Abstract
Lead (Pb) is the most common heavy metal contaminant in the environment. Pb is not an essential element for plants, but they absorb it when it is present in their environment, especially in rural areas when the soil is polluted by automotive exhaust and in fields contaminated with fertilizers containing heavy metal impurities. To investigate lead effects on nutrient uptake and metabolism, two plant species, spinach (Spinacia oleracea) and wheat (Triticum aestivum), were grown under hydroponic conditions and stressed with lead nitrate, Pb(NO3)2, at three concentrations (1.5, 3, and 15 mM).
Lead is accumulated in a dose-dependent manner in both plant species, which results in reduced growth and lower uptake of all mineral ions tested. Total amounts and concentrations of most mineral ions (Na, K, Ca, P, Mg, Fe, Cu and Zn) are reduced, although Mn concentrations are increased, as its uptake is reduced less relative to the whole plant’s growth. The deficiency of mineral nutrients correlates in a strong decrease in the contents of chlorophylls a and b and proline in both species, but these effects are less pronounced in spinach than in wheat. By contrast, the effects of lead on soluble proteins differ between species; they are reduced in wheat at all lead concentrations, whereas they are increased in spinach, where their value peaks at 3 mM Pb.
The relative lead uptake by spinach and wheat, and the different susceptibility of these two species to lead treatment are discussed.
Keywords: Chlorophyll, Heavy metal stress, Nutrient elements, Proline, Soluble protein, Spinach, Wheat
1. Introduction
Heavy metal contamination has disastrous effects on plant productivity and threatens human and animal health (Adriano, 2001). Lead in the environment can cause serious problems to plants and animals. It has become a major environmental contaminant following rapid industrialization and urbanization. Lead is not amongst the essential elements for plants, but they absorb this metal if it is present in their environment, especially in rural areas where the soil is polluted by automotive exhaust and in fields contaminated with fertilizers which contain heavy metals as impurities (Adriano, 2001).
According to the US Environmental Protection Agency, lead is one of the most common heavy metal contaminants in aquatic and terrestrial ecosystems and can have adverse effects on the growth and metabolism of plants, owing to its direct release into the atmosphere (Watanabe, 1997). Lead effects on plants have been described in several reviews (Sharma and Dubey, 2005; Sengar et al., 2008; Seregin and Kosevnikova, 2008).
Plants absorb Pb from solution in the soil through their roots and, subsequently, the largest proportion of Pb2+ is accumulated within roots in an insoluble form (Wierzbicka et al., 2007). Lead accumulation in plants increases with an increase in the exogenous lead level. Lead can cause a broad range of physiological and biochemical dysfunctions on seed germination, plant growth, water status and nitrate assimilation (Sharma and Dubey, 2005; Seregin and Kosevnikova, 2008; Lamhamdi et al., 2011). Although lead transport from plant roots to shoots is usually limited (Huang and Cunningham, 1996), photosynthesis is especially affected by lead exposure (Bazzaz et al., 1975); chlorophyll and carotenoid contents, photosynthetic rate and CO2 assimilation are strongly decreased. Ca, Fe and Zn levels decrease in the root tips after lead exposure (Eun et al., 2002). In Norway spruce, lead application inhibits growth, and this effect is related to a decrease of Ca2+ and Mn2+ levels (Rout and Das, 2003). A decreased uptake of K+, Ca2+, Mg2+, Fe3+ and Na+ was also observed in Picea abies treated with lead (Haussling et al., 1998). Thus, the inhibition of mineral ion uptake appears to be a general consequence of lead exposure. Conversely, increased provision of certain inorganic salts can antagonize lead effects to some extent (Javis and Leung, 2002). Other heavy metal ions can evoke the same effects as lead; thus, Pandy and Sharma (2002) reported that the accumulation of Co2+, Ni2+ or Cd2+ in cabbage plants also resulted in growth inhibition.
Despite the importance of lead contamination in North Africa, it remains unclear as to which economical species are able to resist Pb-stress. Wheat is grown on 17% of all crop areas and represents the staple food for 40% of the world’s population, and is the primary food staple in North Africa (MacCaferri et al., 2009), spinach is an important dietary crop and it has significant antioxidant activity, mainly related to the presence of flavonoids, which constitute its major water-soluble polyphenols (Aehle et al., 2004). Wheat and spinach are two important agricultural species, so it appeared to be of interest to compare the effects of lead exposure on these two species. In the present study, we have investigated the effects of lead stress on mineral content (Na, K, Ca, P, Mg, Fe, Cu, Zn and Mn), and its consequences on biomass, chlorophyll, soluble proteins and proline contents in leaves and roots of spinach and wheat.
