Abstract
This study was initiated to screen the antioxidant activities, tyrosinase inhibitory effects on the fruiting bodies of Pleurotus ferulae extracted with acetone, methanol and hot water. The antioxidant activities were performed on β-carotene–linoleic acid, reducing power, DPPH, ferrous ions chelating abilities, and xanthine oxidase. In addition to this, phenolic compounds were also analyzed. The methanolic extract showed the strongest β-carotene–linoleic acid inhibition and high reducing power as compared to other extracts. The scavenging effects on DPPH radicals, the acetonic and methanolic extracts were more effective than hot water extracts. The strongest chelating effect was obtained from the methanolic extract as compared to the tested synthetic antioxidant. Gallic acid, protocatechuic acid, caffeic acid, vanillin, ferulic acid, naringin, resveratrol, naringenin, hesperetin, formononetin and biochanin-A were detected from acetonitrile and hydrochloric acid (5:1) solvent extract. Xanthine oxidase and tyrosinase inhibitory activities of acetonic, methanolic, and hot water extracts of P. ferulae increased with increasing concentration. The results suggested that consumption of P. ferulae might be beneficial to the antioxidant, xanthine oxidase, and tyrosinase protection system of the human body against oxidative damage and others complications.
Keywords: Antioxidant, Phenolic compounds, Pleurotus ferulae, Tyrosinase inhibition, Xanthine oxidase
1. Introduction
Pleurotus ferulae is an edible mushroom, belongs to the family Pleurotaceae and order Agaricales. It is mostly pathogenic and grows on the roots of Ferula communis, distributed throughout the Mediterranean region (Urbanelli et al., 2002). This mushroom has been known to produce various biologically active molecules and novel enzymes (Choi et al., 2005). Laccase is a ligninolytic enzyme, typically produced as multiple isoenzymes, which have been isolated and characterized in different strains of P. ferulae (Soden and Dobson, 2001). Laccase enzymes have also been shown to play an important role in the development of the disease of some wood-decaying fungi (Schouten et al., 2008).
Oxidation is essential to many living organisms for the production of energy to fuel biological processes. However, oxygen-centered free radicals and other reactive oxygen species that are continuously produced in vivo, result in cell death and tissue damage. Oxidative damage caused by free radicals may be related to aging and diseases, such as atherosclerosis, diabetes, cancer and cirrhosis (Halliwell and Gutteridge, 1984). Almost all organisms are well protected against free radical damage by enzymes, such as superoxide dismutase and catalase, or compounds such as ascorbic acid, tocopherols and glutathione. Although almost all organisms possess antioxidant defence and repair systems that have evolved to protect them against oxidative damage, these systems are insufficient to prevent the damage entirely (Simic, 1988). However, antioxidant supplements, or foods containing antioxidants, may be used to help the human body reduce oxidative damage (Yanga et al., 2002). Allopurinol is the clinically used xanthine oxidase inhibitor, which also suffers from many side effects such as hypersensitivity syndrome and renal toxicity (Alam et al., 2011a). Thus, there is a need to develop compounds with xanthine oxidase inhibitor activity which is devoid of the undesirable side effects of allopurinol. A potential source of such a compound can be obtained from mushrooms. Flavonoids and polyphenolic crude extracts have been reported to possess xanthine oxidase inhibitory activity (Zhou et al., 2001; Alam et al., 2011b).
Tyrosinase, also known as polyphenol oxidase, is a copper-containing enzyme, which is widely distributed in mushrooms, animals, and plants. Nowadays mushroom tyrosinase has become popular because it is readily available and useful in a number of applications (Yoon et al., 2011). Despite the clinical importance of P. ferulae or the therapeutic potential, there have not been many studies on physiologically beneficial components. However, the antioxidant properties of this mushroom are not available. Accordingly our objective was to evaluate and compare the antioxidant and antityrosinase properties of acetonic, methanolic, and hot water extracts from the fruiting bodies of P. ferulae. The profiles of phenolic compounds were also determined.
2. Materials and methods
2.1. Chemicals and reagents
β-Carotene, linoleic acid, chloroform, polyoxyethylene sorbitan monopalmitate (Tween 40), butylated hydroxytoluene (BHT), α-tocopherol (TOC), 1,1-diphenyl-2-picrylhydrazyl (DPPH), l-ascorbic acid, potassium ferricyanide, trichloroacetic acid, ferrous chloride, ferric chloride, ferrozine, Folin–Ciocalteu reagent, gallic acid, methanol, 3,4-dihydroxy-l-phenylalanine (l-DOPA), xanthine, allopurinol, mushroom tyrosinase, and dimethyl sulfoxide (DMSO) were obtained from Sigma–Aldrich (St. Louis, MO, USA). All chemicals and solvents were used as HPLC or analytical grade.
