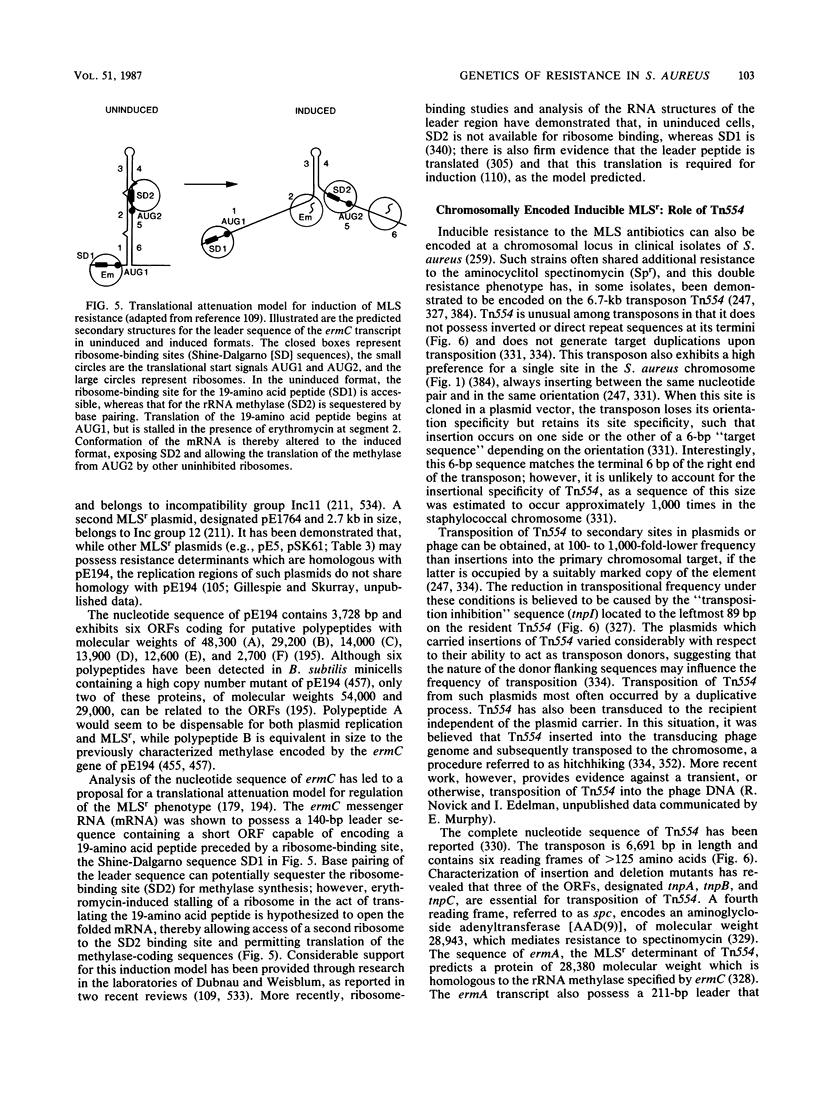
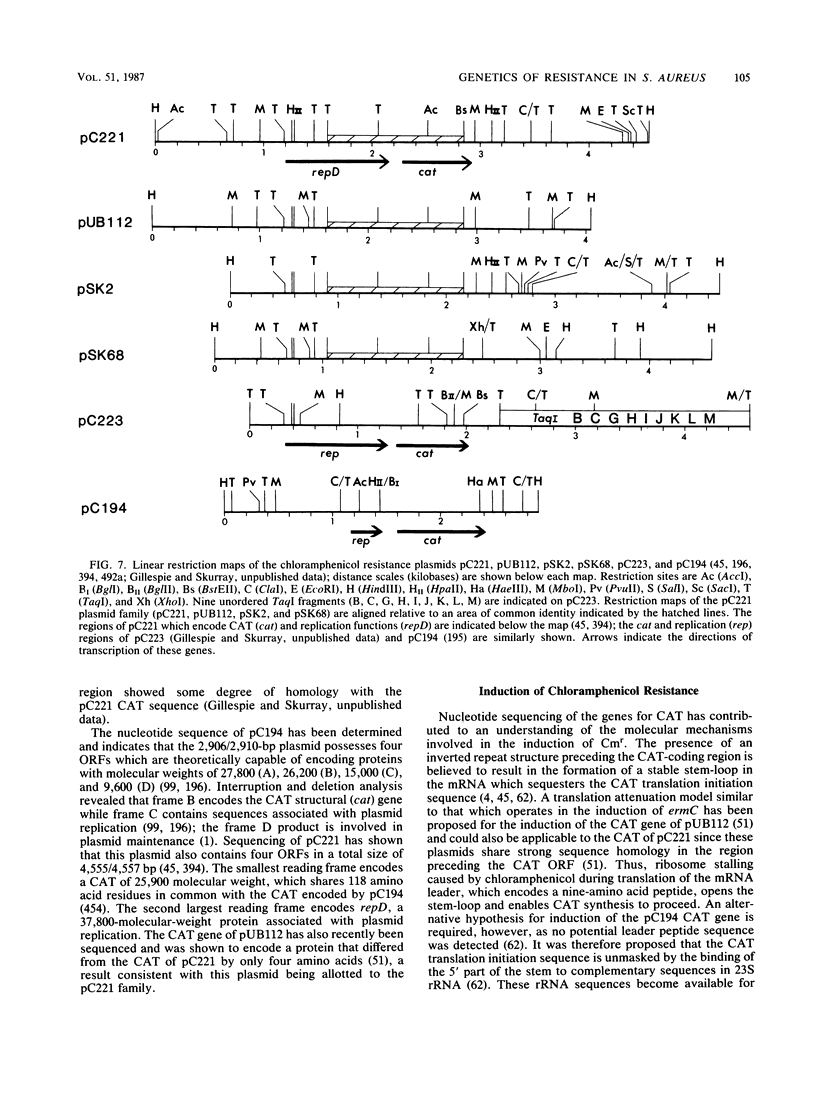
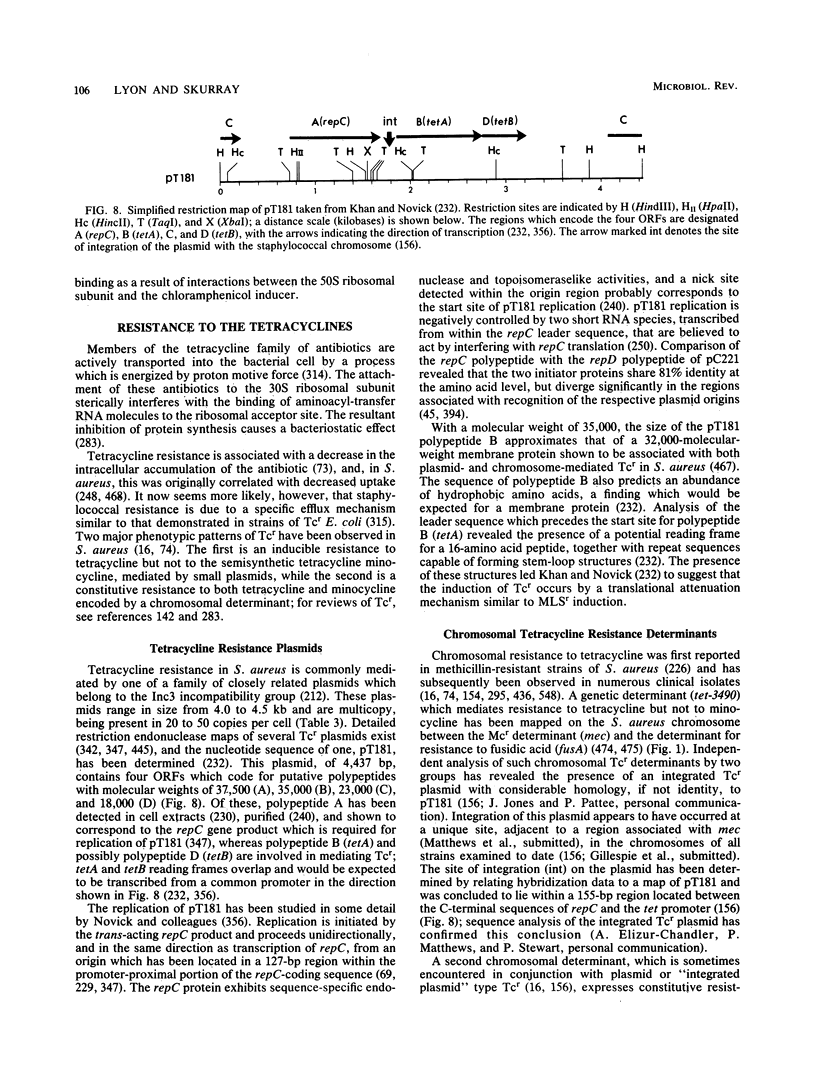
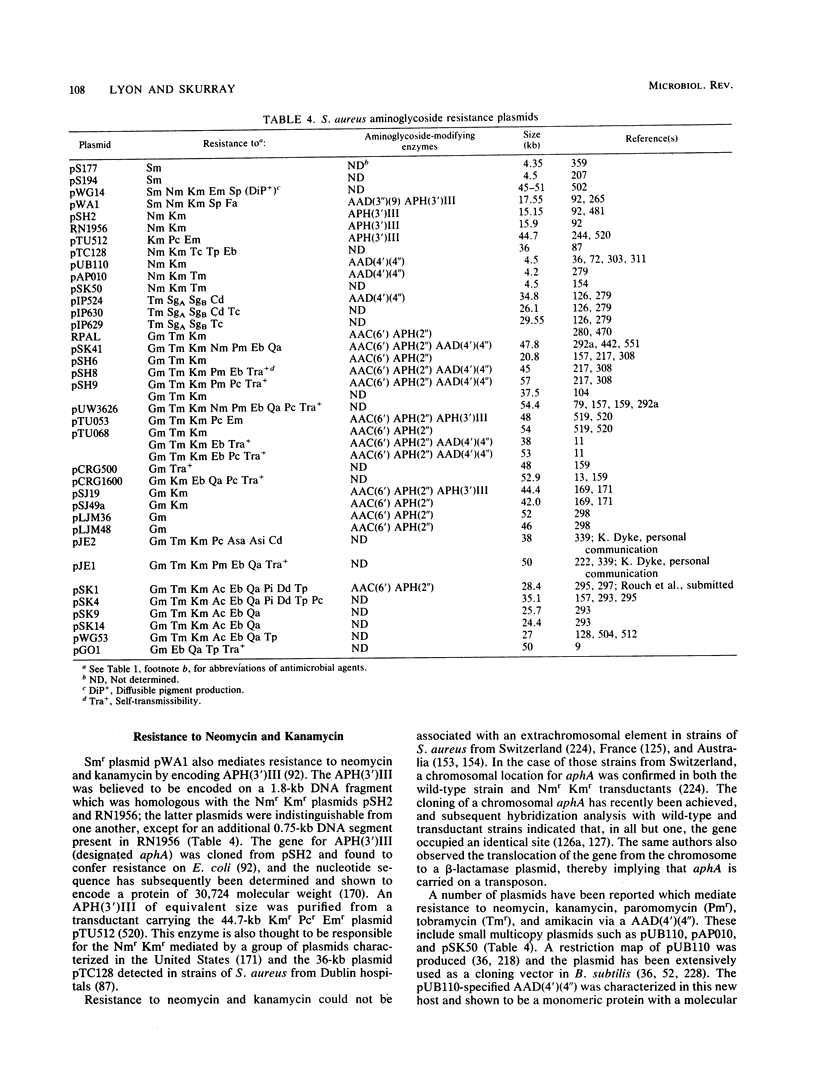
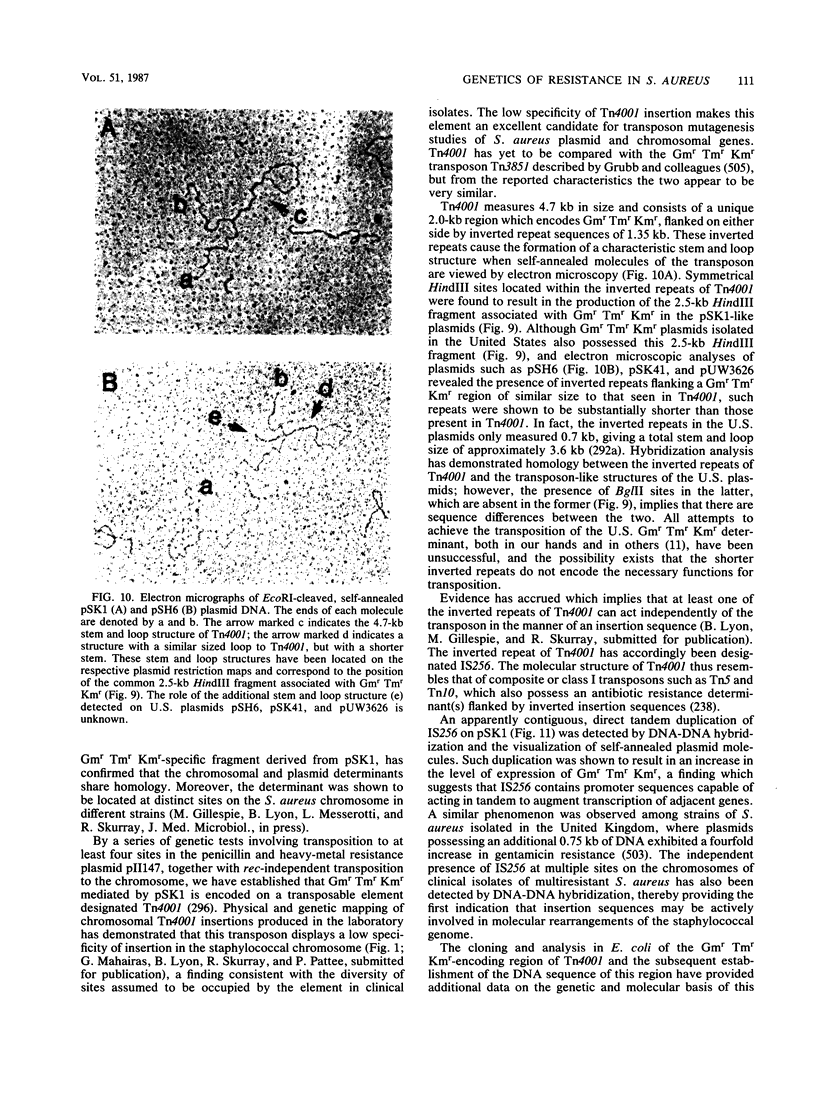
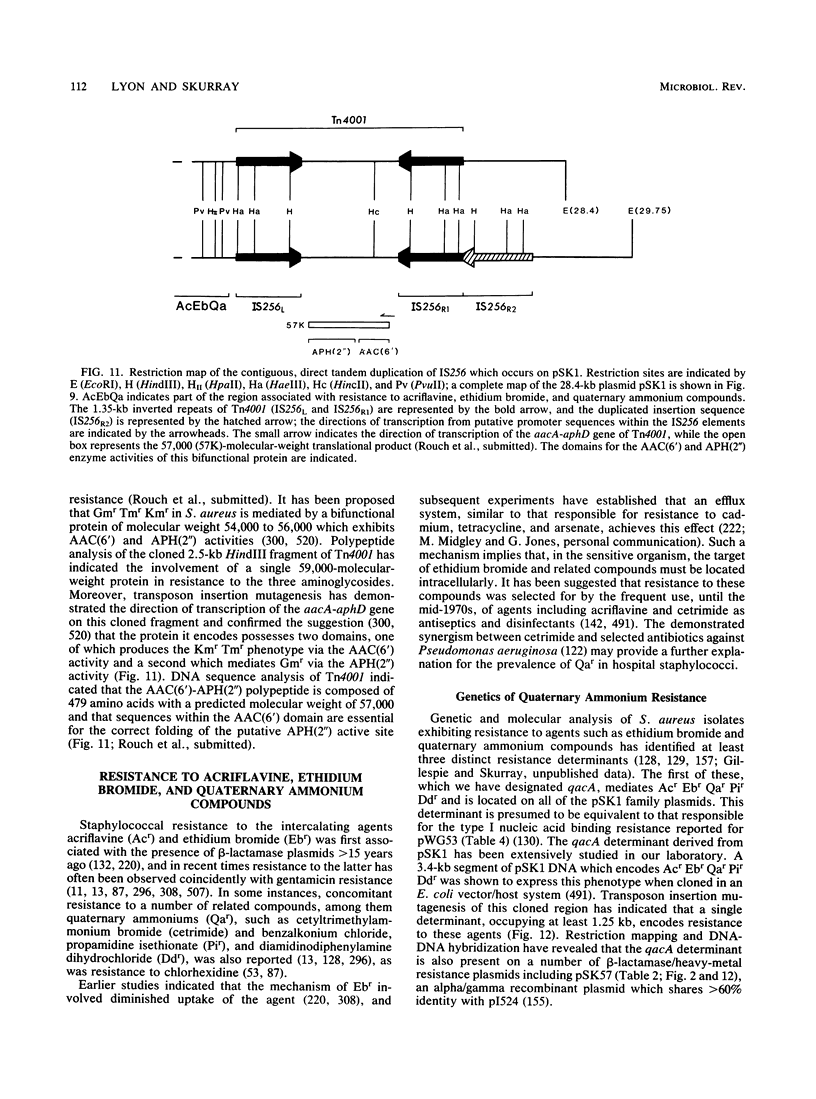
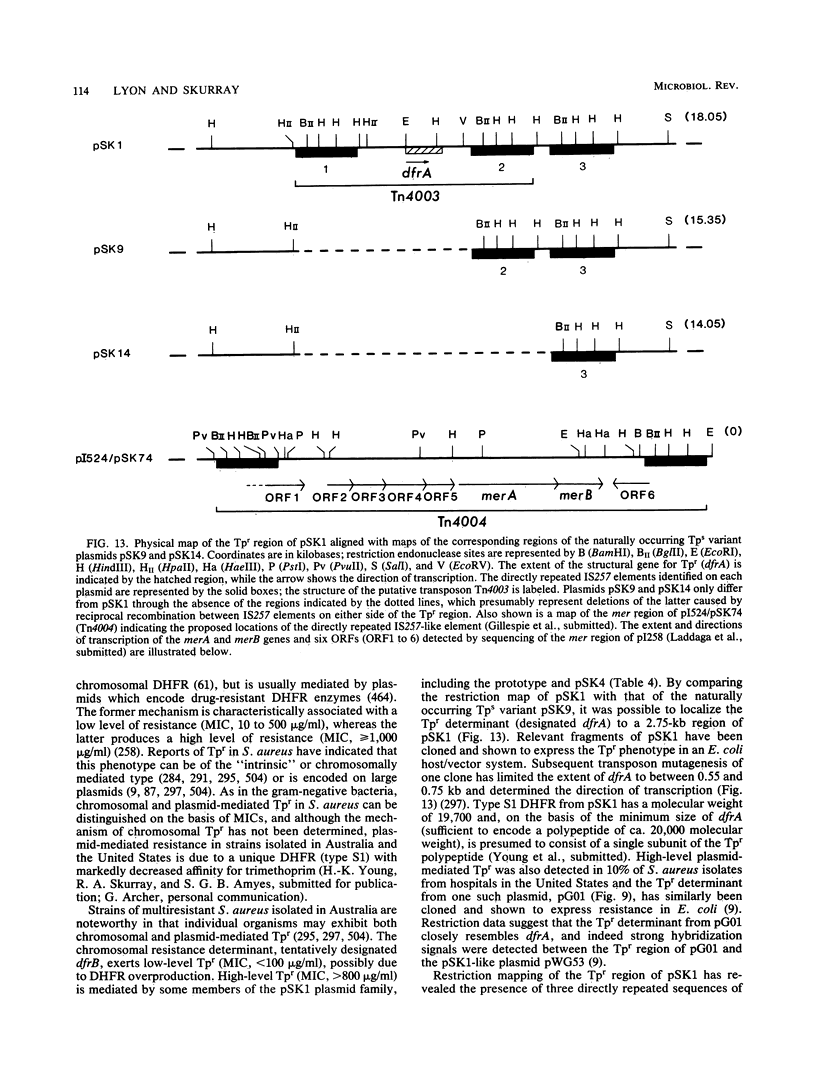
Full text
PDF





































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. C., Trautner T. A. A gene controlling segregation of the Bacillus subtilis plasmid pC194. Mol Gen Genet. 1985;198(3):427–431. doi: 10.1007/BF00332934. [DOI] [PubMed] [Google Scholar]
- Altboum Z., Hertman I., Sarid S. Penicillinase plasmid-linked genetic determinants for enterotoxins B and C1 production in Staphylococcus aureus. Infect Immun. 1985 Feb;47(2):514–521. doi: 10.1128/iai.47.2.514-521.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P. The amino acid sequence of Staphylococcus aureus penicillinase. Biochem J. 1975 Nov;151(2):197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambulos N. P., Jr, Chow J. H., Mongkolsuk S., Preis L. H., Vollmar W. R., 2nd, Lovett P. S. Constitutive variants of the pC194 cat gene exhibit DNA alterations in the vicinity of the ribosome binding site sequence. Gene. 1984 May;28(2):171–176. doi: 10.1016/0378-1119(84)90254-3. [DOI] [PubMed] [Google Scholar]
- Annear D. I., Grubb W. B. Methicillin-sensitive variants in ageing broth cultures of methicillin-resistant Staphylococcus aureus. Pathology. 1976 Jan;8(1):69–72. doi: 10.3109/00313027609094426. [DOI] [PubMed] [Google Scholar]
- Annear D. I., Grubb W. B. Unstable resistance to kanamycin, lincomycin and penicillin in a methicillin resistant culture of Staphylococcus aureus. Pathology. 1972 Oct;4(4):247–252. doi: 10.3109/00313027209068949. [DOI] [PubMed] [Google Scholar]
- Annear D. I. The effect of temperature on resistance of Staphylococcus aureus to methicillin and some other antibioics. Med J Aust. 1968 Mar 16;1(11):444–446. [PubMed] [Google Scholar]
- Archer G. L., Coughter J. P., Johnston J. L. Plasmid-encoded trimethoprim resistance in staphylococci. Antimicrob Agents Chemother. 1986 May;29(5):733–740. doi: 10.1128/aac.29.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Dietrick D. R., Johnston J. L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985 Feb;151(2):243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Mayhall C. G. Comparison of epidemiological markers used in the investigation of an outbreak of methicillin-resistant Staphylococcus aureus infections. J Clin Microbiol. 1983 Aug;18(2):395–399. doi: 10.1128/jcm.18.2.395-399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch D. K., Goering R. V., Ruff E. A. Isolation and preliminary characterization of a plasmid mutant derepressed for conjugal transfer in Staphylococcus aureus. Plasmid. 1984 Nov;12(3):197–202. doi: 10.1016/0147-619x(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H. Chromosomal location of the genetic elements controlling penicillinase production in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):804–806. doi: 10.1038/210804a0. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H. The genetics of penicillinase production in Staphylococcus aureus strain PS80. J Gen Microbiol. 1969 Dec;59(3):289–301. doi: 10.1099/00221287-59-3-289. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H. The genetics of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1975 May;88(1):132–140. doi: 10.1099/00221287-88-1-132. [DOI] [PubMed] [Google Scholar]
- Ayliffe G. A., Green W., Livingston R., Lowbury E. J. Antibiotic-resistant Staphylococcus aureus in dermatology and burn wards. J Clin Pathol. 1977 Jan;30(1):40–44. doi: 10.1136/jcp.30.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBER M. Staphylococcal infection due to penicillin-resistant strains. Br Med J. 1947 Nov 29;2(4534):863–865. doi: 10.1136/bmj.2.4534.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banai M., LeBlanc D. J. Streptococcus faecalis R plasmid pJH1 contains an erythromycin resistance transposon (Tn3871) similar to transposon Tn917. J Bacteriol. 1984 Jun;158(3):1172–1174. doi: 10.1128/jb.158.3.1172-1174.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna J. C., Williams D. H. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- Barr V., Barr K., Millar M. R., Lacey R. W. Beta-lactam antibiotics increase the frequency of plasmid transfer in Staphylococcus aureus. J Antimicrob Chemother. 1986 Apr;17(4):409–413. doi: 10.1093/jac/17.4.409. [DOI] [PubMed] [Google Scholar]
- Barrett F. F., McGehee R. F., Jr, Finland M. Methicillin-resistant Staphylococcus aureus at Boston City Hospital. Bacteriologic and epidemiologic observations. N Engl J Med. 1968 Aug 29;279(9):441–448. doi: 10.1056/NEJM196808292790901. [DOI] [PubMed] [Google Scholar]
- Bastos M. C., Bonaldo M. C., Penido E. G. Constitutive erythromycin resistance plasmid in Staphylococcus aureus. J Gen Microbiol. 1980 Dec;121(2):513–516. doi: 10.1099/00221287-121-2-513. [DOI] [PubMed] [Google Scholar]
- Beard-Pegler M. A., Vickery A. M. Lysogenicity of methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1985 Oct;20(2):147–155. doi: 10.1099/00222615-20-2-147. [DOI] [PubMed] [Google Scholar]
- Beck W. D., Berger-Bächi B., Kayser F. H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986 Feb;165(2):373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B. Increase in transduction efficiency of Tn551 mediated by the methicillin resistance marker. J Bacteriol. 1983 Apr;154(1):533–535. doi: 10.1128/jb.154.1.533-535.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard K., Schrempf H., Goebel W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J Bacteriol. 1978 Feb;133(2):897–903. doi: 10.1128/jb.133.2.897-903.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill D. L., Pattee P. A. Buoyant density analysis of staphylococcal bacteriophage 80 transducing particles. J Virol. 1969 Nov;4(5):804–806. doi: 10.1128/jvi.4.5.804-806.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best G. K., Best N. H., Koval A. V. Evidence for participation of autolysins in bactericidal action of oxacillin on Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Dec;6(6):825–830. doi: 10.1128/aac.6.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham A. H., Atkinson T., Bruton C. J. Construction and characterization of a chimeric plasmid in Bacillus subtilis. Plasmid. 1982 Sep;8(2):119–125. doi: 10.1016/0147-619x(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Bingham A. H., Bruton C. J., Atkinson T. Isolation and partial characterization of four plasmids from antibiotic-resistant thermophilic bacilli. J Gen Microbiol. 1979 Oct;114(2):401–408. doi: 10.1099/00221287-114-2-401. [DOI] [PubMed] [Google Scholar]
- Bint A. J., George R. H., Healing D. E., Wise R., Davies M. An outbreak of infection caused by a gentamicin-resistant Staphylococcus aureus. J Clin Pathol. 1977 Feb;30(2):165–167. doi: 10.1136/jcp.30.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham V. A., Pattee P. A. Genetic transformation in Staphylococcus aureus: isolation and characterization of a competence-conferring factor from bacteriophage 80 alpha lysates. J Bacteriol. 1981 Oct;148(1):301–307. doi: 10.1128/jb.148.1.301-307.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard T. J., Poston S. M., Reynolds P. J. Recipient characteristics in the transduction of methicillin resistance in Staphylococcus epidermidis. Antimicrob Agents Chemother. 1986 Mar;29(3):539–541. doi: 10.1128/aac.29.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. M., White R. L., Causey W. A., Lockwood W. R. Burn units as a source of methicillin-resistant Staphylococcus aureus infections. JAMA. 1983 May 27;249(20):2803–2807. [PubMed] [Google Scholar]
- Bradley H. E., Wetmur J. G., Hodes D. S. Tolerance in Staphylococcus aureaus: evidence for bacteriophage role. J Infect Dis. 1980 Feb;141(2):233–237. doi: 10.1093/infdis/141.2.233. [DOI] [PubMed] [Google Scholar]
- Bradley J. M., Noone P., Townsend D. E., Grubb W. B. Methicillin-resistant Staphylococcus aureus in a London hospital. Lancet. 1985 Jun 29;1(8444):1493–1495. doi: 10.1016/s0140-6736(85)92263-9. [DOI] [PubMed] [Google Scholar]
- Brenner D. G., Shaw W. V. The use of synthetic oligonucleotides with universal templates for rapid DNA sequencing: results with staphylococcal replicon pC221. EMBO J. 1985 Feb;4(2):561–568. doi: 10.1002/j.1460-2075.1985.tb03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson D. L., Pattee P. A. Genetic analysis of resistance to erythromycin and oleandomycin in Staphylococcus aureus. Can J Microbiol. 1972 Apr;18(4):429–434. doi: 10.1139/m72-067. [DOI] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Choi C. L., Grinsted J., Richmond M. H., Whitehead P. R. Nucleotide sequences at the ends of the mercury resistance transposon, Tn501. Nucleic Acids Res. 1980 May 10;8(9):1933–1945. doi: 10.1093/nar/8.9.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Dixson S., Hamilton-Miller J. M. Resistance to antiseptics in methicillin and gentamicin resistant Staphylococcus aureus. Lancet. 1985 Jun 22;1(8443):1442–1443. doi: 10.1016/s0140-6736(85)91863-x. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner R., Matzura H. Regulation of the inducible chloramphenicol acetyltransferase gene of the Staphylococcus aureus plasmid pUB112. EMBO J. 1985 Sep;4(9):2295–2300. doi: 10.1002/j.1460-2075.1985.tb03929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner R., Zyprian E., Matzura H. Expression of a chloramphenicol-resistance determinant carried on hybrid plasmids in gram-positive and gram-negative bacteria. Gene. 1984 Dec;32(1-2):151–160. doi: 10.1016/0378-1119(84)90043-x. [DOI] [PubMed] [Google Scholar]
- Buckwold F. J., Albritton W. L., Ronald A. R., Lertzman J., Henriksen R. Investigations of the occurrence of gentamicin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1979 Feb;15(2):152–156. doi: 10.1128/aac.15.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger R. J., Sherris J. C. Decreased incidence of antibiotic resistance among Staphylococcus aureus. A study in a university hospital over a 9-year period. Ann Intern Med. 1968 Dec;69(6):1099–1108. doi: 10.7326/0003-4819-69-6-1099. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Elwell L. P., Fling M. E. Molecular mechanisms of resistance to trimethoprim. Rev Infect Dis. 1982 Mar-Apr;4(2):246–254. doi: 10.1093/clinids/4.2.246. [DOI] [PubMed] [Google Scholar]
- Byeon W. H., Weisblum B. Post-transcriptional regulation of chloramphenicol acetyl transferase. J Bacteriol. 1984 May;158(2):543–550. doi: 10.1128/jb.158.2.543-550.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi B. Physical mapping of the BglI, BglII, PstI and EcoRI restriction fragments of staphylococcal phage phi 11 DNA. Mol Gen Genet. 1980;180(2):391–398. doi: 10.1007/BF00425853. [DOI] [PubMed] [Google Scholar]
- Bülow P. Prevalence of extrachromosomal drug resistance. Staphylococci in Danish hospitals during the last decade: factors influencing some properties of predominant epidemic strains. Ann N Y Acad Sci. 1971 Jun 11;182:21–39. doi: 10.1111/j.1749-6632.1971.tb30640.x. [DOI] [PubMed] [Google Scholar]
- COURTIEU A. L., GUILLERMET F. N., LONGERAY C., MAKA G., CHABBERT Y. A. FR'EQUENCE DES STAPHYLOCOQUES PR'ESENTANT UNE R'ESISTANCE H'ET'EROG'ENE A LA M'ETHICILLINE ET L'OXACILLINE EN MILIEU HOSPITALIER. Ann Inst Pasteur (Paris) 1964 Nov;107:691–697. [PubMed] [Google Scholar]
- Cafferkey M. T., Hone R., Coleman D., Pomeroy H., McGrath B., Ruddy R., Keane C. T. Methicillin-resistant Staphylococcus aureus in Dublin 1971-84. Lancet. 1985 Sep 28;2(8457):705–708. doi: 10.1016/s0140-6736(85)92942-3. [DOI] [PubMed] [Google Scholar]
- Cafferkey M. T., Hone R., Falkiner F. R., Keane C. T., Pomeroy H. Gentamicin and methicillin resistant Staphylococcus aureus in Dublin hospitals: clinical and laboratory studies. J Med Microbiol. 1983 May;16(2):117–127. doi: 10.1099/00222615-16-2-117. [DOI] [PubMed] [Google Scholar]
- Cafferkey M. T., Hone R., Keane C. T. Severe staphylococcal infections treated with vancomycin. J Antimicrob Chemother. 1982 Jan;9(1):69–74. doi: 10.1093/jac/9.1.69. [DOI] [PubMed] [Google Scholar]
- Canawati H. N., Witte J. L., Sapico F. L. Temperature effect on the susceptibility of methicillin-resistant Staphylococcus aureus to four different cephalosporins. Antimicrob Agents Chemother. 1982 Jan;21(1):173–175. doi: 10.1128/aac.21.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari P., Varaldo P. E., Fontana R., Satta G. Different staphylococcal species contain various numbers of penicillin-binding proteins ranging from four (Staphylococcus aureus) to only one (Staphylococcus hyicus). J Bacteriol. 1985 Aug;163(2):796–798. doi: 10.1128/jb.163.2.796-798.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Carleton S., Projan S. J., Highlander S. K., Moghazeh S. M., Novick R. P. Control of pT181 replication II. Mutational analysis. EMBO J. 1984 Oct;3(10):2407–2414. doi: 10.1002/j.1460-2075.1984.tb02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Hartman B. J., Tomasz A. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest. 1985 Jul;76(1):325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Bennett P. M., Lacey R. W. A variety of Staphylococcal plasmids present as multiple copies. J Gen Microbiol. 1973 Dec;79(2):343–345. doi: 10.1099/00221287-79-2-343. [DOI] [PubMed] [Google Scholar]
