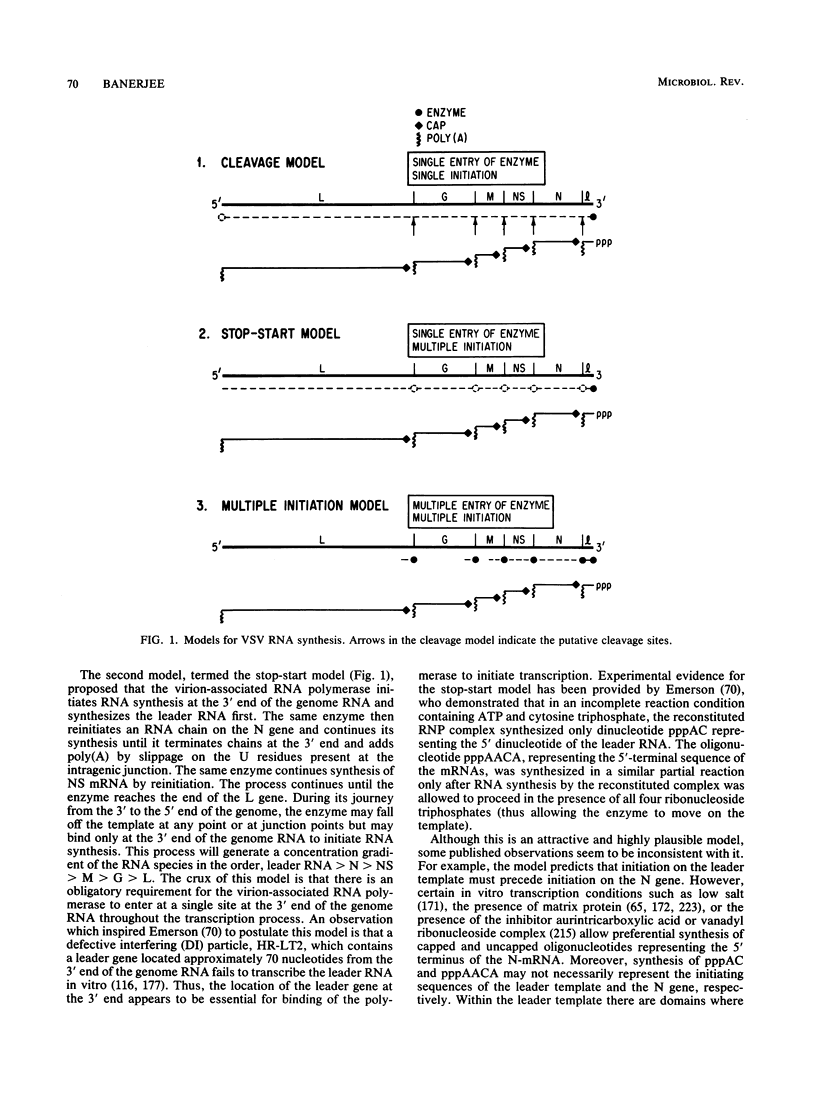
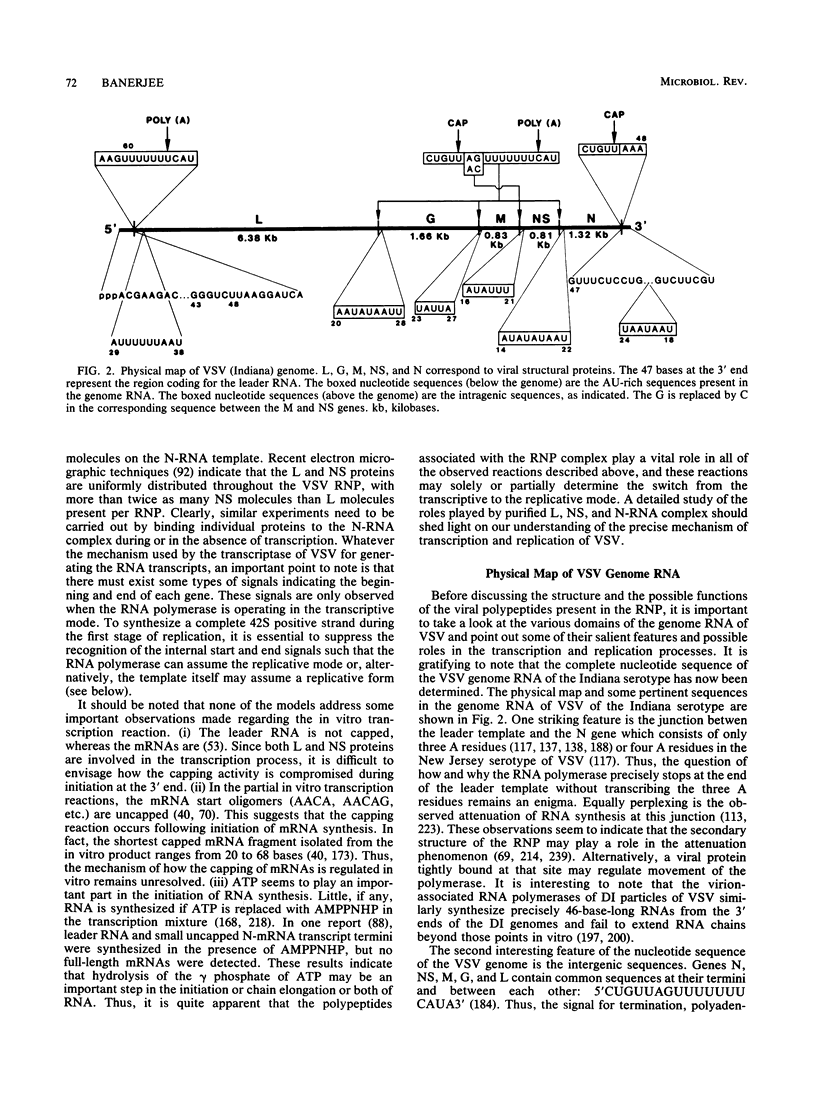
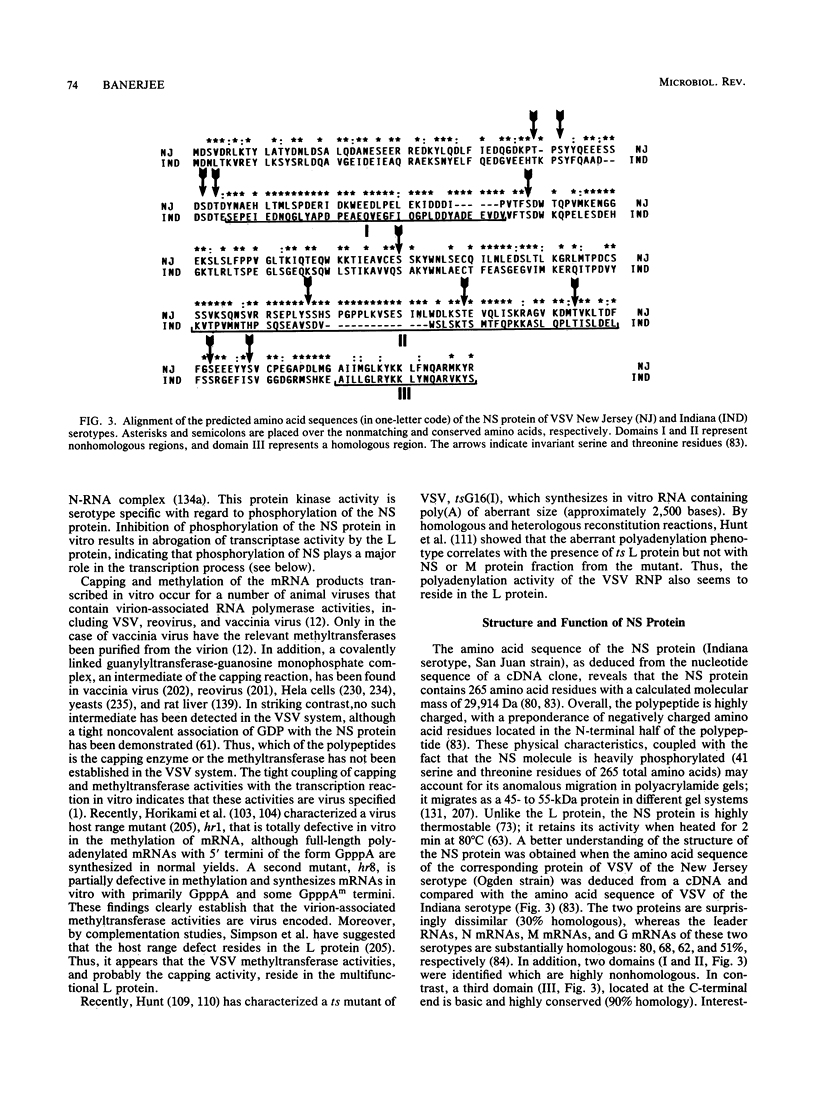
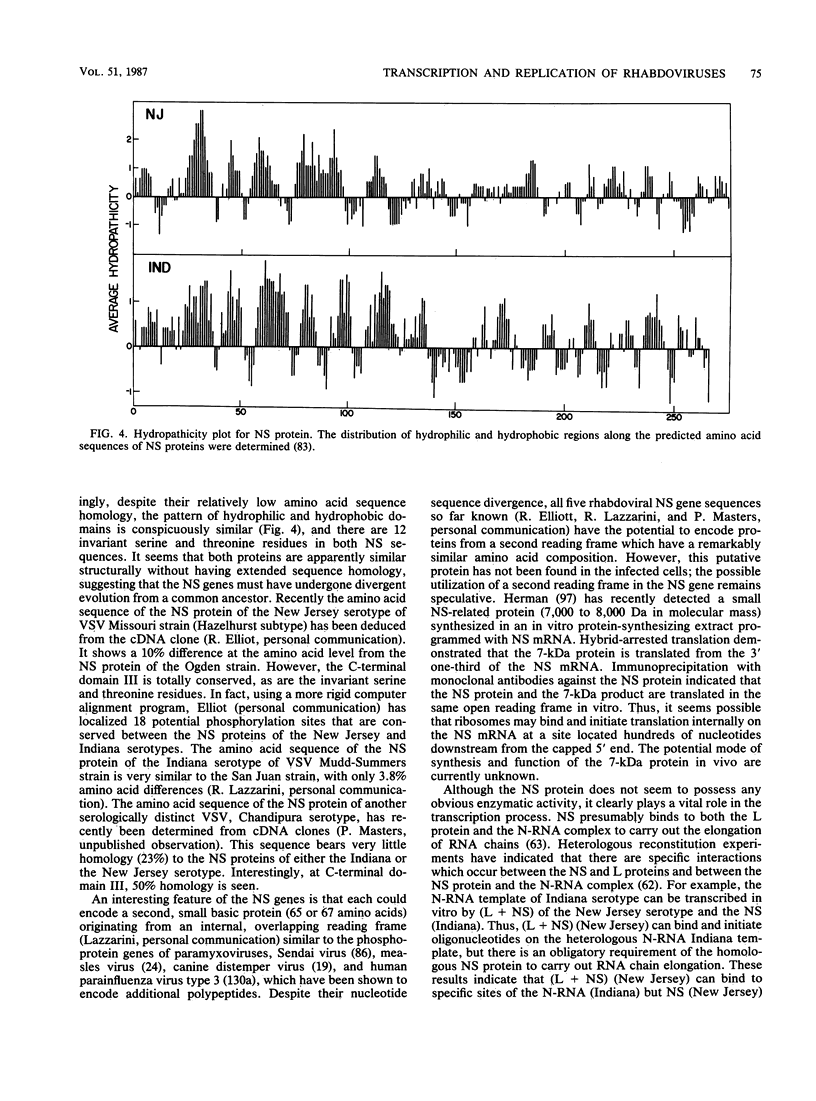
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham G., Banerjee A. K. The nature of the RNA products synthesized in vitro by subviral components of visicular stomatitis virus. Virology. 1976 May;71(1):230–241. doi: 10.1016/0042-6822(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Abraham G., Rhodes D. P., Banerjee A. K. Novel initiation of RNA synthesis in vitro by vesicular stomatitis virus. Nature. 1975 May 1;255(5503):37–40. doi: 10.1038/255037a0. [DOI] [PubMed] [Google Scholar]
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Davis N. L., Wertz G., Schubert M., Lazzarini R. A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985 May;41(1):259–267. doi: 10.1016/0092-8674(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Ball L. A., Wertz G. W. VSV RNA synthesis: how can you be positive? Cell. 1981 Oct;26(2 Pt 2):143–144. doi: 10.1016/0092-8674(81)90297-x. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Coupled transcription and translation in mammalian and avian cell-free systems. Virology. 1978 Feb;84(2):479–495. doi: 10.1016/0042-6822(78)90264-7. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Moyer S. A., Rhodes D. P. Studies on the in vitro adenylation of RNA by vesicular stomatitis virus. Virology. 1974 Oct;61(2):547–558. doi: 10.1016/0042-6822(74)90289-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Rhodes D. P. 3'-Terminal sequence of vesicular stomatitis virus genome RNA. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1387–1394. doi: 10.1016/0006-291x(76)90349-1. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Rhodes D. P., Gill D. S. Complete nucleotide sequence of the mRNA coding for the N protein of vesicular stomatitis virus (New Jersey serotype). Virology. 1984 Sep;137(2):432–438. doi: 10.1016/0042-6822(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Rhodes D. P. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., Shrimpton S. B., Russell S. E. Nucleotide sequence of the entire protein coding region of canine distemper virus polymerase-associated (P) protein mRNA. Virus Res. 1985 Nov;3(4):367–372. doi: 10.1016/0168-1702(85)90436-8. [DOI] [PubMed] [Google Scholar]
- Batt-Humphries S., Simonsen C., Ehrenfeld E. Full-length viral RNA synthesized in vitro by vesicular stomatitis virus-infected HeLa cell extracts. Virology. 1979 Jul 15;96(1):88–99. doi: 10.1016/0042-6822(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Bell J. C., Brown E. G., Takayesu D., Prevec L. Protein kinase activity associated with immunoprecipitates of the vesicular stomatitis virus phosphoprotein NS. Virology. 1984 Jan 30;132(2):229–238. doi: 10.1016/0042-6822(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Bell J. C., Prevec L. Phosphorylation sites on phosphoprotein NS of vesicular stomatitis virus. J Virol. 1985 Jun;54(3):697–702. doi: 10.1128/jvi.54.3.697-702.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H. Complete transcription by the transcriptase of vesicular stomatitis virus. J Virol. 1971 Apr;7(4):486–490. doi: 10.1128/jvi.7.4.486-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Preparation and analysis of the nucleocapsid proteins of vesicular stomatitis virus and sendai virus, and analysis of the sendai virus leader-NP gene region. J Gen Virol. 1984 Apr;65(Pt 4):769–779. doi: 10.1099/0022-1317-65-4-769. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Kolakofsky D. Intracellular vesicular stomatitis virus leader RNAs are found in nucleocapsid structures. J Virol. 1981 Nov;40(2):568–576. doi: 10.1128/jvi.40.2.568-576.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboche M., La Bonnardiere C. Vesicular stomatitis virus mRNA methylation in vivo: effect of cycloleucine, an inhibitor of S-adenosylmethionine biosynthesis, on viral transcription and translation. Virology. 1979 Mar;93(2):547–557. doi: 10.1016/0042-6822(79)90257-5. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Reversal by certain polyanions of an endogenous inhibitor of the vesicular stomatitis virus-associated transcriptase. J Biol Chem. 1978 May 25;253(10):3361–3363. [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Identification of promoter-proximal oligonucleotides and a unique dinucleotide, pppGpC, from in vitro transcription products of vesicular stomatitis virus. J Virol. 1981 Jul;39(1):93–103. doi: 10.1128/jvi.39.1.93-103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Inhibition of vesicular stomatitis virus transcriptase in vitro by phosphonoformate and ara-ATP. Virology. 1980 Dec;107(2):562–566. doi: 10.1016/0042-6822(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Two distinct populations of vesicular stomatitis virus ribonucleoprotein cores with differential sensitivities to micrococcal nuclease. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1337–1345. doi: 10.1016/0006-291x(79)91213-0. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Kang C. Y., Banerjee A. K. Synthesis in vitro of the full-length complement of defective-interfering particle RNA of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3927–3931. doi: 10.1073/pnas.77.7.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda P. K., Roy J., Banerjee A. K. In vitro synthesis of genome length complementary RNA of vesicular stomatitis virus in the presence of inosine 5'-triphosphate. Virology. 1983 Aug;129(1):225–229. doi: 10.1016/0042-6822(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Hefti E., Obijeski J. F., Bishop D. H. RNA transcription by the virion polymerases of five rhabdoviruses. J Virol. 1974 Mar;13(3):652–661. doi: 10.1128/jvi.13.3.652-661.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchar V. G., Amesse L. S., Portner A. Linked transcripts of the genes for leader and N message are synthesized in vitro by vesicular stomatitis virus. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1296–1302. doi: 10.1016/0006-291x(82)90927-5. [DOI] [PubMed] [Google Scholar]
