Abstract
The increased use of natural product in the pharmaceutical industry has led to an increase in demand for screening for bioactive compounds in marine algae. An important economic algae, through chemical composition analysis and their antioxidant activities were investigated in this study. Chemical composition analysis of three algal samples from the Chlorophyta Ulva lactuca (U), Phaeophyta Sargassum crassifolia (S) and Rhodophyta Digenea simplex (D) was tested. Main components were sugars (57.40–185.13 mg/g dry weight), uronic acids (29.3–45.26 mg/g dry weight), sulfate (94.7–181.2 mg/g dry weight), amino acids (7.6–16.7 mg/g dry weight) and small amounts of betaines (2.38–8.47 mg/g dry weight). Hydrolyzed chemical composition analysis fractions of algal extract was shown a great proportion of sugars plus sulfate (as polysaccharide composed) ranges between 332 and 538.2 mg/g dry weight with trace amounts of uronic acids (⩽9%). All three algal extract showed antioxidant activities on lipoxygenase, DPPH and on Ames test. Two of aqueous extracts (U and D) inhibited lipoxygenase activity by less than 50%, where as the methanolic extract (S) caused 76% inhibition of the control. In all cases, the methanolic extract were more inhibitory than the aqueous extract. The (S) showed the highest antioxidant activity with DPPH (69%) in aqueous extract and in methanol extract with Ames test (85%). Both U and D showed antioxidant activity with DPPH in hexane by less of 25% where as in both aqueous and methanolic extracts by less than 50% of the control. Aqueous and methanolic extracts of U and D showed high inhibition by Ames test which caused 70% and 75% respectively. IR spectra of algal extracts (U; D and S) range from 1450 to 750 cm−1 were very similar absorption band at 1430, 1370, 1250, 1130, 1110, 1050 and 1020 cm−1. Absorption bands were due to uronic acids, glucosides and sulfate. The presence of sulfated polysaccharide material in the fractions UF2, DF2 and SF2 were found as cell wall storage of marine algae, confirmed by 13C NMR spectroscopy. It is concluded that the algal species probably have a different components and can be used in the activities of antioxidant enzymes as reduced the risks of enzymes. But the correlation between the chemical composition and antioxidant activities of algal extracts needs further investigation.
Abbreviations: (U), Ulva lactuca; (S), Sargassum crassifolia; (D), Digenea simplex; DPPH, α-diphenyl-β-picrylhydrazyl; HPLC, high performance liquid chromatographic
Keywords: Seaweeds, Algae, Antioxidant activity, Lipoxygenase activity
1. Introduction
During the last years, many studies have been made on biological activities of the seaweed (Ehresmann et al., 1977), and could be potential rich sources of natural antioxidants (Matanjun et al., 2008). Traditionally, seaweeds have been used in the treatment of various infectious diseases (Hoppe, 1999), and reports of many active compounds have been isolated and their structure determined (Vairappan et al., 2001, Mundt et al., 2003). Among the features of marine algae and their substance, several extracts were screened on an antioxidant capability (Latham, 2008), and their inhibitory activity on lipoxygenase enzyme (Mori et al., 2003), as well as by radical scavenging activity, using a stable free radical (Matsukawa et al., 1997). Until now, however, no screening for antioxidant activities has been done with Jeddah corniche algae, even though the abundance and diversity of algae in the coastal waters of the Jeddah corniche are very high (Mutawie, 2006). We therefore decided to incorporate these studies into our test protocols to antioxidant activities of selected algae.
2. Materials and methods
2.1. Algal materials
Algal samples were collected along Jeddah corniche, about 22 km through the longitudinal direction of N–W. Samples were randomly selected at depths of about 20–100 cm and three different samples belong to different classes of algae were collected. Samples of algae were cleaned of barnacle, gastropod and other contaminants at the site then immediately transported to the laboratory in polyethylene container. Part of that they were air-dried at room temperature and other part were stored polyethylene plastic bags as fresh material in freezer until used.
2.2. Extract preparation
Dry algal material (100 g) was extracted with methanol (absolute) in a Soxhlet apparatus for 8 h according to the method of Blunden et al. (1981). The extract was concentrated under reduced pressure at 60 °C using rotary evaporator then filtered, washed with about 25 ml distilled water and stored in the dark at 4 °C according to the method of Nascimento et al. (1993).