2. Materials and methods
2.1. Plant growth and lead treatment
A variety of wheat (Triticum aestivum L. cv. Achtar) was provided by the National Institute of Agronomical Research (INRA), Tangier, Morocco, and spinach (Spinacia oleracea L., var. “Géant d’hiver”) was purchased from Truffaut (France). Prior to germination, seeds were surface-sterilized with 5% (v/v) sodium hypochlorite for 10 min and rinsed several times with distilled water. The seeds were then germinated in Petri dishes containing two sheets of Whatman no. 1 filter paper moistened initially with 6 mL water. After germination, when the cotyledons had fully emerged (after 6 days for spinach, and 4 days for wheat), the seedlings were grown in glass test-tubes containing 5 mL Hoagland’s solution (pH 5.5), at 25 °C in a 16-h light/8-h dark photoperiod regime at 45 μmol m−2 s−1 from cool white fluorescent tubes. Nutrient solution was changed twice a week. Different concentrations of Pb(NO3)2 solutions (0, 1.5, 3 and 15 mM) were added 25 days after germination. Plants were harvested after 30 days, and roots and shoots were separated to determine their biomass, dry weight and nutrient content.
2.2. Determination of lead accumulation and nutrition elements uptake
After being washed four times with deionized water, the harvested samples were immediately blotted and oven-dried at 105 °C for 24 h. The dried material was ground in an agate mortar. Powdered samples (whole plants or leaves and roots) of each plant material were weighed, then 500 mg aliquots were transferred to Pyrex tubes and digested for 12 h with (7.5 mL) 65% HNO3 and (2.5 mL) 36% HCl at 25 °C, then heated for 2 h at 105 °C. The contents of Pb, Ca, Mg, Fe, Cu, Zn and Mn were determined by atomic absorption spectroscopy with a Thermo-Elemental Solar spectrometer. K and Na were detected by flame emission spectroscopy. P was determined by inductively-coupled plasma atomic emission. All measurements were performed in triplicate, as described in Nóvoa-Muñoz et al. (2008).
2.3. Chlorophyll a and b determination
Weighed leaf samples from controls and the different Pb treatments were homogenized in 1 mL 80% acetone and filtered through Whatman paper. The absorbance was measured at two wavelengths (645 and 663 nm), and the quantification of chlorophylls was made according to Moran (1982).
2.4. Soluble protein and proline determination
Soluble protein was quantified according to Bradford (1976). Samples (leaves and roots) were homogenized in 0.1 M Na-phosphate buffer (pH 7; 1:5 w/v). After adding the reagent, absorbance was recorded at 595 nm and the concentration was calculated using a calibration curve made with bovine serum albumin.
Proline content was measured according to the method of Bates et al. (1973). Fresh seedlings (0.5 g) were ground in 1.5 mL of aqueous sulfosalicylic acid 3% (w/v), and proline was estimated by ninhydrin reagent (0.125 g of ninhydrin in 2 mL orthophosphoric acid 6 M, and 3 mL of acetic acid). The ninhydrin reaction mixture was partitioned against toluene and the absorbance of the toluene phase was read at 520 nm. Proline concentrations were determined after the realization of a standard curve, and are expressed in μmol g−1 fresh weight.
2.5. Statistical analyses
In all experiments, three replicates were performed for each sample, and each treatment was repeated three times. Data presented here are mean values and standard deviation (±SD). One-way analysis of variance (ANOVA) was carried out using post hoc multiple comparison from the Tukey test to determine the difference between the levels of Pb-stress in each studied parameter (a significance level of 0.05 was used for all statistical tests).
3. Results and discussion
3.1. Effect of lead on plant growth
Lead exposure results in a dose-dependent damage to both plant species. In wheat plants exposed to 15 mM Pb (Table 1), there is a clear growth inhibition, whereas spinach fresh weight (F.W.) and dry weight (D.W.) decrease by only 28% and 29%, respectively, at 15 mM Pb, when compared with controls. The growth inhibition under Pb-stress was similar to that previously reported by Mesmar and Jaber (1991) in wheat and lentils. Similar phenomena were also described in Plantago major (Kosobrukhov et al., 2004), where a considerable reduction in the dry weight of plant parts was observed under lead treatment. Similarly, in tomato seedlings, fresh and dry biomass of roots, shoots and leaves were negatively affected by increasing lead concentrations (Akinci et al., 2010). These symptoms can be essentially attributed to a deficiency of macroelements (especially K, P, Ca and Mg), which results from an inhibition of their uptake under Pb exposure (see below).