- Chow J. M., Schnitzlein W. M., Reichmann M. E. Expression of genetic information contained in the RNA of a defective interfering particle of vesicular stomatitis virus. Virology. 1977 Apr;77(2):579–588. doi: 10.1016/0042-6822(77)90483-4. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Phosphoproteins of vesicular stomatitis virus: identity and interconversion of phosphorylated forms. Virology. 1979 Nov;99(1):84–94. doi: 10.1016/0042-6822(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. In vitro RNA transcription by the New Jersey serotype of vesicular stomatitis virus. II. Characterization of the leader RNA. J Virol. 1978 Apr;26(1):188–194. doi: 10.1128/jvi.26.1.188-194.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Nucleotide sequence of the leader RNA of the New Jersey serotype of vesicular stomatitis virus. Nucleic Acids Res. 1978 Nov;5(11):4165–4176. doi: 10.1093/nar/5.11.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Lazzarini R. A., Keene J. D., Banerjee A. K. In vitro synthesis of messenger RNA by a defective interfering particle of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 May;74(5):1884–1888. doi: 10.1073/pnas.74.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combard A., Printz Ane C. Inhibition of vesicular stomatitis virus transcriptase complex by the virion envelope M protein. Biochem Biophys Res Commun. 1979 May 14;88(1):117–123. doi: 10.1016/0006-291x(79)91704-2. [DOI] [PubMed] [Google Scholar]
- Condra J. H., Lazzarini R. A. Replicative RNA synthesis and nucleocapsid assembly in vesicular stomatitis virus-infected permeable cells. J Virol. 1980 Dec;36(3):796–804. doi: 10.1128/jvi.36.3.796-804.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. L., Peters D. Protein composition of the virions of five plant rhabdoviruses. Intervirology. 1981;16(2):86–94. doi: 10.1159/000149252. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Wertz G. W. Synthesis of vesicular stomatitis virus negative-strand RNA in vitro: dependence on viral protein synthesis. J Virol. 1982 Mar;41(3):821–832. doi: 10.1128/jvi.41.3.821-832.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Requirements and functions of vesicular stomatitis virus L and NS proteins in the transcription process in vitro. Biochem Biophys Res Commun. 1985 Jan 16;126(1):40–49. doi: 10.1016/0006-291x(85)90568-6. [DOI] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Specific binding of guanosine 5'-diphosphate with the NS protein of vesicular stomatitis virus. Biochem Biophys Res Commun. 1983 Jul 18;114(1):138–147. doi: 10.1016/0006-291x(83)91605-4. [DOI] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Specific interactions of vesicular stomatitis virus L and NS proteins with heterologous genome ribonucleoprotein template lead to mRNA synthesis in vitro. J Virol. 1984 Sep;51(3):628–634. doi: 10.1128/jvi.51.3.628-634.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Tahara S. M., Banerjee A. K. Production and characterization of a monoclonal antibody to the N protein of vesicular stomatitis virus (Indiana serotype). Virology. 1982 Oct 30;122(2):510–514. doi: 10.1016/0042-6822(82)90255-0. [DOI] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch V., Banerjee A. K. Effect of temperature on the enzymatic activities present in purified virions of vesicular stomatitis virus. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1360–1367. doi: 10.1016/0006-291x(79)91130-6. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Cox J. H., Schneider L. G. Rabies virus strains: a comparison study by polypeptide analysis of vaccine strains with different pathogenic patterns. Virology. 1979 Oct 15;98(1):63–75. doi: 10.1016/0042-6822(79)90525-7. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. Polyadenylation of vesicular stomatitis virus mRNA. J Virol. 1974 May;13(5):1055–1060. doi: 10.1128/jvi.13.5.1055-1060.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U. Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell. 1982 Dec;31(3 Pt 2):635–642. doi: 10.1016/0092-8674(82)90319-1. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Delagneau J. F., Bussereau F. An RNA polymerase activity in purified rabies virions. J Gen Virol. 1978 Jul;40(1):233–238. doi: 10.1099/0022-1317-40-1-233. [DOI] [PubMed] [Google Scholar]
- Flamand A., Delagneau J. F. Transcriptional mapping of rabies virus in vivo. J Virol. 1978 Nov;28(2):518–523. doi: 10.1128/jvi.28.2.518-523.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francki R. I., Randles J. W. RNA-dependent RNA polymerase associated with particles of lettuce necrotic yellows virus. Virology. 1972 Feb;47(2):270–275. doi: 10.1016/0042-6822(72)90262-0. [DOI] [PubMed] [Google Scholar]
