Abstract
Thermopsis turcica, endemic to Turkey, is in danger of extinction. Studies on this species are very few due to the fact that it was only discovered in 1983 and grows in a small circumscribed area in Turkey. In this study, free radical scavenging activity, total phenolic content, total oxidant status (TOS), and total antioxidant status (TAS) of methanol (TTM) and acetone (TTA) extracts of T. turcica were measured spectroscopically. Free radical scavenging activity was determined according to the elimination of DPPH radicals and total phenol content was determined by the Folin–Ciocalteu reaction. Total oxidant status (TOS) and total antioxidant status (TAS) were measured with commercially available kits. Methanol and acetone extracts of T. turcica were found to have a specific radical scavenging effect. This effect was found to be related to the total phenolic content of the extracts. Since the TTA had a higher phenolic content than the methanol extract, it had a stronger radical scavenging effect. In addition, the total antioxidant capacity of the methanol extract was observed to be higher than that of its acetone counterpart. As a result, due to its antioxidative properties, T. turcica is thought to be a natural source of antioxidants.
Keywords: Thermopsis turcica, Radical scavenging activity, Total phenolic content, DPPH, TAS, TOS
1. Introduction
All aerobic organisms have antioxidant defence systems to offset harmful effects caused by free radicals. In the case of failure of the antioxidant defence system, antioxidants need to be supplemented from outside sources. Antioxidants can be found naturally in foods (Kedare and Singh, 2011). A majority of antioxidants naturally present in foods occur in phenolic structures and especially in flavonoid structures. In addition, antioxidants are added to nutrients to prevent deterioration in their taste, smell, and colour. Butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and propyl gallate (PG) can be included in this group, which are known as synthetic antioxidants (Koksal and Gulcin, 2008). The high cost of natural antioxidants has led to the use of synthetic antioxidants. However, studies conducted subsequently have demonstrated that synthetic antioxidants have toxic effects and, consequently, restrictions have been imposed on their use. Therefore, researchers have focused their studies on plant-derived natural antioxidants (Kulisic et al., 2004).
Discovered in 1983 by Turkish botanists Thermopsis turcica, is a perennial herbaceous plant belonging to the Thermopsis genus. Members of this genus generally grow in the United States of America and Asia (Chen et al., 1994). T. turcica was recorded as endemic to Turkey. T. turcica is the only species of the Thermopsis genus grown in the province of Afyonkarahisar in Turkey. T. turcica was reported to be among the species that will become extinct in nature in a short time and was listed in the Critically Endangered (CR) category (Cenkci et al., 2009).
Some species of the Thermopsis genus are used for medical purposes. However, many species include phytochemicals that may cause poisoning. Keller, et al. found in a study that Thermopsis Montana induced myopathy owing to the anagyrine, thermopsine, N-methycytisine, and cytosine alkaloids present in it. In another study conducted later, the authors mention toxic and teratogenic effects of anagyrine and ammodendrine alkaloids on animals (Lee et al., 2008).
There are very few studies on T. turcica and one of these is a micropropagation study aimed at continuation of the generation. Two other studies are on the mutagenic potential, morphology, and anatomy of T. turcica. In another study examining the chemical composition of T. turcica, the plant was found to include alkaloids, flavonoids, coumarin, and steroidal content in its structure. The total alkaloid content in its structure was found to be 1.48% and, among the alkaloids, the proportion of anagyrine was determined to be high (Ozdemir et al., 2008; Cenkci et al., 2008; Liman et al., 2012).
In this study we determined the free radical scavenging activity, total phenolic content, total oxidant level, and total antioxidant level in methanol and acetone extracts of T. turcica, which is in danger of extinction even though it is a newly discovered species. Moreover, BHT, which is a synthetic antioxidant, was evaluated as a positive control and compared with acetone and methanol extracts of the plant.
2. Material and methods
2.1. Plant material
T. turcica was collected in May 2011 around Lake Eber (38°36′N, 31°14′E) in Afyonkarahisar and identified by Dr. Mustafa Kargioglu. A sample of the plant is stored in the Herbarium of the Faculty of Science and Arts at the University of Afyon.