2.2. Mushroom and extraction
Fresh and mature fruiting bodies of P. ferulae were obtained from Mushmaru mushroom farm at Cheonan in Korea. A pure culture was deposited in Culture Collection DNA Bank of Mushroom (CCDBM), Division of Life Sciences, University of Incheon, Korea and acquired accession number, IUM-4402. Fruiting bodies were dried with hot air at 40 °C for 48 h and finely pulverized. Five grams of powdered samples was extracted with 100 ml of 60% acetone and 80% methanol with stirring at 150 rpm for 24 h at 25 °C to obtain acetonic and methanolic extracts. The mixture was filtered through two layers of Whatman no. 1 filter paper (Whatman, Maidstone, UK). The same quantity of sample was boiled at 100 °C for 3 h with 100 ml deionized distilled water to obtain a hot water extract. The mixture was cooled to room temperature and filtered through Whatman no. 1 filter paper. The residues were then extracted with two additional 100 ml aliquots of acetone, methanol, and deionized water, as described above. Then the combined extracts were evaporated with a rotary evaporator (Eyela, Saitama, Japan) at 40 °C, and the remaining solvent was removed with a freeze-drier (Optizen, Daejeon, Korea). The yields from the acetonic, methanolic and hot water extracts of P. ferulae were 23.18%, 23.80%, and 16.18% (w/w), respectively.
2.3. Antioxidant activity by β-carotene–linoleic acid
Antioxidant activity was determined by measuring the inhibition of volatile organic compounds and the conjugated diene hydroperoxides arising from linoleic acid oxidation (Dapkevicius et al., 1998). A stock solution of a β-carotene–linoleic acid mixture was prepared as follows: 0.5 mg β-carotene was dissolved in 1 ml of chloroform, and 25 μl of linoleic acid and 200 mg of Tween 40 was added. The chloroform was removed completely using a vacuum evaporator. Then, 100 ml of oxygenated distilled water was added with vigorous shaking; 2.5 ml of this reaction mixture was dispensed to test tubes, 0.5 ml of various concentrations (0.5–20.0 mg/ml) of the extracts in methanol was added, and the reaction mixture was incubated for up to 2 h at 50 °C. The same procedure was repeated with the positive controls BHT and TOC, and a blank. After the incubation, the absorbance of the mixtures was measured at 490 nm using a spectrophotometer (Optizen POP; Mecasys Co. Ltd., Daejeon, Korea). The absorbance was measured until the β-carotene color disappeared. The β-carotene bleaching rate (R) was calculated according to Eq. (1):
| (1) |
where, ln = natural log, a = absorbance at time t (0), b = absorbance at time t (120 min). The antioxidant activity (AA) was calculated as the percent inhibition relative to the control using Eq. (2):
| (2) |
Antioxidant activities of the extracts were compared with those of BHT and TOC at 0.5 mg/ml and a blank consisting of 0.5 ml methanol.
2.4. Reducing power
Reducing power was determined according to the method of Gülçin et al. (2003). Each extract (1–8 mg/ml) in methanol (2.5 ml) was mixed with 2.5 ml of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide, and the mixture was incubated at 50 °C for 20 min. Then, 2.5 ml of 10% trichloroacetic acid was added, and the mixture was centrifuged at 200g (6 K 15; Sigma, Munich, Germany) for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml of deionized water and 0.5 ml of 0.1% ferric chloride. Finally, the absorbance was measured at 700 nm against a blank. BHT and TOC were used as positive controls.
2.5. Scavenging effect on 1,1-diphenyl-2-picrylhydrazyl radicals
The hydrogen atoms or electron donation ability of the corresponding extracts and some pure compounds were measured from the bleaching of the purple colored DPPH methanol solution (Cuendet et al., 1997). Four milliliters of various concentrations (0.125–2.0 mg/ml) of the extracts in methanol was added to 1 ml of DPPH radical solution in methanol (final concentration of DPPH was 0.2 mM). The mixture was shaken vigorously and allowed to stand for 30 min, and the absorbance of the resulting solution was measured at 517 nm using a spectrophotometer. Inhibition of the DPPH free radical in percent (I%) was calculated as:
where, Acontrol is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. BHT, TOC, and l-ascorbic acid were used as positive controls.
2.6. Chelating effects on ferrous ions
The chelating effect was determined according to the method of Dinis et al. (1994). Briefly, 2 ml of various concentrations (0.063–1.0 mg/ml) of the extracts in methanol was added to a solution of 2 mM FeCl2 (0.05 ml). The reaction was initiated by adding 5 mM ferrozine (0.2 ml). The total volume was adjusted to 5 ml with methanol, and the mixture was shaken vigorously and left at room temperature for 10 min. The absorbance of the solution was measured spectrophotometrically at 562 nm. The inhibition percentage of the ferrozine–Fe2+ complex formation was calculated using the following formula:
where, Acontrol is the absorbance of the control (control contained FeCl2 and ferrozine; complex formation molecules), and Asample is the absorbance of the test compound. BHT and TOC were used as positive controls.
