means ± SD of 6 replicates, plants harvested at 50 days after sowing; different letters indicate significant differences between treatment means according to Duncan’s multiple range test (p ⩽ 0.05).
Data in Fig. 1(b) show that the nodulation varied significantly among genotypes (p ⩽ 0.05) and was increased by AMF inoculation (p ⩽ 0.01). Thus, the combination of AMF and CIAT899 induced a significant increase in nodulation whatever the genotypes. The increases of about 63% and 70% were observed in nodule number of CocoT and Flamingo, respectively, in comparison to that in control plants (Fig. 1b).
Also, inoculation with AMF increased significantly the nodule mass per plant of 40% with CocoT and 43% with Flamingo without significant difference among genotypes (Fig. 1c). Although, the highest nodule number per plant was observed with AMF in Coco T. However, the lowest nodule biomass was observed in control plants of Flamingo.
3.2. Shoot, root growth and correlation with nodulation
Data in Fig. 2 show that plant-growth was significantly increased by AMF inoculation (p ⩽ 0.01) since higher dry weight was observed for plants inoculated with AMF than for control plants whatever the genotypes. Thus, inoculation with AMF induced a significant increase in shoot dry weight of 23% and 24% for CocoT and Flamingo, respectively, in comparison to that in control plants. On the other hand, CocoT was distinct by the highest shoot dry weight of plants inoculated with AMF or not inoculated in comparison to that in Flamingo (Fig. 2a).
Figure 2.
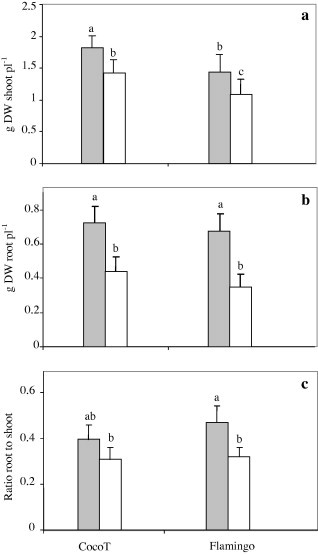
Effect of G. intraradices on dry weight of shoot (a), root (b) and ratio: root to shoots (c) of common bean genotypes CocoT and Flamingo grown in sand–soil culture after inoculation with R. tropici CIAT899, and with G. intraradices (grey bars) or not as control (open bars). Data are means ± SD of 6 replicates, plants harvested at 50 days after sowing; different letters indicate significant differences between treatment means according to Duncan’s multiple range test (p ⩽ 0.05).
Also, the root biomass was affected by inoculation with AMF (p = 0.03), but not by genotypic variation (p = 0.54). Though, in comparison to control plants, inoculation with AMF induced a significant increase of 39% and 48% in root-growth for CocoT and Flamingo, respectively (Fig. 2b). On the other hand, there was no significant difference between both genotypes in root biomass for inoculated or control plants.
Biomass partitioning between roots and shoots was significantly affected by AMF inoculation (p = 0.04). The root to shoot ratio was lower in control plants with both genotypes (Fig. 2c). Thus, inoculation with AMF induced a significant increase in the range of 24–35% in CocoT and Flamingo, respectively and Flamingo showed the highest ratio root to shoot than CocoT.
The efficiency in utilization of the rhizobial symbiosis (EURS) was assessed by the relation of shoot growth as a function of nodule biomass for both genotypes. The slope of the linear regression in Fig. 3 was found to vary with genotypes and AMF treatment. Thus, the EURS was significantly higher with AMF than in control plants for both genotypes. Flamingo, inoculated with Glomus, was characterized by the significantly highest EURS of 4.3 g shoot dry weight g−1 nodule dry weight. Also, control plants of CocoT were distinct with lowest EURS of 2.8 g shoot dry weight g−1 nodule dry weight, but not significant (Fig. 3).
Figure 3.
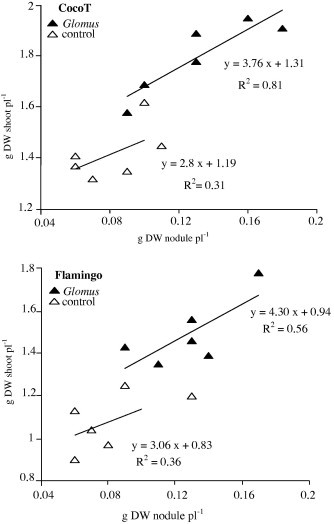
Effect of G. intraradices on relation between shoot growth (g plant−1) and nodule biomass (g plant−1). Plants harvested at 50 days after sowing.
