Abstract
Conventional treatments for breast cancer are costly and have serious side effects. Non-conventional natural treatments have gained wide acceptance due to their promise of a cure with minimal or no side effects, but little scientific evidence exists. One such common remedy is the seed of the Lepidium sativum plant. Presented here is the first reported use of the aqueous extract of Lepidium sativum seeds on breast cancer cells. The ability of the extract to induce apoptosis and necrosis in the human breast cancer cell line MCF-7, compared to normal human skin fibroblasts (HFS), was determined by morphological changes in the cells using light microscopy, DNA fragmentation assay, and florescent stains (Annexin V and propidium iodide) using flow cytometry and fluorescent microscopy. Apoptosis was induced in both cells, and more in MCF-7, when they were treated with 25% and 50% extract, while necrosis was observed mainly after exposure to elevated extract concentrations (75%). DNA fragmentation resulted for both cells, in a time and dose-dependent manner. Both cells, at all extract concentrations, showed no significant differences in the number of living, dead, apoptotic, and necrotic cells. Finally, the results may indicate that apoptotic changes in MCF-7 may be independent of caspase-3, which is involved in apoptosis and is lacking in MCF-7 cells.
Keywords: Apoptosis, Necrosis, Human breast cancer cells, Aqueous extract, Garden cress seeds, Lepidium sativum seeds
1. Introduction
Cancer is a major cause of death worldwide with breast cancer being the most common cancer and a leading cause of death in females in many parts of the world. Both conventional and non-conventional treatments of cancer are widely used. Non-conventional treatments are attractive due to their claim to counteract the problems of conventional treatments, such as high cost and serious side effects.
Many natural dietary agents, including vegetables, fruits, herbs, and spices have been used in traditional medicines, as non-conventional treatments, for thousands of years, but without sufficient scientific proofs. If effective, natural agents might lead to the development of natural and novel drugs with low or no side effects.
Numerous epidemiological, biological and clinical studies (American Cancer Society, 2010; Amin and Mousa, 2007; Anand et al., 2008; Berquin et al., 2008; Campbell et al., 2007; Cao et al., 2010; Conforti et al., 2008, 2009) indicate a strong correlation between dietary factors and lower risk for developing cancer. Dietary factors can prevent cancers, and they, on the other hand, can induce cancers.
Many dietary agents, such as curcumin (in turmeric) and epigallocatechin gallate (in green tea), have been shown (Berquin et al., 2008; Czene et al., 2002) to cause induction of apoptosis and cell cycle arrest in many types of cancer cells without affecting normal cells. Cruciferae or Brassicaceae vegetables, such as garden cress (Lepidium sativum, [L. sativum]), and their active ingredients have been found to stimulate apoptosis in cancer cells (Das et al., 2000; Divisi et al., 2006; Diwakara et al., 2008), thereby killing cancer cells specifically without harming normal healthy cells.
The seeds of the L. sativum plant, which are used in folk remedies, have many activities including thermogenic, depurative, rubefacient, tonic, aphrodisiac, abortive, ophthalmic, diuretic, and contraceptive (Dugasani et al., 2009; Gokavi et al., 2005). They are useful as poultices for sprains and in leprosy, ophthalopathy, leucorrhoea, scurvy, seminal weakness, bronchial asthma, cough, and hemorrhoids (Gokavi et al., 2005; Güvenç et al., 1998). L. sativum seeds are recommended in the treatment of various ailments, but in therapeutic doses because of their known toxicity if used in high doses, although there is no scientific evidence.
The healing effects of natural dietary agents are partially due to the constituent phytochemicals. Many studies (Hail et al., 2008; Anand et al., 2008; Berquin et al., 2008) demonstrate and describe the various therapeutic effects of plant phytochemicals, which include the treatment and/or prevention of cancer. The most important phytochemicals (phenolic compounds, terpenoids, alkaloids, and organosulfur compounds) are all found in L. sativum seeds.
L. sativum also contains plant phytosterols and their derivatives, which have been shown (Hardman et al., 2001; Conforti et al., 2008) to possess antioxidant potential, anti-inflammatory activity, and to protect against some illnesses and cancers. Phenolic compounds, most importantly the flavonoids, may protect the human body from oxidative stress that may lead to cancer, aging, and cardiovascular diseases (Hudaib et al., 2008; Jänicke et al., 1998; Conforti et al., 2008).
The chemopreventive and anti-cancer effects of Cruciferous vegetables have also been attributed to the presence of high levels of organosulfur compounds (Diwakara et al., 2008; Johansson et al., 2003; Jourdan et al., 2007; Kaefer and Milner, 2008; Karazhiyan et al., 2009), which have been shown to exert diverse biological effects, including induction of carcinogen detoxification, inhibition of tumor cell proliferation, free radical scavenging, induction of cell cycle arrest, and induction of apoptosis.
The oil of the L. sativum seeds is rich in alpha linolenic acid, and contains an ideal ratio of ω-3 fatty acids (n-3) and ω-6 fatty acids (n-6) Kassie et al., 2002; Kassie et al., 1999. Recent studies (Kassie et al., 2002; Kassie et al., 2003; Khan et al., 2008; Matthäus and Angelini, 2005; Mc Gee et al., 2002) proved the preventive effect of ω-3 polyunsaturated fatty acids, especially alpha linolenic acid, on different types of cancer, including breast, in both animals or cell line models, and in the treatment of cancer (Matthäus and Angelini, 2005; Mc Gee et al., 2002; Merlin et al., 2010).