- Chopra I., Howe T. G. Bacterial resistance to the tetracyclines. Microbiol Rev. 1978 Dec;42(4):707–724. doi: 10.1128/mr.42.4.707-724.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Lacey R. W., Connolly J. Biochemical and genetic basis of tetracycline resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):397–404. doi: 10.1128/aac.6.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. Mechanisms of resistance to fusidic acid in Staphylococcus aureus. J Gen Microbiol. 1976 Oct;96(2):229–238. doi: 10.1099/00221287-96-2-229. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986;40:635–659. doi: 10.1146/annurev.mi.40.100186.003223. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Wong E. S., Falkow S. Common R-plasmids in Staphylococcus aureus and Staphylococcus epidermidis during a nosocomial Staphylococcus aureus outbreak. Antimicrob Agents Chemother. 1982 Feb;21(2):210–215. doi: 10.1128/aac.21.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N. Transposable genetic elements and plasmid evolution. Nature. 1976 Oct 28;263(5580):731–738. doi: 10.1038/263731a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M., Basu S. K. Mutations in prophage phi11 that impair the transducibility of their Staphylococcus aureus lysogens for methicillin resistance. J Bacteriol. 1977 Jan;129(1):237–245. doi: 10.1128/jb.129.1.237-245.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Constitutive penicillinase formation in Staphylococcus aureus owing to a mutation unlinked to the penicillinase plasmid. J Bacteriol. 1968 Apr;95(4):1368–1374. doi: 10.1128/jb.95.4.1368-1374.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Effect of the prophage and penicillinase plasmid of the recipient strain upon the transduction and the stability of methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):803–811. doi: 10.1128/jb.116.2.803-811.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Modulation of protein A formation in Staphylococcus aureus by genetic determinants for methicillin resistance. J Bacteriol. 1979 Dec;140(3):1028–1035. doi: 10.1128/jb.140.3.1028-1035.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Vernon E. G., Sweeney H. M. Differential depression of staphylococcal plasmid and chromosomal penicillinase genes by a class of unlinked chromosomal mutations (R2-). J Bacteriol. 1970 Sep;103(3):616–621. doi: 10.1128/jb.103.3.616-621.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Pomeroy H., Estridge J. K., Keane C. T., Cafferkey M. T., Hone R., Foster T. J. Susceptibility to antimicrobial agents and analysis of plasmids in gentamicin- and methicillin-resistant Staphylococcus aureus from Dublin hospitals. J Med Microbiol. 1985 Oct;20(2):157–167. doi: 10.1099/00222615-20-2-157. [DOI] [PubMed] [Google Scholar]
- Collatz E., Carlier C., Courvalin P. The chromosomal 3',5"-aminoglycoside phosphotransferase in Streptococcus pneumoniae is closely related to its plasmid-coded homologs in Streptococcus faecalis and Staphylococcus aureus. J Bacteriol. 1983 Dec;156(3):1373–1377. doi: 10.1128/jb.156.3.1373-1377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey R. C., Baldwin J. N. Relatedness of tetracycline resistance plasmids among species of coagulase-negative staphylococci. Antimicrob Agents Chemother. 1985 Feb;27(2):234–238. doi: 10.1128/aac.27.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Fiandt M. Aminoglycoside-modifying enzymes of Staphylococcus aureus; expression in Escherichia coli. Gene. 1980 May;9(3-4):247–269. doi: 10.1016/0378-1119(90)90326-m. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Ounissi H., Arthur M. Multiplicity of macrolide-lincosamide-streptogramin antibiotic resistance determinants. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):91–100. doi: 10.1093/jac/16.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Reed C., Kollisch N., DeMaria A., Lichtenberg D., Shen K., McCabe W. R. A large outbreak of infections caused by a strain of Staphylococcus aureus resistant of oxacillin and aminoglycosides. Am J Med. 1981 Jul;71(1):53–58. doi: 10.1016/0002-9343(81)90258-8. [DOI] [PubMed] [Google Scholar]
- Crossley K., Loesch D., Landesman B., Mead K., Chern M., Strate R. An outbreak of infections caused by strains of Staphylococcus aureus resistant to methicillin and aminoglycosides. I. Clinical studies. J Infect Dis. 1979 Mar;139(3):273–279. doi: 10.1093/infdis/139.3.273. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. Self defence in antibiotic-producing organisms. Br Med Bull. 1984 Jan;40(1):61–67. doi: 10.1093/oxfordjournals.bmb.a071949. [DOI] [PubMed] [Google Scholar]
- Dagert M., Jones I., Goze A., Romac S., Niaudet B., Ehrlich S. D. Replication functions of pC194 are necessary for efficient plasmid transduction by M13 phage. EMBO J. 1984 Jan;3(1):81–86. doi: 10.1002/j.1460-2075.1984.tb01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damper P. D., Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981 Dec;20(6):803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Davis B. D., Chen L. L., Tai P. C. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester C., Rondelet J. Microbial acetylation of M factor of virginiamycin. J Antibiot (Tokyo) 1976 Dec;29(12):1297–1305. doi: 10.7164/antibiotics.29.1297. [DOI] [PubMed] [Google Scholar]
- Della Latta P., Bouanchaud D., Novick R. P. Partition kinetics and thermosensitive replication of pT169, a naturally occurring multicopy tetracycline resistance plasmid of Staphylococcus aureus. Plasmid. 1978 Jun;1(3):366–375. doi: 10.1016/0147-619x(78)90052-5. [DOI] [PubMed] [Google Scholar]
- Docherty A., Grandi G., Grandi R., Gryczan T. J., Shivakumar A. G., Dubnau D. Naturally occurring macrolide-lincosamide-streptogramin B resistance in Bacillus licheniformis. J Bacteriol. 1981 Jan;145(1):129–137. doi: 10.1128/jb.145.1.129-137.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O., Löfquist F. Extrachromosomal control of methicillin resistance and toxin production in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):351–358. doi: 10.1128/jb.98.2.351-358.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O. Transduction of penicillinase production and methicillin resistance-enterotoxin B production in strains of Staphylococcus aureus. J Gen Microbiol. 1973 May;76(1):1–11. doi: 10.1099/00221287-76-1-1. [DOI] [PubMed] [Google Scholar]
- Dowd G., Cafferkey M., Dougan G. Gentamicin and methicillin resistant Staphylococcus aureus in Dublin hospitals: molecular studies. J Med Microbiol. 1983 May;16(2):129–138. doi: 10.1099/00222615-16-2-129. [DOI] [PubMed] [Google Scholar]
- Dubnau D. Induction of ermC requires translation of the leader peptide. EMBO J. 1985 Feb;4(2):533–537. doi: 10.1002/j.1460-2075.1985.tb03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. Translational attenuation: the regulation of bacterial resistance to the macrolide-lincosamide-streptogramin B antibiotics. CRC Crit Rev Biochem. 1984;16(2):103–132. doi: 10.3109/10409238409102300. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., Rock M. I., Iandolo J. J. Autogenous transduction of phi 11de in Staphylococcus aureus: transfer and genetic properties. J Bacteriol. 1984 May;158(2):689–695. doi: 10.1128/jb.158.2.689-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., Rock M. I., Lee C. Y., Iandolo J. J. Generation of transducing particles in Staphylococcus aureus. J Bacteriol. 1985 Jan;161(1):91–95. doi: 10.1128/jb.161.1.91-95.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K. G., Noble W. C. Plasmids of phage-Group-II Staphylococcus aureus. J Med Microbiol. 1984 Jun;17(3):325–334. doi: 10.1099/00222615-17-3-325. [DOI] [PubMed] [Google Scholar]
- Dyke K. G., Parker M. T., Richmond M. H. Penicillinase production and metal-ion resistance in Staphylococcus aureus cultures isolated from hospital patients. J Med Microbiol. 1970 Feb;3(1):125–136. doi: 10.1099/00222615-3-1-125. [DOI] [PubMed] [Google Scholar]
- Dyke K. G. Penicillinase production and intrinsic resistance to penicillins in methicillin-resistant cultures of Staphylococcus aureus. J Med Microbiol. 1969 Aug;2(3):261–278. doi: 10.1099/00222615-2-3-261. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D., Niaudet B., Michel B. Use of plasmids from Staphylococcus aureus for cloning of DNA in Bacillus subtilis. Curr Top Microbiol Immunol. 1982;96:19–29. doi: 10.1007/978-3-642-68315-2_2. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E. S., Mandel L. J., Kaback H. R., Miller M. H. Quantitative association between electrical potential across the cytoplasmic membrane and early gentamicin uptake and killing in Staphylococcus aureus. J Bacteriol. 1984 Mar;157(3):863–867. doi: 10.1128/jb.157.3.863-867.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Solh N., Bismuth R., Allignet J., Fouace J. M. Résistance à la pristinamycine (ou virginiamycine) des souches de Staphylococcus aureus. Pathol Biol (Paris) 1984 May;32(5):362–368. [PubMed] [Google Scholar]
- El Solh N., Ehrlich S. D. A small cadmium resistance plasmid isolated from Staphylococcus aureus. Plasmid. 1982 Jan;7(1):77–84. doi: 10.1016/0147-619x(82)90029-4. [DOI] [PubMed] [Google Scholar]
- El Solh N., Fouace J. M., Pillet J., Chabbert Y. A. Plasmid DNA content of multiresistant Staphylococcus aureus strains. Ann Microbiol (Paris) 1981 Sep-Oct;132B(2):131–156. [PubMed] [Google Scholar]
- Emslie K. R., Townsend D. E., Grubb W. B. A resistance determinant to nucleic acid-binding compounds in methicillin-resistant Staphylococcus aureus. J Med Microbiol. 1985 Oct;20(2):139–145. doi: 10.1099/00222615-20-2-139. [DOI] [PubMed] [Google Scholar]
- Emslie K. R., Townsend D. E., Grubb W. B. Isolation and characterisation of a family of small plasmids encoding resistance to nucleic acid-binding compounds in Staphylococcus aureus. J Med Microbiol. 1986 Aug;22(1):9–15. doi: 10.1099/00222615-22-1-9. [DOI] [PubMed] [Google Scholar]
- Engel H. W., Soedirman N., Rost J. A., van Leeuwen W. J., van Embden J. D. Transferability of macrolide, lincomycin, and streptogramin resistances between group A, B, and D streptococci, Streptococcus pneumoniae, and Staphylococcus aureus. J Bacteriol. 1980 May;142(2):407–413. doi: 10.1128/jb.142.2.407-413.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINEGOLD S. M., GAYLOR D. W. Enterocolitis due to phage type 54 staphylococci resistant to kanamycin, neomycin, paromomycin and chloramphenicol. N Engl J Med. 1960 Dec 1;263:1110–1116. doi: 10.1056/NEJM196012012632204. [DOI] [PubMed] [Google Scholar]
- Farber B. F., Moellering R. C., Jr Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother. 1983 Jan;23(1):138–141. doi: 10.1128/aac.23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faville R. J., Jr, Zaske D. E., Kaplan E. L., Crossley K., Sabath L. D., Quie P. G. Staphylococcus aureus endocarditis. Combined therapy with vancomycin and rifampin. JAMA. 1978 Oct 27;240(18):1963–1965. doi: 10.1001/jama.240.18.1963. [DOI] [PubMed] [Google Scholar]
- Feitelson J. S., Lederberg J. Crude lysates of Staphylococcus aureus can transform Bacillus subtilis. J Gen Microbiol. 1980 Feb;116(2):545–547. doi: 10.1099/00221287-116-2-545. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton J. E., Shaw W. V. Comparison of chloramphenicol acetyltransferase variants in staphylococci. Purification, inhibitor studies and N-terminal sequences. Biochem J. 1979 Feb 1;177(2):575–582. doi: 10.1042/bj1770575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. F., Clewell D. B. A conjugative transposon (Tn919) in Streptococcus sanguis. Infect Immun. 1985 Feb;47(2):415–420. doi: 10.1128/iai.47.2.415-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes B. A., Schaberg D. R. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J Bacteriol. 1983 Feb;153(2):627–634. doi: 10.1128/jb.153.2.627-634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouace J. Transfer of resistance plasmids in Staphylococcus aureus. Ann Microbiol (Paris) 1974 May-Jun;125(4):517–520. [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y., Weisblum B. A family of r-determinants in Streptomyces spp. that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1981 May;146(2):621–631. doi: 10.1128/jb.146.2.621-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARROD L. P. The erythromycin group of antibiotics. Br Med J. 1957 Jul 13;2(5036):57–63. doi: 10.1136/bmj.2.5036.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedney J., Lacey R. W. Properties of methicillin-resistant staphylococci now endemic in Australia. Med J Aust. 1982 May 29;1(11):448–450. doi: 10.5694/j.1326-5377.1982.tb132412.x. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Bonner D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):172–175. doi: 10.1128/aac.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellou H., Papapetropoulou M., Daikos G. K. 'Methicillin resistant' Staphylococcus aureus infections during 1978-79: clinical and bacteriologic observations. J Antimicrob Chemother. 1981 Jun;7(6):649–655. doi: 10.1093/jac/7.6.649. [DOI] [PubMed] [Google Scholar]
- Gilbert G. L., Asche V., Hewstone A. S., Mathiesen J. L. Methicillin-resistant Staphylococcus aureus in neonatal nurseries. Two years' experience in special-care nurseries in Melbourne. Med J Aust. 1982 May 29;1(11):455–459. [PubMed] [Google Scholar]