2.7. Analysis of phenolic compounds
Fifteen standard phenolic compounds, including gallic acid, pyrogallol, homogentisic acid, protocatechuic acid, (+) catechin, chlorogenic acid, caffeic acid, vanillin, ferulic acid, naringin, resveratrol, naringenin, hesperetin, formononetin, and biochanin-A were purchased from Sigma–Aldrich and used for calibration curves. The standard stock solutions (50, 100, 250, and 500 ppm) were prepared in DMSO. Sample compounds were identified based on retention times of authentic standards and were quantified by comparing their peak areas with those of the standard curves.
Sample preparation for the phenolic compound analysis followed by Alam et al. (2011b). Two grams of dried mushroom powder were mixed with 10 ml of acetonitrile and 2 ml of 0.1 N hydrochloric acid and stirred at 150 rpm for 2 h at room temperature. The suspension was filtered through Whatman no. 42 filter paper. The extract was freeze-dried, and the residues were redissolved in 10 ml of 80% aqueous methanol (HPLC grade) and filtered through a 0.45 μm nylon membrane filter (Titan, Rockwood, TN, USA). Then 20 μl filtrate was loaded onto an Agilent-1100 series liquid chromatography HPLC system (Agilent Technologies, Waldbronn, Germany). Separation was achieved on a 250 nm × 4.6 mm i.d., 5 μm, YMC-Pack ODS AM (YMC Co. Ltd., Kyoto, Japan) column. The mobile phase was distilled water with 0.1% glacial acetic acid (solvent A) and acetonitrile with 0.1% glacial acetic acid (solvent B). The gradient was 0 min, 92% A; 0–2 min, 90% A; 2–27 min, 70% A; 27–50 min, 10% A; 50–51 min, 0% A; 51–60 min, 0% A; 60–63 min, 92% A. The run time was 60 min using a flow rate of 1 ml/min. Detection was performed with a diode array detector at a wavelength of 280 nm.
2.8. Xanthine oxidase inhibition
In vitro xanthine oxidase (XO) inhibitory activity of various extracts from the fruiting bodies of P. ferulae was assayed spectrophotometrically under aerobic conditions using xanthine as the substrate (Owen and Johns, 1999). The assay mixture consisted of 1 ml extract of the different concentrations (0.5–8.0 mg/ml), 2.9 ml of phosphate buffer (pH 7.5), and 0.1 ml of xanthine oxidase enzyme solution (0.1 units/ml in phosphate buffer, pH 7.5), which was prepared immediately before use. After pre incubation at 25 °C for 15 min, the reaction was initiated by the addition of 2 ml of the substrate solution (150 μM xanthine in the same buffer). The assay mixture was incubated at 25 °C for 30 min. The reaction was then stopped by the addition of 1 ml of 1 N hydrochloric acid and the absorbance was measured at 290 nm using a spectrophotometer. Different concentrations of the extracts were dissolved in DMSO and the final concentration of DMSO was 5%, which did not affect the enzyme assay. Proper controls with DMSO were carried out. Allopurinol (0.5–8.0 mg/ml), a known inhibitor of XO, was used as positive control. One unit of XO is defined as the amount of enzyme required to produce 1 mmol of uric acid/min at 25 °C. Xanthine oxidase inhibitory activity was expressed as the percentage inhibition of XO in the above assay system calculated as
where, A is the activity of the enzyme without the extraction, B is the control of A without the extraction and enzyme; C and D are the activities of the extraction with and without XO, respectively.
2.9. Tyrosinase inhibition
Tyrosinase inhibition activity was determined using the modified dopachrome method with l-DOPA as the substrate (Masuda et al., 2005). A 96-well microtiter plate was used to measure absorbance at 475 nm with 700 nm as reference. Extract fractions were dissolved in 50% DMSO. Each well contained 40 μl of sample with 80 μl of phosphate buffer (0.1 M, pH 6.8), 40 μl of tyrosinase (31 units/ml), and 40 μl of l-DOPA (2.5 mM). The mixture was incubated for 10 min at 37 °C, and absorbance was measured at 475 nm using a UVM 340 microplate reader (Asys, Eugendrof, Austria). Each sample was accompanied by a blank containing all components except l-DOPA. l-Ascorbic acid and kojic acid were used as positive controls. The results were compared with a control consisting of 50% DMSO in place of the sample. The percentage of tyrosinase inhibition was calculated as follows:
2.10. Statistical analysis
Data were expressed as means ± SD of three replicate determinations and were analyzed by SPSS V.13 (SPSS Inc., Chicago, IL, USA). A one way analysis of variance and Duncan’s new multiple-range test were used to determine the differences among the means.