2.2. Chemicals
Butylated hydroxytoluene (BHT), Folin–Ciocalteu’ phenol reagent, the stable free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH•), gallic acid, and sodium carbonate (Na2CO3) were obtained from Sigma (Sigma–Aldrich GmbH, Sternheim, Germany). Acetone and methanol were purchased from Merck. All other chemicals used were of analytical grade.
2.3. Preparation of plant extract
In this study, a mixture of plant stems, leaves, and flowers of T. turcica was used. These parts were dissected into small pieces and dried in the shade at room temperature. The dried materials were stored in the dark until analysis. To prepare methanol and acetone extracts of the plant, the mixture was first pulverised by grinding in a blender. To prepare the acetone extract, 400 mL of acetone was added to 20 g of powdered T. turcica. To prepare the methanol extract, the same process was performed but 400 mL of methanol was added as the solvent. The mixture was stirred on a magnetic stirrer under a reflux condenser. The resulting extracts obtained were filtered through filter paper and the solvents were removed by a rotary evaporator (Gulcin, 2005). Free radical scavenging activity and total phenolic content were determined in these prepared extracts.
Ten millilitres of solvent was added to 1 g of material derived from the dried plant parts and pulverised for extraction in order to determine the total antioxidant status and total oxidant status; then the mixture (1 g powdered plant + 10 mL solvent) was sonicated. After filtering though filter paper, the mixture was then centrifuged for 15 min at 10,000 rpm. The supernatant was removed and the remaining mixture was centrifuged again and used in the analyses (Dikilitas et al., 2011).
2.4. Determination of free radical scavenging activity, total phenolic content, total antioxidant status, total oxidant status and oxidative stress index
Free radical scavenging activities of solutions of the plant extracts and synthetic antioxidant substances used in the study prepared in methanol at concentrations of 50, 100 and 200 μg/mL were determined in accordance with the Shimada (1992) method, which is based on the principle of scavenging the DPPH (1,1-diphenyl-2-picrylhydrazyl) radical. DPPH was added to the solutions prepared with plant extracts and standard antioxidant substances and stirred. Each mixture was kept in the dark for 30 min and the absorbance was measured at 517 nm against a blank (Shimada et al., 1992).
The total phenolic content of the plant extracts and the standard antioxidant materials was determined according to the Folin–Ciocalteu method (Gamez-Meza et al., 1999). Folin–Ciocalteu reagent was added to the extract and BHT solutions. After 5 min, Na2CO3 was added and the mixture was stored at room temperature for 2 h. The absorbance of the mixture was measured at 760 nm against water on a UV spectrophotometer. The results were calculated using the standard calibration curve of gallic acid (R2 = 0.9236) and expressed as gallic acid equivalents (GAE mg/g).
Absorbance (λ760) = 0.0026 × [Phenols (μg)]
The total antioxidant capacity of the extracts was measured using commercial kits. In this method, the 2,2′-azinobis-3-ethylbenzothiazoline-6-sulphonic acid radical (ABTS) reacts with hydrogen peroxide and is oxidised to the ABTS+ molecule. The ABTS radical loses its original blue and green colour. The intensity of the colour varies according to the quantity of antioxidants and their antioxidant capacity. The absorbance of this colour is measured spectrophotometrically at 660 nm (Dikilitas et al., 2011).
The total oxidant capacity of the extracts was measured using commercially available kits. The basis of this method depends on the oxidation of Fe with valence +2 to an iron complex with valence +3. Fe+3 forms a coloured complex with xylenol orange. Colour intensity varies according to the amount of oxidant in the sample. The absorbance of this colour is measured spectrophotometrically at 530 nm (Dikilitas et al., 2011).
Oxidative stress index (OSI) is an indicator of oxidative stress. It is calculated by dividing total oxidant level (TOS) by total antioxidant level (TAS).
2.5. Statistical analysis
All data were reported as mean ± standard deviation of four replicates. The data were analysed using one-way variance analysis (One Way ANOVA) and Tukey’s Post-test. Correlations between the antioxidant activity and total phenolic content were examined using Pearson’s correlation. A statistical analysis was performed using the SPSS 10.0 software package.
