Abstract
Camel milk (CM) has good nutritive value, in addition to its antigenotoxic and anticytotoxic effects. Therefore the aim of this investigation was to evaluate the capacity of CM to inhibit the micronucleated polychromatic erythrocytes (MnPCEs) in the bone marrow and improve the mitotic activity produced by cisplatin. Cisplatin is one of the most widely used antineoplastic drugs in the treatment of cancer. The 70 adult male Swiss albino mice were divided into seven groups:
Gr. I: treated with distilled water and considered as a control group.
Gr. II: treated with camel milk (33 ml/kg, b.w).
Gr. III: treated previously with cisplatin (0.5 mg/kg, b.w).
Gr. IV: treated with camel milk and followed after 2 h. with cisplatin (33 ml/kg → 0.5 mg/kg, b.w).
Gr. V: treated with camel milk and cisplatin at the same time (33 ml/kg + 0.5 mg/kg, b.w).
Gr. VI: treated with an acute single dose of cisplatin (2.5 mg/kg, b.w).
Gr. VII: treated with camel milk prior and followed with an acute single dose of cisplatin (33 ml/kg → 2.5 mg /kg, b.w). The animals were sacrificed 24 h after cisplatin injection. The pretreatment with CM dose caused a significant decrease (P < 0.001) in the frequency of MnPCEs and increase (P < 0.001) in the mitotic index (MI) induced by cisplatin when compared with the groups treated with cisplatin alone. The possible explanation for the antigenotoxic and anticytotoxic effects observed in the pretreatment with CM is ascribed to its contents. In conclusion, from the findings we suggest that this milk has some antioxidant effect, and the antigenotoxic mechanism of this milk needs to be explored further before their use during cisplatin chemotherapy.
Keywords: Cisplatin, Micronucleus, Mitotic index, Camel milk
1. Introduction
Antitumor agents are used in the common therapy against many forms of human cancers. However, as with many agents that have mammalian cell toxicity as a target, physiological side effects can occur and genotoxic effects can be induced in non-tumor cells that can give rise to secondary tumors (Beretta, 1991). The intake of food with chemopreventive constituents has been recommended as an effective strategy for strengthening our defense against the deleterious effect of genotoxins and carcinogens (Ramel et al., 1986).
Cisplatin is one of the most widely used cytostatic agents in the treatment of patients with solid tumor which induced nephropathy, hearing loss and neurotoxicity (Rosenberg, 1985). The clastogenic potential of cisplatin has been of great interest because of its serious effects on the chromosomes of non-tumor cells. In patients treated with cisplatin for a long-term, genetic damage can be observed during chemotherapy or many years later (Elsendoorn et al., 2001). The clastogenicity of this drug in humans and experimental animals has been well documented. Cisplatin induced the micronuclei in bone marrow cells and chromosomal aberrations in the germinal cells of mice (e.g. Kliesch and Adler, 1987; Adler and El-Tarras, 1989), and the micronuclei in peripheral blood lymphocytes of testicular patients with various type of cancer (Osanto et al., 1991; Elsendoorn et al., 2001).
The available evidence has shown that many dietary products are anticlastogen agents (Ferguson, 1999).
Camel’s milk (CM) is an excellent source of well balanced nutrients and also exhibits a range of biological activities that influence digestion, metabolic responses to absorbed nutrients, growth and development of specific organs and resistance to diseases. These biological activities are mainly due to the presence of peptides and protein in milk (Yagil et al., 1984; Korhonen and Pihlanto, 2001). Casein is the principal protein component in most of the mammalian milk. Besides casein CM also contains lactoferrin protein. CM is low in fat, high in protein and vitamin C than cow’s milk. It also contains fat with a relatively large amount of polyunsaturated fatty acids and linoleic acids, which are essential for human nutrition (Gorban and Izzeldin, 2001). There are high levels of linoleic acids (18:2) among the polyunsaturated fatty acids in camel milk (Crawford et al., 1976). The anticytoxic and antigenotoxic effects of most of the CM constituents against the genotoxic effects of chemicals are being investigated (e.g. vitamin C: Krishna et al., 1986; Vijayalaxmi and Venu, 1999; Rao et al., 2001; Selenium: Hurná et al., 1997; Cabrera et al., 2003; Hassan et al., 2006, Zinc: Hurná and Hurná, 2000; Casein: Van Boekel et al., 1993; Goeptar et al., 1997; Lactoferrin: Konuspayeva et al., 2004). Therefore, the aim of this work is to study the possible protective role of camel milk against the genotoxic effects of cisplatin.
2. Materials and methods
2.1. Animals
Male Swiss albino mice (Mus musculus), MFI strain, 8–9 weeks old, weighed 25–30 g, were obtained from the animal house of King Fhad Medical Research Center. Animals were housed in polyplastic cages with steel wire tops in an air conditioned room (22 ± 1 °C, 45–75% relative humidity) maintained in a controlled atmosphere of 12 h light/12 h dark cycles. Food and water were provided ad libitum.
