Abstract
In the present study, in vitro anticariogenic potential of ethyl acetate, hexane and methanol and aqueous extracts of plant leaves of Eucalyptus globules Labill. were evaluated by using four cariogenic bacteria, Lactobacillus acidophilus, Lactobacillus casei, Staphylococcus aureus and Streptococcus mutans. Agar well diffusion method and minimum inhibitory concentration (MIC) were used for this purpose. The ethyl acetate extracted fraction of plant leaves showed good inhibitory effects against all selected bacteria. In Eucalyptus globules, hexane and ethyl acetate extracts found highly effective against, Lactobacillus acidophilus with MIC value of 0.031 and 0.062 mg/mL, respectively. Qualitative phytochemical investigation of above extracts showed the presence of alkaloids, phenolic compounds, steroids, cardiac glycosides and terpenes. Based on the MIC value and bioautography, ethyl acetate of plant leaf was selected for further study. Further investigation on the structure elucidation of the bioactive compound using IR, GC-MS and NMR techniques revealed the presence of alpha-farnesene, a sesquiterpene. Eucalyptus globules plant leaf extracts have great potential as anticariogenic agents that may be useful in the treatment of oral disease.
Keywords: Eucalyptus globules, Anticariogenic, Phytochemistry, Bioautography, α-Farnesene
1. Introduction
Dental caries is a multifactorial human disease that has widely affected many populations all over the world. According to WHO report on dental disorders, dental caries affects about 60% of the world adult population (Petersen, 2003). Until fairly recently, it was considered that early childhood caries, a particularly rampant form of caries manifested in young children, had a different etiology. There are mainly many different types of bacteria involved in dental caries process. Plaque is found preferentially at protected and stagnant surfaces, and these are at the greatest risk of disease (Scheie, 1994). Two major groups of bacteria produce such acids, namely, the mutans streptococci (including Streptococcus mutans and Streptococcus sobrinus) and the lactobacilli species (Loesche, 1986; Leverett et al., 1993). Streptococcus mutans are the primary species associated with the early dental caries process (Loesche, 1986). There are undoubtedly other acidogenic organisms involved in dental caries. However, it is now obvious that the same bacteria are involved, but the reasons for the rapid progression of the disease in these children are still uncertain (Alaluusua et al., 1987; Caufield et al., 1993).
There are an overwhelming number of studies on the biological activities of plants and their natural product derivatives (Hebber et al, 2004; Cowan, 1999). It is well known to add to toothpastes and mouth rinses an extract of Chamomille folia in a dosage of 0.1-2.0% by weight (Kitagaki et al., 1983). This extract is anti-bacterial on micro biota of the mouth and can therefore be used against certain inflammations in the mouth. The increasing resistance to available antimicrobials has attracted the attention of the scientific community regarding a search for new cost-effective drugs of natural or synthetic origin (Pai et al., 2004; Fine et al, 2000). The Eucalyptus is used to control several diseases derived from microbial infections.
A Eucalyptus globules Labill. under the family of Myrtaceae. A large tree attains a height of 60 m or more with a straight, clean bole and smooth bark peeling off in long thin strips or sheets Leaves on young twigs opposite, sessile, cordate-ovate, glaucous gray; adult leaves alternate, lanceolate or ovate-lanceolate, acuminate, falcate. Flowers large, axillary, solitary or 2 to 3 together; calyx tube broadly turbinate, thick and woody. Fruits (capsules) semi-globular, containing numerous minute seeds (Pandey et al., 2005). It is reported to be antiseptic, astringent, deodorant, diaphoretic, expectorant, febrifuge, insect repellant, rubefacient. The bluegum eucalyptus is a folk remedy for abscess, arthritis, asthma, bronchitis, burns, cough, fever, flu, inflammation, leprosy, malaria, sores, sore throat and wounds (Duke and Wain, 1981; List and Horhammer, 1969–1979; Morton, 1981). Leaves contain 70–80% eucalyptol (cineol). Also includes terpineol, sesquiterpene alcohols, aliphatic aldehydes, isoamyl alcohol, ethanol, and terpenes (Morton, 1981). The main objective of the present study was to investigate the effects of leaf extracts of Eucalyptus globules for anticariogenic activity and phytochemical study.