- Gillespie M. T., May J. W., Skurray R. A. Antibiotic resistance in Staphylococcus aureus isolated at an Australian hospital between 1946 and 1981. J Med Microbiol. 1985 Apr;19(2):137–147. doi: 10.1099/00222615-19-2-137. [DOI] [PubMed] [Google Scholar]
- Gillespie M. T., May J. W., Skurray R. A. Antibiotic susceptibilities and plasmid profiles of nosocomial methicillin-resistant Staphylococcus aureus: a retrospective study. J Med Microbiol. 1984 Jun;17(3):295–310. doi: 10.1099/00222615-17-3-295. [DOI] [PubMed] [Google Scholar]
- Gillespie M. T., May J. W., Skurray R. A. Detection of an integrated tetracycline resistance plasmid in the chromosome of methicillin-resistant Staphylococcus aureus. J Gen Microbiol. 1986 Jun;132(6):1723–1728. doi: 10.1099/00221287-132-6-1723. [DOI] [PubMed] [Google Scholar]
- Gillespie M. T., Skurray R. A. Plasmids in multiresistant Staphylococcus aureus. Microbiol Sci. 1986 Feb;3(2):53–58. [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessens W. H., Fontijne P., van Raffe M., Michel M. F. Tolerance percentage as a criterion for the detection of tolerant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1984 May;25(5):575–578. doi: 10.1128/aac.25.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffic F. L., Capmau M. L., Bonnet D., Cerceau C., Soussy C., Dublanchet A., Duval J. Plasmid-mediated pristinamycin resistance. PAC IIA: a new enzyme which modifies pristinamycin IIA. J Antibiot (Tokyo) 1977 Aug;30(8):665–669. doi: 10.7164/antibiotics.30.665. [DOI] [PubMed] [Google Scholar]
- Gopal V., Bisno A. L., Silverblatt F. J. Failure of vancomycin treatment in Staphylococcus aureus endocarditis. In vivo and in vitro observations. JAMA. 1976 Oct 4;236(14):1604–1606. [PubMed] [Google Scholar]
- Goullet P., Hieng H. S. Progression de la résistance à la gentamicine et à la tobramycine des souches de Staphylococcus aureus isolées d'infections hospitalières. Nouv Presse Med. 1981 Sep 12;10(32):2645-6, 2651-2. [PubMed] [Google Scholar]
- Goze A., Ehrlich S. D. Replication of plasmids from Staphylococcus aureus in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7333–7337. doi: 10.1073/pnas.77.12.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. Y., Weisblum B. 23S ribosomal ribonucleic acid of macrolide-producing streptomycetes contains methylated adenine. J Bacteriol. 1979 Mar;137(3):1464–1467. doi: 10.1128/jb.137.3.1464-1467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S. Characterization of plasmids in aminocyclitol-resistant Staphylococcus aureus: electron microscopic and restriction endonuclease analysis. Plasmid. 1983 Mar;9(2):159–181. doi: 10.1016/0147-619x(83)90018-5. [DOI] [PubMed] [Google Scholar]
- Gray G. S., Fitch W. M. Evolution of antibiotic resistance genes: the DNA sequence of a kanamycin resistance gene from Staphylococcus aureus. Mol Biol Evol. 1983 Dec;1(1):57–66. doi: 10.1093/oxfordjournals.molbev.a040298. [DOI] [PubMed] [Google Scholar]
- Gray G. S., Huang R. T., Davies J. Aminocyclitol resistance in Staphylococcus aureus: presence of plasmids and aminocyclitol-modifying enzymes. Plasmid. 1983 Mar;9(2):147–158. doi: 10.1016/0147-619x(83)90017-3. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Lacey R. W. Genetic variation of streptomycin resistance in clinical strains of Staphylococcus aureus. J Med Microbiol. 1973 Aug;6(3):351–361. doi: 10.1099/00222615-6-3-351. [DOI] [PubMed] [Google Scholar]
- Groves D. J. Interspecific relationships of antibiotic resistance in Staphylococcus sp.: isolation and comparison of plasmids determining tetracycline resistance in S. aureus and S. epidermidis. Can J Microbiol. 1979 Dec;25(12):1468–1475. doi: 10.1139/m79-227. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., Annear D. I. Spontaneous loss of methicillin resistance in Staphylococcus aureus at room-temperature. Lancet. 1972 Dec 9;2(7789):1257–1257. doi: 10.1016/s0140-6736(72)92315-x. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., Annear D. I. Unstable drug resistance in Staphylococcus aureus M4. Genet Res. 1981 Dec;38(3):217–223. doi: 10.1017/s0016672300020565. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., O'Reilly R. J. Joint transduction of separate extrachromosomal drug resistance determinants in Staphylococcus aureus E169. Biochem Biophys Res Commun. 1971 Feb 5;42(3):377–383. doi: 10.1016/0006-291x(71)90381-0. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., O'Reilly R. J., May J. W. Segregation o co-transduced streptomycin and tetracycline resistance in Staphylococcus aureus. Genet Res. 1972 Aug;20(1):43–50. doi: 10.1017/s0016672300013574. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Grandi G., Hahn J., Grandi R., Dubnau D. Conformational alteration of mRNA structure and the posttranscriptional regulation of erythromycin-induced drug resistance. Nucleic Acids Res. 1980 Dec 20;8(24):6081–6097. doi: 10.1093/nar/8.24.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Hahn J., Contente S., Dubnau D. Replication and incompatibility properties of plasmid pE194 in Bacillus subtilis. J Bacteriol. 1982 Nov;152(2):722–735. doi: 10.1128/jb.152.2.722-735.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T., Israeli-Reches M., Del Bue M., Dubnau D. DNA sequence and regulation of ermD, a macrolide-lincosamide-streptogramin B resistance element from Bacillus licheniformis. Mol Gen Genet. 1984;194(3):349–356. doi: 10.1007/BF00425543. [DOI] [PubMed] [Google Scholar]
- Götz F., Ahrné S., Lindberg M. Plasmid transfer and genetic recombination by protoplast fusion in staphylococci. J Bacteriol. 1981 Jan;145(1):74–81. doi: 10.1128/jb.145.1.74-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz F., Zabielski J., Philipson L., Lindberg M. DNA homology between the arsenate resistance plasmid pSX267 from Staphylococcus xylosus and the penicillinase plasmid pI258 from Staphylococcus aureus. Plasmid. 1983 Mar;9(2):126–137. doi: 10.1016/0147-619x(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Haley R. W., Bregman D. A. The role of understaffing and overcrowding in recurrent outbreaks of staphylococcal infection in a neonatal special-care unit. J Infect Dis. 1982 Jun;145(6):875–885. doi: 10.1093/infdis/145.6.875. [DOI] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Hall B. M. Mercury resistance of Staphylococcus aureus. J Hyg (Lond) 1970 Mar;68(1):121–129. doi: 10.1017/s0022172400028576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action-with special reference to streptomycin and gentamicin. II. Effects of aminoglycosides on cells. J Antimicrob Chemother. 1981 Dec;8(6):429–445. doi: 10.1093/jac/8.6.429. [DOI] [PubMed] [Google Scholar]
- Harmon S. A., Baldwin J. N., Tien W. C., Critz D. B. Co-transduction of the genetic determinants of synthesis of penicillinase and methionine in Staphylococcus aureus. Can J Microbiol. 1966 Oct;12(5):973–977. doi: 10.1139/m66-131. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone R., Cafferkey M., Keane C. T., Harte-Barry M., Moorhouse E., Carroll R., Martin F., Ruddy R. Bacteraemia in Dublin due to gentamicin-resistant Staphylococcus aureus. J Hosp Infect. 1981 Jun;2(2):119–126. doi: 10.1016/0195-6701(81)90020-7. [DOI] [PubMed] [Google Scholar]
- Horaud T., Le Bouguenec C., Pepper K. Molecular genetics of resistance to macrolides, lincosamides and streptogramin B (MLS) in streptococci. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):111–135. doi: 10.1093/jac/16.suppl_a.111. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Ikeda T., Furukawa K., Tomizuka N. Genetic relationship between pUB110 and antibiotic-resistant plasmids obtained from thermophilic bacilli. Can J Microbiol. 1985 Jul;31(7):614–619. doi: 10.1139/m85-116. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Ikeda T., Narushima H., Tomizuka N. Isolation and characterization of antibiotic-resistance plasmids in thermophilic bacilli. Can J Microbiol. 1985 Apr;31(4):339–345. doi: 10.1139/m85-065. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Ikeda T., Tomizuka N., Furukawa K. Nucleotide sequence of the tetracycline resistance gene of pTHT15, a thermophilic Bacillus plasmid: comparison with staphylococcal TcR controls. Gene. 1985;37(1-3):131–138. doi: 10.1016/0378-1119(85)90265-3. [DOI] [PubMed] [Google Scholar]
- Hotta K., Yamamoto H., Okami Y., Umezawa H. Resistance mechanisms of kanamycin-, neomycin-, and streptomycin-producing streptomycetes to aminoglycoside antibiotics. J Antibiot (Tokyo) 1981 Sep;34(9):1175–1182. doi: 10.7164/antibiotics.34.1175. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aiba S. Isolation and characterization of antibiotic resistance plasmids from thermophilic bacilli and construction of deletion plasmids. J Bacteriol. 1981 Jun;146(3):1091–1097. doi: 10.1128/jb.146.3.1091-1097.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J. Genetic regulation of penicillinase synthesis in Gram-positive bacteria. Microbiol Rev. 1978 Mar;42(1):67–83. doi: 10.1128/mr.42.1.67-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J., Lilleholm J. L. Characterization of mutations in the penicillinase operon Staphylococcus aureus. Mol Gen Genet. 1976 Aug 10;147(1):23–27. doi: 10.1007/BF00337931. [DOI] [PubMed] [Google Scholar]
- Imsande J., Lilleholm J. L. Nature of the plasmid-linked penicillinase regulatory region in Staphylococcus aureus. Mol Gen Genet. 1977 Jun 8;153(2):153–157. doi: 10.1007/BF00264730. [DOI] [PubMed] [Google Scholar]
- Imsande J. Repressor and antirepressor in the regulation of staphylococcal penicillinase synthesis. Genetics. 1973 Sep;75(1):1–17. doi: 10.1093/genetics/75.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanescu S., Surdeanu M., Della Latta P., Novick R. Incompatibility and molecular relationships between small Staphylococcal plasmids carrying the same resistance marker. Plasmid. 1978 Sep;1(4):468–479. doi: 10.1016/0147-619x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Iordanescu S., Surdeanu M. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol. 1976 Oct;96(2):277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- Iordănescu S. Recombinant plasmid obtained from two different, compatible staphylococcal plasmids. J Bacteriol. 1975 Nov;124(2):597–601. doi: 10.1128/jb.124.2.597-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S. Relationships between cotransducible plasmids in Staphylococcus aureus. J Bacteriol. 1977 Jan;129(1):71–75. doi: 10.1128/jb.129.1.71-75.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S., Surdeanu M. New incompatibility groups of Staphylococcus aureus plasmids. Plasmid. 1980 Nov;4(3):256–260. doi: 10.1016/0147-619x(80)90064-5. [DOI] [PubMed] [Google Scholar]
- Iordănescu S. Three distinct plasmids originating in the same Staphylococcus aureus strain. Arch Roum Pathol Exp Microbiol. 1976 Jan-Jun;35(1-2):111–118. [PubMed] [Google Scholar]
- Israeli-Reches M., Weinrauch Y., Dubnau D. Evolutionary relationships of the Bacillus licheniformis macrolide-lincosamide-streptogramin B resistance elements. Mol Gen Genet. 1984;194(3):362–367. doi: 10.1007/BF00425545. [DOI] [PubMed] [Google Scholar]
- Jackson M. P., DeSena J., Lednicky J., McPherson B., Haile R., Garrison R. G., Rogolsky M. Isolation and characterization of a bacteriophage factor that confers competence for genetic transformation to an exfoliative toxin-producing strain of Staphylococcus aureus. Infect Immun. 1983 Feb;39(2):939–947. doi: 10.1128/iai.39.2.939-947.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Nathan C., Weinstein R. A., Kabins S. A., Cohen S. Identity and interspecific transfer of gentamicin-resistance plasmids in Staphylococcus aureus and Staphylococcus epidermidis. J Infect Dis. 1980 Jun;141(6):738–747. doi: 10.1093/infdis/141.6.738. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Weinstein R. A., Kabins S. A., Nathan C., Cohen S. Structural and phenotypic varieties of gentamicin resistance plasmids in hospital strains of Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1982 May;21(5):773–779. doi: 10.1128/aac.21.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalanko A., Palva I., Söderlund Restriction maps of plasmids pUB110 and pBD9. Gene. 1981 Sep;14(4):325–328. doi: 10.1016/0378-1119(81)90165-7. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Dyke K. G. Ethidium bromide resistance, a new marker on the staphylococcal penicillinase plasmid. J Bacteriol. 1969 Dec;100(3):1413–1414. doi: 10.1128/jb.100.3.1413-1414.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. H., Dyke K. G. Staphylococcal penicillinase plasmids: studies on the reversion of a temperature-sensitive replication mutant to temperature stability. J Gen Microbiol. 1974 Jun;82(2):309–317. doi: 10.1099/00221287-82-2-309. [DOI] [PubMed] [Google Scholar]
- Kayser F. H., Berger-Bächi B., Beck W. D. Genetics of multiply-resistant Staphylococcus aureus. J Hosp Infect. 1986 Mar;7 (Suppl A):19–27. doi: 10.1016/0195-6701(86)90004-6. [DOI] [PubMed] [Google Scholar]
- Kayser F. H., Homberger F., Devaud M. Aminocyclitol-modifying enzymes specified by chromosomal genes in Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):766–772. doi: 10.1128/aac.19.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Mak T. M. Methicillin-resistant staphylococci. Am J Med Sci. 1972 Sep;264(3):197–205. [PubMed] [Google Scholar]
