Abstract
Halobacteria, members of the domain Archaea that live under extremely halophilic conditions, are often considered as dependable source for deriving novel enzymes, novel genes, bioactive compounds and other industrially important molecules. Protein antibiotics have potential for application as preserving agents in food industry, leather industry and in control of infectious bacteria. Halocins are proteinaceous antibiotics synthesized and released into the environment by extreme halophiles, a universal characteristic of halophilic bacteria. Herein, we report the production of halocin (SH10) by an extremely halophilic archeon Natrinema sp. BTSH10 isolated from salt pan of Kanyakumari, Tamilnadu, India and optimization of medium for enhanced production of halocin. It was found that the optimal conditions for maximal halocin production were 42 °C, pH 8.0, and 104 h of incubation at 200 rpm with 2% (V/V) inoculum concentration in Zobell’s medium containing 3 M NaCl, Galactose, beef extract, and calcium chloride as additional supplements. Results indicated scope for fermentation production of halocin for probable applications using halophilic archeon Natrinema sp. BTSH10.
Keywords: Haloarcheon, Natrinema sp., Halocin, Production, Medium optimization
1. Introduction
Halobacteria, members of the domain Archaea, live under extremely halophilic conditions (4–5 M NaCl) and lower concentrations of sodium chloride generally cause cell lysis (Meral et al., 2007). They produce organic solutes which maintain the concentration of ions inside and outside the cell in order to keep themselves intact and survive in high saline environments (Gonzalo et al., 2002; Jan et al., 2007; Torsten et al., 2007). They are often considered as dependable source for deriving novel enzymes, novel genes, bioactive compounds and other industrially important molecules. Protein antibiotics have potential for application as preserving agents in food industry, leather industry and in control of infectious bacteria and hence there is great interest in isolation of potential proteinaceous bioactive substances. Haloarchaea were the first members of Archaea found to produce proteinaceous bacteriocins which are released into the environment, a universal characteristic of halophilic bacteria (Rodríguez et al., 1982). These proteinaceous bacteriocins, termed as halocins, act against related species and are universally produced by halophilic archaea (Torreblanca et al., 1994). In spite of the fact that several halophilic archaea are being explored, only very few halocins have been studied up to their molecular level and their mode of action whether they kill or inhibit other haloarchaeons as a defence is yet to be understood clearly (Tamar and Aharon, 2000; Meseguer and Rodrı´guez, 1986). Moreover, all haloarchaea are not sensitive to any particular halocin, and a “sensitive” strain is the one which elaborates a zone of inhibition on a double-agar overlay plate in response to the presence of halocin (Paši et al., 2008).
Halocins have been reported to generally kill the indicator organisms by cell swelling followed by cell lysis (O’Connor and Shand, 2002; Sun et al., 2005; Paši et al., 2008). Halocin A4, G1, R1, H1, H2 (O’Connor and Shand, 2002); H3, H5 (Rodríguez et al., 1982; O’Connor and Shand, 2002); H4 (Sun et al.,2005; Gonzalo et al., 2002; O’Connor and Shand, 2002); H6/H7 (O’Connor and Shand, 2002; Li et al., 2003); S8 (O’Connor and Shand, 2002); C8 (Li et al., 2003; Sun et al., 2005) and Sech7a (Paši et al., 2008) are few halocins reported till date and only few of them are studied up to molecular level. They are generally produced at the mid exponential phase (O’Connor and Shand, 2002; Paši et al., 2008). The mechanism of action of halocin may involve modification of cell permeability or inhibition of Na+/H+ antiporter and Proton flux. Few halocins are said to be salt dependent since the protein loses its activity when the concentration of salts decreases beyond a minimum level (Rodríguez et al., 1982; Price and Shand, 2000). Halocin H6 produced by haloarchaea Haloferax gibbonsii was reported to inhibit Na+/H+ exchanger (NHE) in mammalian cells (Meseguer et al., 1995). Studies have been conducted up to gene level for halocins H4 (Cheung et al., 1997), C8 (Sun et al., 2005), and S8 (Price and Shand, 2000), and for identification of the mRNA responsible for production of halocin (Cheung et al., 1997; Price and Shand, 2000).
Though several species of Haloarchaeons were studied for halocin production, reports on halocin production by Natrinema sp. are rather limited. In this context, herein we report the production of halocin by Natrinema sp. an extreme halophile isolated from saltern ponds of south India, which are well known as source of salt prepared for human consumption.
2. Materials and methods
2.1. Microorganisms
Soil samples were collected from salt pans of Kanyakumari district, Tamilnadu, India. One gram of soil was mixed with 10 ml of saturated solution of sodium chloride, homogenized and 100 μl of the prepared sample was spread plated on Zobell’s agar medium containing 3 M sodium chloride and incubated for a week at 37 °C. Later, the single cell colonies formed on the plates were picked, purified and stored as glycerol stocks in −80 °C deep freezer. All the isolated cultures were tested for production of antimicrobial compounds (halocins) against other halophilic bacterial cultures. Potential strains that showed intense bioactivities were screened against selected halobacteria isolated from saltern pond. Both strains that produced bioactive substances and showed inhibition were identified based on their morphological, biochemical, physiological characteristics (Bergey’s Manual of Systematic bacteriology) and 16S ribotyping.