Glucosinolates, a class of thioglycosides, are major secondary metabolites of L. sativum leaves and seeds (Kassie et al., 2002; Miyoshi et al., 2004; Moghadasian, 2000) and have been shown to inhibit carcinogenesis and have chemopreventive effects against the development and proliferation of cancers (Diwakara et al., 2008; Pledgie-Tracy et al., 2007; Shao et al., 1995).
As presented above, some researchers have shown that certain constituents of the L. sativum plant and the alcoholic extracts of its different parts have chemopreventive and anti-cancer effects, but, to our knowledge, no studies exist on the effects of the aqueous extract of L. sativum seeds on the viability and growth of cancer cells. Therefore, the potential of L. sativum to induce death of human breast cancer cells in tissue culture is investigated here in the hope of finding a natural treatment.
2. Materials and methods
2.1. Cell lines
The human breast cancer cell line MCF-7 (Michigan Cancer Foundation–7) (National Cancer Institute, Cairo University, Cairo, Egypt), is an epithelial invasive breast ducal carcinoma cell line, which is estrogen and progesterone receptor positive. Normal human skin fibroblasts (HFS) were acquired from foreskin circumcision operations, at the King Abdulaziz University Hospital, Jeddah, Kingdom of Saudi Arabia (KSA).
Both MCF-7 and HFS were cultivated in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (both GIBCO, Grand Island, NY, USA), 1% l-glutamine, 1% penicillin, and streptomycin and incubated in 5% CO2 atmosphere, at 37 °C, and 96% relative humidity.
2.2. Preparation of the aqueous extract
L. sativum seeds were obtained locally and they were powdered using an electric grinder. The extract was prepared by dissolving 1 g of L. sativum seed powder in 99.87 ml of deionized water and adding 0.14 ml of DMSO, thereby leading to a 0.1% DMSO for the highest used concentration of extract (75%). Several dilutions of the extract in serum-free medium (SFM) were prepared [extract:SFM (vol/vol); 1:3 (25% of extract), 1:1 (50%), and 3:1 (75%)], and were stored at −80 °C.
2.3. Determination of cells’ morphology using light microscopy
To determine the effects of L. sativum seed extract on the morphology of the cells, both MCF-7 and HFS cells were suspended in DMEM containing 10% FBS and then seeded on a cover slip placed in a Petri dish. After 24 h, the original DMEM was replaced with 5 ml of SFM containing one of the different concentrations of L. sativum seed extract (25%, 50%, or 75%), while the control contained only 0.1% DMSO in SFM. After 48 h, the medium was discarded, and the cells were then stained with Coomassie blue. Finally, the cells were observed and photographed on an Eclipse E400 light microscope (Nikon, Tokyo, Japan) attached to a Nikon F-601 camera (Nikon, Tokyo, Japan).
2.4. DNA extraction, purification, and electrophoresis
Both MCF-7 and HFS cells (1.5 × 105 cell/ml) were suspended in DMEM containing 10% FBS, and subsequently cultivated on 6-well plates at 5 ml/well. After 24 h, the original DMEM was replaced with 5 ml of SFM containing a concentration (25%, 50%, or 75%) of L. sativum seed extract. The control used was as described above. The cells were treated in a time- or dose-dependent manner. One group of both cells was exposed to 25%, 50%, or 75% extract and incubated for 72 h, while another group of both cells was treated only with 50% of extract and incubated for 24, 48, or 72 h.
The cells were harvested by adding 1 ml/well of trypsin to separate adherent cells and then 3 ml/well of SFM to stop the effect of trypsin and harvest the cells. The cells were subsequently centrifuged at 1500 rpm for 10 min. Each pellet was washed with PBS and then centrifuged, as above, and the process was repeated. The resultant pellets were stored at −20 °C until the time of DNA extraction.
DNA from both types of cells was extracted and purified using a DNA extraction kit (Qiagen, Chatsworth, CA, USA) according to the manufacturer’s protocol. The resultant purified DNA was stored at −20 °C until DNA electrophoresis.
Isolated DNA samples were subjected to electrophoresis on a 0.8% agarose gel for 45 min at 100 volt. The gel was visualized under UV light following ethidium bromide staining to determine DNA fragmentation.
2.5. Quantitative determination of apoptosis and necrosis using flow cytometry
The two major types of cell death, necrosis and apoptosis, produced by the effects of the extract on MCF-7 and HFS cells were detected by the CF488A-Annexin V and Propidium Iodide kit (Biotium, Inc, Hayward, CA, USA) by using flow cytometry and fluorescent microscopy (below).
MCF-7 and HFS cells (2 × 105 cell/ml) were cultivated in DMEM containing 10% FBS, at 10 ml per Petri dish. Upon formation of a monolayer of cells, 10 ml of a concentration (25%, 50%, or 75%) of L. sativum seed extract were added. As above, the control was SFM containing 0.1% DMSO. After 24 h of incubation, cells were harvested by the addition of trypsin, centrifuged for 5 min at 1000x, and finally washed with PBS. Cells were stained according to the kit’s protocol, and were analyzed by a BD FACSCanto ІІ Flow Cytometer (BD Biosciences, San Jose, CA, USA), at the National Guards Hospital, Jeddah, KSA. The determinations were performed in duplicates.
2.6. Qualitative determination of apoptosis and necrosis using fluorescent microscopy
MCF-7 (1 × 105 cell/ml) and HFS (1.5 × 105 cell/ml) cells in DMEM containing 10% FBS were added into the wells of 96-well microtiter plates, at 100 μl/well. After 24 h, the original medium was replaced with 100 μl of SFM containing different concentrations (25%, 50% or 75%) of L. sativum seed extract, in addition to the usual control, and allowed to incubate for 24 h. Cells were then washed with PBS and then stained with annexin V and PI according to the instructions of the kit used (above). Finally the stained cells were observed and photographed under an Eclipse 50i Nikon fluorescent microscope (Nikon, Tokyo, Japan) equipped with a DS-Fil digital camera (Nikon, Tokyo, Japan).