2.3. Composition analysis
Specimens of extract samples were used for composition analysis, total sugar content was determined by modified method described by Hellebust and Craigie (1978). Hydrolyzed sugar at 105 °C for 2.5 h were also determined with the same technique described by Adams (1965). Uronic acids assays were carried out using the method described by Blumenkrantz and Asboe-Hansen (1973). Amino acid assays were carried out using the method described by Matoh et al. (1980). Betaines estimation were carried out using HPLC assay according to the method of Gorham (1984). Sulfate content was determined according to the method of David and James (1979). Sulfate hydrolyzed with 1 M HCl was calculated and expressed as sulfate equivalents according to the method of Ruperez et al. (2002).
2.4. Infrared spectra
Extract samples were centrifuged at 3000 (rpm) for 30 min and after lyophilization, characterized by infrared spectra (FT-IR/NICOLET–ESPN670).
2.5. Fractionation
Fractionation of extracts by centrifugations yielded two fractions (F1 and F2). Fractions were extracted with MeOH–CHCl3 and soluble fraction (F1) as a major containing fraction. The process was repeated twice and the combined supernatants were used. The residue was then sequentially extracted with a successive MeOH–CHCl3 and the final volume was measured and noted as fraction (F2). Samples of each fractions from the three algal samples (F1 and F2) were tested for hydrolyzed chemical composition. Fraction (F2) was run on silica gel column chromatographed using chloroform and methanol as described by Vairappan et al. (2001), and after lyophilization, characterized by 13H NMR according to the method of Pengzhan et al. (2003).
2.6. Lipoxygenase test
The assay for enzyme (lipoxygenase) activity was carried out as described by Matsukawa et al. (1997). The reaction mixture contained 0.2 M borate buffer pH 9.0, Tween 20, linolic acid, an enzyme solution (0.1 U/ml dissolved in ice cold borate buffer). The enzyme reaction was carried out in the cuvette and monitored at 234 nm using UV–visible recording spectrophotometer (160A-Shimadzu, Japan), The percentage inhibition was defined by the presence and absence of fraction material.
2.7. DPPH test
Radical scavenging activity of the fraction material was determined by the use of a stable free radical DPPH (α-diphenyl-β-picrylhydrazyl). The oxidation and decolorization of DPPH was followed in absorbance at 540 nm. according to Matsukawa et al. (1997).
2.8. Ames test
Plate test consist of 100 μl of the fraction, 100 μl of bacterial strain (TA/104) and 100 μl H2O2 incubated for 30 min. Enzyme (0.5 ml) was added to the top agar (0.5 mM histidine/biotin solution). Mixture was transformed to the glucose plate and incubated at 37 °C for 48 h. Colonies were counted and the percentage inhibition was defined as described by Maron and Ames (1983).
3. Statistical analysis
The experimental data were analyzed for statistical significance between control and mean values of treated groups, using Origin 5.0 software. Values were presented as means (±) Standard Error (SE). Data were analyzed by Student’s t-test. Differences with P < 0.05 were considered significant.
4. Results and discussion
4.1. Chemical analysis
The chemical compositions analysis of algal extract of three chosen species Ulva lactuca (U), Digenea simplex (D) and Sargassum crassifolia (S) from green, red and brown algae respectively were shown (Table 1). Total recovery (21.1–42.4%) of the algal dry weight corresponded to nondialyzable compounds, as free minerals and low-molecular-weight substances were removed during extraction and centrifugation process. The result was consistent with infrared analysis. Main and highest components were sugars (57.40–185.13 mg/g dry weight), uronic acids (29.3–45.26 mg/g dry weight), sulfate (94.7–181.2 mg/g dry weight), whereas amino acids had very low (7.6–16.7 mg/g dry weight) and small amounts of betaines (2.38–8.47 mg/g dry weight), as lowest content components in all algal extracts. Studies on chemical compositions from brown algae, showed their relatively high sulfate content (Haroun-Bouhedia et al., 2000).
Table 1.
Chemical composition analysis of algal extract (mg/g dry weight).
| Algal sample | Yield (%) | Sugar | Uronic acids | Sulfate | Betaines | Amino acid | Total recovery (%) |
|---|---|---|---|---|---|---|---|
| Ulva lactuca (U) | 21.79 ± 0.6 | 68.40 ± 0.06∗ | 29.3 ± 0.04∗ | 94.7 ± 0.21∗ | 8.47 ± 0.62 | 7.6 ± 0.1 | 21.1 |
| Digenea simplex (D) | 23.52 ± 0.8 | 185.13 ± 0.12∗ | 45.26 ± 0.2∗ | 173.1 ± 0.42 | 6.35 ± 0.32 | 14.30 ± 0.24 | 42.4 |
| Sargassum crassifolia (S) | 25.11 ± 1.2 | 57.40 ± 0.22 | 36.06 ± 0.04 | 181.2 ± 0.41∗ | 2.38 ± 0.1 | 16.7 ± 0.22 | 29.4 |
Data are mean value of triplicate determinations (±) SD = standard deviation.