3. Results and discussion
3.1. Antioxidant activity on β-carotene–linoleic acid
The results indicate that the antioxidant activities of P. ferulae lower than the synthetic antioxidant, BHT and TOC, respectively at 0.5 mg/ml. At 0.5–20.0 mg/ml concentration, antioxidant activities of the acetonic, methanolic, and hot water extracts of P. ferulae ranged from 52.71% to 95.63%, 69.51% to 96.52%, and 34.88% to 93.22%, respectively (Table 1). The antioxidant activities on β-carotene–linoleic acid of the acetonic, methanolic and hot water extracts from the fruiting bodies of P. ferulae gradually increased with increasing concentration. However, the methanolic and acetonic extracts showed good, hot water extract showed moderate activities at the concentration tested.
Table 1.
Antioxidant activity against β-carotene–linoleic acid of different concentrations of various extracts from the fruiting bodies of Pleurotus ferulae.
| Solvent and control | Sample concentration (mg/ml) |
|||
|---|---|---|---|---|
| 0.5 | 2.0 | 8.0 | 20.0 | |
| Acetone | 52.71 ± 0.07 | 88.64 ± 0.03 | 92.26 ± 0.06 | 95.63 ± 0.05 |
| Methanol | 69.51 ± 0.19 | 89.16 ± 0.55 | 94.31 ± 0.71 | 96.52 ± 0.62 |
| Hot water | 34.88 ± 0.28 | 57.38 ± 0.16 | 85.62 ± 0.17 | 93.22 ± 0.18 |
| BHT | 95.21 ± 0.17 | – | – | – |
| TOC | 96.02 ± 0.18 | – | – | – |
Values expressed as means ± SD (n = 3), –, not analyzed; BHT, butylated hydroxytoluene; TOC, α-tocopherol.
The linoleic acid free radical attacks the highly unsaturated β-carotene models. The presence of carotenoid shows, not only a decrease of the free radical concentration, but the reduction of Fe3+ to Fe2+ by carotenoids. It is probable that the antioxidative components in the mushroom extracts can reduce the extent of β-carotene destruction by neutralizing the linoleate free radical and other free radicals formed in the reaction process (Yoon et al., 2011). Barros et al. (2007) reported that antioxidant activities of Leucopaxillus giganteus, Sarcodon imbricatus and Agaricus arvensis in various extracts increased with increasing concentration. Their antioxidant activities were 61.4%, 54.3% and 46.7% at 5 mg/ml. It seems that the antioxidant activity of P. ferulae was more effective than those mentioned above.
3.2. Reducing power
The reducing power of acetonic, methanolic, and hot water extracts from the fruiting bodies of P. ferulae increased with increasing concentration. At 1.0–8.0 mg/ml, the reducing power of the acetonic, methanolic, and hot water extracts of P. ferulae ranged from 0.37 to 1.39, 0.50 to 1.62, and 0.38 to 1.37, while reducing power of BHT and TOC at 1.0 mg/ml were 3.21and 2.16, respectively (Table 2).
Table 2.
Reducing power of different concentrations of various extracts from the fruiting bodies of Pleurotus ferulae.
| Solvent and control | Sample concentration (mg/ml) |
|||
|---|---|---|---|---|
| 1.0 | 2.0 | 4.0 | 8.0 | |
| Acetone | 0.371 ± 0.06 | 0.586 ± 0.07 | 0.919 ± 0.07 | 1.394 ± 0.32 |
| Methanol | 0.499 ± 0.07 | 0.718 ± 0.08 | 1.087 ± 0.09 | 1.616 ± 0.11 |
| Hot water | 0.380 ± 0.02 | 0.524 ± 0.03 | 0.802 ± 0.07 | 1.367 ± 0.11 |
| BHT | 3.212 ± 0.49 | – | – | – |
| TOC | 2.162 ± 0.32 | – | – | – |
Values expressed as means ± SD (n = 3), –, not analyzed; BHT, butylated hydroxytoluene; TOC, α-tocopherol.
With regard to ethanolic extracts, the reducing power of Pleurotus citrinopileatus was 1.03 at 5 mg/ml (Lee et al., 2007a) whereas, Agricus bisporus and Pleurotus ostreatus showed reducing powers of 0.76 and 0.61 at 20 mg/ml, respectively (Lee et al., 2007b). It can be seen that the reducing power of P. ferulae was higher than those of P. citrinopileatus, A. bisporus, and P. ostreatus. It was reported that the reducing power properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Shimada et al., 1992). Reducing power of a compound may be serving as a significant indication of its potential antioxidant activity.