2.2. Test compound
2.2.1. Cisplatin
Cis-diamminedichloroplatinum-II Cisplatin (Cis-DDP) was purchased in the form of a solution dissolved in distilled water (CAs No. 781520-03) under the trade name cisplatinum (EBEWE Pharma, Austria). The cisplatin dose was determined according to the human therapeutic dose (sub-acute dose 20 mg/m2 and acute dose 100 mg/m2), taking into consideration the relative body weight and surface area of the mice relative to that of an adult human being.
2.2.2. Camel milk
2.2.2.1. Sampling of milk
Camel milk samples were collected from three lactating camels of the same breed, the camels were in 3.5–4 months of lactation. Camels were of the C. Hamra breed dromedaries. All lactating camels consumed the same type of feed (barley and Lucerne). Female camels were selected from a local farm in Usfaan region, Jeddah. The milk was collected in the morning.
Samples were collected in bottles and kept on ice during transportation to the laboratory where they were stored at 3 °C.
2.3. Treatment, doses and route of administration
Cisplatin was administered both sub-acutely by a single intraperitoneal injection (i.p.) for five consecutive days and acutely by a single intraperitoneal injection (i.p.). In sub-acute treatment the therapeutic dose of cisplatin was 20 mg/m2, while in sub-acute treatment was 100 mg/m2. The doses were adjusted for mice according to Paget and Barnes (1964). The doses for mice were 0.5 mg/kg and 2.5 mg/kg, respectively.
The control animals received an equal volume of the solvent by intraperitoneal injection (i.p.) for five consecutive days.
The route of administration for camel milk was oral intubation (o.i.) for five consecutive days. The camel milk dose was found to be 33 ml/kg after some preliminary experiments.
2.4. Experiment design
Male mice were randomly divided into seven groups of ten animals each. Animals in group one (Gr. I) were given the solvent (i.p.), in group two (Gr. II) the camel milk (o.i) 33 ml/kg, in group three (Gr. III) the cisplatin 0.5 mg/kg (i.p.), in group four (Gr. IV) the sub-acute pretreatment of camel milk (o.i.) 2 h before cisplatin (0.5 mg/kg) for five consecutive days, in group five (Gr. IV) the simultaneous treatment with camel milk (o.i.) and cisplatin (0.5 mg/kg) (i.p.), in group six (Gr. VI) the acute treatment of cisplatin (2.5 mg/kg) and in group seven (Gr. VII) sub-acute treatment of camel milk (o.i.) followed by an acute treatment with cisplatin (2.5 mg/kg) 2 h after the fifth administration of camel milk.
2.5. Procedure
2.5.1. Slides preparation for micronucleus (MN) and mitotic index (MI)
The mouse micronucleus test was carried out according to Schmid (1973, 1975) with some modification in fixation and staining based on the method of Heddle et al. (1984).
Animals were scarified 24 h after the last treatment either for sub-acute or acute treatment. Both the femora were removed and stripped clean of muscles. The bone marrow was pushed out with a pin, placed on a microscope slide and mixed with drops of fetal calf serum. The cells were then smeared and allowed to dry. The slides were fixed in absolute methanol for 5 min and stained for 10 min in a 5% solution of Giemsa in 0.01 phosphate buffer adjusted to PH 6.8 and mounted in DPX.
2.5.2. Scoring
Stained preparations were coded and scored by a light microscope at 1000× magnification.
2.5.2.1. Micronucleus (MN)
One thousand polychromatic erythrocytes (PCEs) were scored per animal for determining the frequency of micronucleated poly-chromatid erythrocytes (MnPCEs).
Micronuclei were identified according to Schmid (1975), Hayashi et al. (1984) and Albanese and Middleton (1987) criteria. They were morphologically identical to the normal nuclei but smaller than them (their diameter 1/5 of the main nuclei) Fig. 1.
Figure 1.
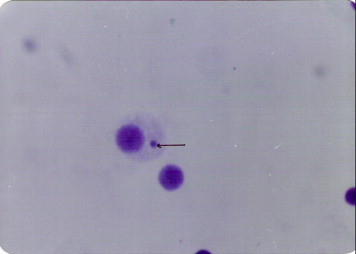
The micronucleated polychromatic erythrocyte.
2.5.2.2. Mitotic index (MI)
The MI was scored in the same slides of MN by calculating the number of dividing cells (including prophase, metaphase and anaphase) in a population of 1000 cells.
2.6. Statistical analysis
The student t-test analysis of variance (ANOVA) followed by low significant difference (LSD) were used for statistical analysis of the data. The results were expressed as mean ± standard deviation (SD) for MN and MI data and were compared with controls (saline and camel milk) by student’s t-test and the minimum level of significance accepted being at p < 0.05.