2. Materials and Methods
2.1. Collection of plants material
Eucalyptus globules Labill. plant leaves were collected between January to February, 2010 from the surroundings of Vallabh Vidyanagar, Gujarat, India. The leaves of all the healthy and disease free plants were used to test the antibacterial activity. Plant specimens were identified by Dr. Kalpesh Ishnava (Plant Taxonomist) at Ashok and Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences (ARIBAS), New Vallabh Vidyanagar, Gujarat, India.
2.2. Extraction of leaves
First of all the leaves of E. globules were thoroughly washed with tap water, blotted and dried under sunlight after cutting them into small pieces and subjected to oven drying at 60°C for 12 hours. For the purpose of making powder it was ground in a grinder. From this, 50 grams of powdered material was soaked in 250 mL of ethyl acetate for 24 hours at room temperature. The extract was filtered with the help of Whatman filter paper number-1. The filtrate was collected in petridish and dried at room temperature. The dried extract from petridish was scraped and transferred to eppendorf tube.
The residual material from the funnel was dried again and resuspended in 250 mL hexane for 24 hours at room temperature. The extract was filtered and collected in the petri dish. It was dried at room temperature.
Similarly, the residual materials from the funnel are preserved and reextracted with a same volume (250 mL) of methanol and distilled water respectively. In both cases, the resultant culture filtrate was air dried at room temperature. The dried extract from petri dish was scraped and transferred to eppendorf tube.
2.3. Cariogenic bacteria
A group of bacteria known to cause dental caries were selected and purchased from Microbial Type Culture Collection (MTCC) bank, Chandigarh as a freeze dried pure culture. The bacterial cultures were revived by using MTCC specified selective growth medium and preserved as glycerol stocks. The bacteria responsible for dental caries Lactobacillus acidophilus (MTCC-∗447), Lactobacillus casei (MTCC-1423), Streptococcus mutans (MTCC-890) and Staphylococcus aureus (MTCC-96) were used for the study.
2.4. Inoculums Preparation
Fresh inoculums were prepared by streaking a loopful of bacterial suspension into the bacteria specific selective media (Hi-media) and incubated at an optimal temperature in order to maintain an approximately uniform growth rate. The bacterial cultures were compared with 0.5 McFarland turbidity standard, which is equivalent to approximately 1X108 bacterial cell count per mL (Perilla, 2003), was maintained throughout the experimentation.
2.5. Bioassay for anticarcinogenic activity of E. globules leaf extracts
2.5.1. Agar Well Diffusion Method
In the present study, to test anticariogenic activity, E. globules plant leaf extracts were used. The anticariogenic activity was studied by agar well diffusion method (Perez et al., 1990). From the stock, 100 mg of leaf extracts was suspended in one milliliter of each of ethyl acetate, hexane, methanol and distilled water. Selective agar medium plates were marked and divided in to 4 equal parts, labeled for specific organism and extract name. A fresh bacterial culture of 100μL having 108 CFU/mL was spread on agar plates with a glass spreader. A well of 10 mm diameter was punched off at previously marked petriplates into agar medium with sterile cup borer and then it was filled with 100 μL of E. globules leaf supernatant. Plates were placed for 30 minutes in a refrigerator for diffusion of extracts and then incubated at 37°C (or specified temperature) for 24 hours or more depending upon the bacterial species, until appearances of the inhibition zone. The zone of inhibition (including well diameter) was measured as a property of anticariogenic activity. Antibiotic, ampicillin was used as a standard at a concentration of 10 μg/mL and all the organic solvents were used as positive control and negative control respectively. Bioassay was performed in duplicate and repeated twice.