Significant at P < 0.05.
4.2. Antioxidant activities
Antioxidant activities of algal extracts (Table 2) was estimated from their ability to inhibit lipoxygenase activity or to oxidized and decolorized the DPPH and to determine the inhibition of bacterial colonies as by Ames test (Maron and Ames, 1983). Two of aqueous (U and D) extracts and one of methanol (S) extract inhibited lipoxygenase activity by less than 50% of the control. The most potent methanol extract was that of (S), which caused 76% inhibition. In all cases, the methanol extract (hexane not determined) were more inhibitory than the aqueous extract. The sequence of antioxidant activity as assayed by lipoxygenase inhibition by the methanol extract was as follows: (S) > (D) > (U). Of the seaweed extracts tested, from (S) showed the highest antioxidant activity with DPPH (69%) in aqueous extract and in methanol extract with Ames (85%) test (hexane not determined). The current literature reports that many different in vitro methods are being used to evaluate antioxidants of interest in many biological systems (Frankel and Meyer, 2000). In some of these protocols, samples were extracted with organic solvents (Yan et al., 1998) and in aqueous (Matsukawa et al., 1997); however, on these conditions one single test being used to evaluate inhibition A good efficiency in the vitro inhibition of LDL oxidation was reported by Jimenez-Escrig et al. (2001). Extracts (U and D) showed antioxidant activity with DPPH in hexane by less of 25% where as in both aqueous and methanol extracts by less than 50% of the control. Both aqueous and methanol extracts (U and D) were also examined by Ames test and showed high inhibition (70–75%). The antioxidant activity of brown was attributed to their phloroglucinol content (Ruperez et al., 2002). Our results are in agreement with Matsukawa et al. (1997), who found that the antioxidant activity of brown algae was superior to that of red or green groups. Antioxidant potential of sulfated polysaccharides from the brown algae was higher than that of agar-like sulfated galactans from the red algae (Ruperez, 2001). Fujimoto and Kaneda (1980) reported the chloroform-soluble phospholipids fraction of Eisenia bicyclis showed high antioxidant activity.
Table 2.
Antioxidant activities of algal extract.
| Algal extract | (% Inhibition) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Aqueous |
Methanola |
Hexaneb |
|||||||
| Lt | At | Dt | Lt | At | Dt | Lt | At | Dt | |
| Ulva lactuca (U) | 24 | n.d. | 34.9 | 39 | 70 | 30.2 | n.d. | n.d. | 18.7 |
| Digenea simplex (D) | 48 | n.d. | 42.3∗ | 52 | 75 | 41.0 | n.d. | n.d. | 21.5 |
| Sargassum crassifolia (S) | 65∗ | n.d. | 69.3∗ | 76 | 85 | 49.7∗ | n.d. | n.d. | 24.9∗ |
Lt = lipoxygenase test; At = Ames test; Dt = DPPH test; n.d. = not determined.
Correlation between soluble extract in aqueous and methanol (% inhibition), r = 0.802, P < 0.05; and between aqueous and hexane.
r = 0.70, P < 0.05.
Significant at P < 0.05.
4.3. Fractions analysis
Fractions (F1 and F2) contained a high proportion of sulfate, sugars and quite low content of uronic acids in tested hydrolyzed fractions of the three algae. Hydrolyzed chemical composition analysis fractions of algal extract were shown in Table 3. F1 and F2 contained sugars, uronic acids and sulfate as the main constituent of all algae. The low level of soluble sugar (SF1) in (S) could be related to the high values of insoluble (SF2 – 123.8 mg/g dry weight) associated to lignin or dietary fiber of alga. A great proportion of three algae could be sugars plus sulfate (as polysaccharide composed) ranges (332–538.2 mg/g dry weight) with trace amounts of uronic acids (⩽9%). The amounts and type of compounds separated after centrifugation procedure into different fractions would, of course, vary from algal yield. Fraction values obtained to be (14.93 g) in (SF1), (13.41 g) in (DF1) and (12.28 g) in (UF1). This would mainly contain the majority of quaternary alkaloids and N-oxides (Blunden et al., 1982). On the other hand, fraction yield was found (6.96 g) in (SF2), (5.16 g) in (DF2) and (4.78 g) in (UF2). These fractions (F2) would mainly presented the polysaccharide as insoluble residue which contained may be small amounts of free sugar from cellulose (Pengzhan et al., 2003) with slight amount of alkaloids (Dragendorff-positive compounds) such as betaines (Blunden et al., 1982).