The difference in the total number of MN and MI was analysed statistically by (ANOVA) with calculation of the “F” statistics and its p value.
2.7. Protective effect
The protective index of camel milk (CM) against the clastogenic and cytotoxic effects of cisplatin on the induction of MN and MI was calculated according to the equation of Shukla and Taneja (2002) as follows:
3. Results
3.1. Micronucleus (MN)
The results obtained are shown in Tables 1 and 2. Table 1 presents the effect of CM (Gr. II) dose on the number of micronucleated polychromatic erythrocytes (MnPCEs) in the bone marrow cells of male mice treated with cisplatin (Gr. III and Gr. VI).
Table 1.
Effect of camel milk (CM) pretreatment on MN induced by cisplatin in bone marrow polychromatic erythrocytes (PCEs) of male mice.
| Groups | Treatment and dose | No. of examined mice | No. of examined PCEs | No. of MnPCEs | Mean ± SD | % of MN | % Protective index |
|---|---|---|---|---|---|---|---|
| Gr. I | Control | 10 | 1000 | 235 | 23.50 ± 2.72 | 2.35 | – |
| Gr. II | CM (33 ml/kg) | 10 | 1000 | 288 | 28.80 ± 1.80 | 2.88 | – |
| Gr. III | Cis-DDP (0.5 mg/kg) | 10 | 1000 | 861 | 86.10 ± 5.24a⁎⁎⁎ | 8.61 | – |
| Gr. IV | CM → Cis-DDP (0.5 mg/kg) | 10 | 1000 | 428 | 42.80 ± 5.14b⁎⁎⁎ | 4.28 | 50.29 |
| Gr. V | CM + Cis-DDP (0.5 mg/kg) | 10 | 1000 | 629 | 62.90 ± 5.16b⁎⁎ | 6.29 | 26.95 |
| Gr. VI | Cis-DDP (2.5 mg/kg) | 10 | 1000 | 677 | 97.70 ± 5.68a⁎⁎⁎ | 9.77 | – |
| Gr. VII | CM → Cis-DDP (2.5 mg/kg) | 10 | 1000 | 372 | 37.20 ± 3.41c⁎⁎⁎ | 3.72 | 61.92 |
∗p < 0.05.
p < 0.01.
p < 0.001.
Statistically compared with Gr. I.
Statistically compared with Gr. III.
Statistically compared with Gr. VI.
Table 2.
Effect of pretreatment of camel milk (CM) on the mitotic index of male mice bone marrow cells treated with cisplatin.
| Groups | Treatment and dose | No. of examined mice | No. of examined cells | Total no. of dividing cells | Mean ± SD | % of Mitotic index | % Protective index |
|---|---|---|---|---|---|---|---|
| Gr. I | Control | 10 | 1000 | 549 | 54.90 ± 2.80 | 5.49 | – |
| Gr. II | CM (33 ml/kg) | 10 | 1000 | 520 | 52.00 ± 1.99 | 5.20 | – |
| Gr. III | Cis-DDP (0.5 mg/kg) | 10 | 1000 | 213 | 21.30 ± 1.93a⁎⁎⁎ | 2.13 | – |
| Gr. IV | CM → Cis-DDP (0.5 mg/kg) | 10 | 1000 | 433 | 43.30 ± 1.72b⁎⁎⁎ | 4.33 | −103.29 |
| Gr. V | CM + Cis-DDP (0.5 mg/kg) | 10 | 1000 | 380 | 38.00 ± 2.43b⁎⁎⁎ | 3.80 | −78.4 |
| Gr. VI | Cis-DDP (2.5 mg/kg) | 10 | 1000 | 171 | 17.10 ± 1.35a⁎⁎⁎ | 1.71 | – |
| Gr. VII | CM → Cis-DDP (2.5 mg/kg) | 10 | 1000 | 348 | 34.80 ± 1.99c⁎⁎⁎ | 3.48 | −103.51 |
∗p < 0.05.
∗∗p < 0.01.
p < 0.001.
Statistically compared with Gr. I.
Statistically compared with Gr. III.
Statistically compared with Gr. VI.
Table 1 shows that a sub-acute pretreatment of CM (33 ml/kg) slightly increases the number of MnPCEs, however, this increase did not reach the significant level when compared with the control group (Gr. I).
Both sub-acute (Gr. III) and acute (Gr. VI) treatments of therapeutic doses, 0.5 mg/kg and 2.5 mg/kg, respectively, of cisplatin induced significant increase (p < 0.001) in the numbers of MnPCEs in comparison with the control group.
With respect with the inhibitor effect of MN on MnPCEs induced either by sub-acute dose (0.5 mg/kg) or acute dose (2.5 mg/kg) of cisplatin. Table 1 shows that the dose of CM produced a statistically significant reduction of the micronuclei number along the evaluated schedule.