- Franze-Fernandez M. T., Banerjee A. K. In vitro RNA transcription by the New Jersey serotype of vesicular stomatitis virus. I. Characterization of the mRNA species. J Virol. 1978 Apr;26(1):179–187. doi: 10.1128/jvi.26.1.179-187.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galet H., Prevec L. Polyadenylate synthesis by extracts from L cells infected with vesicular stomatitis virus. Nat New Biol. 1973 Jun 13;243(128):200–203. doi: 10.1038/newbio243200a0. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983 Apr;46(1):162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P. Synthesis in vitro of full length genomic RNA and assembly of the nucleocapsid of vesicular stomatitis virus in a coupled transcription-translation system. Nucleic Acids Res. 1982 Oct 25;10(20):6341–6351. doi: 10.1093/nar/10.20.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Banerjee A. K. Complete nucleotide sequence of the matrix protein mRNA of vesicular stomatitis virus (New Jersey serotype). Virology. 1986 Apr 15;150(1):308–312. doi: 10.1016/0042-6822(86)90293-x. [DOI] [PubMed] [Google Scholar]
- Gill D. S., Banerjee A. K. Vesicular stomatitis virus NS proteins: structural similarity without extensive sequence homology. J Virol. 1985 Jul;55(1):60–66. doi: 10.1128/jvi.55.1.60-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Chattopadhyay D., Banerjee A. K. Identification of a domain within the phosphoprotein of vesicular stomatitis virus that is essential for transcription in vitro. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8873–8877. doi: 10.1073/pnas.83.23.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B., Kolakofsky D. Sequence determination of the (+) leader RNA regions of the vesicular stomatitis virus Chandipura, Cocal, and Piry serotype genomes. J Virol. 1983 Apr;46(1):125–130. doi: 10.1128/jvi.46.1.125-130.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna Y., Lenard J. Sequence alterations in temperature-sensitive M-protein mutants (complementation group III) of vesicular stomatitis virus. J Virol. 1985 Dec;56(3):655–659. doi: 10.1128/jvi.56.3.655-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. L., Emerson S. U. Effect of the beta-gamma phosphate bond of ATP on synthesis of leader RNA and mRNAs of vesicular stomatitis virus. J Virol. 1984 Apr;50(1):255–257. doi: 10.1128/jvi.50.1.255-257.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Roy P. Alternate capping mechanisms for transcription of spring viremia of carp virus: evidence for independent mRNA initiation. J Virol. 1980 Jan;33(1):292–303. doi: 10.1128/jvi.33.1.292-303.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Roy P. Stimulation of transcription by S-adenosyl-L-homocysteine and virion-encapsidated methyl donor in spring viraemia of carp virus. J Gen Virol. 1981 Mar;53(Pt 1):183–187. doi: 10.1099/0022-1317-53-1-183. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Roy P. Synthesis of capped and uncapped methylated oligonucleotides by the virion transcriptase of spring viremia of carp virus, a rhabdovirus. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4758–4762. doi: 10.1073/pnas.78.8.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. A., Robinson E. N., Jr, Summers D. F. Ultrastructural localization of L and NS enzyme subunits on vesicular stomatitis virus RNPs using gold sphere-staphylococcal protein A-monospecific IgG conjugates. Virology. 1985 Apr 30;142(2):406–410. doi: 10.1016/0042-6822(85)90348-4. [DOI] [PubMed] [Google Scholar]
- Harmon S. A., Summers D. F. Characterization of monospecific antisera against all five vesicular stomatitis virus-specific proteins: anti-L and anti-NS inhibit transcription in vitro. Virology. 1982 Jul 15;120(1):194–204. doi: 10.1016/0042-6822(82)90017-4. [DOI] [PubMed] [Google Scholar]
- Hefti E., Bishop D. H. The 5' sequence of VSV viral RNA and its in vitro transcription product RNA. Biochem Biophys Res Commun. 1975 Sep 16;66(2):785–792. doi: 10.1016/0006-291x(75)90578-1. [DOI] [PubMed] [Google Scholar]
- Hefti E., Bishop D. H. The 5' sequences of VSV in vitro transcription product RNA (+/-SAM). Biochem Biophys Res Commun. 1976 Jan 26;68(2):393–400. doi: 10.1016/0006-291x(76)91158-x. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Adler S., Lazzarini R. A., Colonno R. J., Banerjee A. K., Westphal H. Intervening polyadenylate sequences in RNA transcripts of vesicular stomatitis virus. Cell. 1978 Oct;15(2):587–596. doi: 10.1016/0092-8674(78)90027-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Internal initiation of translation on the vesicular stomatitis virus phosphoprotein mRNA yields a second protein. J Virol. 1986 Jun;58(3):797–804. doi: 10.1128/jvi.58.3.797-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Lazzarini R. A. Aberrant glycoprotein mRNA synthesized by the internal deletion mutant of vesicular stomatitis virus. J Virol. 1981 Oct;40(1):78–86. doi: 10.1128/jvi.40.1.78-86.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Lazzarini R. A. Vesicular stomatitis virus RNA polymerase can read through the boundary between the leader and N genes in vitro. J Virol. 1981 May;38(2):792–796. doi: 10.1128/jvi.38.2.792-796.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill V. M., Marnell L., Summers D. F. In vitro replication and assembly of vesicular stomatitis virus nucleocapsids. Virology. 