3.3. N and P accumulation and P use efficiency
Data in Fig. 4(a) show that shoot nitrogen percentage (TN) varied among genotypes (p ⩽ 0.05) and was altered by AMF inoculation (p = 0.01). Thus, the AMF in combination with CIAT899 induced a significant increase in shoot nitrogen percentage in comparison to control plants, whereas, the highest nitrogen percentage was observed in CocoT inoculated with AMF with a mean of 2.6 ± 0.3% (Fig. 4a). Inoculation with AMF induced a significant increase of nitrogen percentage of 42% and 29% with CocoT and Flamingo, respectively, in comparison to that in control plants.
Figure 4.
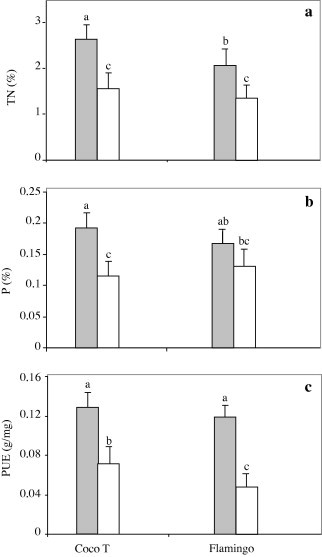
Effect of G. intraradices on shoot nitrogen percentage (a), shoot phosphorus percentage (b) and phosphorus use efficiency (c) of common bean genotypes CocoT and Flamingo grown in sand–soil culture after inoculation with R. tropici CIAT899, and with G. intraradices (grey bars) or not as control (open bars). Data are means ± SD of 6 replicates, plants harvested at 50 days after sowing; different letters indicate significant differences between treatment means according to Duncan’s multiple range test (p ⩽ 0.05).
Regarding phosphorus percentage, significant differences between treatments (p ⩽ 0.001) and genotypes (p ⩽ 0.05) were recorded (Fig. 4b). The highest phosphorus percentage was observed in CocoT inoculated with AMF with a mean of 0.20 ± 0.02%. Also, the lowest phosphorus percentage was obtained in control plants in CocoT with mean of 0.12% ± 0.02. Thus, the inoculation with AMF induced a significant increase of 40% and 30% for CocoT and Flamingo, respectively (Fig. 4b).
As a consequence, the P use efficiency (PUE) was strongly affected by inoculation with AMF (p ⩽ 0.01). Fig. 4(c) showed a significant increase in PUE with AMF in comparison to control plants. Indeed, the inoculation with AMF increased significantly the PUE of 46% and 58% with CocoT and Flamingo respectively. Though, the highest PUE was observed in CocoT with a mean of 0.13 ± 0.02 g shoot DW mg−1 P which did not significantly differ from that of 0.12 ± 0.01 g DW mg−1P in Flamingo. Whereas, the lowest PUE was obtained in control plants for Flamingo with a mean of 0.05 ± 0.01 (Fig. 4c).
4. Discussion
The highest phosphorus use efficiency and nitrogen accumulation in the present work was obtained in the plants in combination with AMF for both genotypes (Fig. 3a and c) illustrates the relationship between P use efficiency and symbiotic nitrogen fixation (Tang et al., 2001) and demonstrates that the mechanisms affecting the efficiency of absorption and utilization of phosphorus in plants are related to colonization by AMF (Bucher et al., 2001; Jia et al., 2004). It was also indicated that the shoot growth and N2 fixation were determined mainly by the efficiency in P utilization (Rodino et al., 2009). In addition, inoculation with AM fungi improves plant growth parameters and nutrient uptake, but this effect was more pronounced in CocoT than in Flamingo, expressing that the colonization of AM fungi also significantly reduced the negative effect of P deficiency (Figs. 2(a) and 4). Also, significant correlation between growth and nodulation (Fig. 3) proves a synergistic effect between rhizobia and AM fungi on common bean growth in this study and that this effect is dependent upon nutrient status.