A statistical decrease (p < 0.001) in the number of MnPCEs was detected after the sub-acute pretreatment of CM dose (33 ml/kg) before sub-acute dose (0.5 mg/kg) of cisplatin (Gr. IV) from 8.61% to 4.28%, whereas a statistically significant decrease (p < 0.01) was recorded after the simultaneous treatment of CM dose and sub-acute dose of cisplatin from 8.61% to 6.29% when compared with Gr. III (0.5 mg/kg of cisplatin). On the other hand the sub-acute treatment of CM dose followed by the acute dose of cisplatin (2.5 mg/kg) (Gr. VII) induced a significant decrease (p < 0.001) in the number of MnPCE from 9.77% to 3.72%.
Therefore, the administration of CM resulted in a decrease in cisplatin induced increase in the number of MnPCEs, indicating a protective effect of about 50.29% in Gr. IV, 26.95% in Gr. V and 61.92 in Gr. VII.
3.2. Mitotic index (MI)
The results obtained for MI are shown in Table 2.
Sub-acute pretreatment with a single dose of CM at the dose of 33 mg/kg (Gr. II) was not cytotoxic since no significant difference was observed between the mitotic indices from the pretreatment and control group (Table 3).
Table 3.
Analysis of variance “ANOVA” and low significant differences “LSD” between the effects of sub-acute pretreatment of camel milk (CM) or sub-acute dose (0.5 mg/kg) or acute dose (2.5 mg/kg) of cisplatin and pre-simultaneous treatments of CM and sub-acute dose and acute dose of cisplatin on the frequency of MN induction in bone marrow polychromatic erythrocytes (MnPCEs) of male mice.
| Treatments (dose mg/kg) | Control group | ANOVA |
LSD |
|||
|---|---|---|---|---|---|---|
| F | Groups | Treatment of dose | Mean difference | Sig | ||
| Sub-acute treatment (0.5 mg/kg) | I | 36.56⁎⁎⁎ | II | CM | −5.30 | |
| III | Cis-DDP (0.5 mg/kg) | −62.60 | ⁎⁎⁎ | |||
| IV | CM → Cis-DDP | −19.30 | ⁎⁎ | |||
| V | CM + Cis-DDP | −39.40 | ⁎⁎⁎ | |||
| Acute treatment (2.5 mg/kg) | I | 86.90⁎⁎⁎ | II | CM | −5.30 | |
| VI | Cis-DDP (2.5 mg/kg) | −74.20 | ⁎⁎⁎ | |||
| VII | CM + Cis-DDP | −13.70 | ⁎ | |||
p < 0.05.
p < 0.01.
p < 0.001.
In Gr. III and Gr. VI a statistically significant decrease (p < 0.001) in MI was observed when compared to Gr. I indicating a cytotoxic response of a sub-acute dose of 0.5 mg/ kg and an acute dose of 2.5 mg/kg of cisplatin (Table 3). The mean ± SD of mitotic indices were found to be 21.30 ± 1.93 and 17.10 ± 1.35 in Gr. III and Gr. VI, respectively, and elevated to 43.30 ± 1.72, 38.00 ± 2.43, 34.80 ± 1.99 in Gr. IV, Gr. V and Gr. VII, respectively. The calculated protective effect was 103.29%, 87.4% and 103.51% in Gr. IV, Gr. V and Gr. VII, respectively.
Tables 3 and 4 show the results of ANOVA and LSD obtained with respect to the rate of MnPCEs and MI produced by CM along the experiment.
Table 4.
Analysis of variance “ANOVA” and low significant differences “LSD” between the effects of sub-acute pretreatment of camel milk (CM) or sub-acute dose (0.5 mg/kg) or acute dose (2.5 mg/kg) of cisplatin and the pre- and simultaneous treatments of CM and sub-acute dose on the mitotic index in bone marrow cells of male mice.
| Treatments (dose mg/kg) | Control group | ANOVA |
LSD |
|||
|---|---|---|---|---|---|---|
| F | Groups | Treatment of dose | Mean difference | Sig | ||
| Sub-acute treatment (0.5 mg/kg) | I | 36.49⁎⁎⁎ | II | CM | 2.90 | |
| III | Cis-DDP (0.5 mg/kg) | 33.60 | ⁎⁎⁎ | |||
| IV | CM → Cis-DDP | 11.60 | ⁎⁎ | |||
| V | CM + Cis-DDP | 16.90 | ⁎⁎⁎ | |||
| Acute treatment (2.5 mg/kg) | I | 69.42⁎⁎⁎ | II | CM | 2.90 | |
| VI | Cis-DDP (2.5 mg/kg) | 37.80 | ⁎⁎⁎ | |||
| VII | CM + Cis-DDP | 20.10 | ⁎⁎⁎ | |||
∗p < 0.05.
p < 0.01.
p < 0.001.