Table 1.
Effect of Pb on mineral ion uptake by wheat and spinach. Concentrations are given as mg (or μg) per gram dry weight. Results are the means of three replicates ± SD. Biomass fresh weight (F.W.) and dry weight (D.W.) results are the mean of six replicates ± SD. Different letters indicate significant differences at p < 0.05.
| Pb (mM) | F.W. (mg) | D.W. (mg) | Na (mg g−1) | K (mg g−1) | Ca (mg g−1) | P (mg g−1) | Mg (μg g−1) | Fe (μg g−1) | Cu (μg g−1) | Zn (μg g−1) | Mn (μg g−1) | Pb (mg g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | ||||||||||||
| 0 | 200.86 ± 11.00a | 78.14 ± 6.83a | 10.74 ± 0.89a | 57.85 ± 5.83a | 2.01 ± 0.11a | 3.54 ± 0.121a | 292.7 ± 16.3 | 77.4 ± 5.1a | 11.5 ± 1.3a | 46.1 ± 2.3a | 43.6 ± 1.5a | 0 ± 0a |
| 1.5 | 121.60 ± 12.00b | 43.27 ± 6.00b | 7.54 ± 1.50b | 47.25 ± 0.56b | 1.74 ± 0.16a | 3.07 ± 0.180a | 130.5 ± 15.6a | 41.7 ± 1.5b | 5.8 ± 0.2b | 37.3 ± 4.1b | 53.4 ± 7.0a | 0.20 ± 0.02b |
| 3 | 94.94 ± 5.00b | 33.48 ± 3.30b | 6.51 ± 1.10b | 38.87 ± 3.65c | 1.05 ± 0.04b | 2.28 ± 0.270b | 75.9 ± 6.8b | 34.7 ± 3.6b | 4.9 ± 0.4bc | 28.5 ± 4.2b | 66.4 ± 11.2b | 0.36 ± 0.07c |
| 15 | 56.84 ± 13.00c | 22.14 ± 2.70c | 2.36 ± 0.35c | 21.14 ± 1.27d | 0.56 ± 0.02c | 1.63 ± 0.112c | 71.4 ± 7.2b | 27.3 ± 1.2c | 3.7 ± 0.27c | 9.9 ± 1.0c | 77.3 ± 5.5b | 1.22 ± 0.04d |
| Spinach | ||||||||||||
| 0 | 247.50 ± 13.32a | 79.31 ± 3.90a | 33.98 ± 1.32a | 81.33 ± 8.02a | 1.15 ± 0.22a | 4.16 ± 0.062a | 429.2 ± 11.9a | 450.3 ± 23.1a | 11.8 ± 1.2a | 82.6 ± 1.7a | 609.2 ± 10.5a | 0 ± 0a |
| 1.5 | 200.56 ± 19.08b | 65.11 ± 4.50b | 22.58 ± 3.98b | 63.66 ± 3.82b | 0.96 ± 0.05a | 3.59 ± 0.177a | 250.6 ± 9.7b | 417.5 ± 17.6a | 10.4 ± 0.3a | 76.1 ± 5.5a | 710.8 ± 21.9b | 0.17 ± 0.01b |
| 3 | 163.80 ± 16.91b | 55.93 ± 7.80b | 15.53 ± 4.91bc | 50.43 ± 3.12c | 0.75 ± 0.09a | 2.78 ± 0.184b | 135.8 ± 12.4c | 350.7 ± 20.7b | 7.9 ± 0.15b | 51.4 ± 1.1b | 729.4 ± 27.1b | 0.32 ± 0.01c |
| 15 | 108.27 ± 23.56c | 29.21 ± 8.10c | 10.73 ± 1.56c | 50.37 ± 2.86c | 0.66 ± 0.06b | 2.77 ± 0.272b | 117.2 ± 8.3c | 227.4 ± 15.2c | 7.1 ± 0.6b | 45.5 ± 6.0b | 753.4 ± 24.7b | 0.99 ± 0.03d |
3.2. Lead uptake and accumulation
Table 1 shows that plant lead concentrations increase significantly with increased lead supply, reaching 1.22 mg g−1 dry matter in wheat plants and 0.99 mg g−1 dry matter in spinach at 15 mM Pb. From the data for lead concentrations and of dry and fresh weights, we can calculate lead concentrations relative to the plant water content, which shows that plant concentrations are always lower than those of the medium (Fig. 1A). Of course this is a mean value, and it remains possible that lead concentrations in roots are in fact much higher than in aerial parts and closer to those of the medium. Such a situation was indeed observed in tomato (Akinci et al., 2010). The extent of lead accumulation seems to vary with plant age; thus when exposed to 3 mM Pb, 6 day-old seedlings accumulated lead at 1.2 mg g−1 dry weight (Lamhamdi et al., 2011), compared with 0.36 mg g−1 in the present experiments using 30 day-old plants Table 2.