2.5.2. Determination of Minimum Inhibitory Concentration (MIC)
Minimum inhibitory concentration was evaluated by the two fold serial broth dilution method (Chattopadhyay et al., 2001). Leaf extracts showing more than 10 mm inhibition zone were selected for MIC. Selective broth medium was used for dilutions as well as preparing inoculums. The bacterial cell density was maintained uniformly throughout the experimentation at 1X108 CFU/mL by comparing with 0.5 McFarland turbidity standards. Leaf extracts of 100μL from the stock solution (10mg/mL) was taken into a first dilution tube containing 900μL of the selective medium broth and mixed well. From this, 500 μL were transferred to second tubes containing 500μL broth. This step was repeated nine times and from the last tube 500μL of the solution was discarded. 100μL of test organisms was added in each tube. The final volume of solution in each tube was made up to 0.6 mL. The MIC was tested in the concentration range between 10 μg/mL and 0.039 μg/mL. Tubes were incubated at an optimal temperature and time in an incubator. Growth indicator 2, 3, 5-Triphenyl tetrazolium chloride solution (100μL of 0.1%) was incorporated in each tube to find out the bacterial growth inhibition. Tubes were further incubated for 30 minutes under dark conditions. Bacterial growth was visualized when colorless 2, 3, 5-Triphenyl tetrazolium chloride was converted into red color formazan in the presence of bacteria. Each assay was repeated thrice by using DMSO and selective medium as control.
2.6. Phytochemical Characterization
2.6.1. Preliminary phytochemical analysis
Qualitative phytochemical analysis of E. globules crude leaf extracts selected based on MIC analysis was performed as per the standard methodology to determine the presence of Tannins, Alkaloids, Saponis, cardiac glycosides, Steroids, Terpenoides and Phenolic compounds (Parekh and Chanda, 2008).
2.6.2. Analytical thin layer chromatography
Analytical TLC was performed to find out a suitable solvent system for the development of chromatogram. Different solvent system was tried on precoated TLC plates (Merck, Silica gel 60 F254 plate, 0.25mm) for the development of chromatogram. Among all, Toluene: ethyl acetate: (93:7) solvent system was found to be the best and used for subsequent analysis.
2.6.3. Bioautography
By using capillaries 10μL of hexane extract of E. globules leaf extracts (100mg/mL stock solution) was spotted on to 0.25mm thick precoated silica gel 60 F254 plate (Merck, Germany). The band length was 2 mm thick. After air drying the TLC plate was run using pre-standardized solvent system, Toluene: ethyl acetate (93:7). The chromatogram was observed under UV illumination and used for bioautography. Specific growth medium (Tomato juice: 100 mL, Yeast extract: 5.0gm, Skimmed milk: 100gm per 1000 mL of distilled water, pH: 7.2), seeded with Lactobacillus acidophilus, was overlaid onto the silica gel plate loaded with sample and incubated at 37°C for 24 hrs. The next day, the plate was flooded with 2, 3, 5-Tri phenyl tetrazolium chloride (0.1%) to visualize growth inhibition. The area of inhibition zone appeared transparent against a red background (lawn of living bacteria).
2.6.4. Preparative thin layer chromatography (PTLC)
The preparative thin layer chromatography was performed at the final step of the purification of the pure compound prior to the structure elucidation. Bands that showed antibacterial activity were pulled together and further purified by preparative thin layer chromatography (PTLC). For the PTLC, sample aliquots were loaded onto TLC plates and developed in Toluene: ethyl acetate (93:7) solvent system. Bioautography of the TLC plate was used to confirm the position of the compound showing antibacterial activity. The compound was eluted from the developed plate by scrapping off silica gel and mixed well with hexane and centrifuged at 10,000 rpm for 10 minutes. The supernatant was collected and used for further analysis.
2.6.5. Fourier Transformer Infra Red (FTIR) Spectroscopy
A thin film of E. globules plant leaf active eluted fraction in hexane was applied on the glass and IR spectra were recorded by using Perkin Elmer spectrophotometer, Spectrum Instrument (Germany) with FTIR paragon 1000 PC software at the Sophisticated Instrumentation Centre for Applied Research and Testing (SICART), Vallabh Vidyanagar, Gujarat.