2.2. Media for halocin production
Medium that supported maximal biomass and halocin production was selected from among six different media namely Eimhjellen medium (EM) (Catherine et al., 2001), Sehgal and Gibbons (SG) (Sehgel and Gibbons, 1960), MH medium (Ventosa et al., 1982), HE medium (Torreblanca et al., 1986; Catherine et al., 2001) DSM 97 (ATCC Manual) and Zobell’s medium (Hi-media). The compositions of the media are as given below:
2.2.1. Eimhjellen medium (modified)
Yeast extract – 5 g, MgSO4·7H2O – 2.0 g, CaCl2.2H2O – 0.5 g, NaCl – 3.0 M, Distilled water – 100 ml (Catherine et al., 2001).
2.2.2. Sehgel and gibbons medium (modified)
Yeast extract – 1 g, Casamino acids – 0.75 g, Sodium citrate – 0.3 g, MgSO4·7H2O – 2.0 g, KCl – 0.2 g, FeCl2 – 0.0023 g, NaCl – 3.0 M, Distilled water – 100 ml (Sehgel and Gibbons, 1960).
2.2.3. DSM 97
Casamino acids – 7.5 g, KCl – 2 g, NaCl – 3.0 M, Trisodium citrate – 3 g, MgSO4 – 20 g, MnSO4 – 0.05 g, Ferrous sulphate – 0.5 g, Yeast extract – 10 g, Agar – 10 g, Distilled water – 1000 ml (ATCC manual).
2.2.4. MH medium (modified)
Protease peptone – 0.5 g, Yeast extract – 1.0 g, Glucose – 0.1 g with 25% (w/v) of total salts (Ventosa et al.,1982).
2.2.5. HE medium (modified)
Yeast extract – 0.5 g, Glucose – 0.1 g with 25% (w/v) of total salts (Torreblanca et al., 1986).
The total salts solution was prepared with NaCl – 3.0 M, MgCl2·6H2O – 4.2 g, MgSO4·7H2O – 6.0 g, CaCl2·2H2O – 0.1 g, KCl – 0.6 g, NaCO3H – 0.02 g, NaBr – 0.07 g, FeCl3 – 0.0005 g, Distilled water – 100 ml (Subov, 1931).
2.2.6. Zobell’s medium
Peptic digest of animal tissues – 5 g, Yeast extract – 1 g, Ferric citrate – 0.1 g, NaCl – 3.0 M, MgCl2·6H2O – 8.8 g, Sodium sulphate – 3.24 g, CaCl2·2H2O – 1.8 g, KCl – 0.55 g, NaCO3H – 0.16 g, KBr – 0.08 g, Strontium chloride – 0.034 g, Boric acid – 0.022 g, Sodium silicate – 0.004 g, Sodium fluorate – 0.0024 g, Ammonium nitrate – 0.0016 g, Disodium phosphate – 0.008 g, Distilled water – 1000 ml supplemented with NaCl (4.5 M).
Irrespective of the medium, the final concentration of sodium chloride in the medium was adjusted to 3 M unless otherwise specified. The pH of the medium was maintained at 7.4 ± 2. Solid medium was prepared by the addition of 2% (w/v) of agar (Hi-media) to broth. The bacterial growth was measured in terms of OD at 600 nm in a UV–Visible spectrophotometer (Shimadzu Model 160A).
2.3. Preliminary screening of halocin activity
All the halophilic bacterial strains isolated from the salt ponds were screened for halocin activity by testing each strain against the other strain. The presence of zone of inhibition on double layer agar plates was used as indicator for halocin production (Shand et al., 1999; O’Connor and Shand, 2002). 10 μl aliquots of broth culture of each strain grown in Zobell’s medium were spotted onto top-agar lawn culture of the other halophilic strains and incubated at 42 °C for 7 days. Appropriate controls were maintained using uninoculated media. Cultures that showed inhibition were selected and subjected to further studies.
2.4. Culture conditions
A preculture of the selected bacterial strain that showed halocin activity was prepared initially by growing the strain in 10 ml of Zobell’s medium at 37 °C for 24 h. After growth the culture obtained was centrifuged at 10,000 rpm at 4 °C for 15 min, under aseptic conditions, and the cells were harvested, washed with brine solution (15% NaCl), and suspended in the same solution. The concentration of the cell suspension was adjusted to 0.2 OD at 600 nm and used as inoculum at 2% (V/V) level. 100 ml of Zobell’s medium taken in a 250 ml conical flask was inoculated with the prepared inoculums and incubated for 96 h. Similarly the selected indicator organism was also grown in Zobell’s medium and a cell suspension was prepared in the same manner as was done with the halocin producing strain.