- Kayser F. H. Methicillin-resistant staphylococci 1965-75. Lancet. 1975 Oct 4;2(7936):650–653. doi: 10.1016/s0140-6736(75)90129-4. [DOI] [PubMed] [Google Scholar]
- Kayser F. H., Wüst J., Corrodi P. Transduction and elimination of resistance determinants in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Sep;2(3):217–223. doi: 10.1128/aac.2.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Wüst J., Santanam P. Genetic and molecular characterisation of resistance determinants in methicillin-resistant Staphylococcus-aureus. J Med Microbiol. 1976 May;9(2):137–148. doi: 10.1099/00222615-9-2-137. [DOI] [PubMed] [Google Scholar]
- Keggins K. M., Lovett P. S., Duvall E. J. Molecular cloning of genetically active fragments of Bacillus DNA in Bacillus subtilis and properties of the vector plasmid pUB110. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1423–1427. doi: 10.1073/pnas.75.3.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Adler G. K., Novick R. P. Functional origin of replication of pT181 plasmid DNA is contained within a 168-base-pair segment. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4580–4584. doi: 10.1073/pnas.79.15.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Carleton S. M., Novick R. P. Replication of plasmid pT181 DNA in vitro: requirement for a plasmid-encoded product. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4902–4906. doi: 10.1073/pnas.78.8.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid. 1983 Nov;10(3):251–259. doi: 10.1016/0147-619x(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Terminal nucleotide sequences of Tn551, a transposon specifying erythromycin resistance in Staphylococcus aureus: homology with Tn3. Plasmid. 1980 Sep;4(2):148–154. doi: 10.1016/0147-619x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- King K., Brady L., Thomson M., Harkness J. L. Antibiotic-resistant staphylococci in a teaching hospital. Med J Aust. 1982 Nov 13;2(10):461–465. doi: 10.5694/j.1326-5377.1982.tb132522.x. [DOI] [PubMed] [Google Scholar]
- Kirby W. M. EXTRACTION OF A HIGHLY POTENT PENICILLIN INACTIVATOR FROM PENICILLIN RESISTANT STAPHYLOCOCCI. Science. 1944 Jun 2;99(2579):452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- Kirby W. M. Vancomycin therapy of severe staphylococcal infections. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):73–78. doi: 10.1093/jac/14.suppl_d.73. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Beumer J., Daneau D. Bacteriophage types and antibiotic susceptibility of Staphylococcus aureus. Appl Microbiol. 1971 Dec;22(6):1000–1007. doi: 10.1128/am.22.6.1000-1007.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Evolution of antibiotic resistance gene function. Microbiol Rev. 1981 Jun;45(2):355–378. doi: 10.1128/mr.45.2.355-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsel R. R., Murray R. W., Rosenblum W. D., Khan S. A. The replication initiator protein of plasmid pT181 has sequence-specific endonuclease and topoisomerase-like activities. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6845–6849. doi: 10.1073/pnas.82.20.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I., Ishikawa T., Nakahara H. Mercury and cadmium resistances mediated by the penicillinase plasmid in Staphylococcus aureus. J Bacteriol. 1974 Jan;117(1):1–7. doi: 10.1128/jb.117.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Sasatsu M. Association of a penicillin resistance gene with a tetracycline resistance plasmid (PTP-2) in Staphylococcus aureus. Antimicrob Agents Chemother. 1976 Apr;9(4):706–712. doi: 10.1128/aac.9.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Sasatsu M., Ubukata K., Konno M., Fujii R. New plasmid (pTU512), mediating resistance to penicillin, erythromycin, and kanamycin, from clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Apr;13(4):691–694. doi: 10.1128/aac.13.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- Krogstad D. J., Smith R. M., Moellering R. C., Jr, Parquette A. R. Visualization of cell-cell contact during conjugation in Streptococcus faecalis. J Bacteriol. 1980 Feb;141(2):963–967. doi: 10.1128/jb.141.2.963-967.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski J. J., Murphy E., Novick R. P., Rush M. G. Site-specificity of the chromosomal insertion of Staphylococcus aureus transposon Tn554. J Mol Biol. 1981 Oct 15;152(1):19–33. doi: 10.1016/0022-2836(81)90093-0. [DOI] [PubMed] [Google Scholar]
- Kuck N. A., Forbes M. Uptake of minocycline and tetracycline by tetracycline-susceptible and -resistant bacteria. Antimicrob Agents Chemother. 1973 Jun;3(6):662–664. doi: 10.1128/aac.3.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Pattee P. A., Baldwin J. N. Chromosomal map location of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1978 Aug;135(2):460–465. doi: 10.1128/jb.135.2.460-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar C. C., Novick R. P. Plasmid pT181 replication is regulated by two countertranscripts. Proc Natl Acad Sci U S A. 1985 Feb;82(3):638–642. doi: 10.1073/pnas.82.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWBURY E. J., BABB J. R., BROWN V. L., COLLINS B. J. NEOMYCIN-RESISTANT STAPHYLOCOCCUS AUREUS IN A BURNS UNIT. J Hyg (Lond) 1964 Jun;62:221–228. doi: 10.1017/s0022172400039942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance in Staphylococcus aureus and streptococci. Br Med Bull. 1984 Jan;40(1):77–83. doi: 10.1093/oxfordjournals.bmb.a071951. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance plasmids of Staphylococcus aureus and their clinical importance. Bacteriol Rev. 1975 Mar;39(1):1–32. doi: 10.1128/br.39.1.1-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Evidence for mutation to streptomycin resistance in clinical strains of Staphylococcus aureus. J Gen Microbiol. 1972 Nov;73(1):175–180. doi: 10.1099/00221287-73-1-175. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Genetic studies of a multi-resistant strain of Staphylococcus aureus. J Med Microbiol. 1974 May;7(2):285–297. doi: 10.1099/00222615-7-2-285. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Do sulphonamide-trimethoprim combinations select less resistance to trimethoprim than the use of trimethoprim alone? J Med Microbiol. 1982 Nov;15(4):403–427. doi: 10.1099/00222615-15-4-403. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Evidence for two mechanisms of plasmid transfer in mixed cultures of Staphylococcus aureus. J Gen Microbiol. 1980 Aug;119(2):423–435. doi: 10.1099/00221287-119-2-423. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Genetic control in methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1972 Nov;5(4):497–508. doi: 10.1099/00222615-5-4-497. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Genetic analysis of methicillin-resistant strains of Staphylococcus aureus; evidence for their evolution from a single clone. J Med Microbiol. 1973 Nov;6(4):511–526. doi: 10.1099/00222615-6-4-511. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Linkage of fusidic acid resistance to the penicillinase plasmid in Staphylococcus aureus. J Gen Microbiol. 1972 Dec;73(3):501–508. doi: 10.1099/00221287-73-3-501. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. High-frequency transfer of neomycin resistance between naturally occurring strains of Staphylococcus aureus. J Med Microbiol. 1971 Feb;4(1):73–84. doi: 10.1099/00222615-4-1-73. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Keyworth N., Lincoln C. Staphylococci in the U.K.: a review. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):19–25. doi: 10.1093/jac/14.suppl_d.19. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Lewis E., Rosdahl V. T. Evolution of plasmids in vivo in a strain of Staphylococcus aureus. J Med Microbiol. 1974 Feb;7(1):117–125. doi: 10.1099/00222615-7-1-117. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Lord V. L. Sensitivity of staphylococci to fatty acids: novel inactivation of linolenic acid by serum. J Med Microbiol. 1981 Feb;14(1):41–49. doi: 10.1099/00222615-14-1-41. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Lord V. L. Transfer of gentamicin resistance between cultures of Staphylococcus aureus in nutrient broth, serum and urine. J Med Microbiol. 1980 Aug;13(3):411–421. doi: 10.1099/00222615-13-3-411. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Mitchell A. A. Gentamicin-resistant Staphylococcus aureus. Lancet. 1969 Dec 27;2(7635):1425–1426. doi: 10.1016/s0140-6736(69)90967-2. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Richmond M. H. The genetic basis of antibiotic resistance in S. aureus: the importance of gene transfer in the evolution of this organism in the hospital environment. Ann N Y Acad Sci. 1974 Jul 31;236(0):395–412. doi: 10.1111/j.1749-6632.1974.tb41506.x. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Rosdahl V. T. An unusual "penicillinase plasmid" in staphylococcus aureus; evidence for its transfer under natural conditions. J Med Microbiol. 1974 Feb;7(1):1–9. doi: 10.1099/00222615-7-1-1. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Stokes A. Studies on recently isolated cultures of methicillin-resistant Staphylococcus aureus. J Gen Microbiol. 1979 Oct;114(2):329–339. doi: 10.1099/00221287-114-2-329. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Transfer of chromosomal genes between staphylococci in mixed cultures. J Gen Microbiol. 1972 Jul;71(2):399–401. doi: 10.1099/00221287-71-2-399. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Transfer of tetracycline-resistance between strains of Staphylococcus aureus in mixed cultures. J Gen Microbiol. 1971 Dec;69(2):229–237. doi: 10.1099/00221287-69-2-229. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Dahlberg J. E., Weisblum B. Structure of an inducibly methylatable nucleotide sequence in 23S ribosomal ribonucleic acid from erythromycin-resistant Staphylococcus aureus. Biochemistry. 1973 Jan 30;12(3):457–460. doi: 10.1021/bi00727a015. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H. P. Impact of bacterial resistance to antibiotics on therapy. Br Med Bull. 1984 Jan;40(1):102–106. doi: 10.1093/oxfordjournals.bmb.a071938. [DOI] [PubMed] [Google Scholar]
- Lampson B. C., Parisi J. T. Naturally occurring Staphylococcus epidermidis plasmid expressing constitutive macrolide-lincosamide-streptogramin B resistance contains a deleted attenuator. J Bacteriol. 1986 May;166(2):479–483. doi: 10.1128/jb.166.2.479-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy M., Larkum N. W., Oswald E. J., Streightoff F. INCREASED SYNTHESIS OF p-AMINOBENZOIC ACID ASSOCIATED WITH THE DEVELOPMENT OF SULFONAMIDE RESISTANCE IN STAPHYLOCOCCUS AUREUS. Science. 1943 Mar 19;97(2516):265–267. doi: 10.1126/science.97.2516.265. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Capmau M. L., Abbe J., Cerceau C., Dublanchet A., Duval J. Plasmid mediated pristinamycin resistance: PH 1A, a pristinamycin 1A hydrolase. Ann Microbiol (Paris) 1977 Nov-Dec;128B(4):471–474. [PubMed] [Google Scholar]
- Le Goffic F., Martel A., Capmau M. L., Baca B., Goebel P., Chardon H., Soussy C. J., Duval J., Bouanchaud D. H. New plasmid-mediated nucleotidylation of aminoglycoside antibiotics in Staphlococcus aureus. Antimicrob Agents Chemother. 1976 Aug;10(2):258–264. doi: 10.1128/aac.10.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic F., Moreau N., Masson M. Are some aminoglycoside antibiotics inactivating enzymes polyfunctional? Ann Microbiol (Paris) 1977 Nov-Dec;128B(4):465–469. [PubMed] [Google Scholar]
- Levy S. B. Microbial resistance to antibiotics. An evolving and persistent problem. Lancet. 1982 Jul 10;2(8289):83–88. doi: 10.1016/s0140-6736(82)91701-9. [DOI] [PubMed] [Google Scholar]
- Lewis E. L., Lacey R. W. Present significance of resistance to trimethoprim and sulphonamides in coliforms, Staphylococcus aureus, and Streptococcus faecalis. J Clin Pathol. 1973 Mar;26(3):175–180. doi: 10.1136/jcp.26.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Novick R. P. Plasmid-specific transformation in Staphylococcus aureus. J Bacteriol. 1973 Jul;115(1):139–145. doi: 10.1128/jb.115.1.139-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Sjöström J. E., Johansson T. Transformation of chromosomal and plasmid characters in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):844–847. doi: 10.1128/jb.109.2.844-847.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowbury E. J., Jackson D. M., Ricketts C. R., Davis B. Topical chemoprophylaxis for burns: trials of creams containing silver sulphadiazine and trimethoprim. Injury. 1971 Jul;3(1):18–24. doi: 10.1016/s0020-1383(71)80131-6. [DOI] [PubMed] [Google Scholar]
- Luchansky J. B., Pattee P. A. Isolation of transposon Tn551 insertions near chromosomal markers of interest in Staphylococcus aureus. J Bacteriol. 1984 Sep;159(3):894–899. doi: 10.1128/jb.159.3.894-899.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Iuorio J. L., May J. W., Skurray R. A. Molecular epidemiology of multiresistant Staphylococcus aureus in Australian hospitals. J Med Microbiol. 1984 Feb;17(1):79–89. doi: 10.1099/00222615-17-1-79. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Marshall J. H., Skurray R. A. Plasmid-mediated antibiotic resistance in methicillin-resistant Staphylococcus aureus. Med J Aust. 1982 May 29;1(11):468–469. doi: 10.5694/j.1326-5377.1982.tb132418.x. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Analysis of plasmids in nosocomial strains of multiple-antibiotic-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Jun;23(6):817–826. doi: 10.1128/aac.23.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. A vector for recombinant DNA in Staphylococcus aureus. Gene. 1978 Apr;3(2):161–172. doi: 10.1016/0378-1119(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. Characterization of small plasmids from Staphylococcus aureus. Gene. 1978 Apr;3(2):145–159. [PubMed] [Google Scholar]