2.6.6. Gas Chromatography-Mass Spectroscopy (GC-MS)
The GC-MS analysis was done by the electron impact ionization (EI) method on Auto system XL gas chromatography (Perkin Elmer Instrument, Germany) coupled to a Turbo Mass Spectrophotometer (Perkin Elmer Instrument, Germany) at Sophisticated and Instrumentation Centre for Applied Research and Testing (SICART), Vallabh Vidyanagar, Gujarat. The column was fused silica capillary column, 30 x 0.25 mm ID; coated with D-I, 0.25 μm film thickness. The temperature of the column was programmed at 70 to 2500C at the rate of 100C/min increase, injection port temperature at 2500C. Helium was used as carrier gas at a constant pressure of 100 kpa and a flow rate of 20mL/min. Samples which dissolved in chloroform were run fully at a range of 60-550 amu and the results were compared by using NIST 107 Spectral library search programme.
2.6.7. NMR Spectroscopy
1H NMR spectra were recorded in CDCl3 using a BRUKER and 400 MHz for proton NMR spectrometer at the Department of chemistry, Sardar Patel University, Vallabh Vidynagar, Gujarat, India.
3. Result and discussion
The results of anticariogenic activity of the extracts and their efficacy are quantitatively assessed by the presence or absence of the zone of inhibition and diameter (in mm) respectively (Table 1). Four different solvents were used for the extraction of anticariogenic substances (Table 1). The solvents used were hexane, ethyl acetate, methanol and distilled water. Among all the solvents, ethyl acetate proved to be the most prominent solvent for the extraction of anticariogenic substances from the selected plant.
Table 1.
Anticariogenic activity of extracts of Eucalyptus globules leaves.
| Plant extracts | Zone of Inhibition in mm (MIC in mg/ml) | |||
|---|---|---|---|---|
| L. acidophilus | L. casei | S. aureus | S. mutans | |
| Distilled water | 03 | - | - | - |
| Ethyl acetate | 13(0.033) | 11(0.25) | 04 | 13 |
| Hexane | 12(0.062) | 10(0.25) | 04 | 08 |
| Methanol | 12(01) | 12(0.5) | 08 | 13(02) |
| Cefadroxil | 36 | 31 | 31 | 12 |
| Erythromycin | 23 | 15 | 19 | 15 |
| Tetracycline | 28 | 31 | 24 | 28 |
Distilled water extract of E. globules leaf extracts is only active against L. acidophilus. Ethyl acetate extracts are active against all the selected bacterial strain. Maximum activity against L. acidophilus, S. mutans and L. casei recorded with the zone of inhibition is 13mm, 13mm and 11 mm respectively (Table 1). Hexane extracts of E. globules are active against all selected microorganisms. The maximum zone of inhibition against L. acidophilus, S. mutans and L. casei is 12mm, 8mm and 10 mm respectively (Table 1). The maximum activity of methanolic extract of plant leaves against L. acidophilus, L. casei and S. mutans is 12mm, 12mm and 13 mm respectively (Table 1) showed. Bachir and Mohamed reported E. globules activity against Staphylococcus aureus Gram (+) and Escherichia coli Gram (-) bacteria (Bachir and Mohamed, 2008). Sirivan Athikomkulchai reported the volatile oils showed good antimicrobial activity against Propionibacterium acnes (MIC, MBC = 9.38 mg/ml for eucalyptus oil) (Sirivan et al., 2008). Methanolic extract of Eucalyptus globules leaves is very much effective in comparison to those which were carried out by Kachhiya (2008) on E. globules methanolic extract of E. globules stem on selected anticariogenic bacteria (Kachhiya, 2008).