2.5. Halocin production and assay
Zobell’s medium was used for the production of halocin. The sterile medium was inoculated with the selected strain BTSH10, incubated at 37 °C for 96 h, and the bacterial free supernatant was used for halocin assay after centrifugation (10,000 rpm for 10 min) of the culture broth.
Halocin activity was checked by agar well diffusion method (John et al., 1966) using the culture supernatant. The indicator strain BTSH03 was grown up to 0.3 OD at 600 nm and 200 μl of the indicator strain was mixed with 20 ml of Zobell’s medium containing half strength (1%) agar, and overlaid on Zobell agar plates containing 2% agar. Halocin activity was checked by the addition of 50 μl of cell free supernatant of BTSH10 into the well (0.5 cm diameter) made on the plate containing the top agar and performing the halocin assay after incubation for 72 h.
The halocin activity was determined using serial twofold critical end point dilutions to extinction (Meseguer et al., 1986) and expressed as arbitrary units (AU), which are defined as the reciprocal of the first dilution at which all traces of inhibitory activity disappears (Cheung et al., 1997). The two fold dilution ratio of halocin follows a geometric progression where the halocin activity can be calculated by
where “a” denotes the scale factor, “q” is the common ratio and “n” being the first dilution at which all traces of inhibitory activity disappears.
2.6. Cell lysis assay
Activity of halocin SH10 on the cells of indicator bacteria BTSH03 was studied by monitoring the cell lysis under phase contrast microscope. Stationary phase cells of indicator strain Halorubrum sp. BTSH03 was mixed with halocin SH10 (1024 AU/ml) on a microtitre plate and incubated for different time intervals. Samples were drawn at regular intervals and observed under the microscope. Images were captured and presented as photomicrographs.
2.7. Optimization of medium and process variables for halocin production
Various constituents of the selected medium and process parameters that influence halocin production by BTSH10 was optimized by adopting ‘one factor at a time’ approach. Strategy adopted for the optimization was to evaluate the effect of each variable for its optimum level for maximal halocin production, and incorporate the same variable at its optimized level in the subsequent experiment while evaluating the next variable. The variables studied included the following in the sequential order: incubation temperature (27–47 °C), pH (2–13), NaCl concentration (0.5–4 M), carbon sources at 0.1 M concentrations (dextrin, galactose, fructose, lactose, sucrose, sorbitol, xylose, maltose and glycerol), nitrogen source at 1% (w/v) concentration (peptone, yeast extract, malt extract, soybean meal, tryptone, casein, urea and beef extract) and different inorganic salts at 0.1 M concentration (ammonium nitrate, sodium fluorate, sodium silicate, potassium chloride, magnesium chloride, calcium chloride, sodium bicarbonate, potassium bromide and strontium chloride), agitation (50–250 rpm), and incubation time (0–144 h). Preparation of inoculum, inoculation and culture conditions were same as mentioned earlier unless otherwise mentioned.
3. Results and discussion
3.1. Identification of halophiles
The halophilic bacterial strain BTSH10 that showed strong halocin activity from among the isolates obtained as halophiles from the saltern pond was selected for further studies. Strain BTSH03 which showed sensitivity against halocin SH10 was considered as the indicator organism for determining halocin activity during the course of the studies. Based on the morphological, biochemical, physiological characteristics, and molecular 16S ribotyping (data not shown) the halocin producing strain BTSH10 and the indicator strain BTSH03 were identified as Natrinema sp. BTSH10 and Halorubrum sp. BTSH03, respectively. The nucleotide sequences obtained for the 16S rDNA of the selected bacterial strains were submitted to NCBI and the allotted accession numbers are JN228202 and JF830242, for Natrinema sp. BTSH10 and Halorubrum sp BTSH03, respectively. The genus Natrinema was proposed by McGenity et al., 1998) to accommodate Natrinema pellirubrum (formerly Halobacterium salinarum NCIMB 786T) and Natrinema pallidum (formerly Halobacterium halobium NCIMB 777T). In a phylogenetic tree based on 16S rRNA gene sequences, Natrinema species formed an independent cluster with respect to Halobacterium species. Natrinema species could be cultured at low salt concentrations, and possessed a specific protein profile and polar lipid composition. Subsequently, a novel species of this genus, Natrinema versiforme, was described (Xin et al., 2000). From the results (data not shown) obtained for the standardization of optimal cultivation media for halocin production, it was observed that Zobell’s medium supported enhanced growth and halocin production compared to other media evaluated and hence the same was selected for further cultivation of the halophiles. Further the halocin was named as halocin SH10 since it was produced by Natrinema sp. BTSH10.