In sand–soil culture where P limited conditions are more pronounced, the expression of mycorrhizal benefits was more obvious in both genotypes (Fig. 1a). It is believed that mycorrhizae especially benefit plants grown in soils where P is likely to limit plant growth by increasing the soil volume explored by AM hyphae relative to that of root hairs of non-AM plants. This would agree with previous studies showing highest mycorrhizal benefits to plant growth under moderate P deficiency (Mathimaran et al., 2005; Tajini et al., 2009; Xiurong et al., 2011), especially with leguminous plants harbouring a coarser root system with less extension of root hairs than graminaceous (Isobe and Tsuboki, 1998). Also, the colonization by Glomus was higher under low P. So that means that there may be signal coming from the plants, in relation to their P deficiency, that aim at increasing the level of micorrhization.
It has been demonstrated for increased N2 fixation in mycorrhizal plants that when both nitrogen and phosphorus are limiting, AM fungi can improve phosphorus uptake by the plant which in turn would result in more energy available for nitrogen fixation by rhizobia (Fitter and Garbaye, 1994). In the same way and in order to ground one’s argument for our hypothesis, Karandashov and Bucher (2005) found that the enhanced N2-fixing ability in mycorrhizal plants compared with non-mycorrhizal plants, usually disappears if the non-mycorrhizal plants are supplied with a readily available P source.
Plants deficient in P show decreased nodule-number (Vadez et al., 1996), and biomass when grown in soil, sand and alkaline solution, or hydroaeroponics (Araújo et al., 1997; Vadez et al., 1996). The present study provides additional evidence that nodulation could be improved in common bean by double inoculation with AMF and rhizobia in comparison to control (Fig. 1b and c).
Dual inoculation showed synergistic effects on nodulation and N2 fixation in deficiency P soils. The uptake of other essential micronutrients from the soil by the AM fungal hyphae might also play a role in general plant growth improvement as well as in more indirect effects upon the N2-fixing system.
The obtained results indicated that AMF inoculation of common bean plants significantly increased nitrogen accumulation in the shoot and increased the phosphorus content in both genotypes, compared with their controls which were not dually inoculated (Fig. 4a and b). These findings are in agreement with that of Aysan and Demir (2009), Askar and Rashad (2010) and Xiurong et al. (2011). It is well known that AM fungi can improve the nutrient status of their host plants (Smith and Read, 2008; Kim et al., 2010). It is also thought that the plant–rhizobium system benefits from the presence of AM fungi because the mycorrhizae ameliorate not only P deficiency but also any other nutrient deficiencies that might be limiting to rhizobium (Smith, 2002). Increased mineral nutrient levels in the plants would not only benefit rhizobium directly, but would also lead to increased photosynthesis, making a greater proportion of photosynthates available to the rhizobium nodules (Mortimer et al., 2008). Also, Nautiyal et al. (2010) found that the dual inoculation of Cicer arietinum L. with rhizobia and AMF significantly enhanced the number of nodules and the dry weight per plant. Thus, the results reported also were confirmed in this study, that AM symbiosis improved nodulation and N2 fixation and phosphorus use efficiency, causing augmentation of plant growth and indicating intimate interactions between all three partners during co-evolution (Demir and Akkopru, 2007; Stancheva et al., 2006; Xiao et al., 2010). This conclusion disagrees with works in other conditions with other AM fungus and other rhizobial strains that related inhibition of nodule development and N2 fixation, causing diminution of plant growth (Franzini et al., 2010). Lisette et al. (2003) reported that co-inoculation with rhizobia and compatible AM fungi could dramatically enhance pea growth, and N and P uptake. Therefore, the AM fungi we used for the present study are compatible with the rhizobial strain and common bean genotypes, which might have potential for agricultural application.
5. Conclusion
In conclusion, our results have verified the important effects of co-inoculation with rhizobia and AM fungi on common bean growth and the synergistic relationship between rhizobia and AM fungi. These results show an interesting practical application for agricultural development in marginal lands that are often deficient in P. The symbiosis with rhizobia and mycorrhiza under P limitation is a biological technology that may improve symbiotic nitrogen fixation in leguminous plants for an agricultural development that would be friendly to the environment and the consumers.
Acknowledgement
Financial support from Aquarhiz project of the European Union is gratefully acknowledged.
Contributor Information
Fatma Tajini, Email: fatmatajini@yahoo.fr.
Mustapha Trabelsi, Email: mus.trabelsi@yahoo.fr.
Jean-Jacques Drevon, Email: drevonjj@supagro.inra.fr.