3.3. Scavenging effect on DPPH
Scavenging effects of the acetonic, methanolic, and hot water extracts from the fruiting bodies of P. ferulae on DPPH radicals increased with increasing concentration. At 0.125–2.0 mg/ml, the scavenging activities of acetonic, methanolic, and hot water extracts of P. ferulae on DPPH radical ranged from 14.02% to 91.32%, 18.39% to 88.85%, and 9.96% to 77.78%, respectively (Fig. 1). The results indicated that the acetonic, methanolic, and hot water extracts, respectively showed good, moderate, and poor activities at the concentration tested. However, at 0.125–2.0 mg/ml, BHT, TOC, and l-ascorbic acid showed the excellent scavenging activities of 85.25–98.74, 67.37–97.78, and 96.74–98.23%, respectively.
Figure 1.
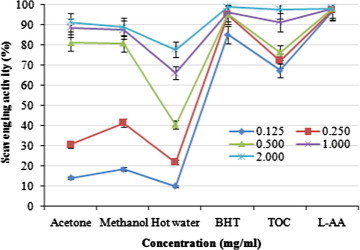
Scavenging activity of various extracts from the fruiting bodies of Pleurotus ferulae against 1,1-diphenyl-2-picrylhydrazyl. Values expressed as means ± SD (n = 3). BHT, butylated hydroxytoluene; TOC, α-tocopherol; l-AA, l-ascorbic acid.
DPPH is a stable free radical and possesses a characteristic absorbance at 517 nm, which decreases significantly on exposure to radical scavengers by providing hydrogen atom or electron to become a stable diamagnetic molecule (Herraiz et al., 2003). With regard to ethanolic extracts of Hypsizigus marmoreus, A. bisporus and P. citrinopileatus fruiting bodies could scavenge DPPH radicals by 46.6–68.4% at 5 mg/ml (Lee et al., 2007a). For cold and hot water extracts, at 20 mg/ml, the scavenging activities of fruiting bodies, mycelia and filtrate were 20.7–52.3, 37.6–48.3, and 19.6–23.3%, respectively. It seems that the scavenging activity of P. ferulae fruiting bodies was more effective than those mentioned above. Various extracts might react with free radicals, particularly the peroxy radicals, which are the major propagators of the autoxidation chain of fat, thereby terminating the chain reaction (Shahidi and Wanasundara, 1992). Antioxidant activity of natural antioxidants has been shown to be involved in the termination of free radical reaction (Shimada et al., 1992). The presence of l-tryptophan in various extracts might most likely account for the scavenging activity on DPPH radicals. However, the better activity of acetonic extract might be due to more hydrogen-donating components contained within the extracts.
3.4. Chelating effects on ferrous ions
The chelating activity of the acetonic, methanolic, and hot water extracts at five different concentrations (0.063, 0.125, 0.25, 0.50, and 1.0 mg/ml) from the fruiting bodies of P. ferulae toward ferrous ions was investigated. BHT and TOC were used as reference standards on ferrous ions. The strongest chelating effect (87.80%) obtained from the methanolic extract, while the lowest chelating effect was exhibited by hot water extract (82.12%) at 1.0 mg/ml (Fig. 2). All of the extracts evaluated here showed significantly higher chelating effects on ferrous ions than those of the standards, BHT and TOC at the concentrations of 0.063, 0.125, and 0.25 mg/ml, respectively.
Figure 2.
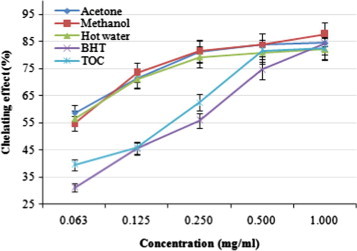
Chelating effect of various extracts from the fruiting bodies of Pleurotus ferulae. Values expressed as means ± SD (n = 3). BHT, butylated hydroxytoluene; TOC, α-tocopherol.
With regard to hot water extracts at 20 mg/ml, Ganoderma tsugae and Agrocybe cylindracea chelated ferrous ions by 42.6% and 45.8%, respectively (Mau et al., 2005; Tsai et al., 2006). At 1–5 mg/ml, chelating abilities of H. marmoreus and P. citrinopileatus were 75.6–92.6% (Lee et al., 2007b). It seems that the chelating ability of P. ferulae on ferrous ions was similar to that of H. marmoreus and P. citrinopileatus, while being more effective than those of G. tsugae and A. cylindracea. Chelating agents may serve as secondary antioxidants because they reduce the redox potential thereby stabilizing the oxidized form of the metal ions. Since ferrous ions were the most effective pro-oxidants on auto oxidation of oil compounds in foods (Alam et al., 2010), the high ferrous-ion chelating abilities of the various extracts from the fruiting bodies of P. ferulae would be beneficial to human health.