Figure 1.
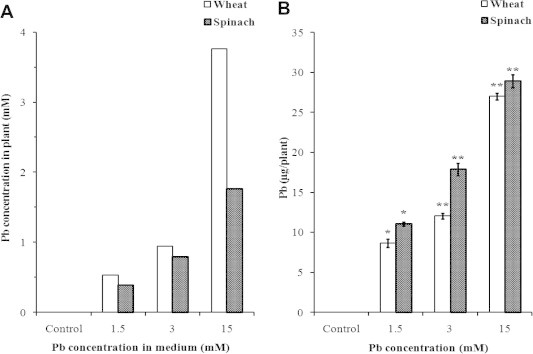
Concentration of Pb in plants (expressed relative to their water content) as a function of Pb concentration in the medium (A), and the content of Pb per plant (B). Results are the mean of three replicates ± SD. ∗Asterisks indicate significant differences between the Pb-stressed and control plants (∗p < 0.05; ∗∗p < 0.01).
Table 2.
Importance of changes induced by lead treatment of wheat and spinach shown as changes in nutrients given as percentage of concentrations ([%] conc.) and percentage changes of total amounts per plant (%), as well as effects on changes of the total amount of chlorophyll (% Chl.).
| Pb (mM) | Na | K | Ca | P | Mg | Fe | Cu | Zn | Mn | Chl.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [%] conc. | % | [%] conc. | % | [%] conc. | % | [%] conc. | % | [%] conc. | % | [%] conc. | % | [%] conc. | % | [%] conc. | % | [%] conc. | % | ||
| Wheat | |||||||||||||||||||
| 0 | |||||||||||||||||||
| 1.5 | −30 | −61 | −18 | −55 | −13 | −52 | −13 | −52 | −55 | −75 | −46 | −70 | −50 | −72 | −19 | −55 | 22 | −32 | −62 |
| 3 | −39 | −74 | −33 | −71 | −48 | −78 | −36 | −72 | −74 | −89 | −55 | −81 | −57 | −82 | −38 | −74 | 52 | −35 | −81 |
| 15 | −78 | −94 | −63 | −90 | −72 | −92 | −54 | −87 | −76 | −93 | −65 | −90 | −68 | −91 | −79 | −94 | 77 | −50 | −93 |
| Spinach | |||||||||||||||||||
| 0 | |||||||||||||||||||
| 1.5 | −34 | −45 | −22 | −36 | −17 | −31 | −14 | −29 | −42 | −52 | −7 | −24 | −12 | −28 | −8 | −24 | 17 | −4 | −48 |
| 3 | −54 | −68 | −38 | −56 | −35 | −54 | −33 | −53 | −68 | −78 | −22 | −45 | −33 | −53 | −38 | −56 | 20 | −16 | −75 |
| 15 | −68 | −88 | −38 | −77 | −43 | −79 | −33 | −75 | −73 | −90 | −50 | −81 | −40 | −78 | −45 | −80 | 24 | −54 | −90 |
In the case of wheat, there is a linear relationship between lead concentrations in the plant and the medium. In spinach, lead concentrations are almost the same as in wheat for 1.5 and 3 mM Pb, but they are much lower (by 50%) for 15 mM Pb, which means that spinach could have a better capacity to control lead entry, even if in fact, when calculated for whole plants, spinach plants contain more lead than wheat (Fig. 1B). Thus, we have to be cautious in how lead uptake is expressed. Species differences were already observed by Mesmar and Jaber (1991), who showed that T. aestivum seedlings take up more lead than Lens culinaris, and this difference was tentatively explained by the presence of nitrogen fixing nodules on leguminous roots, which would prevent lead uptake by lentil plants. Bazzaz et al. (1974) used a similar argument to explain the differences in Pb uptake between corn and soybean. This difference in Pb uptake between wheat and spinach could be related to a higher capacity of spinach to resist oxidative stress generated by Pb, as observed in our previous work (Lamhamdi et al., 2010), but such an explanation should be regarded as only tentative.