Table 3.
Hydrolyzed chemical composition analysis in fractions (F1 & F2) of algal extract (mg/g dry weight).
| Fraction | Yield (g) | Sugar | Uronic acids | Sulfate |
|---|---|---|---|---|
| UF1 | 12.28 | 27.5 ± 0.26∗ | 14.3 ± 0.13 | 39.4 ± 0.02 |
| UF2 | 4.78 | 136.69 ± 0.04 | 14.8 ± 0.01 | 127.9 ± 0.01 |
| DF1 | 13.41 | 75.32 ± 0.16 | 17.03 ± 0.2 | 58.02 ± 0.55 |
| DF2 | 5.16 | 212.21 ± 0.01 | 24.43 ± 0.2 | 192.6 ± 0.26 |
| SF1 | 14.93 | 21.7 ± 0.22 | 15.09 ± 0.21 | 45.07 ± 0.22 |
| SF2 | 6.96 | 123.8 ± 0.03 | 20.4 ± 0.19 | 215.7 ± 0.31 |
Data are mean value of triplicate determinations (±) SD = standard deviation.
Significant at P < 0.05. UF1 & UF2 = Ulva lactuca; DF1 & DF2 = Digenea simplex; SF1 & SF2 = Sargassum crassifolia.
4.4. Infrared spectrum
IR spectra of algal extract from the three algal species (U; D and S) are shown in Fig. 1. Wavenumbers range from 1450 to 750 cm−1 and the extracts (U, D and S) were very similar absorption band (at 1430, 1370, 1250, 1130, 1110, 1050 and 1020 cm−1). A large absorption band at 1430 cm−1 in with a small shoulder at 1450 cm−1 was due to uronic acids, in agreement with a higher uronic acids content (Ruperez et al., 2002). Two important bands were assigned at 1370 and 1050 cm−1 corresponding respectively to the stretching of C O of uronic acids and the vibration of the C–O–C bridge of glucosides (Pengzhan et al., 2003). The absorption at 1230–1020 cm−1 was due to sulfate and attributed to stretching of C–O–S (Pantakar et al., 1993, Ruperez et al., 2002). The band close to 1250 cm−1 was quite similar to that previously used for calculating total ester sulfate content of carrageenan and agar (Melo et al., 2002).
Figure 1.
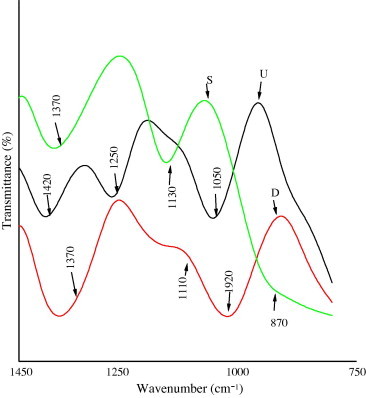
Infrared absorption spectrum of algal extracts from; Ulva lactuca (U); Digenea simplex (D); Sargassum crassifolia (S). Wavenumbers range from 1450 to 750 cm−1.
4.5. NMR analysis
The 13C NMR spectrum of (UF2, DF2 and SF2) from Ulva lactuca, Digenea simplex and Sargassum crassifolia respectively are shown (Table 4). The signals assignment were done by comparison with the previously published data (Lahaye et al., 1998, Melo et al., 2002). The major signals type G6M-4S (6-0-methyl-d-galactose-4 sulfate) was prominently observed in the spectrum from (UF2); G′-L6S (1–3) β-d-galactose (1-4)-α-l-galactose-6-sulfate was prominently observed in the spectrum from (DF2) and A as basic repeating structures of (1–4) linked 3,6 anhydrogalactose was prominently observed in the spectrum from (SF2). The major signals for algal fractions (UF2, DF2 and SF2) were observed in the spectrum corresponding to polysaccharides materials as expected, their cell wall storage of marine algae sulfated polysaccharides, as the same as those of other (Pengzhan et al., 2003). In summary, polysaccharides which are associated as expected with sulfate from algal extracts and especially (F2) which exhibited antioxidant potential. Nevertheless, at present, the mechanisms by which sulfated polysaccharides from the marine algae exert their antioxidant power are still unknown.