3.5. Analysis of phenolic compound
Gallic acid, pyrogallol, homogentisic acid, protocatechuic acid, (+) catechin, chlorogenic acid, caffeic acid, vanillin, ferulic acid, naringin, resveratrol, naringenin, hesperetin, formononetin and biochanin-A were used as standards for the detection of phenolic compounds. Eleven phenolic compounds, gallic acid, protocatechuic acid, caffeic acid, vanillin, ferulic acid, naringin, resveratrol, naringenin, hesperetin, formononetin and biochanin-A were detected from the extract of P. ferulae (Fig. 3). The concentration of total phenolic compound was 190 μg/g. The highest and lowest phenolic compound concentrations were recorded in gallic acid (40 μg/g) and vanillin and formononetin, respectively (9 μg/g).
Figure 3.
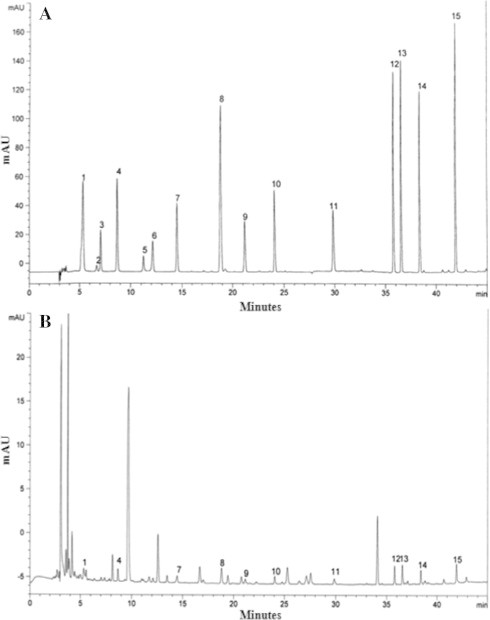
High performance liquid chromatography of phenolic compounds. (A) Standard mixture of 15 phenolic compounds; (B) Pleurotus ferulae extract; 1, gallic acid; 2, pyrogallol; 3, homogentisic acid; 4, protocatechuic acid; 5, (+) catechin; 6, chlorogenic acid; 7, caffeic acid; 8, vanillin; 9, ferulic acid; 10, naringin; 11, resveratrol; 12, naringenin; 13, hesperetin; 14, formononetin; 15, biochanin-A.
These findings are comparable to the previous studies on edible mushrooms (Kim et al., 2008) in which average total phenolic compounds’ concentration was 174 μg/g. Mushroom species also contained varying numbers of phenolic compounds, ranging from 3 to 15, while gallic acid and protocatechuic acid were reported common phenolic compounds found in edible mushrooms. Thus, the content of phenolic compounds could be used as an important indicator of antioxidant capacity. Several reports have convincingly shown a close relationship between antioxidant activity and phenolic content (Alam et al., 2011a; Yoon et al., 2011). Mushroom extracts have high levels of phenolic compounds, which are composed of one or more aromatic rings which are bearing one or more hydroxyl groups, which can exhibit extensive free radical-scavenging activities as hydrogen donors or electron-donating agents, and metal ion-chelating properties. The greater numbers of hydroxyl groups in the phenolics could exhibit higher antioxidant activity (Alam et al., 2011b).
3.6. Xanthine oxidase inhibitory activity
Xanthine oxidase inhibitory activities of various extracts of P. ferulae increased with increasing concentration. At 0.5–8.0 mg/ml, the xanthine oxidase inhibition of acetonic, methanolic, and hot water extracts ranged from 5.89% to 45.82%, 3.21% to 45.56%, and 4.49% to 46.89%, respectively. However, at the same concentrations, allopurinol showed the excellent xanthine oxidase inhibitory activity of 92.31–94.58% (Fig. 4).
Figure 4.
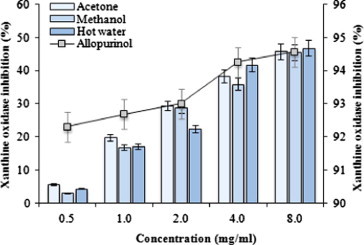
Xanthine oxidase inhibition activities of various extracts from the fruiting bodies of Pleurotus ferulae. Values expressed as means ± SD (n = 3).