1981 Aug;113(1):109–118. doi: 10.1016/0042-6822(81)90140-9. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Simonsen C. C., Summers D. F. Characterization of vesicular stomatitis virus replicating complexes isolated in renografin gradients. Virology. 1979 Nov;99(1):75–83. doi: 10.1016/0042-6822(79)90038-2. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Summers D. F. Synthesis of VSV RNPs in vitro by cellular VSV RNPs added to uninfected HeLa cell extracts: VSV protein requirements for replication in vitro. Virology. 1982 Dec;123(2):407–419. doi: 10.1016/0042-6822(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Horikami S. M., De Ferra F., Moyer S. A. Characterization of the infections of permissive and nonpermissive cells by host range mutants of vesicular stomatitis virus defective in RNA methylation. Virology. 1984 Oct 15;138(1):1–15. doi: 10.1016/0042-6822(84)90142-9. [DOI] [PubMed] [Google Scholar]
- Horikami S. M., Moyer S. A. Host range mutants of vesicular stomatitis virus defective in in vitro RNA methylation. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7694–7698. doi: 10.1073/pnas.79.24.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Constitutively phosphorylated residues in the NS protein of vesicular stomatitis virus. J Biol Chem. 1985 Jul 25;260(15):8990–8995. [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W., Murti K. G. Assembly of vesicular stomatitis virus nucleocapsids in vivo: a kinetic analysis. J Virol. 1979 Oct;32(1):304–313. doi: 10.1128/jvi.32.1.304-313.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. NS phosphoprotein of vesicular stomatitis virus: subspecies separated by electrophoresis and isoelectric focusing. J Virol. 1982 Apr;42(1):342–345. doi: 10.1128/jvi.42.1.342-345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Morgan E. M., Kingsbury D. W. Site-specific phosphorylation regulates the transcriptive activity of vesicular stomatitis virus NS protein. J Virol. 1982 Jul;43(1):104–112. doi: 10.1128/jvi.43.1.104-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Smith E. F., Buckley D. W. Aberrant polyadenylation by a vesicular stomatitis virus mutant is due to an altered L protein. J Virol. 1984 Nov;52(2):515–521. doi: 10.1128/jvi.52.2.515-521.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M. Vesicular stomatitis virus mutant with altered polyadenylic acid polymerase activity in vitro. J Virol. 1983 Jun;46(3):788–799. doi: 10.1128/jvi.46.3.788-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isle H. D., Emerson S. U. Use of a hybrid infectivity assay to analyze primary transcription of temperature-sensitive mutants of the New Jersey serotype of vesicular stomatitis virus. J Virol. 1982 Jul;43(1):37–40. doi: 10.1128/jvi.43.1.37-40.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981 Feb;23(2):477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Sequential synthesis of 5'-proximal vesicular stomatitis virus mRNA sequences. J Virol. 1982 Oct;44(1):356–365. doi: 10.1128/jvi.44.1.356-365.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A. Transcriptase activity associated with rabies virion. J Virol. 1977 Dec;24(3):826–835. doi: 10.1128/jvi.24.3.826-835.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Chien I. M., Lazzarini R. A. Vesicular stomatitis virus defective interfering particle containing a muted internal leader RNA gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2090–2094. doi: 10.1073/pnas.78.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Intervening sequence between the leader region and the nucleopcapsid gene of vesicular stomatitis virus RNA. J Virol. 1980 Feb;33(2):789–794. doi: 10.1128/jvi.33.2.789-794.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Thornton B. J., Emerson S. U. Sequence-specific contacts between the RNA polymerase of vesicular stomatitis virus and the leader RNA gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6191–6195. doi: 10.1073/pnas.78.10.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Rose J. K., Lodish H. F. Translation of individual species of vesicular stomatitis viral mRNA. J Virol. 1975 Apr;15(4):1004–1011. doi: 10.1128/jvi.15.4.1004-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson D. L. Rhabdoviruses. J Gen Virol. 1973 Jun;20(Suppl):105–130. doi: 10.1099/0022-1317-20-Supplement-105. [DOI] [PubMed] [Google Scholar]
- Kurath G., Ahern K. G., Pearson G. D., Leong J. C. Molecular cloning of the six mRNA species of infectious hematopoietic necrosis virus, a fish rhabdovirus, and gene order determination by R-loop mapping. J Virol. 1985 Feb;53(2):469–476. doi: 10.1128/jvi.53.2.469-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Leong J. C. Characterization of infectious hematopoietic necrosis virus mRNA species reveals a nonvirion rhabdovirus protein. J Virol. 1985 Feb;53(2):462–468. doi: 10.1128/jvi.53.2.462-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Cabradilla C. D., Holloway B. P., Keene J. D. Nucleotide sequence and host La protein interactions of rabies virus leader RNA. J Virol. 1984 Jun;50(3):773–778. doi: 10.1128/jvi.50.3.773-778.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Keene J. D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1983 Oct;34(3):837–845. doi: 10.1016/0092-8674(83)90541-x. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Chien I., Yang F., Keene J. D. The metabolic fate of independently initiated VSV mRNA transcripts. J Gen Virol. 1982 Feb;58(Pt 2):429–441. doi: 10.1099/0022-1317-58-2-429. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kolakofsky D. Effect of defective interfering particles on plus- and minus- strand leader RNAs in vesicular stomatitis virus-infected cells. J Virol. 1980 Sep;35(3):704–709. doi: 10.1128/jvi.35.3.704-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Lesnaw J. A., Dickson L. R., Curry R. H. Proposed replicative role of the NS polypeptide of vesicular stomatitis virus: structural analysis of an electrophoretic variant. J Virol. 1979 Jul;31(1):8–15. doi: 10.1128/jvi.31.1.8-15.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk D., Sánchez A., Banerjee A. K. Messenger RNA encoding the phosphoprotein (P) gene of human parainfluenza virus 3 is bicistronic. Virology. 1986 Sep;153(2):318–325. doi: 10.1016/0042-6822(86)90036-x. [DOI] [PubMed] [Google Scholar]
- Lynch K. R., Pennica D., Ennis H. L., Cohen P. S. Separation and purification of the mRNAs for vesicular stomatitis virus NS and M proteins. Virology. 1979 Oct 15;98(1):251–254. doi: 10.1016/0042-6822(79)90543-9. [DOI] [PubMed] [Google Scholar]
- Madore H. P., England J. M. Rabies virus protein synthesis in infected BHK-21 cells. J Virol. 1977 Apr;22(1):102–112. doi: 10.1128/jvi.22.1.102-112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell L. L., Summers D. F. Characterization of the phosphorylated small enzyme subunit, NS, of the vesicular stomatitis virus RNA polymerase. J Biol Chem. 1984 Nov 10;259(21):13518–13524. [PubMed] [Google Scholar]
- Martinet C., Combard A., Printz-Ané C., Printz P. Envelope proteins and replication of vesicular stomatitis virus: in vivo effects of RNA+ temperature-sensitive mutations on viral RNA synthesis. J Virol. 1979 Jan;29(1):123–133. doi: 10.1128/jvi.29.1.123-133.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Phosphoprotein NS of vesicular stomatitis virus: phosphorylated states and transcriptional activities of intracellular and virion forms. Virology. 1986 Oct 30;154(2):259–270. doi: 10.1016/0042-6822(86)90452-6. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Samuel C. E. Detection of in vivo synthesis of polycistronic mRNAs of vesicular stomatitis virus. Virology. 1984 Apr 30;134(2):277–286. doi: 10.1016/0042-6822(84)90297-6. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Virion RNA polymerases of two salmonid rhabdoviruses. J Virol. 1977 Jun;22(3):839–843. doi: 10.1128/jvi.22.3.839-843.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A. Sequence of 200 nucleotides at the 3'-terminus of the genome RNA of vesicular stomatitis virus. Nucleic Acids Res. 1979 Jul 25;6(10):3199–3211. doi: 10.1093/nar/6.10.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J. Structure of the gene N:gene NS intercistronic junction in the genome of vesicular stomatitis virus. Cell. 1979 Jul;17(3):673–681. doi: 10.1016/0092-8674(79)90274-5. [DOI] [PubMed] [Google Scholar]
- Mizumoto K., Kaziro Y., Lipmann F. Reaction mechanism of mRNA guanylyltransferase from rat liver: isolation and characterization of a guanylyl-enzyme intermediate. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1693–1697. doi: 10.1073/pnas.79.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Abraham G., Adler R., Banerjee A. K. Methylated and blocked 5' termini in vesicular stomatitis virus in vivo mRNAs. Cell. 1975 May;5(1):59–67. doi: 10.1016/0092-8674(75)90092-6. [DOI] [PubMed] [Google Scholar]
- Moyer S. A. Alteration of the 5' terminal caps of the mRNAs of vesicular stomatitis virus by cycloleucine in vivo. Virology. 1981 Jul 15;112(1):157–168. doi: 10.1016/0042-6822(81)90621-8. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Banerjee A. K. In vivo methylation of vesicular stomatitis virus and its host-cell messenger RNA species. Virology. 1976 Apr;70(2):339–351. doi: 10.1016/0042-6822(76)90276-2. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Banerjee A. K. Messenger RNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1975 Jan;4(1):37–43. doi: 10.1016/0092-8674(75)90131-2. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Grubman M. J., Ehrenfeld E., Banerjee A. K. Studies on the in vivo and in vitro messenger RNA species of vesicular stomatitis virus. Virology. 1975 Oct;67(2):463–473. doi: 10.1016/0042-6822(75)90447-x. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Kolakofsky C. M., Summers D. F. Comparison o;f vesicular stomatitis virus intracellular and virion ribonucleoproteins. J Virol. 1980 Feb;33(2):856–865. doi: 10.1128/jvi.33.2.856-865.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Summers D. F. Electron microscopy of vesicular stomatitis virus replicative ribonucleoproteins. J Virol. 1980 Jun;34(3):764–771. doi: 10.1128/jvi.34.3.764-771.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Nakashima K., Chanda P. K., Deutsch V., Banerjee A. K., Shatkin A. J. Inactivation of influenza and vesicular stomatitis virion RNA polymerase activities by photoreaction with 4'-substituted psoralens. J Virol. 1979 Dec;32(3):838–844. doi: 10.1128/jvi.32.3.838-844.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongrádi J., Cunningham C., Szilágyi J. F. Temperature sensitivity of the transcriptase of mutants tsB1 and tsF1 of vesicular stomatitis virus New Jersey is a consequence of mutation affecting polypeptide L. J Gen Virol. 1985 Jul;66(Pt 7):1507–1513. doi: 10.1099/0022-1317-66-7-1507. [DOI] [PubMed] [Google Scholar]