Table 4.
Chemical shift assignment for 13C NMR spectra of main units from algal extract.
| Residue unit |
13Carbon chemical shift |
References | |||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | ||
| G | 102.4 | 70.2 | 82.2 | 68.8 | 75.3 | 61.4 | Lahaye et al. (1998) |
| A | 98.3 | 69.9 | 80.1 | 77.4 | 75.7 | 69.4 | |
| G′ | 103.7 | 69.8 | 81.2 | 69.1 | 75.9 | 61.8 | |
| L6S | 101.3 | 69.3 | 71.1 | 79.1 | 70.3 | 67.9 | |
| G | 102.0 | 69.8 | 81.8 | 68.4 | 75.0 | 61.0 | Melo et al. (2002) |
| A | 98.0 | 69.4 | 79.7 | 77.0 | 75.2 | 69.3 | |
| G6M-4S | 102.2 | 70.1 | 80.2 | 71.4 | 70.1 | 68.6 | |
| G′ | 103.1 | 69.9 | 81.8 | 69.0 | 75.5 | 61.3 | |
| L6S | 100.8 | 69.5 | 71.4 | 79.9 | 70.4 | 67.2 | |
| ∗Fraction | |||||||
| UF2 | 100.1 | 72.5 | 81.1 | 71.4 | 71.1 | 70.3 | ∗Observed spectra |
| DF2 | 99.7 | 70.5 | 80.3 | 71.2 | 72.8 | 62.2 | |
| SF2 | 99.8 | 71.3 | 79.1 | 78.4 | 74.3 | 69.3 | |
G = (103) linked β-d-galactose; A = (1–4) linked 3,6 anhydrogalactose; G6M-4S = 6-0-methyl-d-galactose-4 sulfate; G′-L6S = (1–3) β-d-galactose (1–4)-α-l-galactose-6-sulfate.
UF2 = Ulva lactuca, DF2 = Digenea simplex, SF2 = Sargassum crassifolia.
5. Conclusion
Marine algae exert their antioxidant power are still unknown. In this sense, it is of great interest to have available highly purified and well characterized sulfated polysaccharides with which to elucidate their mode of action. Algal species probably have a different components and can be used in the activities of antioxidant enzymes as reduced the risks of enzymes. But the correlation between the chemical composition and antioxidant activities of algal extracts needs further investigation.

Acknowledgements
The authors are grateful to Professor M.J. Wynne, Department of Ecology and Evolutionary Biology, University of Michigan Ann Arbor, MI 48109, USA for his kind help and keen interest for algal identification. We thank Dr. Sufian Al-Assoli, King Fahd Medical Center at King Abdul Aziz University for help in Ames test. H.H. Mutawie acknowledges the receipt of financial support from Umm Al-Qura University which made the studies possible.
References
- Adams G.A. Complete acid hydrolysis. Meth. Carbohydr. Chem. 1965;5:269–276. [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acid. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Blunden G., Barouni M., Sally G., Mclean W.F., Rogers D. Extraction, purification and characterization of Dragendorff-positive compounds from some British marine algae. Bot. Mar. 1981;24:451–456. [Google Scholar]
- Blunden G., Gorden S.M., Mclean W.F.H., Guiry M.D. The distribution and possible taxonomic significance of quaternary ammonium and other Dragendorff-positive compounds in some general of marine algae. Bot. Mar. 1982;25:563–567. [Google Scholar]
- David F.B., James A.H. second ed. vol. 8. Academic Press; New York, USA: 1979. (Colorimetric Determination of Nonmetals). [Google Scholar]
- Ehresmann D.W., Deig E.F., Hatch M.T., Vedros V.A. Antiviral substance from California marine algae. Phycologia. 1977;13:37–40. [Google Scholar]
- Frankel E.N., Meyer A.S. The problem of using one dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000;80:1925–1941. [Google Scholar]
- Fujimoto K., Kaneda T. Screening test for antioxygenic compounds from marine algae and fractionation from Eisenia bicyclis and Undaria pinnatifida. Bull. Japan Soc. Sci. Fish. 1980;46:1125–1130. [Google Scholar]
- Gorham J. Separation of plant betaines and their sulphur analogues by cation exchange High-Performance Liquid Chromatography. J. Chromat. 1984;287:345–351. [Google Scholar]
- Haroun-Bouhedia F., Ellouali M., Sinquin C., Boisson-Videl C. Relationship between sulfate groups and biological activities of fucans. Thromb. Res. 2000;100:453–459. doi: 10.1016/s0049-3848(00)00338-8. [DOI] [PubMed] [Google Scholar]