3.3. Alterations in nutrient element contents
We can first notice that the control values of ion concentrations are higher in spinach than in wheat (Table 1). Table 1 shows that high lead concentrations in culture media cause a reduction of most macroelements (K, Ca, P, Mg and Na). Microelements (Fe, Cu, and Zn) are also reduced in growing plants. By contrast, Mn concentrations are increased, but in fact the total Mn content per plant decreases with increasing lead supply (see below). Thus, we can conclude that the uptake of all the measured nutrients is reduced by lead treatment. In order to better visualize the effects of lead, the data have been expressed as the percentage change relative to control values, both for concentrations relative to dry weight and for total amounts per plant (Table 2). It is known that lead physically blocks the access of many ions to their absorption sites on the roots (Godbold and Kettner, 1991), thus inhibiting their uptake. However, the very large reductions of ionic content observed in the present study can hardly result from just an inhibition of ion uptake, and they probably also result from additional ion leakage from the plants. In every case, spinach appears less disturbed than wheat by lead treatment.
At all lead concentrations, the concentrations of K (the major ion) in wheat and spinach are noticeably lower compared to corresponding control values, and this decrease is already observed with low lead concentrations. For 15 mM Pb, the reduction of K concentrations (Table 2) is much lower in spinach (−38%) than in wheat (−63%). Of course, owing to growth inhibition, the reduction in K amount per plant is even more impressive, especially in wheat (Table 2). Paivoke (2002) in Pisum sativum, Malkowski et al. (2002) in corn, and Akinci et al. (2010) in tomato found the same inverse relationship between lead and K concentrations. Similar conclusions can be made for Na, P and Ca, and again spinach appears more resistant than wheat to lead treatment. Akinci et al. (2010) and Paivoke (2002) observed a negative correlation between P-uptake and lead concentration in the soil. Similarly, Azmat et al. (2009a) also found an inverse relation between Pb2+ and Ca2+ ion accumulation attributed to their ionic similarity which allows lead to replace Ca during specific physiological processes.
Mg ion levels seem very sensitive to lead treatments, and the lowest lead concentration already has very large effects (Table 2). This observation also applies to Fe and Cu ions, at least in the case of wheat, whereas in spinach these levels are only slightly disturbed by 1.5 mM Pb.
Manganese is the only ion for which concentrations in both plants increase together with lead concentrations (Table 1). On the other hand, the total Mn amounts per plant are reduced (Table 2), which means that Mn uptake too is inhibited in the presence of lead, although to a lower extent than other ions. Manganese is involved in the production of oxygen from water in photosynthesis (Haider et al., 2006). The decrease of Mn amounts started at 1.5 mM Pb for wheat and only at 3 mM Pb for spinach. Manganese contents per plant were much higher in spinach (18.6-fold) than in wheat.
3.4. Effects of Pb on chlorophyll a and b content
Chlorosis was associated with reduced leaf chlorophyll content. In wheat seedlings, concentrations of chlorophylls a and b were already significantly lowered at 1.5 mM Pb, and this effect was even more pronounced at 3 and 15 mM Pb (Fig. 2). In addition, chlorophyll a and b contents per plant (shown in Fig. 2C and D) present a larger reduction in wheat seedlings (Fig. 2C) where the diminution is by a factor 13–14 for 15 mM Pb. There is a good correlation between the reduction of chlorophyll and Mg content, especially in the case of spinach (Table 2). Previous authors showed that lead stress results in a heavy reduction of chlorophyll, owing to both chloroplast disorganization and diminution in the amount of thylakoids and grana, and direct inhibition of chlorophyll synthesis, as well as changes of chlorophyll structure owing to replacement of key nutrients (Mg, Fe and Cu) by lead (Sengar and Pandey, 1996; Haider et al., 2006; Akinci et al., 2010). Azmat et al. (2009b) reported that Phaseolus mungo and L. culinaris plants undertake adaptative mechanisms aimed to protect photosynthesis against the damaging effects of lead; foliar morphological modifications were induced by exposure to 1.2 mM Pb, which resulted in an increased number of trichomes and stomata, thus allowing these species to maintain photosystem II efficiency and reduce water evaporation from the leaves during stress.