- Löfdahl S., Zabielski J., Philipson L. Structure and restriction enzyme maps of the circularly permuted DNA of staphylococcal bacteriophage phi 11. J Virol. 1981 Feb;37(2):784–794. doi: 10.1128/jvi.37.2.784-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., HASHIMOTO H., KONO M., MORIMURA M. DRUG RESISTANCE OF STAPHYLOCOCCI. II. JOINT ELIMINATION AND JOINT TRANSDUCTION OF THE DETERMINANTS OF PENICILLINASE PRODUCTION AND RESISTANCE TO MACROLIDE ANTIBIOTICS. J Bacteriol. 1965 Apr;89:988–992. doi: 10.1128/jb.89.4.988-992.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel L. J., Murphy E., Steigbigel N. H., Miller M. H. Gentamicin uptake in Staphylococcus aureus possessing plasmid-encoded, aminoglycoside-modifying enzymes. Antimicrob Agents Chemother. 1984 Oct;26(4):563–569. doi: 10.1128/aac.26.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Moorman D. R. Treatment of experimental staphylococcal infections: effect of rifampin alone and in combination on development of rifampin resistance. Antimicrob Agents Chemother. 1980 Apr;17(4):658–662. doi: 10.1128/aac.17.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A., Masson M., Moreau N., Le Goffic F. Kinetic studies of aminoglycoside acetyltransferase and phosphotransferase from Staphylococcus aureus RPAL. Relationship between the two activities. Eur J Biochem. 1983 Jul 1;133(3):515–521. doi: 10.1111/j.1432-1033.1983.tb07494.x. [DOI] [PubMed] [Google Scholar]
- Martel A., Moreau N., Capmau M. L., Soussy C. J., Duval J. 2"-O-phosphorylation of gentamicin components by a Staphylococcus aureus strain carrying a plasmid. Antimicrob Agents Chemother. 1977 Jul;12(1):26–30. doi: 10.1128/aac.12.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates S. M., Eisenberg E. S., Mandel L. J., Patel L., Kaback H. R., Miller M. H. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6693–6697. doi: 10.1073/pnas.79.21.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates S. M., Patel L., Kaback H. R., Miller M. H. Membrane potential in anaerobically growing Staphylococcus aureus and its relationship to gentamicin uptake. Antimicrob Agents Chemother. 1983 Apr;23(4):526–530. doi: 10.1128/aac.23.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M., Katakura Y., Imanaka T., Aiba S. Enzymatic and nucleotide sequence studies of a kanamycin-inactivating enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J Bacteriol. 1984 Oct;160(1):413–420. doi: 10.1128/jb.160.1.413-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M., Weisblum B. Messenger RNA from Staphylococcus aureus that specifies macrolide-lincosamide-streptogramin resistance. Demonstration of its conformations and of the leader peptide it encodes. J Mol Biol. 1985 Oct 20;185(4):769–780. doi: 10.1016/0022-2836(85)90061-0. [DOI] [PubMed] [Google Scholar]
- Mayhall C. G., Medoff G., Marr J. J. Variation in the susceptibility of strains of Staphylococcus aureus to oxacillin, cephalothin, and gentamicin. Antimicrob Agents Chemother. 1976 Oct;10(4):707–712. doi: 10.1128/aac.10.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald M. I., Graham I., Harvey K. J., Sinclair A. Antibacterial activity of hydrolysed linseed oil and linolenic acid against methicillin-resistant staphylococcus aureus. Lancet. 1981 Nov 7;2(8254):1056–1056. doi: 10.1016/s0140-6736(81)91261-7. [DOI] [PubMed] [Google Scholar]
- McDonnell R. W., Sweeney H. M., Cohen S. Conjugational transfer of gentamicin resistance plasmids intra- and interspecifically in Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1983 Jan;23(1):151–160. doi: 10.1128/aac.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983 Nov-Dec;5(6):1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Terry P. M., Huang T. S., Houk C. L., Davies J. Nosocomial infections with gentamicin-resistant Staphylococcus aureus: plamid analysis as an epidemiologic tool. J Infect Dis. 1979 Dec;140(6):864–872. doi: 10.1093/infdis/140.6.864. [DOI] [PubMed] [Google Scholar]
- McKenzie T., Hoshino T., Tanaka T., Sueoka N. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986 Mar;15(2):93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- McMurry L. M., Cullinane J. C., Petrucci R. E., Jr, Levy S. B. Active uptake of tetracycline by membrane vesicles from susceptible Escherichia coli. Antimicrob Agents Chemother. 1981 Sep;20(3):307–313. doi: 10.1128/aac.20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A. Beta-lactamases. Br Med Bull. 1984 Jan;40(1):18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- Meijers J. A., Stobberingh E. E. Chromosomal penicillin resistance in Staphylococcus aureus strains of phage group II. Antonie Van Leeuwenhoek. 1980;46(6):577–586. doi: 10.1007/BF00394013. [DOI] [PubMed] [Google Scholar]
- Meijers J. A., Winkler K. C., Stobberingh E. E. Resistance transfer in mixed cultures of Staphylococcus aureus. J Med Microbiol. 1981 Feb;14(1):21–39. doi: 10.1099/00222615-14-1-21. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Stobberingh E. E., Winkler K. C. Restriction of resistance transfer in Staphylococcus aureus. Contrib Microbiol Immunol. 1979;6:189–197. [PubMed] [Google Scholar]
- Mickelsen P. A., Plorde J. J., Gordon K. P., Hargiss C., McClure J., Schoenknecht F. D., Condie F., Tenover F. C., Tompkins L. S. Instability of antibiotic resistance in a strain of Staphylococcus epidermidis isolated from an outbreak of prosthetic valve endocarditis. J Infect Dis. 1985 Jul;152(1):50–58. doi: 10.1093/infdis/152.1.50. [DOI] [PubMed] [Google Scholar]
- Miller M. H., Edberg S. C., Mandel L. J., Behar C. F., Steigbigel N. H. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1980 Nov;18(5):722–729. doi: 10.1128/aac.18.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman D. R., Mandell G. L. Characteristics of rifampin-resistant variants obtained from clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 Dec;20(6):709–713. doi: 10.1128/aac.20.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimura M., Watanabe K., Mori H., Mitsuhashi S. Lability of streptomycin resistance in Staphylococcus aureus. Jpn J Microbiol. 1970 Jul;14(4):253–256. doi: 10.1111/j.1348-0421.1970.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Morrow T. O., Harmon S. A. Genetic analysis of Staphylococcus aureus RNA polymerase mutants. J Bacteriol. 1979 Jan;137(1):374–383. doi: 10.1128/jb.137.1.374-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R. E., Ano T., Imanaka T., Aiba S. Complete nucleotide sequences of Bacillus plasmids pUB110dB, pRBH1 and its copy mutants. Mol Gen Genet. 1986 Jan;202(1):169–171. doi: 10.1007/BF00330534. [DOI] [PubMed] [Google Scholar]
- Murphy E., Huwyler L., de Freire Bastos M. do C. Transposon Tn554: complete nucleotide sequence and isolation of transposition-defective and antibiotic-sensitive mutants. EMBO J. 1985 Dec 1;4(12):3357–3365. doi: 10.1002/j.1460-2075.1985.tb04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. Inhibition of Tn554 transposition: deletion analysis. Plasmid. 1983 Nov;10(3):260–269. doi: 10.1016/0147-619x(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Murphy E., Löfdahl S. Transposition of Tn554 does not generate a target duplication. Nature. 1984 Jan 19;307(5948):292–294. doi: 10.1038/307292a0. [DOI] [PubMed] [Google Scholar]
- Murphy E., Novick R. P. Physical mapping of Staphylococcus aureus penicillinase plasmid pI524: characterization of an invertible region. Mol Gen Genet. 1979 Aug;175(1):19–30. doi: 10.1007/BF00267851. [DOI] [PubMed] [Google Scholar]
- Murphy E., Novick R. P. Site-specific recombination between plasmids of Staphylococcus aureus. J Bacteriol. 1980 Jan;141(1):316–326. doi: 10.1128/jb.141.1.316-326.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3") (9). Mol Gen Genet. 1985;200(1):33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of ermA, a macrolide-lincosamide-streptogramin B determinant in Staphylococcus aureus. J Bacteriol. 1985 May;162(2):633–640. doi: 10.1128/jb.162.2.633-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Phillips S., Edelman I., Novick R. P. Tn554: isolation and characterization of plasmid insertions. Plasmid. 1981 May;5(3):292–305. doi: 10.1016/0147-619x(81)90006-8. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Naidoo J. Interspecific co-transfer of antibiotic resistance plasmids in staphylococci in vivo. J Hyg (Lond) 1984 Aug;93(1):59–66. doi: 10.1017/s0022172400060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J., Noble W. C. Transfer of gentamicin resistance between coagulase-negative and coagulase-positive staphylococci on skin. J Hyg (Lond) 1981 Apr;86(2):183–187. doi: 10.1017/s0022172400068893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J., Noble W. C. Transfer of gentamicin resistance between strains of Staphylococcus aureus on skin. J Gen Microbiol. 1978 Aug;107(2):391–393. doi: 10.1099/00221287-107-2-391. [DOI] [PubMed] [Google Scholar]
- Naidoo J., Noble W. C., Weissmann A., Dyke K. G. Gentamicin-resistant staphylococci: genetics of an outbreak in a dermatology department. J Hyg (Lond) 1983 Aug;91(1):7–16. doi: 10.1017/s0022172400059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan C. S., Dubnau D. Evidence for the translational attenuation model: ribosome-binding studies and structural analysis with an in vitro run-off transcript of ermC. Nucleic Acids Res. 1985 Oct 25;13(20):7307–7326. doi: 10.1093/nar/13.20.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W. C., Naidoo J. Evolution of antibiotic resistance in Staphylococcus aureus: the role of the skin. Br J Dermatol. 1978 Apr;98(4):481–489. doi: 10.1111/j.1365-2133.1978.tb06547.x. [DOI] [PubMed] [Google Scholar]
- Noguchi N., Shishido K., Ando T., Kono M. Construction and propagation of deletion derivatives of staphylococcal tetracycline-resistance plasmid pTP-5 in Bacillus subtilis. Gene. 1983 Jan-Feb;21(1-2):105–110. doi: 10.1016/0378-1119(83)90152-x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Adler G. K., Majumder S., Khan S. A., Carleton S., Rosenblum W. D., Iordanescu S. Coding sequence for the pT181 repC product: a plasmid-coded protein uniquely required for replication. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4108–4112. doi: 10.1073/pnas.79.13.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Latta P. D., Swanson E. C., Pattee P. A. Translocatable elements in Staphylococcus aureus. Contrib Microbiol Immunol. 1979;6:41–55. [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Lofdahl S. Small Staphylococcus aureus plasmids are transduced as linear multimers that are formed and resolved by replicative processes. J Mol Biol. 1986 Nov 20;192(2):209–220. doi: 10.1016/0022-2836(86)90360-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Schwesinger M. D., Gruss A. D., Swanson E. C., Pattee P. A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Iordanescu S., Surdeanu M., Edelman I. Transduction-related cointegrate formation between Staphylococcal plasmids: a new type of site-specific recombination. Plasmid. 1981 Sep;6(2):159–172. doi: 10.1016/0147-619x(81)90064-0. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Khan S. A., Murphy E., Iordanescu S., Edelman I., Krolewski J., Rush M. Hitchhiking transposons and other mobile genetic elements and site-specific recombination systems in Staphylococcus aureus. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):67–76. doi: 10.1101/sqb.1981.045.01.013. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Morse S. I. In vivo transmission of drug resistance factors between strains of Staphylococcus aureus. J Exp Med. 1967 Jan 1;125(1):45–59. doi: 10.1084/jem.125.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E. MLS-resistance determinants in Staphylococcus aureus and their molecular evolution. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):101–110. doi: 10.1093/jac/16.suppl_a.101. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Projan S. J., Kumar C. C., Carleton S., Gruss A., Highlander S. K., Kornblum J. Replication control for pT181, an indirectly regulated plasmid. Basic Life Sci. 1985;30:299–320. doi: 10.1007/978-1-4613-2447-8_24. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Projan S. J., Rosenblum W., Edelman I. Staphylococcal plasmid cointegrates are formed by host- and phage-mediated general rec systems that act on short regions of homology. Mol Gen Genet. 1984;195(1-2):374–377. doi: 10.1007/BF00332777. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Plasmid-protein relaxation complexes in Staphylococcus aureus. J Bacteriol. 1976 Sep;127(3):1177–1187. doi: 10.1128/jb.127.3.1177-1187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Novick R., Sanchez-Rivas C., Gruss A., Edelman I. Involvement of the cell envelope in plasmid maintenance: plasmid curing during the regeneration of protoplasts. Plasmid. 1980 May;3(3):348–358. doi: 10.1016/0147-619x(80)90048-7. [DOI] [PubMed] [Google Scholar]