The results presented as photomicrographs in Fig. 1 showing the activity of halocin SH10 on the indicator strain Halorubrum sp. BTSH03 clearly evidence cell lysis. Initially Halorubrum sp. did not show any change on exposure to halocin SH10 (Fig. 1a). However, after 3 h the cells showed shrinkage and were found as a floc forming small islands/colonies (Fig. 1b). After 6 h the cells were found to appear bulged and showed signs of lysis (Fig. 1c). On further incubation for 12 h complete lysis of the cells resulting in cell debris (Fig. 1d) was observed. These observations not only confirmed the antibacterial activity of halocin SH10 against other halophiles but also suggest cell lysis as the possible mechanism of action of halocin SH10. Exposure of sensitive cells to HalH6 was reported to cause increase in the intracellular volume and cellular swelling followed by lysis, suggesting that HalH6 may act at the level of the cell membrane (Torreblanca et al., 1989). The present observations on morphological changes of sensitive cells upon exposure to halocin and swelling of the cells and cell lysis corroborate well with those observations reported in previous studies on halocins Sech7a, H4, H6, and C8, (Meseguer and Rodrı´guez, 1985; Torreblanca et al., 1989; Li et al., 2003).
Figure 1.
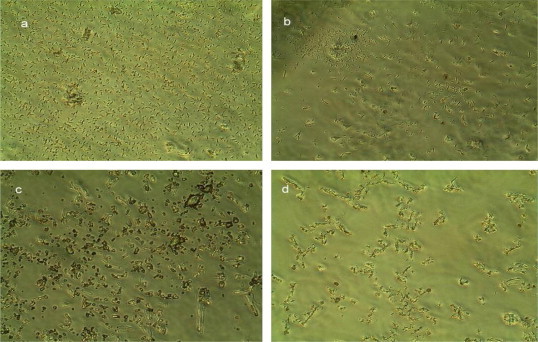
Photomicrographs showing activity of halocin SH10 on indicator strain Halorubrum sp. BTSH03 (a) Normal cells of Halorubrum sp BTSH03 soon after (0 h) halocin SH10 treatment (b) Shrinked cells forming islands after 3 h of treatment (c) Bulged cells after 6 h of treatment (d) Completely lysed cells after 12 h of treatment.
3.2. Optimization of media and process variables
3.2.1. Effect of incubation temperature, pH and NaCl concentration on halocin production
From the results documented in Fig. 2 it was inferred that 42 °C was the optimum temperature for maximum halocin production (1024 AU). Nevertheless appreciable levels of halocin activities could be recorded at other temperatures. Halocin production at high levels observed at 42 °C, and relatively at lesser levels at lower and at slightly higher temperatures (47 °C) indicated that the halocin gene expression might be either temperature dependent or specific temperature of the cultivation medium might act as an antagonist (stress) to the organism that induces this gene expression (Christine et al., 2008; Mirko et al., 2012). Haloarchaeon Sech7a was reported to be thermophilic in character with optimal growth occurring at 45 °C, although the temperature in its native solar saltern crystallizer rarely exceeds 32 °C (Pasic´ et al., 2005). Consistent with the physicochemical properties of a crystallizer, the optimal growth of haloarchaeon Sech7a was observed at pH 8, yet the halocin production reached maximum at neutral pH (Paši et al., 2008). Results presented in Fig. 3 show that the halobacteria could produce halocin in media with a pH varying between pH 4 and pH 10 although maximum halocin production was recorded at pH 8.0 (1024 AU). However, the bacteria did not produce halocin under acidic conditions.
Figure 2.
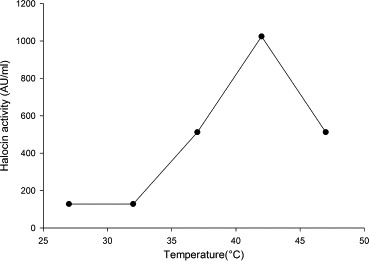
Effect of different incubation temperatures on halocin production by Natrinema sp. BTSH10 in Zobells medium at pH 7.4, 3 M NaCl, 150 rpm, 2% (V/V) inoculum concentration and 96 h of incubation.
Figure 3.
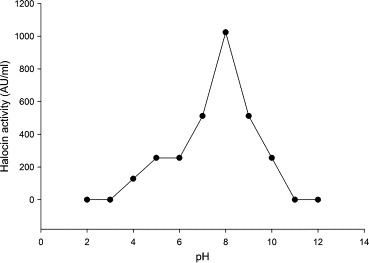
Effect of different pH on halocin production by Natrinema sp. BTSH10 in Zobells medium at 42 °C, 3 M NaCl, 150 rpm, 2% (V/V) inoculum concentration and 96 h of incubation.