Figure 2.
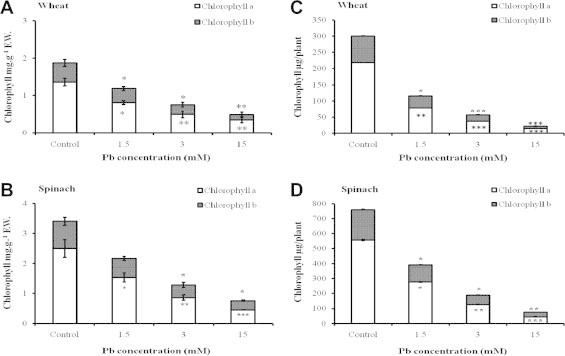
Effect of lead on the concentrations of chlorophyll a and b, in wheat (A) and spinach (B) leaves and on the quantities of chlorophyll a (C) and b (D) per plant. Results are the mean of five replicates ± SD. ∗Asterisks indicate significant differences between the treatments and the control of the same plant species (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
3.5. Effects of lead on soluble protein content
In wheat, lead treatment shows a biphasic effect on soluble protein concentration (Fig. 3A): protein concentrations increase for low lead concentrations and then decrease back to control values at 15 mM Pb. A peak is observed for leaves with 3 mM Pb (190% of control) and for roots with 1.5 mM Pb (330% of control). A similar increase was previously observed with young wheat seedlings (Lamhamdi et al., 2011). In spinach, soluble protein concentrations increase continuously for all lead concentrations in leaves, and peak at 3 mM Pb in roots (Fig. 3B).
Figure 3.
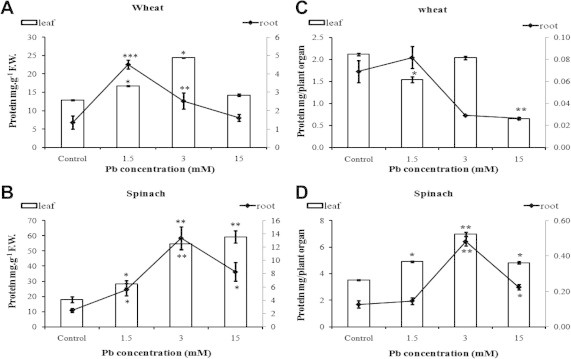
Effect of lead on soluble protein concentrations in wheat (A) and spinach (B) and on total amount of soluble protein in wheat (C) and spinach (D) seedlings. Results are the mean of five replicates ± SD. ∗Asterisks indicate significant differences between the treatments and the control of the same plant species (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
By contrast, total amounts show a different picture (Fig. 3C and D). No increase is observed in wheat, and a heavy reduction is even observed with 15 mM Pb (Fig. 3C). In spinach, a significant increase is observed for 1.5 and even more for 3 mM Pb, and the total protein amount is still increased as compared with controls for 15 mM Pb. This increase of protein under lead stress is possibly a result of the induction of stress proteins, which may comprise various antioxidant enzymes (Lamhamdi et al., 2010). It could also result from the production of phytochelatins aimed to detoxify Pb ions (Rauser, 1995), but additional experiments are required in order to investigate these hypotheses.
3.6. Effects of lead on proline content
Proline is one component of the non-specific defence systems towards lead toxicity. It alleviates metal toxicity by acting as a metal chelator and as a protein stabilizer (Sharma and Dubey, 2005). Proline concentrations (Fig. 4A and B) increase in leaves of wheat and spinach exposed to increasing lead concentrations. In wheat roots, proline concentrations (Fig. 4A) peak significantly at 3 mM Pb and then decrease to control levels at 15 mM Pb. In spinach, proline concentrations were significantly higher than control values for all lead concentrations. We reported previously (Lamhamdi et al., 2011) that proline concentrations were also increased in young wheat seedlings after 6 days of Pb-stress.
Figure 4.