2.7. Statistical analysis
Statistical comparisons of the percentages of living and dead cells were performed using the three-way ANOVA test followed by the Dunnett’s multiple comparisons test. For the differences in the results, the P value was used to determine statistical significance. A difference with a P-value <0.05 was considered statistically significant.
3. Results
3.1. The effects of the extract on the morphology of the cells
Upon the incubation of cells with increasing concentrations of extract, for 48 h, apoptotic cells were more frequently seen. The most common apoptotic morphological changes observed in both cells included chromatin condensation, cytoplasm shrinkage, and loss of normal shape followed by breaking up of the nucleus into discrete fragments by budding of the cell as a whole to produce membrane-bound apoptotic bodies.
Figure 1A shows that an MCF-7 cell treated with 0.1% DMSO retains its normal angular or polygonal shape. Most cells have intact and large vesicular nuclei with prominent nucleoli. HFS cells treated with 0.1% DMSO show (Figure 2A) a normal spindle shape and intact large oval, and vesicular nuclei with two prominent nucleoli. MCF-7 (Figure 1B) and HFS (Figure 2B) cells treated with 25% extract show loss of the normal sheet-like growth and most of the remaining few cells had shrunken cytoplasm, condensed chromatin and loss of normal shape.
Figure 1.
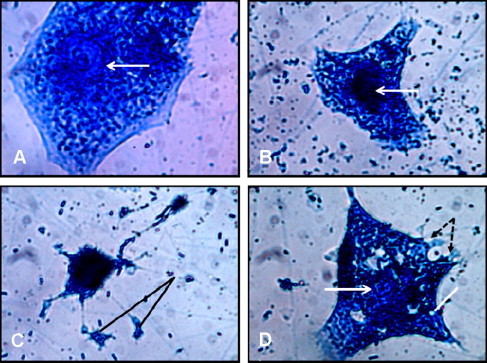
Effects of L. sativum seed extract on the morphology of MCF-7 cells after 48 h of treatment. The cells were examined under an Eclipse E400 light microscope using a 100 × objective lens. (A) Shows an MCF-7 cell treated with 0.1% DMSO only. The cell has normal angular or polygonal shape with intact and large vesicular nucleus and prominent nucleolus (arrow). (B) Represents an MCF-7 cell treated with 25% extract that has lost its normal shape and shows a shrunken cytoplasm and condensed chromatin (arrow). (C) Represents an MCF-7 cell treated with 50% extract that shows evident signs of apoptosis including cytoplasm and chromatin condensation, and fragmentation and formation of apoptotic bodies (lines). (D) Represents an MCF-7 cell treated with 75% extract that show signs of necrosis. The cell has more or less a normal polygonal shape with a poorly defined nucleus (arrow), clear vesicles in the cytoplasm (line) and disrupted cell membrane with leaking cell contents (dashed arrows).
Figure 2.
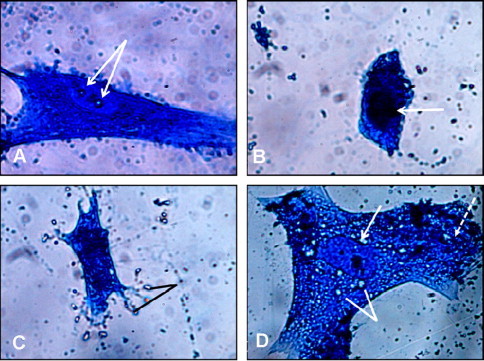
Effects of L. sativum seed extract on the morphology of HFS cells after 48 h of treatment. The cells were examined under Eclipse an E400 light microscope using a 100 × objective lens. (A) Represents an HFS cell treated with 0.1% DMSO that shows a normal spindle shape and intact large, oval, and vesicular nucleus with two prominent nucleoli (arrows). (B) Represents an HFS cell treated with 25% extract that lost its normal shape and shows a shrunken cytoplasm and condensed chromatin (arrow). (C) Represents an HFS cell treated with 50% extract with evident signs of apoptosis (cytoplasm and chromatin condensation and fragmentation) and formation of apoptotic bodies (lines). (D) Represents an HFS cell treated with 75% extract that shows signs of necrosis, including clear nucleus (arrow), clear vesicles in the cytoplasm (lines), and disrupted cell membrane with leakage of cell contents (dashed arrow).
MCF-7 (Figure 1C) and HFS (Figure 2C) cells treated with 50% extract showed a significant loss of cell processes and marked changes in morphology associated with late stage of apoptosis, such as shrinkage, irregular shape, and condensed and fragmented chromatin and cytoplasm, to produce apoptotic bodies. A marked and visible increase in the number of necrotic cells was observed. Necrotic MCF-7 and HFS cells (Figures 1 and 2D, respectively) appeared after incubation with 75% extract and showed clear cytoplasmic vacuoles, disrupted membrane and leakage of cellular contents.
3.2. Chromosomal DNA fragmentation
DNA fragmentation is a hallmark feature of apoptosis. Both MCF-7 (Figure 3A) and HFS (Figure 3B) cells treated with extract in a time- or dose-dependant manner induced a smear pattern of DNA fragmentation with both high molecular weight DNA and smaller DNA fragments compared with the used DNA ladder that extends from 20 kbp to 75 bp. The control for each cell type, treated with 0.1% DMSO for 72 h, showed clear bands of intact DNA and the smear pattern of damaged DNA.