- O'Toole R. D., Drew W. L., Dahlgren B. J., Beaty H. N. An outbreak of methicillin-resistant Staphylococcus aureus infection. Observations in hospital and nursing home. JAMA. 1970 Jul 13;213(2):257–263. [PubMed] [Google Scholar]
- Ohtsubo H., Ohmori H., Ohtsubo E. Nucleotide-sequence analysis of Tn3 (ap): implications for insertion and deletion. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1269–1277. doi: 10.1101/sqb.1979.043.01.144. [DOI] [PubMed] [Google Scholar]
- Parisi J. T. Coagulase-negative staphylococci and the epidemiological typing of Staphylococcus epidermidis. Microbiol Rev. 1985 Jun;49(2):126–139. doi: 10.1128/mr.49.2.126-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi J. T., Robbins J., Lampson B. C., Hecht D. W. Characterization of a macrolide, lincosamide, and streptogramin resistance plasmid in Staphylococcus epidermidis. J Bacteriol. 1981 Nov;148(2):559–564. doi: 10.1128/jb.148.2.559-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariza M. W., Iandolo J. J. Base ratio and deoxyribonucleic acid homology studies of six Staphylococcus aureus typing bacteriophages. Appl Microbiol. 1974 Feb;27(2):317–323. doi: 10.1128/am.27.2.317-323.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariza M. W., Iandolo J. J. Determination of genome size of selected typing bacteriophages of Staphylococcus aureus. Appl Microbiol. 1974 Sep;28(3):510–512. doi: 10.1128/am.28.3.510-512.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. T., Asheshov E. H., Hewitt J. H., Nakhla L. S., Brock B. M. Endemic staphylococcal infections in hospitals. Ann N Y Acad Sci. 1974 Jul 31;236(0):466–484. doi: 10.1111/j.1749-6632.1974.tb41511.x. [DOI] [PubMed] [Google Scholar]
- Parker M. T., Hewitt J. H. Methicillin resistance in Staphylococcus aureus. Lancet. 1970 Apr 18;1(7651):800–804. doi: 10.1016/s0140-6736(70)92408-6. [DOI] [PubMed] [Google Scholar]
- Pattee P. A. Distribution of Tn551 insertion sites responsible for auxotrophy on the Staphylococcus aureus chromosome. J Bacteriol. 1981 Jan;145(1):479–488. doi: 10.1128/jb.145.1.479-488.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A. Genetic linkage of chromosomal tetracycline resistance and pigmentation to a purine auxotrophic marker and the isoleucine-valine-leucine structural genes in Staphylococcus aureus. J Bacteriol. 1976 Sep;127(3):1167–1172. doi: 10.1128/jb.127.3.1167-1172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Neveln D. S. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975 Oct;124(1):201–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Thompson N. E., Haubrich D., Novick R. P. Chromosomal map locations of integrated plasmids and related elements in Staphylococcus aureus. Plasmid. 1977 Nov;1(1):38–51. doi: 10.1016/0147-619x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Pavillard R., Harvey K., Douglas D., Hewstone A., Andrew J., Collopy B., Asche V., Carson P., Davidson A., Gilbert G. Epidemic of hospital-acquired infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust. 1982 May 29;1(11):451–454. [PubMed] [Google Scholar]
- Pearman J. W., Christiansen K. J., Annear D. I., Goodwin C. S., Metcalf C., Donovan F. P., Macey K. L., Bassette L. D., Powell I. M., Green J. M. Control of methicillin-resistant Staphylococcus aureus (MRSA) in an Australian metropolitan teaching hospital complex. Med J Aust. 1985 Jan 21;142(2):103–108. [PubMed] [Google Scholar]
- Perceval A., McLean A. J., Wellington C. V. Emergence of gentamicin resistance in Staphylococcus aureus. Med J Aust. 1976 Jul 10;2(2):74–74. doi: 10.5694/j.1326-5377.1976.tb117630.x. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. A physical and functional analysis of Tn917, a Streptococcus transposon in the Tn3 family that functions in Bacillus. Plasmid. 1984 Sep;12(2):119–138. doi: 10.1016/0147-619x(84)90058-1. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Silver S. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J Bacteriol. 1982 May;150(2):973–976. doi: 10.1128/jb.150.2.973-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyru G., Wexler L. F., Novick R. P. Naturally occurring penicillinase plasmids in Staphylococcus aureus. J Bacteriol. 1969 Apr;98(1):215–221. doi: 10.1128/jb.98.1.215-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I., Shannon K. Aminoglycoside resistance. Br Med Bull. 1984 Jan;40(1):28–35. doi: 10.1093/oxfordjournals.bmb.a071943. [DOI] [PubMed] [Google Scholar]
- Phillips S., Novick R. P. Tn554--a site-specific repressor-controlled transposon in Staphylococcus aureus. Nature. 1979 Mar 29;278(5703):476–478. doi: 10.1038/278476a0. [DOI] [PubMed] [Google Scholar]
- Polak J., Novick R. P. Closely related plasmids from Staphylococcus aureus and soil bacilli. Plasmid. 1982 Mar;7(2):152–162. doi: 10.1016/0147-619x(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Pollock M. R. Origin and function of penicillinase: a problem in biochemical evolution. Br Med J. 1967 Oct 14;4(5571):71–77. doi: 10.1136/bmj.4.5571.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter F. D., Silver S., Ong C., Nakahara H. Selection for mercurial resistance in hospital settings. Antimicrob Agents Chemother. 1982 Nov;22(5):852–858. doi: 10.1128/aac.22.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porthouse A., Brown D. F., Smith R. G., Rogers T. Gentamicin resistance in Staphylococcus aureus. Lancet. 1976 Jan 3;1(7949):20–21. doi: 10.1016/s0140-6736(76)92912-3. [DOI] [PubMed] [Google Scholar]
- Poston S. M. Cellular location of the genes controlling penicillinase production and resistance to streptomycin and tetracycline in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):802–804. doi: 10.1038/210802a0. [DOI] [PubMed] [Google Scholar]
- Price E. H., Brain A., Dickson J. A. An outbreak of infection with a gentamicin and methicillin-resistant Staphylococcus aureus in a neonatal unit. J Hosp Infect. 1980 Sep;1(3):221–228. doi: 10.1016/0195-6701(80)90059-6. [DOI] [PubMed] [Google Scholar]
- Projan S. J., Kornblum J., Moghazeh S. L., Edelman I., Gennaro M. L., Novick R. P. Comparative sequence and functional analysis of pT181 and pC221, cognate plasmid replicons from Staphylococcus aureus. Mol Gen Genet. 1985;199(3):452–464. doi: 10.1007/BF00330758. [DOI] [PubMed] [Google Scholar]
- QUIE P. G., COLLIN M., CARDLE J. B. Neomycin-resistant staphylococci. Lancet. 1960 Jul 16;2(7142):124–126. doi: 10.1016/s0140-6736(60)91266-6. [DOI] [PubMed] [Google Scholar]
- Qoronfleh M. W., Wilkinson B. J. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother. 1986 Feb;29(2):250–257. doi: 10.1128/aac.29.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. DOMINANCE OF THE INDUCIBLE STATE IN STRAINS OF STAPHYLOCOCCUS AUREUS CONTAINING TWO DISTINCT PENICILLINASE PLASMIDS. J Bacteriol. 1965 Aug;90:370–374. doi: 10.1128/jb.90.2.370-374.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. WILD-TYPE VARIANTS OF EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1965 Mar;94:584–593. doi: 10.1042/bj0940584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. J. NEOMYCIN RESISTANCE IN A NEWLY RECOGNIZED STAIN OF STAPHYLOCOCCUS AUREUS. Lancet. 1963 Aug 17;2(7303):333–334. doi: 10.1016/s0140-6736(63)92996-9. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM E. D., TYRONE S. SEROLOGY, DENSITY, AND MORPHOLOGY OF STAPHYLOCOCCAL PHAGES. J Bacteriol. 1964 Dec;88:1737–1742. doi: 10.1128/jb.88.6.1737-1742.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUNTREE P. M., BEARD M. A. THE SPREAD OF NEOMYCIN-RESISTANT STAPHYLOCOCCI IN A HOSPITAL. Med J Aust. 1965 Apr 3;1(14):498–502. doi: 10.5694/j.1326-5377.1965.tb71863.x. [DOI] [PubMed] [Google Scholar]
- ROUNTREE P. M. Changes in the phage-typing patterns of staphylococci following lysogenization. J Gen Microbiol. 1959 Jun;20(3):620–633. doi: 10.1099/00221287-20-3-620. [DOI] [PubMed] [Google Scholar]
- Ranzini A. C., Dubin D. T. The "erythromycin-resistance" methylated sequence of Staphylococcus aureus ribosomal RNA. Plasmid. 1983 Nov;10(3):293–295. doi: 10.1016/0147-619x(83)90044-6. [DOI] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Resistance of the antibiotic target site. Br Med Bull. 1984 Jan;40(1):3–10. doi: 10.1093/oxfordjournals.bmb.a071944. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. A comparison of the exo-penicillinases mediated by a chromosomal gene in a strain of Staphylococcus aureus PS 80 and by a plasmid gene in Staphylococcus aureus 8325. Genet Res. 1972 Apr;19(2):187–189. doi: 10.1017/s0016672300014439. [DOI] [PubMed] [Google Scholar]
- Richmond M. H. Extrachromosomal elements and the spread of antibiotic resistance in bacteria. Biochem J. 1969 Jun;113(2):225–234. doi: 10.1042/bj1130225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Some environmental consequences of the use of antibiotics: or 'what goes up must come down'. J Appl Bacteriol. 1972 Jun;35(2):155–176. doi: 10.1111/j.1365-2672.1972.tb03687.x. [DOI] [PubMed] [Google Scholar]
- Roberts A. N., Hudson G. S., Brenner S. An erythromycin-resistance gene from an erythromycin-producing strain of Arthrobacter sp. Gene. 1985;35(3):259–270. doi: 10.1016/0378-1119(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Tuovinen O. H. Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: physiological, biochemical, and genetic analyses. Microbiol Rev. 1984 Jun;48(2):95–124. doi: 10.1128/mr.48.2.95-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins L. D., Lee L. N., LeBlanc D. J. Evidence for a disseminated erythromycin resistance determinant mediated by Tn917-like sequences among group D streptococci isolated from pigs, chickens, and humans. Antimicrob Agents Chemother. 1985 Apr;27(4):439–444. doi: 10.1128/aac.27.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosdahl V. T. Naturally occurring constitutive -lactamase of novel serotype in Staphylococcus aureus. J Gen Microbiol. 1973 Jul;77(1):229–231. doi: 10.1099/00221287-77-1-229. [DOI] [PubMed] [Google Scholar]
- Rosendal K., Bang J., Rosdahl V. T. Gentamicin-resistant Staphylococcus aureus strains isolated in Denmark in 1979. Acta Pathol Microbiol Scand B. 1981 Jun;89(3):185–191. doi: 10.1111/j.1699-0463.1981.tb00174_89b.x. [DOI] [PubMed] [Google Scholar]
- Rosendorf L. L., Kayser F. H. Transduction and plasmid deoxyribonucleic acid analysis in a multiply antibiotic-resistant strain of Staphylococcus epidermidis. J Bacteriol. 1974 Nov;120(2):679–686. doi: 10.1128/jb.120.2.679-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree P. M., Beard M. A. Hospital strains of Staphylococcus aureus, with particular reference to methicillin-resistant strains. Med J Aust. 1968 Dec 28;2(26):1163–1168. doi: 10.5694/j.1326-5377.1968.tb83502.x. [DOI] [PubMed] [Google Scholar]
- Rountree P. M. History of staphylococcal infection in Australia. Med J Aust. 1978 Dec 2;2(12):543–546. doi: 10.5694/j.1326-5377.1978.tb131715.x. [DOI] [PubMed] [Google Scholar]
- Rountree P. M., Vickery A. M. Further observations on methicillin-resistant staphylococci. Med J Aust. 1973 May 26;1(21):1030–1034. doi: 10.5694/j.1326-5377.1973.tb110903.x. [DOI] [PubMed] [Google Scholar]
- Rudin L., Sjöström J. E., Lindberg M., Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974 Apr;118(1):155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Novick R. P., Warner R. C. Penicillinase plasmid DNA from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1304–1310. doi: 10.1073/pnas.63.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND R., ROLINSON G. N. CHARACTERISTICS OF METHICILLIN-RESISTANT STAPHYLOCOCCI. J Bacteriol. 1964 Apr;87:887–899. doi: 10.1128/jb.87.4.887-899.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Chemical and physical factors influencing methicillin resistance of Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):47–51. doi: 10.1093/jac/3.suppl_c.47. [DOI] [PubMed] [Google Scholar]
- Sabath L. D. Mechanisms of resistance to beta-lactam antibiotics in strains of Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):339–344. doi: 10.7326/0003-4819-97-3-339. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J., Gerstein D. A. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Nov;2(5):350–355. doi: 10.1128/aac.2.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Wallace S. J. The problems of drug-resistant pathogenic bacteria. Factors influencing methicillin resistance in staphylococci. Ann N Y Acad Sci. 1971 Jun 11;182:258–266. doi: 10.1111/j.1749-6632.1971.tb30662.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wheeler N., Laverdiere M., Blazevic D., Wilkinson B. J. A new type of penicillin resistance of Staphylococcus aureus. Lancet. 1977 Feb 26;1(8009):443–447. doi: 10.1016/s0140-6736(77)91941-9. [DOI] [PubMed] [Google Scholar]