Results presented in Fig. 4 indicate that Natrinema sp. BTSH10 required 3 M NaCl as optimum concentration for maximum (1024 AU) production of halocin although NaCl concentration ranging from 1.5 to 4 M in the medium supported enhanced production of halocin. It was also noted that a minimum of 1.5 M NaCl was required for growth, and lesser concentrations did not even support survival of the bacterium. These observations testified the halophilic nature of the isolated bacterium and the impact of higher concentrations of sodium chloride for halocin activity. The halocin of haloarchaeon Sech7a was observed to remain active over a wide NaCl concentration range (0.02–5.2 M) with the highest production observed in high salt media containing 3.4 M NaCl (Paši et al., 2008). The observations made with Natrinema sp. BTSH10 was in agreement with these earlier reports for other species of haloarchaeon although there were marginal differences in optimal temperature and sodium chloride concentrations. It was also noted that sodium chloride concentration was found to have strong influence on the halocin activity of the halobacteria. Another specific observation made during the study was that the maximal halocin activity observed with these three factors namely temperature, pH and NaCl were almost same. This observation could be attributed to the fact that the control medium had 3 M NaCl and pH of the medium was 7.4 which were almost identical with the optimum pH and NaCl concentrations. Hence there was no marked enhancement in halocin activity after optimization of these three variables. Further the results also indicated that these three factors are independent in exerting their influence on halocin production by the bacteria.
Figure 4.
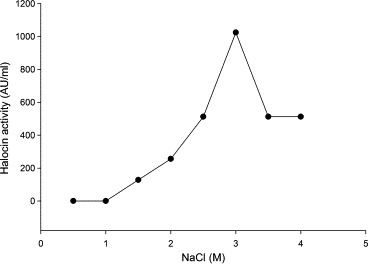
Effect of different concentrations of NaCl on halocin production by Natrinema sp. BTSH10 in Zobells medium at 42 °C, pH 8.0, 150 rpm, 2% (V/V) inoculum concentration and 96 h of incubation.
3.3. Effect of different carbon and nitrogen sources and inorganic salts in the medium on halocin production
From the results presented in Fig. 5 it was inferred that maximal halocin production was supported by the medium supplemented with galactose (2048 AU) followed by sorbitol, maltose, glycerol, glucose, fructose, and lactose. Whereas, the medium supplemented with dextrin, sucrose and xylose supported reduced levels of halocin production. Galactose was observed to enhance halocin production in the medium compared to other carbon sources. Results documented in Fig. 6 indicate that the bacteria could produce maximal halocin in the presence of beef extract (2048 AU) in the medium followed by soybean meal, malt extract, tryptone, peptone, yeast extract, casein and gelatin. Urea did not support halocin production. In fact it was reported earlier that the algae Dunaliella sp. (Avinash et al., 2011) that exist in the natural salt pan ecosystem provides glycerol and galactose for the halobacteria and thus the strain recorded maximal halocin in response to supplementation of galactose under laboratory conditions. In a similar fashion maximal growth rate and halocin activity by haloarchaeon Sech7a was observed in media supplemented with glycerol and yeast extract (Paši et al., 2008). It must be noted that in solar salterns glycerol produced by blooms of unicellular green algae, Dunaliella is considered the most important source of organic carbon for the heterotrophic prokaryotes (Bardavid et al., 2008). Additional salts in the medium were found to exert influence on halocin production by the archaebacteria. Among the inorganic salts used for supplementation of the medium as additional salts calcium chloride (2048 AU) was found to support maximal halocin production in the medium followed by magnesium chloride, sodium fluorate, potassium chloride, and sodium bicarbonate Fig. 7. Whereas aluminium nitrate, potassium bromide, strontium chloride and sodium silicate led to a much reduced level of halocin production when compared to the levels noted with Zobell’s medium in the absence of these particular salts. These observations indicate that the members of archaea have a special nutritional requirements and critical life style in the salt pan environment which needs to be investigated further to have a better understanding of their physiology in hyper saline environments.
Figure 5.
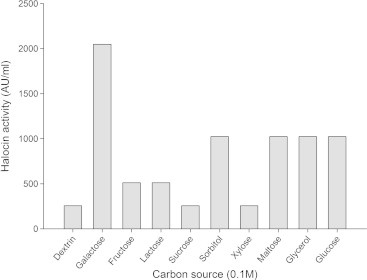
Effect of different carbon sources on halocin production by Natrinema sp. BTSH10 in Zobells medium at 42 °C, pH 8.0, 3 M NaCl, 150 rpm, 2% (V/V) inoculum concentration and 96 h of incubation.
Figure 6.
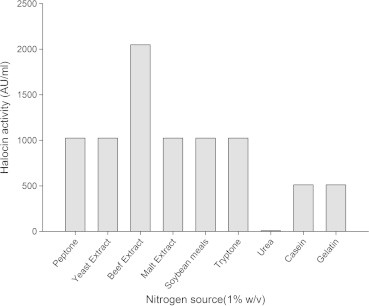
Effect of different nitrogen sources on halocin production by Natrinema sp. BTSH10 in Zobells medium at 42 °C, pH 8.0, 3 M NaCl, Galactose 150 rpm, 2% (V/V) inoculum concentration and 96 h of incubation.
Figure 7.