Figure 3.
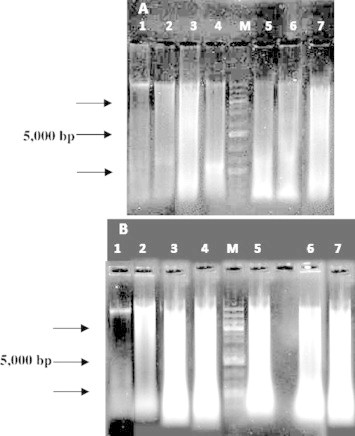
Electrophoresis of DNA extracted from MCF-7 (A) and HFS (B) cells, after treatment with extract at different concentrations and incubation times. Lane 1: DNA from cells of the control, lane 2: DNA treated with 25% extract for 72 h, lane 3: DNA treated with 50% extract for 72 h, lane 4: DNA treated with 75% extract for 72 h, M: 1 kbp DNA marker, lane 5: DNA treated with 50% extract for 24 h, Lane 6: DNA treated with 50% extract for 48 h, and Lane 7: DNA treated with 50% extract for 72 h.
3.3. Determination of apoptosis and necrosis using flow cytometry
Using the three-way ANOVA followed by factorial design, flow cytometry revealed (Table 1) no significant differences between the percentages of living, dead, early and late apoptotic, and necrotic cells for MCF-7 and HFS cells treated with 25%, 50%, or 75% extract compared with the respective (untreated) controls. The majority (∼90%) of detached cells remained unstained, with intact membrane, and no phosphatidylserine translocation suggesting that they were living cells. Flow cytometry also revealed (Table 2) no significant differences between the percentages of both apoptotic (late and early) and necrotic cells for each extract concentration used for the two cell lines.
Table 1.
The percentages of the different types of cell death induced by the extract in both MCF-7 and HFS cells stained with annexin V/propidium iodide as observed by flow cytometry.
| Cell type Concentration | MCF-7 |
HFS |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | 25% | 50% | 75% | Control | 25% | 50% | 75% | |
| % living cells | 89.45 | 91.4 | 92 | 89.9 | 93.2 | 92.5 | 92.9 | 93.8 |
| P | 0.81 | 0.69 | 1 | 0.75 | 0.95 | 0.82 | ||
| % all dead cells | 10.55 | 8.7 | 8.1 | 10.2 | 6.75 | 8.8 | 7.15 | 8.15 |
| P | 0.83 | 0.7 | 1 | 0.71 | 0.94 | 0.83 | ||
| % early apoptotic cells | 2.95 | 3.1 | 1.1 | 2.3 | 1.4 | 2.5 | 0.8 | 1.4 |
| P | 1 | 0.7 | 0.97 | 0.97 | 0.47 | 0.38 | ||
| % late apoptotic cells | 0.9 | 1.35 | 1.05 | 1.95 | 1.85 | 2.2 | 1.5 | 2.6 |
| P | 0.9 | 1 | 0.51 | 0.74 | 0.74 | 0.48 | ||
| % necrotic cells | 6.7 | 4.25 | 5.95 | 5.9 | 3.5 | 4.1 | 4.85 | 4.15 |
| P | 0.21 | 0.85 | 0.83 | 0.89 | 0.5 | 0.86 | ||
Insignificant differences between each concentration and the control for each cell group, where P > 0.05 by using Dunnett’s test (<control).
Table 2.
The percentages of both apoptotic (late and early) and necrotic cells induced by the extract in both MCF-7 and HFS cells stained with annexin V/propidium iodide as observed by flow cytometry.
| Cell type Concentration | MCF-7 |
HFS |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | 25% | 50% | 75% | Control | 25% | 50% | 75% | |
| % apoptotic cells | 0.9 | 1.35 | 1.05 | 1.95 | 1.85 | 2.2 | 1.5 | 2.6 |
| % necrotic cells | 6.7 | 4.25 | 5.95 | 5.9 | 3.5 | 4.1 | 4.85 | 4.15 |
| P | 0.445 | 0.917 | 0.508 | 0.381 | 0.728 | 0.621 | 0.186 | 0.177 |
Insignificant differences were observed between apoptotic and necrotic cells for each concentration, where P > 0.05 by using Dunnett’s test (two-sided).
3.4. Determination of apoptosis and necrosis using fluorescence microscopy
Annexin V is a protein that is conjugated to a green florescent dye to detect apoptosis. Propidium iodide (PI) is a red fluorescent dye that stains DNA of both necrotic and late apoptotic cells with damaged membranes.
Fluorescence microscopy was used to provide qualitative identification of both apoptotic and necrotic deaths of both MCF-7 and HFS cells treated for 24 h with different concentrations (25%, 50% or 75%) of extract (Figure 4). Both MCF-7 and HFS cells that were treated with 0.1% DMSO (Figures 4 and 5A1; and Figures 4 and 5B1, respectively) were viable and negative to annexin V and PI. A few of both cell types treated with 25% of extract were positive to annexin V and PI, indicating the presence of early and late apoptotic cells (Figure 4A2 and B2). A high number of both cells treated with 50% of extract (Figure 4A3 and B3; and Figure 5A2 and B2) were positive to annexin V and PI, indicating the presence of early and late apoptotic cells with few necrotic cells. The effect of 50% of extract was weaker on HFS compared with MCF-7 cells (Figure 4A3 and B3, respectively), but the living HFS cells had abnormal morphology. The effect of 75% of extract (Figure 4A4 and B4) was stronger on both cells, with no living cells and only a few dead cells were observed, and most of these cells were early or late apoptotic cells and necrotic cells.