References
- Andrade G., Mihara K.L., Linderman R.G., Bethlenfalvay G.J. Bacteria from rhizosphere and hyphosphere soils of different arbuscular-mycorrhizal fungi. Plant Soil. 1997;192:71–79. [Google Scholar]
- Araújo A.P., Teixeira M.G., Almeida D.L. Phosphorus efficiency of wild and cultivated genotypes of common bean (Phaseolus vulgaris L.) under biological nitrogen fixation. Soil Biol. Biochem. 1997;29:951–957. [Google Scholar]
- Artursson V., Finlay R.D., Jansson J.K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 2006;8:1–10. doi: 10.1111/j.1462-2920.2005.00942.x. [DOI] [PubMed] [Google Scholar]
- Askar A.A., Rashad Y.M. Arbuscular mycorrhizal fungi: a biocontrol agent against common bean Fusarium root rot disease. J. Plant Pathol. 2010;9:31–38. [Google Scholar]
- Aysan E., Demir S. Using arbuscular mycorrhizal fungi and Rhizobium leguminosarum, Biovar phaseoli against Sclerotinia sclerotiorum (Lib.) de bary in the common bean (Phaseolus vulgaris L.) J. Plant Pathol. 2009;8:74–78. [Google Scholar]
- Barea J.M. Mycorrhiza-bacteria interactions on plant growth promotion. In: Ogoshi, Kobayashi, Homma, Kodama, Kondo, Akino, editors. Plant Growth Promoting Rhizobacteria. OECD Press; Paris, France: 1997. pp. 150–158. [Google Scholar]
- Barea J.M., Azcon R., Azcon-Aguilar C. Mycorrhizosphere interactions to improve plant fitness and soil quality. Anton Van Leeuwen. 2002;81:343–351. doi: 10.1023/a:1020588701325. [DOI] [PubMed] [Google Scholar]
- Bucher M., Rausch C., Daram P. Molecular and biochemical mechanisms of phosphorus uptake into plants. Soil Plant Sci. 2001;164:209–217. [Google Scholar]
- Demir S., Akkopru A. Using of Arbuscular Mycorrhizal Fungi (AMF) for biocontrol of soil-borne fungal plant pathogens. In: Chincholkar, Mukerji, editors. Biological control of plant diseases. Haworth Press; USA: 2007. pp. 17–37. [Google Scholar]
- Edwards S.G., Young J.P.W., Fitter A.H. Interactions between Pseudomonas fluorescens biocontrol agents and Glomusmosseae, an arbuscular mycorrhizal fungus, within the rhizosphere. FEMS Microbiol. Let. 1998;166:297–303. [Google Scholar]
- Fitter A.H., Garbaye J. Interactions between mycorrhizal fungi and other soil organisms. Plant Soil. 1994;159:123–132. [Google Scholar]
- Franzini V.I., Azcón R., Latanze-Mendes F., Aroca R. Interaction between Glomus species and Rhizobium strains affect the nutritional physiology of drought stressed legume hosts. J. Plant Physiol. 2010;167:614–619. doi: 10.1016/j.jplph.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Gamalero E., Martinotti M.G., Trotta A., Lemanceau P., Berta G. Morphogenetic modifications induced by Pseudomonasfluorescens A6RI and Glomusmosseae BEG12 in the root system of tomato differ according to plant growth conditions. New Phytol. 2004;155:293–300. [Google Scholar]
- Isobe K., Tsuboki Y. The relationship between growth promotion by arbuscular mycorrhizal fungi and root morphology and phosphorus absorption in gramineous and leguminous crops. Crop Sci. 1998;67:347–352. [Google Scholar]
- Jansa J., Mozafar A., Anken T., Ruh R., Sanders I.R., Frossard E. Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza. 2002;12:225–234. doi: 10.1007/s00572-002-0163-z. [DOI] [PubMed] [Google Scholar]
- Jebara M., Drevon J.J., Aouani M.E. Effects of hydroponic culture system and NaCl on interactions between common bean lines and native rhizobia from Tunisian soils. Agronomie. 2001;21:601–606. [Google Scholar]
- Jia Y., Gray V.M., Straker C.J. The influence of rhizobium and arbuscular mycorrhizal fungi on nitrogen and phosphorus accumulation by Viciafaba. Ann. Bot. 2004;94:251–258. doi: 10.1093/aob/mch135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandashov V., Bucher M. Symbiotic phosphate transport in arbuscular mycorrhizas. Tren. Plant Sci. 2005;10:22–29. doi: 10.1016/j.tplants.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kim K., Yim W., Trivedi P., Madhaiyan M., Hari P., Boruah D., Rashedul Islam Md., Lee G., Sa T. Synergistic effects of inoculating arbuscular mycorrhizal fungi and Methylobacteriumoryzae strains on growth and nutrient uptake of red pepper (Capsicumannuum L.) Plant Soil. 2010;327:429–440. [Google Scholar]