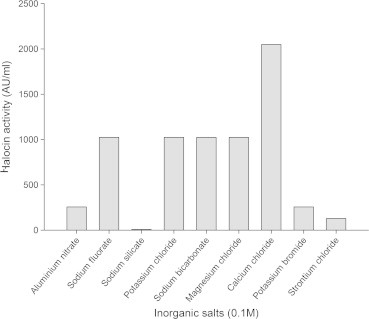
Effect of different inorganic salts on halocin production by Natrinema sp. BTSH10 in Zobells medium at 42 °C, pH 8.0, 3 M NaCl, Galactose beef extract, 150 rpm, 2% (V/V) inoculum concentration and 96 h of incubation.
3.4. Effect of agitation on halocin production
Data documented in Fig. 8 indicate the influence of agitation on the rate of halocin production. It was found that higher agitation rate led to enhanced bacterial growth and production of halocin compared to lesser agitation rates. A maximum of 4096 AU was recorded at 200 and 250 rpm although lower agitation rates (50–150 rpm) also supported considerable levels of halocin production. This might be due to better mixing of the medium which facilitates better mass transfer and also does not support the adherence of the microbes to the surface of the flask. These observations strongly suggested that aerobic conditions are required for enhanced halocin production by the halophilic Natrinema sp. Generally the dissolved oxygen is low in aqueous medium at higher concentrations of NaCl and hence aerobic organisms need provision of adequate oxygen for enhanced electron transport and consequent growth and halocin production. Normally the agitation process facilitates infusion of atmospheric air into the growth medium and provides required oxygen for the bacterium (Feng et al., 2003; Hay et al., 2012).
Figure 8.
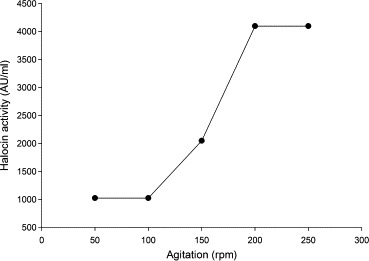
Effect of different agitation rates on halocin production by Natrinema sp. BTSH10 in Zobells medium at 42 °C, pH 8.0, 3 M NaCl, Galactose, beef extract, calcium chloride, 2% (V/V) inoculum concentration and 96 h of incubation.
3.5. Time course experiment for halocin production
Results depicted in Fig. 9 very clearly indicate that Natrinema sp. BTSH10 produces halocin at enhanced levels only during the stationary phase of growth although significant levels of halocin could be noted during late exponential phase. Maximum (8192 AU) production of halocin was observed at 104 h, during the stationary phase. Nevertheless the halocin activity in the medium was also noted even after 144 h. The organism Natrinema sp. is an extreme haloarcheon which produces halocin SH10. The halocin production was reported to register an increase when the culture entered the exponential phase and continued to increase during the course of exponential phase although the maximal level was attained during the stationary phase (Price and Shand, 2000; O’Connor and Shand, 2002). The observations made in the present study indicated that in the case of Natrinema sp. BTSH10 the halocin production is growth associated which gets accumulated in the cell and released during stationary phase. In an earlier study halocin production by haloarchaeal strain Sech7a was reported to be growth dependent (Paši et al., 2008). Although the onset of halocin activity was observed in the early exponential phase of growth, the halocin Sech7a peak activity was observed as the bacteria entered the stationary phase of growth (Paši et al., 2008) in contrast to most other halocins which were first detected when the bacteria entered the stationary phase of growth (Shand et al., 1999; O’Connor and Shand, 2002). Further HalS8 activity was reported to be undetectable in culture supernatants until the culture began the transition into stationary phase (Price and Shand, 2000; Shand et al., 1999) and later the activity reached a maximum within 10 h of onset and was stable for greater than 80 h after reaching maximum values (Price and Shand, 2000). The results observed in the present study for Natrinema sp. BTSH10 were very similar to those observed for most other haloarchaeal bacteria in terms of halocin synthesis during growth in the production medium. It may be noted that during the process of optimization of variables, one after another, the halocin content showed increase and reached a maximum under optimized culture conditions. This observation strongly indicated the need for optimization of production medium towards enhanced level of halocin SH10 production by Natrinema sp. BTSH10 and the significant role of media constituents in inducing halocin synthesis by the bacterium.
Figure 9.
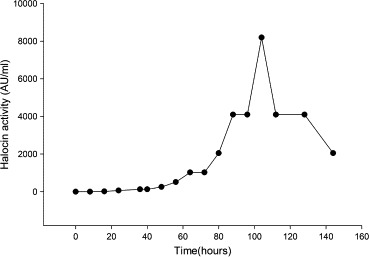
Time course experiment on halocin production by Natrinema sp. BTSH10 in Zobells medium at 37 °C, pH 8, 3 M NaCl, Galactose beef extract, calcium chloride, 2% (V/V) inoculum concentration and 200 rpm.