- Hellebust J.A., Craigie J.S. first ed. Cambridge University Press; Cambridge, UK: 1978. Handbook of Phycological Methods, Physiological and Biochemical Methods. [Google Scholar]
- Hoppe H.A. In: Marine Algae in Pharmaceutical Science. Hoppe H.A., Levering T., Tanaka Y., editors. Walter de Gruyter; Berlin and New York: 1999. p. 26. [Google Scholar]
- Jimenez-Escrig A., Jimenez- Jimenez I., Pulido R., Saura-Calixto F. Antioxidant activity of fresh and processed edible seaweed. J. Sci. Food Agric. 2001;81:530–534. [Google Scholar]
- Lahaye M., Inizan F., Vigouroux J. NMR analysis of the chemical structure of ulvan and of ulvan-boron complex formation. Carbohydr. Polymers. 1998;36:239–249. [Google Scholar]
- Latham H. Temperature stress-induced bleaching of the coralline alga Corallina officinalis: a role for the enzyme bromoperoxidase. Biosci. Horizon. 2008;1(2):104–113. [Google Scholar]
- Maron D., Ames B. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Matanjun P., Mohamed S., Mustapha N.M., Muhammad K., Ming C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008;20(4):367–373. [Google Scholar]
- Matoh T., Shoji I.D.A., Takahashi E. A rapid and sensitive assay for ferredoxin–glutamate synthase. Bull. Res. Inst. Food Sci. Kyoto Univ. 1980;43:1–6. [Google Scholar]
- Matsukawa R., Dubinsky Z., Kishimoto E., Takenchi M., Niki E., Karube I. A comparison of screening methods for antioxidant activity in seaweeds. J. Appl. Phycol. 1997;9:29–35. [Google Scholar]
- Melo M.R.S., Feitosa J.P.A., Freitas A.L.P., de Paula R.C.M. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polymers. 2002;49:491–498. [Google Scholar]
- Mori J., Matsunaga T., Takahashi S., Hsegaula C., Saito H. Inhibitory activity on lipid peroxidation of extracts from marine brown alga. Phytother. Res. 2003;17(5):549–551. doi: 10.1002/ptr.1194. [DOI] [PubMed] [Google Scholar]
- Mundt S., Kretlow S., Jansen R. Fatty acids with antibacterial activity from the cyanobacterium Oscillatoria redekei HUB 051. J. Appl. Phycol. 2003;15:263–267. [Google Scholar]
- Mutawie, H.H., 2006. Studies on biological active compounds from marine algae in Jeddah cornice of Saudi Arabia. Ph.D. Thesis, Umm Al-Qura University.
- Nascimento S.C., Correalima R.N., Diu M.B., Koening M.L. Cytotoxic and antitumoral effects of seaweed extracts from northeastern coast of Brazil. Mem. Soc. Brot. 1993;29:79–84. [Google Scholar]
- Pantakar M.S., Oehninger S., Barnett T., Williams R.L., Clark G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993;268:21770–21776. [PubMed] [Google Scholar]
- Pengzhan Y., Zhang Q., Li N., wang Y., Li Z. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003;15:21–27. [Google Scholar]
- Ruperez, P., 2001. Antioxidant activity of sulphated polysaccharides from the Spanish marine seaweed Nori. In: Proceedings of the COST 916 European Conference on Bioactive Compounds in Plant Foods. Health Effects and Perspectives for the Food Industry, Tenerife, Canary Islands, Spain, April, p. 114.
- Ruperez P., Ahrazem O., Leal J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. Agric. Food Chem. 2002;50:840. doi: 10.1021/jf010908o. [DOI] [PubMed] [Google Scholar]
- Vairappan C., Motonari D., Minoru S., Michio M. Antibacterial halogenated metabolites from the Malaysian Laurencia species. Phytochemistry. 2001;58:291–297. doi: 10.1016/s0031-9422(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Yan X., Nagata T., Fan X. Antioxidative activities in some common seaweeds. Plant Foods Hum. Nutr. (Dordrecht, Netherlands) 1998;52:253–262. doi: 10.1023/a:1008007014659. [DOI] [PubMed] [Google Scholar]