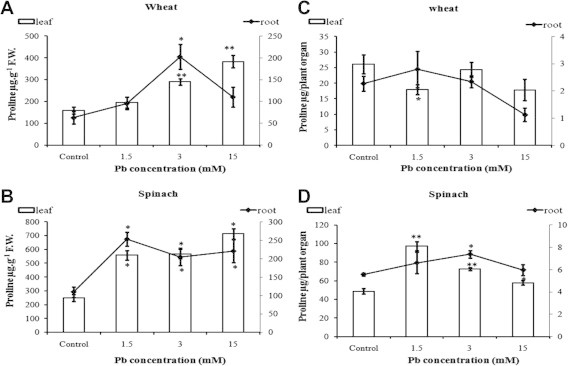
Effect of lead on proline concentrations in wheat (A) and spinach (B) and on total quantities of proline in wheat (C) and spinach (D) seedlings. Results are the mean of five replicates ± SD. ∗Asterisks indicate significant differences between the treatments and the control of the same plant (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
Total proline content (Fig. 4C) stayed constant in wheat seedlings, and decreased only (to 63% of control value) for 15 mM Pb. By contrast, proline amounts per spinach plant (Fig. 4D) peaked at 184% of the control value with 1.5 mM Pb, and remained significantly higher than controls for all lead concentrations, which might provide increased protection to this plant.
4. Conclusion
It is obvious from our results that lead treatment even at low concentrations induces large disturbances in ion uptake by plants, which results in profound metabolic changes (e.g. in photosynthetic capacity), and finally in a strong inhibition of plant growth.
Spinach and wheat exhibit different susceptibilities to lead treatment, and spinach appears far more resistant. Future experiments will be aimed at searching for the mechanisms responsible for the improved protection of spinach against the deleterious effects of lead.
Acknowledgements
The authors are grateful to Dr. L. Dinan for his critical review and language improvement of the manuscript.
Footnotes
Peer review under responsibility of the King Saud University.

References
- Adriano D.C. Springer-Verlag; New York: 2001. Trace Elements in Terrestrial Environments; Biochemistry, Bioavailability and Risks of Metals. pp. 150–159. [Google Scholar]
- Aehle E., Raynaud-Le Grandic S., Ralainirina R., Baltora-Rosset S., Mesnard F., Prouillet C., Maziere J., Fliniaux M. Development and evaluation of an enriched natural antioxidant preparation obtained from aqueous spinach (Spinacia oleracea) extracts by an adsorption procedure. Food Chem. 2004;86:579–585. [Google Scholar]
- Akinci I.E., Akinci S., Yilmaz K. Response of tomato (Solanum lycopersicum L.) to lead toxicity: growth, element uptake, chlorophyll and water content. Afr. J. Agric. Res. 2010;5:416–423. [Google Scholar]
- Azmat R., Haider S., Nasreen H., Aziz F., Riaz M. A viable alternative mechanism in adapting the plants to heavy metal environment. Pak. J. Bot. 2009;41:2729–2738. [Google Scholar]
- Azmat R., Haider S., Riaz M. An inverse relation between Pb2+ and Ca2+ ions accumulation in Phaseolus mungo and Lens culinaris under Pb stress. Pak. J. Bot. 2009;41:2289–2295. [Google Scholar]
- Bates D.H., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39(1):205–207. [Google Scholar]
- Bazzaz F.A., Carlson R.W., Rolfe G.L. The inhibition of corn and sunflower photosynthesis by lead. Physiol. Plant. 1975;34:326–329. [Google Scholar]
- Bazzaz F.A., Rolfe G.L., Windle P. Differing sensitivity of corn and soybean photosynthesis and transpiration to lead contamination. J. Environ. Qual. 1974;3:156–158. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Eun S.O., Youn H.S., Lee Y. Lead disturbs microtubule organization in the root meristem of Zea mays. Physiol. Plant. 2002;110:357–365. [Google Scholar]
- Godbold D.L., Kettner C. Use of root elongation studies to determine aluminium and lead toxicity in Picea abies seedlings. J. Plant Physiol. 1991;138:231–235. [Google Scholar]
- Haider S., Kanwal S., Uddin F., Azmat R. Phytotoxicity of Pb II: changes in chlorophyll absorption spectrum due to toxic metal Pb stress on Phaseolus mungo and Lens culinaris. Pak. J. Biol. Sci. 2006;9:2062–2068. [Google Scholar]
- Haussling M., Jorns C.A., Lehmbecker G., Hecht-Bucholz, Marschner H. Ion and water uptake in relation to root development of Norway spruce (Picea abies (L.) Karst) J. Plant Physiol. 1998;133:486–491. [Google Scholar]