- Ongrádi J., Cunningham C., Szilágyi J. F. The role of polypeptides L and NS in the transcription process of vesicular stomatitis virus New Jersey using the temperature-sensitive mutant tsE1. J Gen Virol. 1985 May;66(Pt 5):1011–1023. doi: 10.1099/0022-1317-66-5-1011. [DOI] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wiener J. R., Volk W. A., Wagner R. R. Monoclonal antibodies to the M protein of vesicular stomatitis virus (Indiana serotype) and to a cDNA M gene expression product. J Virol. 1985 Aug;55(2):298–306. doi: 10.1128/jvi.55.2.298-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. Cell-free synthesis and assembly of vesicular stomatitis virus nucleocapsids. J Virol. 1983 Jan;45(1):155–164. doi: 10.1128/jvi.45.1.155-164.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. Inhibition of vesicular stomatitis virus RNA synthesis by 2',3'-dideoxycytidine 5'-triphosphate. J Gen Virol. 1983 Mar;64(Pt 3):743–748. doi: 10.1099/0022-1317-64-3-743. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Initiation and replication of vesicular stomatitis virus genome RNA in a cell-free system. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3198–3202. doi: 10.1073/pnas.80.11.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Lynch K. R., Cohen P. S., Ennis H. L. Decay of vesicular stomatitis virus mRNAs in vivo. Virology. 1979 Apr 30;94(2):484–487. doi: 10.1016/0042-6822(79)90480-x. [DOI] [PubMed] [Google Scholar]
- Perlman S. M., Huang A. S. RNA synthesis of vesicular stomatitis virus. V. Interactions between transcription and replication. J Virol. 1973 Dec;12(6):1395–1400. doi: 10.1128/jvi.12.6.1395-1400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Clinton G. M., McClure M. A. RNP template of vesicular stomatitis virus regulates transcription and replication functions. Cell. 1983 Nov;35(1):175–185. doi: 10.1016/0092-8674(83)90220-9. [DOI] [PubMed] [Google Scholar]
- Perrault J., Kingsbury D. T. Inhibitor of vesicular stomatitis virus transcriptase in purified virions. Nature. 1974 Mar 1;248(5443):45–47. doi: 10.1038/248045a0. [DOI] [PubMed] [Google Scholar]
- Perrault J., McLear P. W. ATP dependence of vesicular stomatitis virus transcription initiation and modulation by mutation in the nucleocapsid protein. J Virol. 1984 Sep;51(3):635–642. doi: 10.1128/jvi.51.3.635-642.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Semler B. L. Internal genome deletions in two distinct classes of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6191–6195. doi: 10.1073/pnas.76.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. Identification and characterization of a group of discrete initiated oligonucleotides transcribed in vitro from the 3' terminus of the N-gene of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):889–896. doi: 10.1128/jvi.42.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwnica-Worms H., Keene J. D. Sequential synthesis of small capped RNA transcripts in vitro by vesicular stomatitis virus. Virology. 1983 Feb;125(1):206–218. doi: 10.1016/0042-6822(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. The tdCE and hrCE phenotypes: host range mutants of vesicular stomatitis virus in which polymerase function is affected. Cell. 1978 Oct;15(2):597–606. doi: 10.1016/0092-8674(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Schnitzlein W. M., Reichmann M. E. Use of defective interfering particle RNA probes in the determination of the order of in vitro transcription of vesicular stomatitis virus genes. J Virol. 1977 Jan;21(1):432–434. doi: 10.1128/jvi.21.1.432-434.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Abraham G., Colonno R. J., Jelinek W., Banerjee A. K. Characterization of vesicular stomatitis virus mRNA species synthesized in vitro. J Virol. 1977 Mar;21(3):1105–1112. doi: 10.1128/jvi.21.3.1105-1112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Banerjee A. K. 5'-terminal sequence of vesicular stomatitis virus mRNA's synthesized in vitro. J Virol. 1975 Jan;17(1):33–42. doi: 10.1128/jvi.17.1.33-42.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Moyer S. A., Banerjee A. K. In vitro synthesis of methylated messenger RNA by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1974 Dec;3(4):327–333. doi: 10.1016/0092-8674(74)90046-4. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Rose J. K., Knipe D. Nucleotide sequence complexities, molecular weights, and poly(A) content of the vesicular stomatitis virus mRNA species. J Virol. 1975 Apr;15(4):994–1003. doi: 10.1128/jvi.15.4.994-1003.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F., Brock M. L. Giant heterogeneous polyadenylic acid on vesicular stomatitis virus mRNA synthesized in vitro in the presence of S-adenosylhomocysteine. J Virol. 1977 Feb;21(2):683–693. doi: 10.1128/jvi.21.2.683-693.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands D. J. Sequences of vesicular stomatitis virus RNA in the region coding for leader RNA, N protein mRNA, and their junction. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4793–4797. doi: 10.1073/pnas.76.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Clewley J. P. Spring viremia of carp virus RNA and virion-associated transcriptase activity. J Virol. 