- Sadaie Y., Burtis K. C., Doi R. H. Purification and characterization of a kanamycin nucleotidyltransferase from plasmid pUB110-carrying cells of Bacillus subtilis. J Bacteriol. 1980 Mar;141(3):1178–1182. doi: 10.1128/jb.141.3.1178-1182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands L. C., Shaw W. V. Mechanism of chloramphenicol resistance in staphylococci: characterization and hybridization of variants of chloramphenicol acetyltransferase. Antimicrob Agents Chemother. 1973 Feb;3(2):299–305. doi: 10.1128/aac.3.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. R. Genetics and evolution of antibiotic resistance. Br Med Bull. 1984 Jan;40(1):54–60. doi: 10.1093/oxfordjournals.bmb.a071948. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Clewell D. B., Glatzer L. Conjugative transfer of R-plasmids from Streptococcus faecalis to Staphylococcus aureus. Antimicrob Agents Chemother. 1982 Aug;22(2):204–207. doi: 10.1128/aac.22.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Power G., Betzold J., Forbes B. A. Conjugative R plasmids in antimicrobial resistance of Staphylococcus aureus causing nosocomial infections. J Infect Dis. 1985 Jul;152(1):43–49. doi: 10.1093/infdis/152.1.43. [DOI] [PubMed] [Google Scholar]
- Schaefler S. Bacteriophage-mediated acquisition of antibiotic resistance by Staphylococcus aureus type 88. Antimicrob Agents Chemother. 1982 Mar;21(3):460–467. doi: 10.1128/aac.21.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Francois W., Ruby C. L. Minocycline resistance in Staphylococcus aureus: effect on phage susceptibility. Antimicrob Agents Chemother. 1976 Apr;9(4):600–613. doi: 10.1128/aac.9.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Jones D., Perry W., Baradet T., Mayr E., Rampersad C. Methicillin-resistant Staphylococcus aureus strains in New York City hospitals: inter-hospital spread of resistant strains of type 88. J Clin Microbiol. 1984 Sep;20(3):536–538. doi: 10.1128/jcm.20.3.536-538.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S., Jones D., Perry W., Ruvinskaya L., Baradet T., Mayr E., Wilson M. E. Emergence of gentamicin- and methicillin-resistant Staphylococcus aureus strains in New York City hospitals. J Clin Microbiol. 1981 Apr;13(4):754–759. doi: 10.1128/jcm.13.4.754-759.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J., Mandal A., Clark D., Silver S., Hedges R. W. Volatilisation of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature. 1974 Sep 27;251(5473):335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- Schugurensky A., Cahn P., Osatnik G., Zorzopulos J., Ruiz Trevisan A., Denoya C. D. An outbreak of multiply resistant Staphylococcus aureus in Buenos Aires. Clinical observations and plasmid pattern analysis. Medicina (B Aires) 1984;44(1):8–14. [PubMed] [Google Scholar]
- Schwesinger M. D., Novick R. P. Prophage-dependent plasmid integration in Staphylococcus aureus. J Bacteriol. 1975 Aug;123(2):724–738. doi: 10.1128/jb.123.2.724-738.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. F., Wood D. O., Brownell G. H., Carter M. J., Best G. K. Aminoglycoside modification by gentamicin-resistant isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Apr;13(4):641–644. doi: 10.1128/aac.13.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Transduction of staphylococcal enterotoxin B synthesis: establishment of the toxin gene in a recombination-deficient mutant. Infect Immun. 1980 Jan;27(1):280–282. doi: 10.1128/iai.27.1.280-282.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafferman A., Shalita Z., Hertman I. Cleavage maps of a tetracycline plasmid from Staphylococcus aureus. J Bacteriol. 1978 Apr;134(1):345–348. doi: 10.1128/jb.134.1.345-348.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalita Z., Murphy E., Novick R. P. Penicillinase plasmids of Staphylococcus aureus: structural and evolutionary relationships. Plasmid. 1980 May;3(3):291–311. doi: 10.1016/0147-619x(80)90042-6. [DOI] [PubMed] [Google Scholar]
- Shannon K., Phillips I. Mechanisms of resistance to aminoglycosides in clinical isolates. J Antimicrob Chemother. 1982 Feb;9(2):91–102. doi: 10.1093/jac/9.2.91. [DOI] [PubMed] [Google Scholar]
- Shanson D. C., Johnstone D., Midgley J. Control of a hospital outbreak of methicillin-resistant Staphylococcus aureus infections: value of an isolation unit. J Hosp Infect. 1985 Sep;6(3):285–292. [PubMed] [Google Scholar]
- Shanson D. C., Kensit J. C., Duke R. Outbreak of hospital infection with a strain of Staphylococcus aureus resistant to gentamicin and methicillin. Lancet. 1976 Dec 18;2(7999):1347–1348. doi: 10.1016/s0140-6736(76)91986-3. [DOI] [PubMed] [Google Scholar]
- Shanson D. C. Staphylococcal infections in hospital. Br J Hosp Med. 1986 May;35(5):312, 314, 318-20. [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Bacterial resistance to chloramphenicol. Br Med Bull. 1984 Jan;40(1):36–41. doi: 10.1093/oxfordjournals.bmb.a071945. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Brenner D. G., LeGrice S. F., Skinner S. E., Hawkins A. R. Chloramphenicol acetyltransferase gene of staphylococcal plasmid pC221. Nucleotide sequence analysis and expression studies. FEBS Lett. 1985 Jan 1;179(1):101–106. doi: 10.1016/0014-5793(85)80200-3. [DOI] [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Characterization of a plasmid-specified ribosome methylase associated with macrolide resistance. Nucleic Acids Res. 1981 Jun 11;9(11):2549–2562. doi: 10.1093/nar/9.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar A. G., Gryczan T. J., Kozlov Y. I., Dubnau D. Organization of the pE194 genome. Mol Gen Genet. 1980;179(2):241–252. doi: 10.1007/BF00425450. [DOI] [PubMed] [Google Scholar]
- Shivakumar A. G., Hahn J., Dubnau D. Studies on the synthesis of plasmid-coded proteins and their control in Bacillus subtilis minicells. Plasmid. 1979 Apr;2(2):279–289. doi: 10.1016/0147-619x(79)90046-5. [DOI] [PubMed] [Google Scholar]
- Silver S., Budd K., Leahy K. M., Shaw W. V., Hammond D., Novick R. P., Willsky G. R., Malamy M. H., Rosenberg H. Inducible plasmid-determined resistance to arsenate, arsenite, and antimony (III) in escherichia coli and Staphylococcus aureus. J Bacteriol. 1981 Jun;146(3):983–996. doi: 10.1128/jb.146.3.983-996.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Keach D. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6114–6118. doi: 10.1073/pnas.79.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Löfdahl S., Philipson L. Transformation of Staphylococcus aureus by heterologous plasmids. Plasmid. 1979 Oct;2(4):529–535. doi: 10.1016/0147-619x(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Sjöström J. E., Löfdahl S., Philipson L. Transformation reveals a chromosomal locus of the gene(s) for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1975 Sep;123(3):905–915. doi: 10.1128/jb.123.3.905-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Philipson L. Role of the phi 11 phage genome in competence of Staphylococcus aureus. J Bacteriol. 1974 Jul;119(1):19–32. doi: 10.1128/jb.119.1.19-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R., Cundliffe E., Schmidt F. J. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J Biol Chem. 1983 Oct 25;258(20):12702–12706. [PubMed] [Google Scholar]
- Smith K., Novick R. P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972 Nov;112(2):761–772. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Wilkinson B. J. Differential methicillin susceptibilities of peptidoglycan syntheses in methicillin-resistant Staphylococcus aureus. J Bacteriol. 1981 Nov;148(2):610–617. doi: 10.1128/jb.148.2.610-617.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell T. C., Packham D. R., Shanker S., Foldes M., Munro R. Vancomycin therapy for methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):344–350. doi: 10.7326/0003-4819-97-3-344. [DOI] [PubMed] [Google Scholar]
- Soussy C. J., Bouanchaud D. H., Fouace J., Dublanchet A., Duval J. A gentamycin resistance plasmid in Staphylococcus aureus. Ann Microbiol (Paris) 1975 Jul-Aug;126B(1):91–94. [PubMed] [Google Scholar]
- Speller D. C., Raghunath D., Stephens M., Viant A. C., Reeves D. S., Wilkinson P. J., Broughall J. M., Holt H. A. Epidemic infection by a gentamicin-resistant Staphylococcus aureus in three hospitals. Lancet. 1976 Feb 28;1(7957):464–466. doi: 10.1016/s0140-6736(76)91485-9. [DOI] [PubMed] [Google Scholar]
- Spitzy K. H., Rotter M. Resistente Staphylokokken neuerlich im Vormarsch? Infection. 1979;7 (Suppl 2):S220–S224. doi: 10.1007/BF01641127. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Pattee P. A. Computer-assisted chromosome mapping by protoplast fusion in Staphylococcus aureus. J Bacteriol. 1983 Apr;154(1):395–405. doi: 10.1128/jb.154.1.395-405.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L., Pattee P. A. Confirmation of protoplast fusion-derived linkages in Staphylococcus aureus by transformation with protoplast DNA. J Bacteriol. 1983 Apr;154(1):406–412. doi: 10.1128/jb.154.1.406-412.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. C., Rosenblum E. D. Genetic behavior of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1980 Dec;144(3):1200–1202. doi: 10.1128/jb.144.3.1200-1202.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. C., Rosenblum E. D. Transduction of methicillin resistance in Staphylococcus aureus: recipient effectiveness and beta-lactamase production. Antimicrob Agents Chemother. 1980 Sep;18(3):424–432. doi: 10.1128/aac.18.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. R., Waldron H. G., Lee J. S., Matthews P. R. Molecular relationships among serogroup B bacteriophages of Staphylococcus aureus. J Virol. 1985 Jul;55(1):111–116. doi: 10.1128/jvi.55.1.111-116.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Absence of circular plasmid deoxyribonucleic acid attributable to a genetic determinant for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):771–777. doi: 10.1128/jb.116.2.771-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Co-transduction of plasmids mediating resistance to tetracycline and chloramphenicol in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):934–934. doi: 10.1128/jb.120.2.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Schneider M., Cohen S. Isolation and characterization of a kanamycin resistance plasmid from Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):516–520. doi: 10.1128/aac.6.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobberingh E. E., Schiphof R., Sussenbach J. S. Occurrence of a class II restriction endonuclease in Staphylococcus aureus. J Bacteriol. 1977 Aug;131(2):645–649. doi: 10.1128/jb.131.2.645-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobberingh E. E., Winkler K. C. Restriction-deficient mutants of Staphylococcus aureus. J Gen Microbiol. 1977 Apr;99(2):359–367. doi: 10.1099/00221287-99-2-359. [DOI] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Swedberg G., Sköld O. Characterization of different plasmid-borne dihydropteroate synthases mediating bacterial resistance to sulfonamides. J Bacteriol. 1980 Apr;142(1):1–7. doi: 10.1128/jb.142.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. M., Cohen S. Wild-type strain of Staphylococcus aureus containing two genetic linkage groups for penicillinase production. J Bacteriol. 1968 Oct;96(4):920–924. doi: 10.1128/jb.96.4.920-924.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P. Counteracting antibiotic resistance: new drugs. Br Med Bull. 1984 Jan;40(1):96–101. doi: 10.1093/oxfordjournals.bmb.a071954. [DOI] [PubMed] [Google Scholar]
- Tennent J. M., Lyon B. R., Gillespie M. T., May J. W., Skurray R. A. Cloning and expression of Staphylococcus aureus plasmid-mediated quaternary ammonium resistance in Escherichia coli. Antimicrob Agents Chemother. 1985 Jan;27(1):79–83. doi: 10.1128/aac.27.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennent J. M., May J. W., Skurray R. A. Characterisation of chloramphenicol resistance plasmids of Staphylococcus aureus and S. epidermidis by restriction enzyme mapping techniques. J Med Microbiol. 1986 Aug;22(1):79–84. doi: 10.1099/00222615-22-1-79. [DOI] [PubMed] [Google Scholar]
- Tennent J. M., May J. W., Skurray R. A. Multiple antibiotic resistance in Staphylococcus aureus and Staphylococcus epidermidis: plasmids in strains associated with nosocomial infection. Pathology. 1984 Jul;16(3):250–255. doi: 10.3109/00313028409068532. [DOI] [PubMed] [Google Scholar]
- Thabaut A., Meyran M., Huerre M. Evolution and present situation of Staphylococcus aureus sensitivity to MLS in hospital (1975-83). J Antimicrob Chemother. 1985 Jul;16 (Suppl A):205–207. doi: 10.1093/jac/16.suppl_a.205. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Gray G. S. Nucleotide sequence of a streptomycete aminoglycoside phosphotransferase gene and its relationship to phosphotransferases encoded by resistance plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5190–5194. doi: 10.1073/pnas.80.17.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Skinner R. H., Thompson J., Ward J. M., Hopwood D. A., Cundliffe E. Biochemical characterization of resistance determinants cloned from antibiotic-producing streptomycetes. J Bacteriol. 1982 Aug;151(2):678–685. doi: 10.1128/jb.151.2.678-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Pattee P. A. Genetic transformation in Staphylococcus aureus: demonstration of a competence-conferring factor of bacteriophage origin in bacteriophage 80 alpha lysates. J Bacteriol. 1981 Oct;148(1):294–300. doi: 10.1128/jb.148.1.294-300.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Pattee P. A. Transformation in Staphylococcus aureus: role of bacteriophage and incidence of competence among strains. J Bacteriol. 1977 Feb;129(2):778–788. doi: 10.1128/jb.129.2.778-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Cabezudo I., Wenzel R. P. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):309–317. doi: 10.7326/0003-4819-97-3-309. [DOI] [PubMed] [Google Scholar]