There were significant differences (p < 0.001) between the mean of induction of MnPCE (F = 36.56) and MI (F = 36.49) obtained from the sub-acute treatment of CM dose (Gr. II) or sub-acute dose of cisplatin (Gr. III) and pretreatment Gr. IV (33 ml/kg → 0.5 mg/kg) or simultaneous treatment Gr. V (33 ml/kg + 0.5 mg/kg) of CM and the drug in comparison with the control group (Gr. I). In the case of pretreatment of CM dose (Gr. II) followed by the acute dose (2.5 mg/kg) of cisplatin (Gr. VI), the F values were 86.90, 69.42 p < 0.001 for MN and MI, respectively.
According to LSD the arrangement of treatments was:
-
1.
Cis-DDP (0.5 mg/kg) > CM + Cis-DDP > CM → Cis-DDP > CM (33 ml/kg).
-
2.
Cis-DDP (2.5 mg/kg) > CM → Cis-DDP > CM (33 ml/kg) for MN and MI.
4. Discussion
Cisplatin is a heavy metal complex with two labile chloride groups which upon hydrolysis in aqueous solution form various reactive oxygen species, such as superoxide anion, by interaction with DNA (Masuda et al., 1994; Baliga et al., 1998). The clastogenic effect of cisplatin has already been described (Adler and El-Tarras, 1989; Krishnaswamy and Dewey, 1993; Jirsová and Mandys, 1994; Edelweiss et al., 1995; Choudhury et al., 2000).
In the present study, i.p. doses (0.5 mg/kg and 2.5 mg/kg) of cisplatin were clastogenic, which was determined by the cytogenetic parameter, such as an extremely statistically significant increase in the number of micronucleated polychromatic erythrocytes (MnPCEs). These results were corroborated by previous studies on mice and rat bone marrow cells (Ciri et al., 1998; Choudhury et al., 2000; Choudhury and Jagdale, 2002; Khynriam and Prasad, 2003).
The CM administration route was oral, probably most appropriate because it is the same route as that of human exposure. It is worth mentioning that the dose of CM did not cause anincrease in the percentage of MnPCEs or a decrease in the percentage of MI compared with the respective control groups.
The combined treatments of administering CM either before or during simultaneous injection with sub-acute (0.5 mg/kg) or before acute (2.5 mg /kg) therapeutic doses of cisplatin showed a significant (p < 0.001) decrease in the frequency of MnPCEs and increase in the mitotic index when compared with cisplatin alone. The data obtained show that the CM dose has anticlastogenic and anticytotoxic effects against the cisplatin-induced increase in the MnPCEs and decrease in the MI in bone marrow cells. However, the MnPCEs and MI in the animals treated with the combination of cisplatin and CM did not reach the control level or CM-treated groups.
An appropriate explanation of this anticlastogenicity or anticytotoxicity is not easy because the tested agent is a mixture of several constituents that may participate in the observed effect.
Camel’s milk is low in fat, high in protein and vitamin C than cows’ milk.
Farah et al. (1992) reported that the vitamin C content in camel’s milk is about three times higher than that in cows’ milk. The high level of vitamin C in camel milk has been confirmed by several studies (Rao et al., 1970; Kon, 1972; Knoess, 1977; Sohail, 1983; Sawaya et al., 1984; Khanna, 1986; Yagil, 1982; Farah et al., 1992; Mehaia, 1994).
The anticlastogenic and even antimutagenic role of vitamin C (l-ascorbic acid) has been tested in a variety of in vivo and in vitro systems treated or exposed to – mitomycin C (MMC) (Krishna et al., 1986), pesticides (Hoda and Sinha, 1993), bleomycin (BLM) (Povirk and Austin, 1991; Anderson et al., 1995), radiation (Castillo et al., 2000) and rifampicin (RMP) antibiotic (Aly and Donya, 2002). Vitamin C is a strong antioxidant (Rao, 1997; Sato et al., 1997). The detoxification effect of vitamin C is manifested by the removal or minimization of free radicals produced by mercury (Gebhart, 1984; Herbaczyńska et al., 1995). Vitamin C has nucleophilic properties and binds to mercury ions (Hg2+) to reduce the mercury-induced DNA damage (Rao et al., 2001).
Ascorbic acid protects DNA from oxidative damage (Eylar et al., 1996; Antunes and Takahashi, 1999), reduces DNA damage exerted by irradiation (Green et al., 1994) and also reduces micronucleus (MN) frequencies in polychromatic erythrocytes of bone marrow in rodents exposed to heavy metals and radiation (Chorvatovicová et al., 1991; Konopacka et al., 1998).
Al-Awadi and Srikumar (2001) analysed the concentration and distribution of trace elements in camel milk compared to those in human and cow milk. They found that the selenium content of CM was comparable to those of other types of milk.