1978 Mar;25(3):912–916. doi: 10.1128/jvi.25.3.912-916.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio C., Kolakofsky C., Hill V. M., Summers D. F. Replication and assembly of VSV nucleocapsids: protein association with RNPs and the effects of cycloheximide on replication. Virology. 1980 Aug;105(1):123–135. doi: 10.1016/0042-6822(80)90161-0. [DOI] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Richardson C. D., Meier E. Expression of a cDNA encoding a functional 241-kilodalton vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7984–7988. doi: 10.1073/pnas.82.23.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Sprague J., Condra C. S., Lazzarini R. A. In vitro transcription of vesicular stomatitis virus: initiation with GTP at a specific site within the N cistron. J Virol. 1982 Jul;43(1):166–173. doi: 10.1128/jvi.43.1.166-173.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Herman R. C., Lazzarini R. A. Site on the vesicular stomatitis virus genome specifying polyadenylation and the end of the L gene mRNA. J Virol. 1980 May;34(2):550–559. doi: 10.1128/jvi.34.2.550-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Schubert M., Lazzarini R. A. In vitro transcription of vesicular stomatitis virus. Incorporation of deoxyguanosine and deoxycytidine, and formation of deoxyguanosine caps. J Biol Chem. 1982 Mar 25;257(6):2968–2973. [PubMed] [Google Scholar]
- Schubert M., Lazzarini R. A. In vivo transcription of the 5'-terminal extracistronic region of vesicular stomatitis virus RNA. J Virol. 1981 Apr;38(1):256–262. doi: 10.1128/jvi.38.1.256-262.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Abelson J., Holland J. J. Sequence of a RNA templated by the 3'-OH RNA terminus of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4704–4708. doi: 10.1073/pnas.75.10.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Batt-Humphries S., Summers D. F. RNA synthesis of vesicular stomatitis virus-infected cells: in vivo regulation of replication. J Virol. 1979 Jul;31(1):124–132. doi: 10.1128/jvi.31.1.124-132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Hill V. M., Summers D. F. Further characterization of the replicative complex of vesicular stomatitis virus. J Virol. 1979 Aug;31(2):494–505. doi: 10.1128/jvi.31.2.494-505.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Obijeski J. F., Morrongiello M. P. Conditional lethal mutants of vesicular stomatitis virus. III. Host range properties, interfering capacity, and complementation patterns of specific hr mutants. Virology. 1979 Mar;93(2):493–505. doi: 10.1016/0042-6822(79)90252-6. [DOI] [PubMed] [Google Scholar]
- Sinacore M. S., Lucas-Lenard J. The effect of the vesicular stomatitis virus-associated protein kinase on viral mRNA transcription in vitro. Virology. 1982 Sep;121(2):404–413. doi: 10.1016/0042-6822(82)90178-7. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F., Wiktor T. J., McFalls M. L., Bishop D. H., Obijeski J. F. Structural phosphoproteins associated with ten rhabdoviruses. J Gen Virol. 1974 Sep;24(3):433–445. doi: 10.1099/0022-1317-24-3-433. [DOI] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R., Macpherson T. M. Temperature-dependent host range mutation in vesicular stomatitis virus affecting polypeptide L. J Virol. 1977 May;22(2):381–388. doi: 10.1128/jvi.22.2.381-388.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Virion trascriptase activity differences in host range mutants of vesicular stomatitis virus. J Virol. 1975 Oct;16(4):927–936. doi: 10.1128/jvi.16.4.927-936.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Uryvayev L. Isolation of an infectious ribonucleoprotein from vesicular stomatitis virus containing an active RNA transcriptase. J Virol. 1973 Feb;11(2):279–286. doi: 10.1128/jvi.11.2.279-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Talib S., Banerjee A. K. Covalent attachment of psoralen to a single site on vesicular stomatitis virus genome RNA blocks expression of viral genes. Virology. 1982 Apr 30;118(2):430–438. doi: 10.1016/0042-6822(82)90362-2. [DOI] [PubMed] [Google Scholar]
- Talib S., Banerjee A. K. Protamine--a potent inhibitor of vesicular stomatitis virus transcriptase in vitro. Biochem Biophys Res Commun. 1981 Feb 12;98(3):875–883. doi: 10.1016/0006-291x(81)91192-x. [DOI] [PubMed] [Google Scholar]
- Talib S., Hearst J. E. Initiation of RNA synthesis in vitro by vesicular stomatitis virus: single internal initiation in the presence of aurintricarboxylic acid and vanadyl ribonucleoside complexes. Nucleic Acids Res. 1983 Oct 25;11(20):7031–7042. doi: 10.1093/nar/11.20.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. In vitro synthesis of a possible precursor RNA by purified vesicular stomatitis virus. Biochem Biophys Res Commun. 1978 May 30;82(2):655–664. doi: 10.1016/0006-291x(78)90925-7. [DOI] [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Initiation of RNA synthesis in vitro by vesicular stomatitis virus. Role of ATP. J Biol Chem. 1979 Mar 25;254(6):2053–2058. [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Nucleoside diphosphate kinase activity in purified cores of vesicular stomatitis virus. J Biol Chem. 1979 Sep 25;254(18):9075–9079. [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Two methyltransferase activities in the purified virions of vesicular stomatitis virus. J Virol. 1977 Dec;24(3):786–793. doi: 10.1128/jvi.24.3.786-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. In vitro synthesis of the full-length complement of the negative-strand genome RNA of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Jan;77(1):294–298. doi: 10.1073/pnas.77.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton G. B., De B. P., Banerjee A. K. Interaction of L and NS proteins of vesicular stomatitis virus with its template ribonucleoprotein during RNA synthesis in vitro. J Gen Virol. 1984 Mar;65(Pt 3):663–668. doi: 10.1099/0022-1317-65-3-663. [DOI] [PubMed] [Google Scholar]