The Minimum Inhibitory Concentration (MIC) values of leaf extracts of all the selected organic solvents showing the highest activity against selected bacteria are assessed and summarized in Table 1. The maximum MIC value was found to be 2 mg/mL and the minimum value as 0.031 mg/mL (Table 1). The MIC value of hexanolic extract of E. globules against LA and LC was 0.062 mg/mL and 0.25 mg/mL respectively (Table 1). The MIC value of ethyl acetate extract of E. globules was most significant against LA i.e 0.031 mg/mL and that against LC as 0.25 mg/mL (Table 1). As compared to above solvents, methanolic extracts exhibited MIC values ranging from 0.5 to 2 mg/mL against selected cariogenic bacteria (Table 1).
The presence of common phytochemical constituents such as alkaloids, tannins, saponins, terpenoids, steroids, phenolic compounds and cardiac glycosides was tested qualitatively as per the methodology of Ahmad and Beg (2001) (Table 2). The bioactive compound found in the hexane extract of E. globules includes alkaloids, terpenoids, steroids, phenolic compounds and cardiac glycosides (Table 2). A similar observation was made in the ethyl acetate extract of E. globules except there is absence of alkaloids (Table 2). Methanolic extracts of E. globules also exhibited similar phytochemical profile.
Table 2.
Phytochemical analysis of Eucalyptus globules leaves extracts.
| Phytochemical Test | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| E. globules(Hexane) | - | - | + | + | + | + | + |
| E. globules (Ethyl acetate) | - | - | + | + | + | + | - |
| E. globules (Methanol) | + | + | + | + | + | + | - |
1-Tannins, 2-Saponins, 3-Cardiac glycosides, 4-Steroids, 5 –Terpenoids, 6-Phenolic compounds, 7-Alkaloids, Absent = (-), Present = (+)
In order to find out the active principles present in E. globules of hexane, ethyl acetate and methanolic extracts, TLC solvent system was standardized (Toluene: Ethyl acetate (93:7)) and used for subsequent analysis. The bioactive compounds were separated from crude extracts by using TLC technique.
To locate the major active compounds responsible for the anticariogenic activity in E. globules, chromatogram was used for TLC – bioautography against SMU, LA and LC.
The UV analysis of TLC plate of E. globules crude hexane extracts showed orange fluorescence bands at 254 nm. Red and blue fluorescence bands were observed in hexane extracts of E. globules at 365 nm. E. globules ultraviolet spectra of hexanolic extracts of various intensities are plotted in Fig. 1. Spectral analysis shows the total nine numbers of active bands with Rf Value 0.32. The chromatogram was used for bioautography against Lactobacillus acidophilus.
Figure 1.
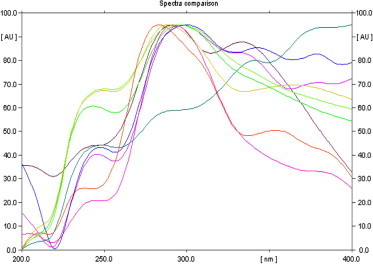
Ultraviolet spectra of various intensities from hexanolic leaf extract of E. globules.
The analysis of TLC plate run from eluted sample showed red fluorescence at 254 nm and blue florescence at 366 nm, respectively, the single band was confirmed by using iodine vapor. The study of infrared spectra (IR) revealed the presence of H-bonded, Alkene C = C, NO2 Nitro group, C–N stretch vib aliphatic, Alkene bending vib and C-Cl chloride as a major functional group (Fig. 2). The peak showing a maximum percentage area at RT 17.75 in GC–MS analysis and scan 1.87e4 through mass spectrophotometer and NMR, revealed the presence of alpha-farnesene (C15H24) and has a molecular weight of 204.35, pK is 17.75 (Fig. 2). The compound identified as alpha-farnesene, a sesquiterpene (Fig. 3). Claudia et al (2003) reported a similar type of compound in different types of chopped leaves of Eucalyptus dunnii, E. citriodora, and E. saligna (Claudia et al., 2003). It is also an active compound present in the leaf oils of E. globules a taxonomically very close species.