4. Conclusion
Based on the results obtained in the present study it is concluded that Natrinema sp. BTSH10, relatively a new genus of archaebacteria that is less explored, exists as a native flora in the highly saline salt pan of coastal areas of southern India known for the harvest of commercial salt used for human consumption. This bacterium is capable of synthesizing halocin SH10 which has scope for probable application as preservative against those bacteria that cause spoilage in food products, leather products during processing and in control of infectious bacteria and few other applications. Results of the present study indicated that halocin could be easily produced under submerged fermentation. Further studies could reveal the mode of gene expression under the influence of various nutrients and inorganic salts which were found to induce halocin under differential environmental conditions.
Acknowledgements
The authors gratefully acknowledge the financial support from Ministry of Earth Sciences – Centre for Marine Living Resources and Ecology (CMLRE), Government of India (Sanction Order No. MOES/10-MLR/2/2007 dt13.02.2008.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
P. Karthikeyan, Email: kaarthi.p@gmail.com.
Sarita G. Bhat, Email: saritagbhat@gmail.com.
M. Chandrasekaran, Email: chansek88@yahoo.com.
References
- Avinash M., Kumari K., Bhavanath J. Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr. Polym. 2011;83:852–857. [Google Scholar]
- Bardavid E.R., Khristo P., Oren A. Interrelationships between Dunaliella and halophilic prokaryotes in saltern crystallizer ponds. Extremophiles. 2008;12(1):5–14. doi: 10.1007/s00792-006-0053-y. [DOI] [PubMed] [Google Scholar]
- Catherine L., Mercedes M.S., Bernardo P., Alberto R.C., Jurgen W., Victoriano C. Taxonomic study of extreme halophilic archaea isolated from the “Salar de Atacama”, Chile. Syst. Appl. Microbiol. 2001;24(3):464–474. doi: 10.1078/0723-2020-00053. [DOI] [PubMed] [Google Scholar]
- Cheung J., Danna K.J., O’Connor E.M., Price L.B., Shand R.F. Isolation, sequence, and expression of the gene encoding halocin H4, a bacteriocin from the halophilic archaeon haloferax mediterranei R4. J. Bacteriol. 1997;179:548–551. doi: 10.1128/jb.179.2.548-551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine A., White-Ziegler, Suzin U., Natalie M.P., Abby L.B., Amy J.M., Sarah Y. Low temperature (236 °C) increases expression of biofilm- cold-shock- and RpoS-dependent genes in Escherichia coli K-12. Microbiology. 2008;154:148–166. doi: 10.1099/mic.0.2007/012021-0. [DOI] [PubMed] [Google Scholar]
- Feng Y., He Z., Ong S.L., Hu J., Zhang Z., Ng W.J. Optimization of agitation, aeration, and temperature conditions for maximum β-mannanase production. Enzyme Microb. Technol. 2003;32(2):282–289. 2003. [Google Scholar]
- Gonzalo P., Inmaculada M., Ricardo A. Purification and biological characterization of halocin H1 from haloferax mediterranei M2a. Int. Microbiol. 2002;5:15–19. doi: 10.1007/s10123-002-0053-4. [DOI] [PubMed] [Google Scholar]
- Hay J.X.W., Wu T.Y., Teh C.Y., Jahim J.M. Optimized growth of Rhodobacter sphaeroides O.U.001 using response surface methodology (RSM) JSIR. 2012;71(02):149–154. [Google Scholar]
- Jan B., Antonio J.P., Nathalie P., Erhard B. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J. Biol. Chem. 2007;282:31147–31155. doi: 10.1074/jbc.M704023200. [DOI] [PubMed] [Google Scholar]
- John V.B., Jean L.B., Ernest J.B., Kirby W.M.M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl. Microbiol. 1966;14(2):170. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xiang H., Liu J., Zhou M., Tan H. Purification and biological characterization of halocin C8, a novel peptide antibiotic from Halobacterium strain AS7092. Extremophiles. 2003;7(5):401–407. doi: 10.1007/s00792-003-0335-6. [DOI] [PubMed] [Google Scholar]
- McGenity T.J., Gemmell R.T., Grant W.D. Proposal of a new halobacterial genus Natrinema gen nov., with two species Natrinema pellirubrum nom.nov and Natrinema pallidum nom.nov. Int. J. Syst. Bacteriol. 1998;48:1187–1196. doi: 10.1099/00207713-48-4-1187. [DOI] [PubMed] [Google Scholar]
- Meral B., Baris C., Bulent M., Rahel E.B., Aharon O., Mehmet N.O., Ayse O. Extremely halophilic archaea from Tuz Lake, Turkey, and the adjacent Kaldirim and Kayacik salterns. World J. Microbiol. Biotechnol. 2007;23:309–316. [Google Scholar]