3. Results and discussion
Since the antioxidant compounds found in plants have different polarities, different solvents are used to isolate antioxidants. Water, methanol, ethanol, and acetone are solvents commonly used in extraction processes. The antioxidant activity of the extract and the yield depends on the selected solvent (Gong et al., 2012). In this study, methanol and acetone were preferred as solvents for the extracts to be prepared.
A number of methods and modifications have been proposed to determine antioxidant activity. Total antioxidant activity, metal chelation, radical scavenging (DPPH) effects and reducing power as well as activities destructive to active oxygen species such as the superoxide anion radical, hydroxyl radical, and hydrogen peroxide are widely used for this purpose (Shimada et al., 1992). In this study, total antioxidant status, radical scavenging effect, and the amounts of phenolic compounds as well as the total oxidant status were determined.
A number of methods are used to determine the radical scavenging effects of antioxidants. The DPPH method is a preferred method because it is fast, easy and reliable and does not require a special reaction and device. DPPH is a stable, synthetic radical that does not disintegrate in water, methanol, or ethanol. The free radical scavenging activities of extracts depend on the ability of antioxidant compounds to lose hydrogen and the structural conformation of these components (Shimada et al., 1992; Fukumoto and Mazza, 2000). The DPPH free radical, which is at its maximum wavelength at 517 nm, can easily receive an electron or hydrogen from antioxidant molecules to become a stable diamagnetic molecule (Soares et al., 1997). Owing to the DPPH radical’s ability to bind H, it is considered to have a radical scavenging property. A solution of DPPH radicals prepared in methanol is converted into DPPH-H (diphenylhydrazine) molecules in the presence of an antioxidant agent, as shown in the following equation. Discoloration occurs due to the decreasing quantity of DPPH radicals in the environment. The discoloration of the DPPH therefore reflects the radical scavenging activity of the analysed extract (Guo et al., 2007; Molyneux, 2004).
In Fig. 1, the radical scavenging effects of different concentrations of methanol and acetone extracts of T. turcica and BHT are demonstrated. The antioxidant activities of the extracts were compared with BHT, which is a synthetic antioxidant. Both acetone and methanol extracts had radical scavenging effects at all concentrations. However, this effect was not as great as the radical scavenging effect of BHT. The radical scavenging effect of the acetone extract appeared to be closer to that of BHT in comparison to the methanol extract. At a concentration of 50 μg/mL, TTA has a radical scavenging effect similar to that of BHT. The magnitude of the radical scavenging effect at all concentrations was ranked as BHT > TTA and TTM, respectively.
Figure 1.
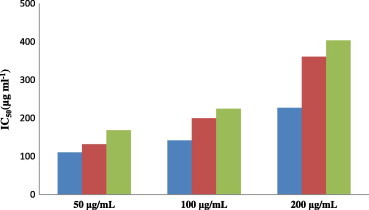
Free radical scavenging effects of methanol and acetone extracts of Thermopsis turcica and BHT at different concentrations.
Phenolic compounds and polyphenols are the most abundant structures in plants. Antioxidant compounds are usually in the phenolic form. The antioxidant properties of phenolic compounds originate from their properties of proton loss, chelate formation, and dismutation of radicals. In fact, in some studies, theoretical methods have been proposed to estimate the antioxidant activities of phenolic substances. Their structure–activity relationships are examined for this purpose. Phenols are compounds that have the ability to destroy radicals because they contain hydroxyl groups. These important plant components give up hydrogen atoms from their hydroxyl groups to radicals and form stable phenoxyl radicals; hence, they play an important role in antioxidant activity. Therefore, determination of the quantity of phenolic compounds is very important in order to determine the antioxidant capacity of plant extracts (Das and Pereira, 1990; De Gaulejac et al., 1999; Hatano et al., 1989). In Fig. 2, the quantity of total phenolic compounds in methanol and acetone extracts of T. turcica and butylated hydroxytoluene (BHT) is shown.
Figure 2.