Figure 2.
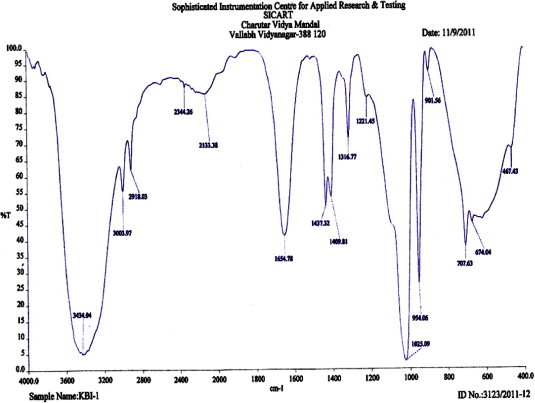
IR spectra of crude leaf extract (in Hexane) of Eucalyptus globules.
Figure 3.
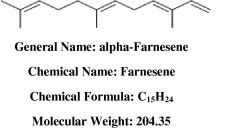
Structure of anticariogenic compound from E. globules active against cariogenic bacteria Lactobacillus acidophilus (Peak 17.75 of GC-MS).
4. Conclusion
Our findings on a broad spectrum activity of various extracts of E. globules against a panel of selected cariogenic bacteria and its phytochemical analysis revealed that it has an anticariogenic substance, one is identified as alpha-farnesene, a sesquiterpene. The good inhibitory potential of hexanolic and ethyl acetate extracts of E. globules plant leaves against Lactobacillus acidophilus and a panel of cariogenic bacteria will be useful in the future development of effective formulations of drugs for the control of dental caries.
Acknowledgements
Authors are thankful to Drs. A.K. Ray, Department of Chemistry, Sardar Patel University, Vallabh Vidyanagar and Jignesh Raval, Ashok and Rita Patel Institute of Integrated Study and Research in Biotechnology and Allied Sciences (ARIBAS), New Vallabh Vidyanagar, Gujarat, India, for the interpretation of IR and NMR data. We also acknowledge the Sophisticated Instrument Centre for Applied Research and Testing (SICART), DST, India for IR and NMR analyses. Authors are also thankful to Charutar Vidya Mandal (CVM), Vallabh Vidyanagar, Gujarat, India and Director of Ashok and Rita Patel Institute of Integrated Studies and Research in Biotechnology and Allied Sciences (ARIBAS), New Vallabh Vidyanagar 388121, Gujarat, India, for providing necessary support research and laboratory facility.
Footnotes
Peer review under responsibility of the King Saud University.

Contributor Information
Kalpesh B. Ishnava, Email: ishnavakb203@yahoo.com.
Jenabhai B. Chauhan, Email: jbc109@yahoo.co.in.
Mahesh B. Barad, Email: maheshbarad@yahoo.com.
References
- Ahmad I., Beg A.Z. Antimicrobial and Phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. Journal of Ethanopharmacology. 2001;74:113–123. doi: 10.1016/s0378-8741(00)00335-4. My paper. [DOI] [PubMed] [Google Scholar]
- Alaluusua S., Kleemola-Kujala E., Nystrom M., Evalahti M., Gronroos L. Caries in the primary teeth and salivary S. mutans and lactobacillus levels as indicators of caries in permanent teeth. Pediat Dent. 1987;9:126–130. [PubMed] [Google Scholar]
- Bachir R.G., Mohamed B. Antibacterial activity of leaf essential oils of Eucalyptus globules and Eucalyptus camaldulensis. African journal of Pharmacy and pharmacology. 2008;2(10):211–215. [Google Scholar]
- Caufield P.W., Cutler G.R., Dasanayake A.P. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. Journal of Dental Research. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Maiti K., Kundu A.P., Chakraborty M.S., Bhadra R., Mandal S.C., Manda A.B. Antimicrobial activity of Alstonia macrophylla - folklore of bay island. Journal of Ethnopharmacology. 2001;77:49–55. doi: 10.1016/s0378-8741(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Claudia A.Zini., Kelen D.Zanin., Eva Christensen., Elina B.Caramao., Janusz Pawliszyn. Solid-phase microextraction of volatile compounds from the chopped leaves of three species of Eucalyptus. Journal of Agriculture Chemistry. 2003;51(9):2679–2686. doi: 10.1021/jf026047g. [DOI] [PubMed] [Google Scholar]
- Cowan . Miami University; Oxford, Ohio-: 1999. In “Plant Products as Antimicrobial Agents” Department of Microbiology. 45056. [Google Scholar]
- Duke, J.A and Wain, K.K., 1981. Medicinal plants of the world. Computer index with more than 85,000 entries. 3 vols.