Figure 4.
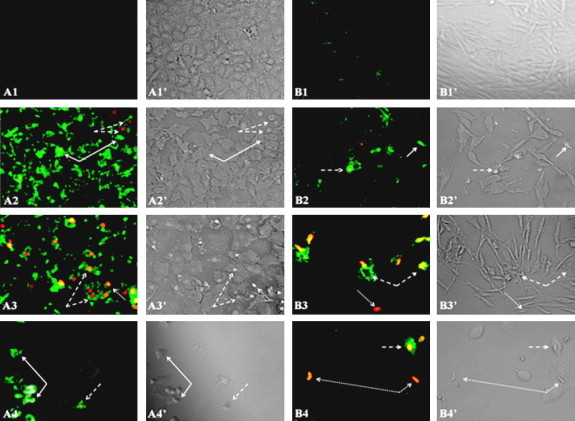
The extract induced apoptosis and necrosis in both MCF-7 and HFS cells after 24 h of incubation. The cells were examined under a Nikon Eclipse 50i Nikon microscope using a 20 × objective lens. Viable cells do not take any color (Annexin V−/PI−), early apoptotic cells (Annexin V+/PI−) are green, late apoptotic cells (Annexin V+/PI+) are green and orange, and necrotic cells (Annexin V−/PI+) are orange. (A1–A4) represent MCF-7 cells and (B1–B4) represent HFS cells that were treated with extract (control, 25%, 50%, and 75% extract, respectively). (A1–A4 and B1–B4) represent fluorescence images and (A1′–A4′ and B1′–B4′) correspond to interference contrast images. Continuous arrows indicate early apoptotic cells, dashed arrows indicate late apoptotic cells, and dotted arrows indicate necrotic cells.
Figure 5.
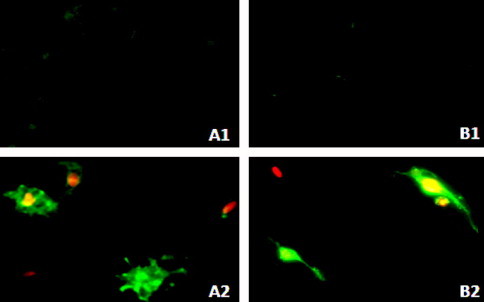
Fluorescent staining of early and late apoptotic cells and necrotic cells of both MCF-7 and HFS cells. (A1 and B1) represent MCF-7 and HFS cells, respectively, treated with 0.1% DMSO only (controls). (A2 and B2) represent MCF-7 and HFS cells, respectively, treated with 50% of extract. Early apoptotic cells (Annexin V+/PI−) are green, late apoptotic cells (Annexin V+/PI+) are green and orange, and necrotic cells (Annexin V−/PI+) are orange.
4. Discussion
When cells become old or damaged, they die by apoptosis, necrosis or a combination of the two and are replaced with new cells. On the other hand, cancer cells are immortal since they are resistant to apoptosis. Chemotherapy kills cancer cells through apoptosis and/or necrosis.
The induction of apoptosis and necrosis in MCF-7 and HFS cells by the aqueous extract of L. sativum seeds was monitored by analysis of morphological changes in the cells using light microscopy, DNA fragmentation assay, and florescent stains (Annexin V and PI) using flow cytometry and fluorescent microscopy. Results demonstrated the occurrence of both types of cell death in MCF-7 and HFS cells following addition of the extract to the culture medium. Apoptosis was induced when cells were treated with 25% and 50% extract, but necrosis was observed mainly after the cells were exposed to elevated concentrations (75%) of extract.
Under light microscopy, the normal angular and spindle shapes of MCF-7 and HFS cells, respectively, treated with 0.1% DMSO, were observed, but they were lost after treatment with the extract. The number of dead cells by apoptosis and necrosis increased with increasing concentrations of extract.
Apoptotic cells with their unique morphology, including cell shrinkage, chromatin condensation and finally nuclear and cytoplasm condensation and fragmentation and formation of apoptotic bodies, were observed after 48 h of exposure to the different concentrations of the extract. At 75% concentration, the majority of cells die and cells with apoptotic and necrotic morphology were observed. This is in contrast to the 25% and 50% concentrations, where apoptosis was the main type of cell death observed.
It is interesting to note that MCF-7 cells are one of the breast cancers that are known to be resistant to currently used chemotherapeutics due to deletion in the CASP-3 gene that leads to an inherited deficiency of caspase-3. Caspase-3 is commonly activated by numerous death signals and cleaves a variety of important cellular proteins. It is responsible for DNA fragmentation and some of the distinct morphological features of apoptotic cells such as shrinkage and budding (Shapiro et al., 2001; Sharief and Gani, 2004).
The results of light microscopy to determine cell morphology proved that apoptotic changes were independent of caspase-3, which is lacking in MCF-7 cells. From these results one can suggest that apoptosis induced by L. sativum seed extract does not correlate with the activation of caspase-3 but may activate different apoptotic pathways and other effector caspases such as caspase-6 or -7 (Shapiro et al., 2001; Shoieb et al., 2003; Shukla and Singh, 2007).