Ianăş et al. (1995) described the all round beneficial action of selenium preparation in rats exposed to carbon tetrachloride (CCl4), as well as a strong antioxidant effect, confirming the essential role of selenium in maintaining cellular integrity. Moreover, the protective effect of selenium against cadmium genotoxicity in the Chinese hamster V79 cells was reported by Hurná et al. (1997). Selenium is a constituent of various oxidant defense selenioproteins and a cofactor of glutathione peroxidase in the elimination of peroxide radicals; selenium also seems to prevent cancer development (Cabrera et al., 2003). The characteristic feature of supplemental selenium to reduce the genotoxic effect of cobalt chloride (CoCl2) was proved by Hassan et al. (2006).
The zinc content of camel’s milk was higher than that of human milk (AL-Awadi and Srikumar, 2001). The protective effect of zinc on cadmium genotoxicity (the number of micronucleated cells decreased) was observed at a lower concentration (5–25 micro M cdcl2) (Hurná and Hurná, 2000). Zinc is an element required for DNA and RNA synthesis and may be a cofactor in the activity of superoxide dismutase (Cabrera et al., 2003).
Furthermore, milk exhibits a range of biological activities. These biological activities are mainly due to peptides and protein in milk. Bioactive peptides are produced during the digestion of milk in the gastrointestinal tract (Korhonen and Pihlanto, 2001). The beneficial health effects of milk proteins can be classified as antimicrobial, antioxidative, antithrombotic, antihypertensive or immuno-modulatory (FitzGerald and Meisel, 2000; Kohonen and Pihlanto, 2003).
The average content of protein (Casein and whey proteins) in camel’s milk is generally similar to that of cows milk, whereas human milk has the lowest protein content among the milk from other mammals (Jenness, 1974). Casein is the principal protein component of the most milk from most of the mammals.
The antimutagenic potential of casein was investigated by Van Boekel et al. (1993) using several mutagens. They found that preincubation increased the antimutagenic potential of casein towards N-nitroquinoline-1-oxide (NQO). They added that the antimutagenic potential of casein increased with pepsin hydrolysis. They postulated that this increase was due to the peptides formed and might be explained by a better accessibility of casein peptides for interaction with mutagens.
In addition, Bosselaers et al. (1994) studied the possible antimutagenic effect of five different proteins including casein. They found that casein significantly inhibited 4-nitroquinoline 1-oxide (4-NQO) and 1-methyl-1-nitroso-3-nitroguanidine (MNNG) induced sister chromatid exchange (SCE). They also reported that pepsin-hydrolysed casein inhibited SCE induction by 4NQO and MNNG. Therefore, they concluded that casein and its pepsin hydrolysis products may protect mammalian cells against certain genotoxic compounds. They added, although the mechanism of antimutagenicity is unknown, it seems believable that the protein acts as a blocking agent by chemical or physical interaction with the mutagens. They added that the accessibility of protein molecules and the presence of nucleophilic binding sites may be significant factors in determining the antimutagenic properties of proteins. Moreover, Goeptar et al. (1997) presented that enzymatic digestion of sodium caseinate greatly improved its antimutagenicity potential. They suggested that the molecular structure of a protein determines the protective effect against mutagens. They added that a stronger protection appears to correspond with a lack of secondary and tertiary structure. Their findings shed new light on the possible prevention of mutagenesis and/or carcinogenesis by food proteins, with a unique role for milk proteins.
Besides casein, camel milk contains high levels of lactoferrin. Lactoferrin is a iron-binding glycoprotein of the transferrin family (Al-Majali et al., 2007). This relatively recent known protein has a number of properties such as antibactericidal activity, antiviral, antifungal, anticarcinogenic, anti inflammatory activity, antioxidant and analgestic properties (Konuspayeva et al., 2004). A comparative survey of lactoferrin concentrations in different milks showed that camel’s milk contain the greatest amount of lactoferrin (Konuspayeva et al., 2004). There are high levels of linoleic acids (18:2) among the polyunsaturated fatty acids in camel milk (Crawford et al., 1976). Conjugated linoleic acid “CLA” was identified as a component of milk and dairy products for over 30 years ago. It is formed as an intermediate in the course of the conversion of linoleic acid into oleic acid in the rumen (Kritchevsky, 2000). CLA compounds could serve as useful food antioxidants and provide additional value because of their potential bioactivity in disease prevention (Badr El-Din and Omaye, 2007). The results obtained by Liew et al. (1995) supported a mechanism involving the inhibition of carcinogen activation by CLA, as opposed to direct interaction with procarcinogen, scavenging of electrophiles or selective induction of phase I detoxification pathways.
Moreover, Yang et al. (2001) concluded that CLA modulate phIP (2-amino-1-methyl-6-phenylimidazol [4,5-b] pyridine) induced mutagenesis in a tissue-specific manner.
In conclusion, our study established a significant decrease in the micronuclei and improvement in the mitotic index of bone marrow cells of male mice pretreated with camel milk, as well as a strong capacity to trap free radicals originating from cisplatin. Thus, from the finding we suggest that camel’s milk has antioxidant effects and the anticlastogenesis mechanisms of this milk should explored further before its use during cisplatin chemotherapy.