- Fine D.H., Furang D., Barnett M.L., Drew C., Steinberg L., Charles C.H. Effect of essential oil containing antiseptic mouth rinse on plaque and salivary Streptococcus mutans levels. Journal of Clinical Periodontal. 2000;27:157–161. doi: 10.1034/j.1600-051x.2000.027003157.x. [DOI] [PubMed] [Google Scholar]
- Hebber S.S., Harsha V.H., Hegde G.R., Shripathi V. Ethnomedicine of Dharwad district in Karnataka, India- plant in oral healthcare. Journal of Ethnopharmacology. 2004;4:261. doi: 10.1016/j.jep.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Kachhiya, J.B., 2008. Screening and evaluation of ethano-botanical plants for their efficacy against cariogenic bacteria. Dissertation Thesis, Sardar Patel University, Vidyanagar.
- Kitagaki K., Natsumae A., Ghoda A. Efficacy of therapeutic agents gingivitis and periodontal disease. Journal of antibacterial Antifungal Agents. 1983;11:451–461. [Google Scholar]
- Leverett D.H., Proskin H.M., Featherstone J.D., Adair S.M., Eisenberg A.D., Mundorff-Shrestha S.A. Caries risk assessment in a longitudinal discrimination study. Journal of Dental Research. 1993;72:538–543. doi: 10.1177/00220345930720021101. [DOI] [PubMed] [Google Scholar]
- Loesche W.J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, J. F., 1981. Atlas of medicinal plants of Middle America. Bahamas to Yucatan. C.C. Thomas, Springfield, IL.
- Pai M.R., Acharya L.D., Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel - a 6-week clinical study. Journal of Ethnopharmacology. 2004;90:99–103. doi: 10.1016/j.jep.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Pandey, C.N., Raval, B.R., Mali, S., Salvi, H., 2005. Medicinal plants of Gujarat, Gujarat Education and Research (Geer) Foundation, Gandhinagar, pp: 1–5.
- Parekh J., Chanda S.V. In vitro antimicrobial and phytochemical analysis of some Indian medicinal plants. Turkish Journal of Biotechnology. 2008;31:53–58. [Google Scholar]
- Perez C.Pau., Bazerque P. Antibiotic assay by agar well diffusion method. Acta Biol Med Exp. 1990;15:113–115. [Google Scholar]
- Perilla, M.J., 2003. Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in this developing world”. WHO. 209–214.
- Peterson P.E. WHO; Geneva, Switzerland: 2003. World Oral Health Report (2003) Oral programme Non-communicable Disease Prevention and Health Promotion. [Google Scholar]
- Scheie A.A. Mechanisms of dental plaque formation. Adv Dent. Res. 1994;8(2):246–253. doi: 10.1177/08959374940080021801. [DOI] [PubMed] [Google Scholar]
- Sirivan Athikomkulchai1., Rith Watthanachaiyingcharoen., Sujimon Tunvichien., Panida Vayumhasuwan., Paisarn Karnsomkiet., Prapan Sae-Jong., Nijsiri Ruangrungsi. The development of anti-acne products from Eucalyptus globules and psidium guajava oil. Journal of Health Research. 2008;22(03):109–113. [Google Scholar]