Therefore, there must be other chemical agents in the extract that induce apoptosis and exert the hallmark features of apoptosis independent of the activation of caspase 3. Another researcher (Shoieb et al., 2003) described typical apoptotic morphological changes in MCF-7 cells treated with the chemical compound pyrrolo-1, 5-benzoxazepine (PBOX-6) in a dose- and time-dependent manner. MCF-7 cells also exhibited apoptotic morphological changes when treated with chloroform extract of Gmelina asiatica (Shukla and Singh, 2007). HFS exposed to staurosporine for 8 h was described by Johansson et al. (Smith et al, 2004) to show typical signs of apoptosis as demonstrated by using light microscopy and electron microscopy.
A typical DNA ladder pattern was not evident in either MCF-7 or HFS cells treated with L. sativum seed extract in a time- or dose-dependent manner. This may indicate that the cells die by apoptosis and/or necrosis, where DNA damage is not a unique feature of apoptosis but can also occur in necrosis (Tisdale and Dhesi, 1990). DNA extracted from apoptotic cells often show a ladder pattern and the presence of a smear pattern may indicate that the apoptotic cells enter into late apoptosis (secondary necrosis) because of the absence of phagocytosis to remove cell remnants (van Breda et al., 2008). Or that some cell lines, such as MCF-7, can undergo apoptosis without showing DNA fragmentation due to lack of caspase-3 which is responsible for this feature (Shapiro et al., 2001; Sharief and Gani, 2004). Another explanation may be that the long incubation time of DNA led to lyses of a substantial part of the cell population. The smear pattern was also observed in the controls for both cell types that were treated with 0.1% DMSO for 72 h, which confirms the previous explanation. The DNAs isolated from the controls of both cell types exhibited one clear band (for each cell types) that pointed to the presence of living cells with intact DNA strand.
DNA fragmentation was induced (Karazhiyan et al., 2009) in four human breast cancer cell lines (MDA-MB-231, MDA-MB-468, MCF-7, and T47D cells) after treatment with sulforaphane, an isothiocyanate found in Cruciferous vegetables, for 96 h. DNA fragmentation was also detected in Jurkat cells exposed to BITC at different concentrations for 20 h, but not detected at a high concentration (Warrier et al., 1996).
Detecting apoptosis and/or necrosis by flow cytometry and fluorescent microscopy after staining with fluorescent dyes, both MCF-7 and HFS cells treated with different concentrations of L. sativum seed extract (25%, 50%, or 75%) for 24 h exhibited both apoptotic and necrotic changes.
Flow cytometry showed that the number of dead MCF-7 cells was not significantly different from that found for HFS cells. The apoptotic and necrotic cells were observed in both types of cells treated with different concentrations of L. sativum seed extract. There were no significant changes in the percentages of different types of dead cells (early and late apoptosis and necrosis) for both MCF-7 and HFS treated with different concentrations of extract, compared with the control cells.
Under fluorescent microscopy, after 24 h of extract exposure, both types of control cells (0.1% DMSO) were viable and did not undergo apoptosis or necrosis. Apoptotic cells decreased and necrotic cells increased with exposure to increasing concentrations of L. sativum seed extract. Early and late apoptotic cells were detected at 25% extract concentration, and early and late apoptotic cells and necrotic cells were observed at 50% concentration. MCF-7 cells were more affected by the extract than HFS at 25% and 50%. For both cell types, a fewer number of cells were observed after treatment with 75% concentration due to the strong effect of this concentration, and most remaining cells were apoptotic or necrotic cells.
Similar results were reported in a study (Wojdyło et al., 2007), using flow cytometry, of HT-29 colorectal cells treated with allyl isothiocyanate, an isothiocyanate found in Cruciferous vegetables, for 24 h, which showed no signs of apoptosis, where the percentage of both apoptotic and necrotic cells in untreated control cells was significantly higher than the cells treated with allyl isothiocyanate. Allyl isothiocyanate increased the percentage of apoptotic cells in a concentration-dependent manner (after 24 h) in both prostate PC-3 and LNCaP cells in comparison with control treated with DMSO (Xiao et al., 2003). A significant increase in the percentage of apoptosis was observed in the prostate cancer cells (Capan-2) that were treated for 24 h with BITC in comparison with control. Flow cytometry and fluorescent microscopy of human brain cancer SHG-44 cell line treated with the water extract of Chinese medicine “Pingliu Keli” (which is composed of nine plants) for 24 h, after being stained with annexin V and PI, showed both apoptotic and necrotic cells that increased with increasing concentrations of extract (Xiao et al., 2006). Fluorescent microscopy indicated (Zhang et al., 2006) that the methanol extract of adenocalymma alliaceum flowers induce time-dependent apoptosis in both MCF-7 and MDA-MB-231 breast cancer cells treated for 6 and 12 h, with less prominent necrosis.
In conclusion, L. Satvium seed extract was equally, and in some experiments more, effective against MCF-7 cells compared to HFS cells. In general, the highest (75%) dose of extract was cytotoxic for both MCF-7 and HFS cells in most assays.
It is recommended that further work be done using the lowest concentration of the extract on MCF-7 and other types of breast cancers and for longer incubation periods. This might help reduce or eliminate the toxic and damaging effects of the high concentrations of the extract on the healthy cells while, at the same time, possibly getting better effects on the cancer cells due to the extended period of incubation. Another area of further research is to work on the active ingredients of the extract with different types of cancer.
Funding
This study was funded by the King Abdulaziz City for Science and Technology.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sawsan Hassan Mahassni, Email: sawsanmahassni@hotmail.com.
Roaa Mahdi Al-Reemi, Email: roaa_bioc@hotmail.com.