- Meseguer I., Rodrı´guez V.F. Production and purification of halocin H4. 1985;28(2):177–182. [Google Scholar]
- Meseguer I., Rodrı´guez V.F. Effect of halocin H4 on cells of halobacterium halobium. J. Gen. Microbiol. 1986;132:3061–3068. [Google Scholar]
- Meseguer I., Rodrı´guez V.F., Ventosa A. Antagonistic interactions among halobacteria due to halocin production. FEMS Microbiol. Lett. 1986;36(2–3):177–182. [Google Scholar]
- Meseguer I., Torreblanca M.T., Konishi Specific inhibition of the halobacterial Na+/H+ antiporter by halocin H6. J. Biol. Chem. 1995;270(12):6450–6455. doi: 10.1074/jbc.270.12.6450. [DOI] [PubMed] [Google Scholar]
- Mirko B., Junsong S., Michael W.W.A. Engineering a hyperthermophilic archaeon for temperaturedependent product formation. ASM J. 2012;3(2):1–8. [Google Scholar]
- O’Connor E.M., Shand R.F. Halocins and sulfolobicins: the emerging story of archaeal protein and peptide antibiotics. J. Ind. Microbiol. Biotechnol. 2002;28(1):23–31. doi: 10.1038/sj/jim/7000190. [DOI] [PubMed] [Google Scholar]
- Paši L., Velikonja B.H., Ulrih N.P. Optimization of the culture conditions for the production of a bacteriocin from halophilic archaeon Sech7a. Prep. Biochem. Biotechnol. 2008;38(3):229–245. doi: 10.1080/10826060802164637. [DOI] [PubMed] [Google Scholar]
- Pasic´ L., Galan B.S., Poklar U.N., Grabnar M., Herzog V.B. Diversity of halophilic archaea in the crystallizers of an adriatic solar saltern. FEMS Microbiol. Ecol. 2005;54(3):491–498. doi: 10.1016/j.femsec.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Price L.B., Shand R.F. Halocin S8: a 36-amino-acid microhalocin from the haloarchaeal strain S8a. J. Bacteriol. 2000;182:4951–4958. doi: 10.1128/jb.182.17.4951-4958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez F.V., Juez G., Kushner D.J. Halocins: salt dependent bacteriocins produced by extremely halophilic rods. Can. J. Microbiol. 1982;28(1):151–154. [Google Scholar]
- Sehgel S.N., Gibbons Effect of some metal ions on the growth of halobacterium cutirubrum. Can. J. Microbiol. 1960;6(2):165–169. doi: 10.1139/m60-018. [DOI] [PubMed] [Google Scholar]
- Shand R.F., Price L.B., O’Connor E.M. Halocins: protein antibiotics from hypersaline environments. In: Oren A., editor. Microbiology and Biogeochemistry of Hypersaline Environments. CRC Press; Boca Raton: 1999. pp. 295–306. [Google Scholar]
- Subov N.N. USSR Oceanographic Institute of Hydrometerological Commission; Moscow: 1931. Oceanographical tables. [Google Scholar]
- Sun C., Li Y., Mei S., Lu Q., Zhou L., Xiang H. A single gene directs both production and immunity of halocin C8 in a haloarchaeal strain AS7092. Mol. Microbiol. 2005;57(2):537–549. doi: 10.1111/j.1365-2958.2005.04705.x. [DOI] [PubMed] [Google Scholar]
- Tamar K.P., Aharon O. Halocins: are they involved in the competition between halobacteria in saltern ponds? Extremophiles. 2000;4(1):35–41. doi: 10.1007/s007920050005. [DOI] [PubMed] [Google Scholar]
- Torreblanca M., Rodríguez-Valera F., Juez G., Ventosa A., Kamekura M., Kates M. Classification of non-alkaliphilic halobacteria based on numerical taxonomy and polar lipid composition, and description of haloarcula gen. nov and haloferax gen. nov. syst. Appl. Microbiol. 1986;8:89–99. [Google Scholar]
- Torreblanca M., Meseguer I., Rodrı´guez F.V. Halocin H6, a bacteriocin from haloferax gibbonsii. J. Gen. Microbiol. 1989;135(10):2655–2661. [Google Scholar]
- Torreblanca M., Meseguer I., Ventosa A. Production of halocin is a practically universal feature of archaeal halophilic rods. Lett. Appl. Microbiol. 1994;19:201–205. [Google Scholar]
- Torsten S., Thomas M., Dirk B., Hauke H., Uta B. Continuous synthesis and excretion of the compatible solute ectoine by a transgenic, nonhalophilic bacterium. Appl. Environ. Microbiol. 2007;73:3343–3347. doi: 10.1128/AEM.02482-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventosa A., Quesada F., Rodrı´guez F.V., Ruiz B.Q.F., Ramos C.A. Numerical taxonomy of moderately gram negative rods. J. Gen. Microbiol. 1982;128:1959–1968. [Google Scholar]
- Xin H., Itoh T., Zhou P., Suzuki K., Kamekura M., Nakase T. Natrinema versiforme sp. nov., an extremely halophilic archaeon from Aibi salt lake, Xinjiang, China. Int. J. Syst. Evol. Microbiol. 2000;50(3):1297–1303. doi: 10.1099/00207713-50-3-1297. [DOI] [PubMed] [Google Scholar]