- Lisette J., Xavier C., Germida J.J. Selective interactions between arbuscular mycorrhizal fungi and Rhizobium leguminosarum bv, Viceae enhance pea yield and nutrition. Biol. Fertil. Soil. 2003;37:261–267. [Google Scholar]
- Mathimaran N., Ruh R., Vullioud P., Frossard E., Jansa J. Glomus intraradices dominates arbuscular mycorrhizal communities in a heavy textured agricultural soil. Mycorrhiza. 2005;16:61–66. doi: 10.1007/s00572-005-0014-9. [DOI] [PubMed] [Google Scholar]
- McGonigle T.P., Miller M.H., Evan D.G., Faichild G.L., Swan J.A. A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fung. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Mortimer P.E., Pérez-Fernández M.A., Valentine A.J. The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol. Biochem. 2008;40:1019–1027. [Google Scholar]
- Nautiyal C.S., Chauhan P.S., DasGupta S.M., Seem K., Varma A., Staddon W.J. Tripartite interactions among Paenibacillus lentimorbus NRRL B-30488, Piriformospora indica DSM 11827, and Cicer arietinum L. World J. Microbiol. Biotechnol. 2010;26:1393–1399. [Google Scholar]
- Rodino A.P., Metrae R., Guglielmi S., Drevon J.J. Variation among common-bean accessions (Phaseolus vulgaris L.) from the Iberian Peninsula for N2-dependent growth and phosphorus requirement. Symbiosis. 2009;47:161–174. [Google Scholar]
- Scheublin T.R., Vander Heijden M.G.A. Arbuscular mycorrhizal fungi colonize nonfixing root nodules of several legume species. New Phytol. 2006;172:732–738. doi: 10.1111/j.1469-8137.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Smith S.E. Soil microbes and plants—raising interest, mutual gains. New Phytol. 2002;156:142–144. doi: 10.1046/j.1469-8137.2002.00514.x. [DOI] [PubMed] [Google Scholar]
- Smith S.E., Read D.J. Academic Press; London, UK: 2008. Mycorrhizal symbioses. [Google Scholar]
- Söderberg K.H., Olsson P.A., Baath E. Structure and activity of the bacterial community in the rhizosphere of different plant species and the effect of arbuscular mycorrhizal colonization. FEMS Microbiol. Ecol. 2002;40:223–231. doi: 10.1111/j.1574-6941.2002.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Stancheva I., Geneva M., Zehirov G., Tsvetkova G., Hristozkova M., Georgiev G. Effects of combined inoculation of Pea plants with arbuscular mycorrhizal fungi and Rhizobium on nodule formation and nitrogen fixing activity. Gen. Appl. Plant Physiol. 2006:61–66. Special Issue. [Google Scholar]
- Tajini F., Suriyakup P., Vailhe H., Jansa J., Drevon J.J. Assess suitability of hydroaeroponic culture to establish tripartite symbiosis between different AMF species, beans, and rhizobia. BMC Plant Biol. 2009;9:73–81. doi: 10.1186/1471-2229-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Hinsinger P., Jaillard B., Rengel Z., Drevon J.J. Effect of phosphorus deficiency on growth, symbiotic N2 fixation and proton release by two bean (Phaseolus vulgaris L.) genotypes. Agronomie. 2001;21:683–689. [Google Scholar]
- Taussky H.H., Shorr E. Microcolorimetric method for the determination of inorganic phosphorus. Biol. Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- Vadez V., Rodier F., Payre H., Drevon J.J. Nodule permeability to O2 and nitrogenase-linked respiration in bean genotypes varying in the tolerance of N2 fixation to P deficiency. Plant Physiol. Biochem. 1996;34:871–878. [Google Scholar]
- Xiao T.J., Yang Q.S., Ran W., Xu G.H., Shen Q.R. Effect of inoculation with arbuscular mycorrhizal fungus on nitrogen and phosphorus utilization in upland rice-mungbean intercropping system. Agric. Sci. 2010;9:528–535. [Google Scholar]
- Xiurong W., Qiang P., Fengxian C., Xiaolong Y., Hong L. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza. 2011;21:173–181. doi: 10.1007/s00572-010-0319-1. [DOI] [PubMed] [Google Scholar]