- Huang J.W., Cunningham S.D. Lead phytoextraction: species variation in lead uptake and translocation. New Phytol. 1996;134:75–84. [Google Scholar]
- Javis M.D., Leung D.W.M. Chelated lead transport in Pinus radiata: an ultrastructural study. Environ. Exp. Bot. 2002;48:21–32. [Google Scholar]
- Kosobrukhov A., Knyazeva I., Mudrik V. Plantago major plants responses to increase content of lead in soil: growth and photosynthesis. Plant Growth Regul. 2004;42:145–151. [Google Scholar]
- Lamhamdi M., Bakrim A., Aarab A., Lafont R., Sayah F. Effects of lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedling growth. C. R. Biol. 2011;334:118–126. doi: 10.1016/j.crvi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Lamhamdi M., Bakrim A., Aarab A., Lafont R., Sayah F. A comparison of lead toxicity using physiological and enzymatic parameters on spinach (Spinacia oleracea) and wheat (Triticum aestivum) growth. Moroccan J. Biol. 2010;6–7:64–73. [Google Scholar]
- Maccaferri M., Sanguineti M.C., Giuliani S., Tuberosa R. Genomics of tolerance to abiotic stress in the Triticeae. In: Feuillet C., Muehlbauer G.J., editors. Plant Genetics and Genomics: Crops and Models, 1. vol. 7. Springer; New York: 2009. pp. 481–558. (Genetics and Genomics of the Triticeae). [Google Scholar]
- Malkowski E., Kita A., Galas W., Karcz W., Kuperberg J.M. Lead distribution in corn seedlings (Zea mays L.) and its effect on growth and the concentrations of potassium and calcium. Plant Growth Regul. 2002;37:69–76. [Google Scholar]
- Mesmar M.N., Jaber K. The toxic effect of lead on seed germination, growth, chlorophyll and protein contents of wheat and lens. Acta Biol. Hung. 1991;42:331–344. [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with N,N dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nóvoa-Muñoz J.C., Simal-Gándara J., Fernández-Calviño D., López-Periago E., Arias-Estévez M. Changes in soil properties and in the growth of Lolium multiforum in an acid soil amended with a soil waste from wineries. Bioresour. Technol. 2008;99:6771–6779. doi: 10.1016/j.biortech.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Paivoke A.E.A. Soil lead alters phytase activity and mineral nutrient balance of Pisum sativum. Environ. Exp. Bot. 2002;48:61–73. [Google Scholar]
- Pandy N., Sharma C.P. Effect of heavy metals Co2+, Ni2+, Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002;163:753–758. [Google Scholar]
- Rauser W.E. Phytochelatins and related peptides: structure, biosynthesis and function. Plant Physiol. 1995;109:1141–1149. doi: 10.1104/pp.109.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout G.R., Das P. Effect of metal toxicity on plant growth and metabolism. Agron. Sustain. Dev. 2003;23:3–11. [Google Scholar]
- Sengar R.S., Gautam M., Sengar R.S., Garg S.K., Sengar K., Chaudhary R. Lead stress effects on physiobiochemical activities of higher plants. Rev. Environ. Contam. Toxicol. 2008;196:73–93. doi: 10.1007/978-0-387-78444-1_3. [DOI] [PubMed] [Google Scholar]
- Sengar R.S., Pandey M. Inhibition of chlorophyll biosynthesis by lead in greening Pisum sativum leaf segment. Biol. Plant. 1996;38:459–462. [Google Scholar]
- Seregin I.V., Kosevnikova A.D. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008;55:1–22. [Google Scholar]
- Sharma P., Dubey R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005;17:35–52. [Google Scholar]
- Watanabe M.A. Phytoremediation on the brink of commercialization. Environ. Sci. Technol. 1997;31:182–186. doi: 10.1021/es972219s. [DOI] [PubMed] [Google Scholar]
- Wierzbicka M.H., Przedpełska E., Ruzik R., Ouerdane L., Połeć-Pawlak K., Jarosz M., Szpunar J., Szakiel A. Comparison of the toxicity and distribution of cadmium and lead in plant cells. Protoplasma. 2007;231:99–111. doi: 10.1007/s00709-006-0227-6. [DOI] [PubMed] [Google Scholar]