- Timoney J. F., Port J., Giles J., Spanier J. Heavy-metal and antibiotic resistance in the bacterial flora of sediments of New York Bight. Appl Environ Microbiol. 1978 Sep;36(3):465–472. doi: 10.1128/aem.36.3.465-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin E., Tomasz A. Beta-lactam-specific resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Oct;30(4):577–583. doi: 10.1128/aac.30.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Vidal L., Baldwin J. N. Penicillin and tetracycline resistance plasmids in Staphylococcus epidermidis. Antimicrob Agents Chemother. 1981 Sep;20(3):359–365. doi: 10.1128/aac.20.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Annear D. I., Grubb W. B. A conjugative plasmid encoding production of a diffusible pigment and resistance to aminoglycosides and macrolides in Staphylococcus aureus. Aust J Exp Biol Med Sci. 1985 Oct;63(Pt 5):573–586. doi: 10.1038/icb.1985.61. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Bradley J. M., Pearman J. W., Grubb W. B. "Australian" methicillin-resistant Staphylococcus aureus in a London hospital? Med J Aust. 1984 Sep 15;141(6):339–340. doi: 10.5694/j.1326-5377.1984.tb132800.x. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Greed L. C., Grubb W. B. Analysis of plasmids mediating gentamicin resistance in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1984 Apr;13(4):347–352. doi: 10.1093/jac/13.4.347. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Greed L. C., Grubb W. B. Transposition of gentamicin resistance to staphylococcal plasmids encoding resistance to cationic agents. J Antimicrob Chemother. 1984 Aug;14(2):115–124. doi: 10.1093/jac/14.2.115. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Grubb W. B. Evolution of Australian isolates of methicillin-resistant Staphylococcus aureus: a problem of plasmid incompatibility? J Med Microbiol. 1985 Aug;20(1):49–61. doi: 10.1099/00222615-20-1-49. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Momoh M., Grubb W. B. Distribution of plasmid-borne resistance to nucleic acid binding compounds in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1985 Apr;15(4):417–434. doi: 10.1093/jac/15.4.417. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Pearman J. W., Annear D. I., Grubb W. B. Genetics and epidemiology of methicillin-resistant Staphylococcus aureus isolated in a Western Australian hospital. Med J Aust. 1985 Jan 21;142(2):108–111. doi: 10.5694/j.1326-5377.1985.tb133045.x. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Bolton S., Ashdown N., Grubb W. B. Transfer of plasmid-borne aminoglycoside-resistance determinants in staphylococci. J Med Microbiol. 1985 Oct;20(2):169–185. doi: 10.1099/00222615-20-2-169. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Bolton S., Ashdown N., Taheri S., Grubb W. B. Comparison of phage-mediated and conjugative transfer of staphylococcal plasmids in vitro and in vivo. J Med Microbiol. 1986 Sep;22(2):107–114. doi: 10.1099/00222615-22-2-107. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Grubb W. B., Annear D. I. A plasmid for diffusible pigment production in Staphylococcus aureus. Aust J Exp Biol Med Sci. 1985 Aug;63(Pt 4):463–472. doi: 10.1038/icb.1985.51. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Grubb W. B., Ashdown N. Genetics of drug resistance in methicillin-resistant Staphylococcus aureus from Australian hospitals. J Hosp Infect. 1983 Dec;4(4):331–337. doi: 10.1016/0195-6701(83)90003-8. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Grubb W. B., Ashdown N. Gentamicin resistance in methicillin-resistant Staphylococcus aureus. Pathology. 1983 Apr;15(2):169–174. doi: 10.3109/00313028309084707. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., den Hollander L., Bolton S., Grubb W. B. Clinical isolates of staphylococci conjugate on contact with dry absorbent surfaces. Med J Aust. 1986 Feb 3;144(3):166–166. doi: 10.5694/j.1326-5377.1986.tb112260.x. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Gerbaud G., Lambert T., Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985 Dec 16;4(13A):3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Durack D. T., Tomasz A. Antibiotic tolerance among clinical isolates of bacteria. Antimicrob Agents Chemother. 1986 Oct;30(4):521–527. doi: 10.1128/aac.30.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Reduced cadmium transport determined by a resistance plasmid in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):305–312. doi: 10.1128/jb.147.2.305-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelaker M. H., Rosenblum E. D. Transduction of plasmid determinants in Staphylococcus aureus and Escherichia coli. J Bacteriol. 1978 Feb;133(2):699–707. doi: 10.1128/jb.133.2.699-707.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Gotoh A., Konno M. Purification and characterization of aminoglycoside-modifying enzymes from Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1984 Jun;25(6):754–759. doi: 10.1128/aac.25.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama H., Weisblum B. N-Methyl transferase of Streptomyces erythraeus that confers resistance to the macrolide-lincosamide-streptogramin B antibiotics: amino acid sequence and its homology to cognate R-factor enzymes from pathogenic bacilli and cocci. Gene. 1985;38(1-3):103–110. doi: 10.1016/0378-1119(85)90208-2. [DOI] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaldo P. E., Cipriani P., Focá A., Geraci C., Giordano A., Madeddu M. A., Orsi A., Pompei R., Prenna M., Repetto A. Identification, clinical distribution, and susceptibility to methicillin and 18 additional antibiotics of clinical Staphylococcus isolates: nationwide investigation in Italy. J Clin Microbiol. 1984 Jun;19(6):838–843. doi: 10.1128/jcm.19.6.838-843.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery A. M., Beard-Pegler M. A., Rountree P. M. Strain differentiation in methicillin-resistant Staphylococcus aureus. Pathology. 1983 Jul;15(3):235–240. doi: 10.3109/00313028309083499. [DOI] [PubMed] [Google Scholar]
- Vickery A. M., Beard-Pegler M. A., Stubbs E. Phage-typing patterns and lysogenicity of methicillin-resistant strains of Staphylococcus aureus from Sydney, Australia, 1965-85. J Med Microbiol. 1986 Nov;22(3):209–216. doi: 10.1099/00222615-22-3-209. [DOI] [PubMed] [Google Scholar]
- Vogel L., Nathan C., Sweeney H. M., Kabins S. A., Cohen S. Infections due to gentamicin-resistant Staphylococcus aureus strain in a nursery for neonatal infants. Antimicrob Agents Chemother. 1978 Mar;13(3):466–472. doi: 10.1128/aac.13.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKLER K. C., GROOTSEN C. The relation of phage pattern and lysogenicity in the phage typing of staphylococci of phage-group II. Antonie Van Leeuwenhoek. 1961;27:225–246. doi: 10.1007/BF02538445. [DOI] [PubMed] [Google Scholar]
- Waldvogel F. A. Treatment of infections due to methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1986 Mar;7 (Suppl A):37–46. doi: 10.1016/0195-6701(86)90006-x. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C. Mode of action and in-vitro activity of vancomycin. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):7–18. doi: 10.1093/jac/14.suppl_d.7. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C. Treatment of infections due to methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):376–378. doi: 10.7326/0003-4819-97-3-376. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Weinstein R. A., Kabins S. A., Nathan C., Sweeney H. M., Jaffe H. W., Cohen S. Gentamicin-resistant staphylococci as hospital flora: epidemiology and resistance plasmids. J Infect Dis. 1982 Mar;145(3):374–382. doi: 10.1093/infdis/145.3.374. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Holder S. B., Halling S. M. Deoxyribonucleic acid sequence common to staphylococcal and streptococcal plasmids which specify erythromycin resistance. J Bacteriol. 1979 Jun;138(3):990–998. doi: 10.1128/jb.138.3.990-998.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B. Inducible erythromycin resistance in bacteria. Br Med Bull. 1984 Jan;40(1):47–53. doi: 10.1093/oxfordjournals.bmb.a071947. [DOI] [PubMed] [Google Scholar]
- Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression--a review. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Silver S., Kinscherf T. G. Cation transport alteration associated with plasmid-determined resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Dec;14(6):856–865. doi: 10.1128/aac.14.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R. P. The emergence of methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):440–442. doi: 10.7326/0003-4819-97-3-440. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Nadakavukaren M. J. Methicillin-resistant septal peptidoglycan synthesis in a methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1983 Apr;23(4):610–613. doi: 10.1128/aac.23.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Williams R. F. Choice of chemotherapy for infection by Staphylococcus aureus. J Antimicrob Chemother. 1982 Jan;9(1):1–3. doi: 10.1093/jac/9.1.1. [DOI] [PubMed] [Google Scholar]
- Wilson C. R., Baldwin N. J. Characterization and construction of molecular cloning vehicles within Staphylococcus aureus. J Bacteriol. 1978 Oct;136(1):402–413. doi: 10.1128/jb.136.1.402-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. R., Skinner S. E., Shaw W. V. Analysis of two chloramphenicol resistance plasmids from Staphylococcus aureus: insertional inactivation of Cm resistance, mapping of restriction sites, and construction of cloning vehicles. Plasmid. 1981 May;5(3):245–258. doi: 10.1016/0147-619x(81)90002-0. [DOI] [PubMed] [Google Scholar]
- Witte W., Dünnhaupt K. Multiple drug-resistant Staphylococcus aureus with resistance to oxytetracycline and minocycline, an example for the distribution of a multiresistant strain-clone. J Hyg Epidemiol Microbiol Immunol. 1982;26(1):49–56. [PubMed] [Google Scholar]
- Witte W., Dünnhaupt K. Occurrence of a nonplasmid-located determinant for gentamicin resistance in strains of Staphylococcus aureus. J Hyg (Lond) 1984 Aug;93(1):1–8. doi: 10.1017/s0022172400060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W., Green L., Misra T. K., Silver S. Resistance to mercury and to cadmium in chromosomally resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):663–669. doi: 10.1128/aac.29.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W. Transfer of drug-resistance-plasmids in mixed cultures of Staphylococci. Zentralbl Bakteriol Orig A. 1977;237(2-3):147–159. [PubMed] [Google Scholar]
- Wood D. O., Carter M. J., Best G. K. Plasmid-mediated resistance to gentamicin in Staphylococcus aureus. Antimicrob Agents Chemother. 1977 Oct;12(4):513–517. doi: 10.1128/aac.12.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B., Hayes M. V., Curtis N. A. A role in vivo for penicillin-binding protein-4 of Staphylococcus aureus. Eur J Biochem. 1981 Oct;119(2):389–393. doi: 10.1111/j.1432-1033.1981.tb05620.x. [DOI] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B., Hayes M. V. Synthesis of peptidoglycan in vivo in methicillin-resistant Staphylococcus aureus. Eur J Biochem. 1982 Oct;127(3):553–558. doi: 10.1111/j.1432-1033.1982.tb06907.x. [DOI] [PubMed] [Google Scholar]
- Wyman L., Novick R. P. Studies on plasmid replication. IV. Complementation of replication-defective mutants by an incompatibility-deficient plasmid. Mol Gen Genet. 1974;135(2):149–161. doi: 10.1007/BF00264782. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]
- Yourassowsky E., van der Linden M. P., Lismont M. J., Crokaert F. Combination of minocycline and rifampicin against methicillin- and gentamicin-resistant Staphylococcus aureus. J Clin Pathol. 1981 May;34(5):559–563. doi: 10.1136/jcp.34.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidenzaig Y., Fitton J. E., Packman L. C., Shaw W. V. Characterization and comparison of chloramphenicol acetyltransferase variants. Eur J Biochem. 1979 Oct 15;100(2):609–618. doi: 10.1111/j.1432-1033.1979.tb04208.x. [DOI] [PubMed] [Google Scholar]
- de Saxe M., Porthouse A. Gentamicin resistance in Staphylococcus aureus. Lancet. 1979 Aug 18;2(8138):370–371. doi: 10.1016/s0140-6736(79)90393-3. [DOI] [PubMed] [Google Scholar]
- el Solh N., Fouace J. M., Shalita Z., Bouanchaud D. H., Novick R. P., Chabbert Y. A. Epidemiological and structural studies of Staphylococcus aureus R plasmids mediating resistance to tobramycin and streptogramin. Plasmid. 1980 Jul;4(1):117–120. doi: 10.1016/0147-619x(80)90087-6. [DOI] [PubMed] [Google Scholar]
- el Solh N., Moreau N., Ehrlich S. D. Molecular cloning and analysis of Staphylococcus aureus chromosomal aminoglycoside resistance genes. Plasmid. 1986 Mar;15(2):104–118. doi: 10.1016/0147-619x(86)90047-8. [DOI] [PubMed] [Google Scholar]
- el-Nima E. I. The synergism between cetrimide and antibiotics against Pseudomonas aeruginosa. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Oct;258(1):120–127. doi: 10.1016/s0176-6724(84)80016-4. [DOI] [PubMed] [Google Scholar]
- van Boven C. P. Restriction and modification of phages in staphylococcal phage typing. Ann N Y Acad Sci. 1974 Jul 31;236(0):376–388. doi: 10.1111/j.1749-6632.1974.tb41504.x. [DOI] [PubMed] [Google Scholar]