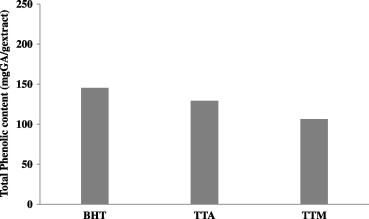
Total phenolic contents of the methanol and acetone extracts of Thermopsis turcica and butylated hydroxytoluene (BHT).
It was observed that the radical scavenging effect of the extracts was positively correlated with their total amount of phenolic compounds (r = 0.9124). The radical scavenging effect of the acetone extract, which had a greater quantity of total phenolic compounds, was also determined to be stronger. Many studies in the literature present positive correlations between the quantity of phenolic compounds and the DPPH free radical scavenging effect (Sagar and Singh, 2011; Liu et al. 2009).
An organism’s metabolism fights against oxidative effects with its own antioxidant defence systems. Elimination and neutralisation of reactive oxygen species is handled by both enzymatic and non-enzymatic antioxidant mechanisms. In practise, TAS represents all of these compounds (Halliwell, 1994). In Table 1, total antioxidant status (TAS), total oxidant status (TOS), and oxidative stress index (OSI) values for methanol and acetone extracts of T. turcica are given. Both the methanol and acetone extracts were observed to have total antioxidant status. The TAS value of the methanol extract was higher than that of the acetone extract. The OSI values of the acetone extract were different in comparison to the methanol extract.
Table 1.
Total antioxidant status (TAS), total oxidant status (TOS), and oxidative stress index (OSI) values of acetone and methanol extracts of Thermopsis turcica.
| TTA | TTM | |
|---|---|---|
| TAS, μmol Trolox Eq/g | 2.06 ± 0.09 | 2.35 ± 0.11 |
| TOS, μmol H2O2 Eq/g | 0.17 ± 0.03 | 0.15 ± 0.03 |
| OSI, Arbitrary Unit | 8.25 ± 0.06 | 6.38 ± 0.07 |
Data were expressed as mean ± S.D. (n = 4) TTA: Thermopsis turcica acetone extract; TTM: Thermopsis turcica methanol extract; TAS: Total antioxidant status; TOS: Total oxidant status; OSI: Oxidative stress index.
There are studies in the literature that report a positive correlation between antioxidant activity and the quantity of phenolic compounds (Faujan et al., 2009; Li et al., 2009; Sun and Ho, 2005). In addition, there are also studies which report that there is no positive relationship (Hesam et al., 2012; Rafat et al., 2010). In our study as well, a positive correlation was not observed between the total antioxidant status and the composition of phenolic substances.
4. Conclusions
In this study, free radical scavenging activity, total phenolic content, total oxidant level, and total antioxidant level of methanol and acetone extracts of T. turcica, an endemic species in danger of extinction, were determined. Both extracts of T. turcica were determined to have a certain level of radical scavenging effect, proportional to their level of total phenolic content. In addition, the acetone extract was determined to have a stronger radical scavenging effect; the methanol extract was observed to have a higher total antioxidant capacity. As a result, T. turcica can be used in pharmaceutical products as a source of natural antioxidants. Therefore, we think studies on how to ensure the existence of this species, which is in danger of extinction, should be emphasised.
Footnotes
Peer review under responsibility of King Saud University.