- Thornton G. B., Kopchick J. J., Stacey D. W., Banerjee A. K. Microinjection of vesicular stomatitis virus ribonucleoprotein into animal cells yields infectious virus. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1160–1167. doi: 10.1016/s0006-291x(83)80264-2. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Characterization and translation of methylated and unmethylated vesicular stomatitis virus mRNA synthesized in vitro by ribonucleoprotein particles from vesicular stomatitis virus-infected L cells. J Virol. 1976 Feb;17(2):477–491. doi: 10.1128/jvi.17.2.477-491.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffereau C., Fischer S., Flamand A. Phosphorylation of the N and M1 proteins of rabies virus. J Gen Virol. 1985 Oct;66(Pt 10):2285–2289. doi: 10.1099/0022-1317-66-10-2285. [DOI] [PubMed] [Google Scholar]
- Tuffereau C., Lafay F., Flamand A. Biochemical analysis of rabies virus proteins in three persistently infected BHK-21 cell lines. J Gen Virol. 1985 Jan;66(Pt 1):159–169. doi: 10.1099/0022-1317-66-1-159. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Moss B. Eukaryotic mRNA capping enzyme-guanylate covalent intermediate. Proc Natl Acad Sci U S A. 1982 Jan;79(2):340–344. doi: 10.1073/pnas.79.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P., Breindl M., Holland J. J. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry. 1976 Apr 20;15(8):1663–1667. doi: 10.1021/bi00653a012. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Holland J. J. Synthesis of poly(A) in vitro by purified virions of vesicular stomatitis virus. Nat New Biol. 1973 Nov 7;246(149):17–19. doi: 10.1038/newbio246017a0. [DOI] [PubMed] [Google Scholar]
- Wang D., Furuichi Y., Shatkin A. J. Covalent guanylyl intermediate formed by HeLa cell mRNA capping enzyme. Mol Cell Biol. 1982 Aug;2(8):993–1001. doi: 10.1128/mcb.2.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Shatkin A. J. Synthesis of Gp4N and Gp3N compounds by guanylyltransferase purified from yeast. Nucleic Acids Res. 1984 Mar 12;12(5):2303–2315. doi: 10.1093/nar/12.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Sakuma S., Tanaka S. A possible biological function of the protein kinase associated with vaccinia and vesicular stomatitis virions. FEBS Lett. 1974 May 1;41(2):331–334. doi: 10.1016/0014-5793(74)81241-x. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Davis N. Characterization and mapping of RNase III cleavage sites in VSV genome RNA. Nucleic Acids Res. 1981 Dec 11;9(23):6487–6503. doi: 10.1093/nar/9.23.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Levine M. RNA synthesis by vesicular stomatitis virus and a small plaque mutant: effects of cycloheximide. J Virol. 1973 Aug;12(2):253–264. doi: 10.1128/jvi.12.2.253-264.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W. Replication of vesicular stomatitis virus defective interfering particle RNA in vitro: transition from synthesis of defective interfering leader RNA to synthesis of full-length defective interfering RNA. J Virol. 1983 May;46(2):513–522. doi: 10.1128/jvi.46.2.513-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Kurilla M. G., Keene J. D. A host protein (La) binds to a unique species of minus-sense leader RNA during replication of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5827–5831. doi: 10.1073/pnas.80.19.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Youngner J. S., Keene J. D. Base mutations in the terminal noncoding regions of the genome of vesicular stomatitis virus isolated from persistent infections of L cells. Virology. 1985 Jan 30;140(2):249–256. doi: 10.1016/0042-6822(85)90363-0. [DOI] [PubMed] [Google Scholar]
- Witt D. J., Summers D. F. Relationship between virion-associated kinase-effected phosphorylation and transcription activity of vesicular stomatitis virus. Virology. 1980 Nov;107(1):34–49. doi: 10.1016/0042-6822(80)90270-6. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Dietzschold B., Smith C. L., Lafon M., Golub E. Antigenic variants of CVS rabies virus with altered glycosylation sites. Virology. 1985 Jan 15;140(1):1–12. doi: 10.1016/0042-6822(85)90440-4. [DOI] [PubMed] [Google Scholar]
- Ye Z., Pal R., Ogden J. R., Snyder R. M., Wagner R. R. Monoclonal antibodies to the matrix protein of vesicular stomatitis virus (New Jersey serotype) and their effects on viral transcription. Virology. 1985 Jun;143(2):657–662. doi: 10.1016/0042-6822(85)90408-8. [DOI] [PubMed] [Google Scholar]
- Zuidema D., Heaton L. A., Hanau R., Jackson A. O. Detection and sequence of plus-strand leader RNA of sonchus yellow net virus, a plant rhabdovirus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5019–5023. doi: 10.1073/pnas.83.14.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]