References
- Adler I.D., El-Tarras A. Clastogenic effects of cis-diamminedichloroplatinum. I. Induction of chromosomal aberrations in somatic and germinal cells of mice. Mutat. Res. 1989;211:131–137. doi: 10.1016/0027-5107(89)90113-9. [DOI] [PubMed] [Google Scholar]
- Al-Awadi F.M., Srikumar T.S. Trace elements and their distribution in protein fractions of camel milk in comparison to other commonly consumed milks. J. Dairy Res. 2001;68:463–469. doi: 10.1017/s0022029901005003. [DOI] [PubMed] [Google Scholar]
- Albanese R., Middleton B.J. Inhibition of adrimycin – induced micro-nuclei by desferrioxamine in Swiss albino mice. Mutat. Res. 1987;301:107–111. doi: 10.1016/0165-7992(93)90032-q. [DOI] [PubMed] [Google Scholar]
- Al-Majali A.M., Bani Ismail Z., Al-Hami Y., Nour A.Y. Lactoferrin concentration in milk from camel (Camelus dromedarius) with and without subclinical mastitis. Int. J. Appl. Res. Vet. Med. 2007;5:120–124. [Google Scholar]
- Aly F.A., Donya S. In vivo antimutagenic effect of vitamins C and E against rifampicin-induced chromosomal aberrations in mouse bone-marrow cells. Mutat. Res. 2002;518:1–7. doi: 10.1016/s1383-5718(02)00037-2. [DOI] [PubMed] [Google Scholar]
- Anderson D., Basaran N., Blowers S.D., Edwards A.J. The effect of antioxidants on bleomycin treatment in in vitro and in vivo genotoxicity assays. Mutat. Res. 1995;329:37–47. doi: 10.1016/0027-5107(95)00017-d. [DOI] [PubMed] [Google Scholar]
- Antunes L.M., Takahashi C.S. Protective and induction of chromosomal damage by vitamin C in human lymphocyte cultures. Terato. Carcino. Mutagen. 1999;19:53–59. [PubMed] [Google Scholar]
- Badr El-Din N.K., Omaye S.T. Concentration dependent anti-oxidant activities of conjugated Linoleic acid and α-tocopherol in corn oil. Br. J. Nutr. 2007;60:32–37. doi: 10.1002/jsfa.3040. [DOI] [PubMed] [Google Scholar]
- Baliga R., Zhang Z., Baliga M., Ueda N., Shah S.V. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- Beretta G. 10th ed. Farmitalia Carlo Erba-Erbamont; Milan: 1991. Cancer Treatment Medical Guide. [Google Scholar]
- Bosselaers I.E., Caessens P.W., Van Boekel M.A., Alink G.M. Differential effects of milk proteins, BSA and soy protein on 4NQO- or MNNG-induced SCEs in V79 cells. Food Chem. Toxicol. 1994;32:905–909. doi: 10.1016/0278-6915(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Cabrera C., Jiménez R., Lopez C. Determination of tea component with antioxidant activity. J. Agric. Food Chem. 2003;51:4427–4435. doi: 10.1021/jf0300801. [DOI] [PubMed] [Google Scholar]
- Castillo J., Benavente-García O., Lorente J., Alcaraz M., Redondo A., Ortuño A., Del Rio J.A. Antioxidant activity and radioprotective effects against chromosomal damage induced in vivo by X-rays of flavan-3-ols (Procyanidins) from grape seeds (Vitis vinifera): comparative study versus other phenolic and organic compounds. J. Agric. Food Chem. 2000;48:1738–1745. doi: 10.1021/jf990665o. [DOI] [PubMed] [Google Scholar]
- Chorvatovicová D., Ginter E., Kosinová A., Zloch Z. Effect of vitamins C and E on toxicity and mutagenicity of hexavalent chromium in rat and guinea pig. Mutat. Res. 1991;262:41–46. doi: 10.1016/0165-7992(91)90104-c. [DOI] [PubMed] [Google Scholar]
- Choudhury R.C., Jagdale M.B. Vitamin E protection from/potentiation of the cytogenetic toxicity of cisplatin in Swiss mice. J. Chemother. 2002;14:397–405. doi: 10.1179/joc.2002.14.4.397. [DOI] [PubMed] [Google Scholar]
- Choudhury R.C., Jagdale M.B., Misra S. Cytogenetic toxicity of cisplatin in bone marrow cells of Swiss mice. J. Chemother. 2000;12:173–182. doi: 10.1179/joc.2000.12.2.173. [DOI] [PubMed] [Google Scholar]
- Ciri A., Khynriam D., Prasad S.B. Vitamin C mediated protection on cisplatin induced mutagenicity in mice. Mutat. Res. 1998;421:139–148. doi: 10.1016/s0027-5107(98)00158-4. [DOI] [PubMed] [Google Scholar]