References
- Cenkci S., Kargioglu M., Dayan S., Konuk M. In vitro propagation of an endangered plant species, Thermopsis turcica (Fabaceae) Biologia. 2008;63:652–657. [Google Scholar]
- Cenkci S., Temel M., Kargioğlu M., Dayan S. Propagation of endangered Thermopsis turcica Kit Tan, Vural & Küçüködük using conventional and in vitro techniques. Turk. J. Biol. 2009;33:327–333. [Google Scholar]
- Chen C.J., Mendenhall M.G., Turner B.L. Taxonomy of Thermopsis (Fabaceae) in North America. Ann. Missouri Bot. Gard. 1994;81:714–742. [Google Scholar]
- Das N.P., Pereira T.A. Effects of flavonoids on thermal autooxidation of palm oil: structure–activity relationship. J. Am. Oil Chem. Soc. 1990;67:255–258. [Google Scholar]
- De Gaulejac N.S.C., Glories Y., Vivas N. Free radical scavenging effect of anthocyanins in red wines. Food Res. Int. 1999;32:327–333. [Google Scholar]
- Dikilitas M., Güldür M.E., Deryaoglu A., Erel O. Antioxidant and oxidant levels of pepper (Capsicum annuum cv. ‘Charlee’) infected with pepper mild mottle virus. Not. Bot. Horti Agrobo. 2011;39:58–63. [Google Scholar]
- Faujan N.H., Noriham A., Norrakiah A.S., Babji A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009;8:484–489. [Google Scholar]
- Fukumoto L.R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Gamez-Meza N., Noriega-Rodriguez J., Medina-Juarez L., Ortega-Garcia J., Cazarez-Casanova R., Angulo-Guerrero O. Antioxidant activity in soybean oil of extracts from Thompson grape bagasse. JAOCS. 1999;76:1445–1447. [Google Scholar]
- Gong Y., Liu X., He W.H., Xu H.G., Yuan F., Gao Y.X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia. 2012;83:481–489. doi: 10.1016/j.fitote.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Gulcin İ. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005;56:491–499. doi: 10.1080/09637480500450248. [DOI] [PubMed] [Google Scholar]
- Guo X.Y., Wang J., Wang N.L., Kitanaka S., Yao X.S. 9, 10-Dihydrophenanthrene derivatives from Pholidota yunnanensis and scavenging activity on DPPH free radical. J. Asian Nat. Prod. Res. 2007;9:165–174. doi: 10.1080/10286020500480522. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Hatano T., Edamatsu R., Hiramatsu M., Mori A., Fujita Y. Effects of the interaction of tannins with co-existing substances. VI: effects of tannins and related polyphenols on superoxide anion radical and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989;37:2016–2021. [Google Scholar]
- Hesam F., Balali G.R., Tehrani R.T. Evaluation of antioxidant activity of three common potato (Solanum tuberosum) cultivars in Iran. AJP. 2012;2:79–85. [PMC free article] [PubMed] [Google Scholar]
- Kedare S.B., Singh R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksal E., Gulcin l. Antioxidant activity of cauliflower (Brassica oleracea L) Turk. J. Agric. For. 2008;32:65–78. [Google Scholar]
- Kulisic T., Radonic A., Katalinic V., Milosa M. Use of different methods for testing antioxidative activity of oregano essential oil, analytical, nutritional and clinical methods. Food Chem. 2004;85:633–640. [Google Scholar]
- Lee S.T., Panter K.E., Pfister J.A., Gardner D.R., Welch K.D. The effect of body condition on serum concentrations of two teratogenic alkaloids (anagyrine and ammodendrine) from lupines (Lupinus species) that cause crooked calf disease. Anim. Sci. 2008;86:2771–2778. doi: 10.2527/jas.2007-0610. [DOI] [PubMed] [Google Scholar]
- Li X., Wu X., Huang L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui) Molecules. 2009;14:5349–5361. doi: 10.3390/molecules14125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman R., Eren Y., Akyil D., Konuk M. Determination of mutagenic potencies of aqueous extracts of Thermopsis turcica by Ames test. Turk. J. Biol. 2012;36:85–92. [Google Scholar]
- Liu S.C., Lin J.T., Wang C.K., Chen H.Y., Yang D.J. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009:577–581. [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl(DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004;26:211–219. [Google Scholar]
- Ozdemır C., Dural H., Ertuğrul K., Küçüködük M., Baran P., Şanda1 M.A. Morphology and anatomy of endemıc thermopsıs turcıca Kıt Tan, Vural & Küçüködük. Bangladesh J. Bot. 2008;37:105–114. [Google Scholar]
- Rafat A., Philip K., Muniandy S. Antioxidant potential and phenolic content of ethanolic extract of selected Malaysian plants. Res. J. Biotechnol. 2010;5:16–19. [Google Scholar]
- Sagar B.K., Singh R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J. Agr. Food Chem. 1992;40:945–948. [Google Scholar]
- Soares J.R., Dins T.C.P., Cunha A.P., Almeida L.M. Antioxidant activity of some extracts of Thymus zygis. Free Radic. Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- Sun T., Ho C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90:743–749. [Google Scholar]