The results indicate that hot water extract showed good, while acetonic and methanolic extracts showed moderate activities at the concentration tested. However, at higher doses of the extract, xanthine oxidase would be significantly inhibited. Flavonoids are a group of polyphenolic compounds, which have been reported to possess xanthine oxidase inhibitory activity (Costantino et al., 1992). Hence, the presence of phenolic and flavonoid content in the extract would have contributed toward xanthine oxidase inhibition.
3.7. Tyrosinase inhibition
Tyrosinase inhibitory activities of acetonic, methanolic, and hot water extracts from the fruiting bodies of P. ferulae increased with increasing concentration. At 0.125–1.0 mg/ml, the tyrosinase inhibition of acetonic, methanolic, and hot water extracts ranged from 19.81% to 60.10%, 13.12% to 59.87%, and 15.92% to 55.07%, respectively (Fig. 5). Results indicate that the acetonic and methanolic extracts showed good, while hot water extract showed moderate activities at the concentration tested. However, at 0.125–1.0 mg/ml, l-ascorbic acid and kojic acid showed excellent tyrosinase inhibitory activities of 75.12–92.74% and 91.23–99.00%, respectively.
Figure 5.
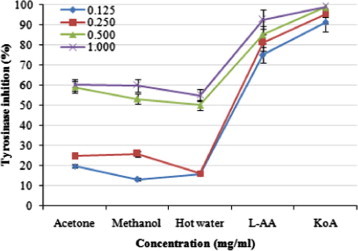
Tyrosinase inhibition activities of various extracts from the fruiting bodies of Pleurotus ferulae. Values expressed as means ± SD (n = 3). l-AA, l-ascorbic acid; KoA, kojic acid.
The inhibition of tyrosinase ability might depend on the hydroxyl groups of the phenolic compounds of the mushroom extracts that could form a hydrogen bond at active site of the enzyme, leading to a lower enzymatic activity. Some tyrosinase inhibitors act through hydroxyl groups that bind to the active site on tyrosinase, resulting in steric hindrance or changed conformation (Baek et al., 2008). Gallic acid proved to be an effective inhibitor of tyrosinase activity, as reported by many researchers (Momtaz et al., 2008). The antioxidant activity may also be one of the important mechanisms for tyrosinase inhibitory activity.
4. Conclusions
P. ferulae is a popular edible mushroom and commercially available in Korea. It is also recognized as a nutritious food as well as an important source of biologically active compounds of medicinal purposes. On the basis of the results, it is suggested that three different extracts from the fruiting bodies of P. ferulae evaluated here could be used as an easily accessible source of natural antioxidants for nourishment. P. ferulae contained various phenolic compounds that have shown good potential, which can be used for functional foods and medicinal industries. Therefore, high level of phenols and good antioxidant and antityrosinase activities indicated that the P. ferulae fruiting bodies could be used as a natural food source of antioxidants.
Acknowledgment
This research was supported by a research grant from University of Incheon in 2011.
References
- Alam N., Yoon K.N., Lee K.R., Shin P.G., Cheong J.C., Yoo Y.B., Shim M.J., Lee M.W., Lee U.Y., Lee T.S. Antioxidant activities and tyrosinase inhibitory effects of different extracts from Pleurotus ostreatus fruiting bodies. Mycobiology. 2010;38:295–301. doi: 10.4489/MYCO.2010.38.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam N., Yoon K.N., Lee T.S. Evaluation of the antioxidant and antityrosinase activities of three extracts from Pleurotus nebrodensis fruiting bodies. Afr. J. Biotechnol. 2011;10:2978–2986. [Google Scholar]
- Alam N., Yoon K.N., Shin P.G., Cheong J.C., Yoo Y.B., Lee T.S. Antioxidant, phenolic compounds concentration, xanthine oxidase and tyrosinase inhibitory activities of Pleurotus cornucopiae. Aust. J. Basic Appl. Sci. 2011;5:229–239. [Google Scholar]
- Baek H.S., Rho H.S., Yoo J.W., Ahn S.M., Lee J.Y., Lee J., Kim M.K., Kim D.H., Chang I.S. The inhibitory effect of new hydroxamic acid derivatives on melanogenesis. Bull. Korean Chem. Soc. 2008;29:43–46. [Google Scholar]
- Barros L., Ferreira M.J., Queiros B., Ferreira I.C., Bapista P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007;103:413–419. [Google Scholar]
- Choi D., Kang S.H., Song Y.H., Kwun K.H., Jeong K.J., Cha W.S. Exopolysaccharide production in liquid culture of Pleurotus ferulae. J. Microbiol. Biotechnol. 2005;146:209–221. [Google Scholar]