References
- American Cancer Society . American Cancer Society, Inc.; GA: 2010. Cancer Facts & Figures 2010. [Google Scholar]
- Amin A., Mousa M. Merits of anti-cancer plants from the Arabian Gulf region. Cancer Therapy. 2007;5:55–66. [Google Scholar]
- Anand P., Sundaram C., Jhurani S., Kunnumakkara A.B., Aggarwal B.B. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Letters. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Berquin I.M., Edwards I.J., Chen Y.Q. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Letters. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C.T., Prince M., Landry G.M., Kha V., Kleiner H.E. Pro-apoptotic effects of 1′-acetoxychavicol acetate in human breast carcinoma cells. Toxicology Letters. 2007;173:151–160. doi: 10.1016/j.toxlet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Cao P., Cai X., Lu W., Zhou F., Huo J. Growth inhibition and induction of apoptosis in SHG-44 glioma cells by Chinese medicine formula “Pingliu Keli”. Evidence-Based Complementary and Alternative Medicine. 2010;2011:1–9. doi: 10.1155/2011/958243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F., Ioele G., Statti G.A., Marrelli M., Ragno G., Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food and Chemical Toxicology. 2008;46:3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Conforti F., Sosa S., Marrelli M., Menichini F., Statti G.A., Uzunov D., Tubaro A., Menichini F. The protective ability of Mediterranean dietary plants against the oxidative damage: the role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chemistry. 2009;112:587–594. [Google Scholar]
- Czene S., Testa E., Nygren J., Belyaev I., Harms-Ringdahl M. DNA fragmentation and morphological changes in apoptotic human lymphocytes. Biochemical and Biophysical Research Communications. 2002;294:872–878. doi: 10.1016/S0006-291X(02)00588-0. [DOI] [PubMed] [Google Scholar]
- Das S., Tyagi A.K., Kaur H. Cancer modulation by glucosinolates: a review. Current science. 2000;79:1665–1671. [Google Scholar]
- Divisi D., Tommaso S.D., Salvemini S., Garramone M., Crisci R. Diet and cancer. Acta Biomedica. 2006;77:118–123. [PubMed] [Google Scholar]
- Diwakara B.T., Duttaa P.K., Lokeshb B.R., Naidu K.A. Bio-availability and metabolism of n-3 fatty acid rich garden cress (Lepidium sativum) seed oil in albino rats. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;78:123–130. doi: 10.1016/j.plefa.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Dugasani S.L., Balijepalli M.K., Mallikarjuna Rao P. Growth inhibition and induction of apoptosis in estrogen receptor-positive and negative human breast carcinoma cells by Adenocalymma alliaceum flowers. Current Trends in Biotechnology and Pharmacy. 2009;3:1–12. [Google Scholar]
- Gokavi S.S., Malleshi N.G., Guo M. Chemical composition of garden cress (Lepidium sativum) seeds and its fractions and use of bran as a functional ingredient. Plant Foods for Human Nutrition (Formerly Qualitas Plantarum) 2005;59:105–111. doi: 10.1007/s11130-004-4308-4. [DOI] [PubMed] [Google Scholar]
- Güvenç A., Okada Y., Akkol E.K., Duman H., Okuyama T., Çalış İ. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Journal of Ethnopharmacology. 1998;61:161–166. [Google Scholar]
- Hail N., Cortes M., Drake E.N., Spallholz J.E. Cancer chemoprevention: a radical perspective. Free Radical Biology and Medicine. 2008;45:97–110. doi: 10.1016/j.freeradbiomed.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Hardman W.E., Avula C.P.R., Fernandes G., Cameron I.L. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clinical Cancer Research. 2001;7:2041–2049. [PubMed] [Google Scholar]
- Hudaib M., Mohammad M., Bustanji Y., Tayyem R., Yousef M., Abuirjeie M., Aburjai T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib nature reserve and surrounding area. Journal of Ethnopharmacology. 2008;120:63–71. doi: 10.1016/j.jep.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Jänicke R.U., Sprengart M.L., Wati M.R., Porter A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. The Journal of Biological Chemistry. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Johansson A.-C., Steen H., Öllinger K., Roberg K. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death and Differentiation. 2003;10:1253–1259. doi: 10.1038/sj.cdd.4401290. [DOI] [PubMed] [Google Scholar]
- Jourdan M.L., Maheo K., Barascu A., Goupille C., Latour M.P.D., Bougnoux P., Rio P.G. Increased BRCA1 protein in mammary tumours of rats fed marine omega-3 fatty acids. Oncology Reports. 2007;17:713–719. [PubMed] [Google Scholar]
- Kaefer C.M., Milner J.A. The role of herbs and spices in cancer prevention. Journal of Nutritional Biochemistry. 2008;19:347–361. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karazhiyan H., Razavi S.M.A., Phillips G.O., Fang Y., Al-Assaf S., Nishinari K., Farhoosh R. Rheological properties of Lepidium sativum seed extract as a function of concentration, temperature and time. Food Hydrocolloids. 2009;23:2062–2068. [Google Scholar]
- Kassie F., Pool-Zobel B., Parzefall W., Knasmüller S. Genotoxic effects of benzyl isothiocyanate, a natural chemopreventive agent. Mutagenesis. 1999;14:595–604. doi: 10.1093/mutage/14.6.595. [DOI] [PubMed] [Google Scholar]