- Costantino L., Albasini A., Rastelli G., Benvenuti S. Activity of polyphenolic crude extracts as scavengers of superoxide radicals and inhibitors of xanthine oxidase. Planta Med. 1992;58:342–344. doi: 10.1055/s-2006-961481. [DOI] [PubMed] [Google Scholar]
- Cuendet M., Hostettmann K., Potterat O., Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv. Chim. Acta. 1997;80:1144–1152. [Google Scholar]
- Dapkevicius A., Venskutonis R., van Beek T.A., Linssen J.P. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998;77:140–146. [Google Scholar]
- Dinis T.C., Madeira V.M., Almeida L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-amino salicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Gülçin I., Büyükokuroglu M.E., Oktay M., Küfrevioglu O.I. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003;86:51–58. doi: 10.1016/s0378-8741(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz T., Galisteo J., Chamorro C. l-Tryptophan reacts with naturally occurring and food-occurring phenolic aldehydes to give phenolic tetrahydro-β-caroline alkaloids: activity as antioxidants and free radical scavengers. J. Agric. Food Chem. 2003;51:2168–2173. doi: 10.1021/jf0210066. [DOI] [PubMed] [Google Scholar]
- Kim M.Y., Seguin P., Ahn J.K., Kim J.J., Chun S.C., Kim E.H., Seo S.H., Kang E.Y., Kim S.L., Park Y.J., Ro H.M., Chung I.M. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008;56:7265–7270. doi: 10.1021/jf8008553. [DOI] [PubMed] [Google Scholar]
- Lee Y.L., Huang G.W., Liang Z.C., Mau J.L. Antioxidant properties of three extracts from Pleurotus citrinopileatus. LWT-Food Sci. Technol. 2007;40:823–833. [Google Scholar]
- Lee Y.L., Yen M.T., Mau J.L. Antioxidant properties of various extracts from Hypsizigus marmoreus. Food Chem. 2007;104:1–9. [Google Scholar]
- Masuda T., Yamashita D., Takeda Y., Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- Mau J.L., Tsai S.Y., Tseng Y.H., Huang S.J. Antioxidant properties of hot water extracts from Ganoderma tsugae Murrill. LWT-Food Sci. Technol. 2005;38:589–597. [Google Scholar]
- Momtaz S., Mapunya B.M., Houghton P.J., Edgerly C., Hussein A., Naidoo S., Lall N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008;119:507–512. doi: 10.1016/j.jep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Owen P.L., Johns T. Xanthine oxidase inhibitory activity of north-eastern North American plant remedies used for gout. J. Ethnopharmacol. 1999;64:149–160. doi: 10.1016/s0378-8741(98)00119-6. [DOI] [PubMed] [Google Scholar]
- Schouten A., Maksimova O., Cuesta-Arenas Y., van den Berg G., Raaijmakers J.M. Involvement of the ABC transporter BcAtrB and the laccase BcLCC2 in defense of Botrytis cinerea against the broad-spectrum antibiotic 2,4-diacetylphloroglucinol. Environ. Microbiol. 2008;10:1145–1157. doi: 10.1111/j.1462-2920.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Wanasundara P.K. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40:945–948. [Google Scholar]
- Simic M.G. Mechanisms of inhibition of free-radical processes in mutagenesis and carcinogenesis. Mutat. Res. 1988;202:377–386. doi: 10.1016/0027-5107(88)90199-6. [DOI] [PubMed] [Google Scholar]
- Soden D.M., Dobson A.D.W. Differential regulation of laccase gene expression in Pleurotus sajor-caju. Microbiology. 2001;147:1755–1763. doi: 10.1099/00221287-147-7-1755. [DOI] [PubMed] [Google Scholar]
- Tsai S.Y., Huang S.J., Mau J.L. Antioxidant properties of hot water extracts from Agrocybe cylindracea. Food Chem. 2006;98:670–677. [Google Scholar]
- Urbanelli S., Fanelli C., Fabbri A.A., Della-Rosa V., Maddau L., Marras F., Reverberi M. Taxonomic studies on two taxa of the Pleurotus eryngii complex: Pleurotus eryngii var. eryngii and P. eryngii var. ferulae (Quel.:Fr.) Biol. J. Linn. Soc. 2002;75:125–136. [Google Scholar]
- Yanga J.H., Linb H.C., Mau J.L. Antioxidant properties of several commercial mushrooms. Food Chem. 2002;77:229–235. [Google Scholar]
- Yoon K.N., Alam N., Lee K.R., Shin P.G., Cheong J.C., Yoo Y.B., Lee T.S. Antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules. 2011;16:2334–2347. doi: 10.3390/molecules16032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C.X., Kong L.D., Ye W.C., Cheng C.H., Tan R.X. Inhibition of xanthine and monoamine oxidases by stillbenoids from Veratrum taliense. Planta Med. 2001;67:158–161. doi: 10.1055/s-2001-11500. [DOI] [PubMed] [Google Scholar]