- Kassie F., Laky B., Gminski R., Mersch-Sundermann V., Scharf G., Lhoste E., Kansmüller S. Effects of garden and water cress juices and their constituents, benzyl and phenethyl isothiocyanates, towards benzo(a)pyrene-induced DNA damage: a model study with the single cell gel electrophoresis/Hep G2 assay. Chemico-Biological Interactions. 2002;142:285–296. doi: 10.1016/s0009-2797(02)00123-0. [DOI] [PubMed] [Google Scholar]
- Kassie F., Rabot S., Uhl M., Huber W., Qin H.M., Helma C., Schulte-Hermann R., Knasmüller S. Chemoprotective effects of garden cress (Lepidium sativum) and its constituents towards 2-amino-3-methyl-imidazo[4,5-f]quinoline (IQ)-induced genotoxic effects and colonic preneoplastic lesions. Carcinogenesis. 2002;23:1155–1161. doi: 10.1093/carcin/23.7.1155. [DOI] [PubMed] [Google Scholar]
- Kassie F., Uhl M., Rabot S., Grasl-Kraupp B., Verkerk R., Kundi M., Chabicovsky M., Schulte-Hermann R., Knasmüller1 S. Chemoprevention of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced colonic and hepatic preneoplastic lesions in the F344 rat by cruciferous vegetables administered simultaneously with the carcinogen. Carcinogenesis. 2003;24:255–261. doi: 10.1093/carcin/24.2.255. [DOI] [PubMed] [Google Scholar]
- Khan N., Adhami V.M., Mukhtar H. Apoptosis by dietary agents for prevention and treatment of cancer. Biochemical Pharmacology. 2008;76:1229–1333. doi: 10.1016/j.bcp.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthäus B., Angelini L.G. Anti-nutritive constituents in oilseed crops from Italy. Industrial Crops and Products. 2005;21:89–99. [Google Scholar]
- Mc Gee M.M., Hyland E., Campiani G., Ramunno A., Nacci V., Zisterer D.M. Caspase-3 is not essential for DNA fragmentation in MCF-7 cells during apoptosis induced by the pyrrolo-1,5-benzoxazepine, PBOX-6. Federation of European Biochemical Societies. 2002;515:66–70. doi: 10.1016/s0014-5793(02)02440-7. [DOI] [PubMed] [Google Scholar]
- Merlin N.J., Parthasarathy V., Santhoshkumar T.R. Induction of apoptosis in human breast cancer cell line MCF-7 by phytochemicals from Gmelina asiatica. African Journal of Biotechnology. 2010;9:4451–4456. [Google Scholar]
- Miyoshi N., Uchida K., Osawa T., Nakamura Y. A link between benzyl isothiocyanate-induced cell cycle arrest and apoptosis: involvement of mitogen-activated protein kinases in the Bcl-2 phosphorylation. Cancer Research. 2004;64:2134–2142. doi: 10.1158/0008-5472.can-03-2296. [DOI] [PubMed] [Google Scholar]
- Moghadasian M.H. Pharmacological properties of plant sterols: in vivo and in vitro observations. Life Sciences. 2000;67:605–615. doi: 10.1016/s0024-3205(00)00665-2. [DOI] [PubMed] [Google Scholar]
- Pledgie-Tracy A., Sobolewski M.D., Davidson N.E. Sulforaphane induces cell type–specific apoptosis in human breast cancer cell lines. Molecular Cancer Therapeutics. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- Shao Y., Pardini L., Pardini R.S. Dietary menhaden oil enhances mitomycin C antitumor activity toward human mammary carcinoma MX-1. Lipids. 1995;30:1035–1045. doi: 10.1007/BF02536289. [DOI] [PubMed] [Google Scholar]
- Shapiro T.A., Fahey J.W., Wade K.L., Stephenson K.K., Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts. Cancer Epidemiology Biomarkers and Prevention. 2001;10:501–508. [PubMed] [Google Scholar]
- Sharief M., Gani Z.H. Garden cress (Lepidium sativum) seeds as oral contraceptive plant in mice. Saudi Medical Journal. 2004;25:965–966. [PubMed] [Google Scholar]
- Shoieb A.M., Elgayyar M., Dudrick P.S., Bell J.L., Tithof P.K. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. International Journal Of Oncology. 2003;22:107–113. [PubMed] [Google Scholar]
- Shukla Y., Singh M. Cancer preventive properties of ginger: a brief review. Food and Chemical Toxicology. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Smith T.K., Lund1 E.K., Parker M.L., Clarke R.G., Johnson I.T. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis. 2004;25:1409–1415. doi: 10.1093/carcin/bgh149. [DOI] [PubMed] [Google Scholar]
- Tisdale M.J., Dhesi J.K. Inhibition of weight loss by u-3 fatty acids in an experimental cachexia model. Cancer Research. 1990;50:5022–5026. [PubMed] [Google Scholar]
- van Breda S.G.J., de Kok T.M.C.M., van Delft J.H.M. Mechanisms of colorectal and lung cancer prevention by vegetables: a genomic approach. The Journal of Nutritional Biochemistry. 2008;19:139–157. doi: 10.1016/j.jnutbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Warrier P.K., Nambiar V.P.K., Ramankutty C., Nair R.V. Indian Medicinal Plants: A Compendium of 500 Species. In: Varier N.V.K., editor. Orient Blackswan; Kottakkal: 1996. [Google Scholar]
- Wojdyło A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry. 2007;105:940–949. [Google Scholar]
- Xiao D., Srivastava S.K., Lew K.L., Zeng Y., Hershberger P., Johnson C.S., Trump D.L., Singh S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24 doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- Xiao D., Vogel V., Singh S.V. Benzyl isothiocyanate–induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Molecular Cancer Therapeutics. 2006;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- Zhang R., Loganathan S., Humphreys I., Srivastava S.K. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. The Journal Of Nutrition. 2006;136:2728–2734. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]