- Crawford M.A., Hall B., Laaurance B.M., Munhambo A. Milk lipids and their variability. Curr. Med. Res. Opin. 1976;4:33–43. [Google Scholar]
- Edelweiss M.I., Trachtenberg A., Pinheiro E.X., da-Silva J., Riegel M., Lizardo-Daudt H.M., Mattevi M.S. Clastogenic effect of cisplatin on Wistar rat bone marrow cells. Braz. J. Med. 1995;26:762–769. [PubMed] [Google Scholar]
- Elsendoorn T.J., Weijl N.I., Mithoe S., Zwinderman A.H., Van Dam F., De Zwart F.A., Tates A.D., Osanto S. Chemotherapy induced chromosomal damage in peripheral blood lymphocytes of cancer patients supplemented with antioxidants or placebo. Mutat. Res. 2001;498:145–158. doi: 10.1016/s1383-5718(01)00278-9. [DOI] [PubMed] [Google Scholar]
- Eylar E., Baez I., Navas J., Mercado C. Sustained levels of ascorbic acids are toxic and immune-suppressive for human T cells. Proc. Royal Health Sci. J. 1996;15:21–26. [PubMed] [Google Scholar]
- Farah Z., Rettenmaier R., Atkins D. Vitamin content of camel milk. Int. J. Vitam. Nutr. Res. 1992;62:30–33. [PubMed] [Google Scholar]
- Ferguson L.R. Natural and man-made mutagens in human diet. Mutat. Res. 1999;443:1–10. [PubMed] [Google Scholar]
- FitzGerald R.J., Meisel H. Milk protein derived inhibitors of angiotensin-I-converting enzyme. Br. J. Nutr. 2000;84:33–37. doi: 10.1017/s0007114500002221. [DOI] [PubMed] [Google Scholar]
- Gebhart E. The action of anticlastogens on chemically induced SCE. In: Tice R.R., Hollaender A., editors. Sister Chromatid Exchanges – 25 years of Experimental Research-Part A and B. Plenum Press; New York: 1984. pp. 319–332. [Google Scholar]
- Goeptar A.R., Koeman J.H., Van Boekel M.A., Alink G.M. Impact of digestion on the antimutagenic activity of the milk protein casein. Nutr. Res. 1997;17:1363–1379. [Google Scholar]
- Gorban A.M., Izzeldin O.M. Fatty acids and lipids of camel milk and colostrum. Int. J. Food Sci. Nutr. 2001;52:283–287. doi: 10.1080/713671778. [DOI] [PubMed] [Google Scholar]
- Green M.H., Lowe J.E., Waugh A.P., Aldridge K.E., Cole J., Arlett C.F. Effect of diet and vitamin C on DNA strand breakage in freshly-isolated human white blood cells. Mutat. Res. 1994;316:91–102. doi: 10.1016/0921-8734(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Hassan N.H., Fahmy M.A., Farghaly A.A., Hassan E.E. Anti-mutagenic effect of selenium and vitamins against the genotoxicity by cobalt chloride in mice. Cytologia. 2006;71:213–222. [Google Scholar]
- Hayashi M., Sofuni T., Ishidate M.J.R. Kinetics of micronucleus formation in relation to chromosomal aberrations in mouse bone marrow. Mutat. Res. 1984;127:129–137. doi: 10.1016/0027-5107(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Heddle W.F., Brugada P., Wellens H.J. Multiple circus movement tachycardias with multiple accessory path-ways. J. Am. Coll. Cardiol. 1984;4:168–175. doi: 10.1016/s0735-1097(84)80336-8. [DOI] [PubMed] [Google Scholar]
- Herbaczyńska C.K., Ktosiewicz W.B., Cedro K., Wasek W., Panczenko K.B., Wartanowicz M. Supple-mentation with vitamins C and E suppresses leukocyte oxygen free radical production in patients with myocardial infarction. Eur. Health J. 1995;16:1044–1049. doi: 10.1093/oxfordjournals.eurheartj.a061045. [DOI] [PubMed] [Google Scholar]
- Hoda Q., Sinha S.P. Vitamin C mediated minimization of Roger – induced genotoxicity. Mutat. Res. 1993;299:29–36. doi: 10.1016/0165-1218(93)90116-u. [DOI] [PubMed] [Google Scholar]
- Hurná E., Hurná S. Protective effect of zinc on cadmium-induced micronuclei in V79 cells. J. Trace Elem. Med. Biol. 2000;14:55–57. doi: 10.1016/S0946-672X(00)80024-3. [DOI] [PubMed] [Google Scholar]
- Hurná E., Siklenka P., Hurná S. Effect of selenium on cadmium genotoxicity investigated by micronucleus assay. Vet. Med. Czech. 1997;42:339–342. [PubMed] [Google Scholar]
- Ianăş O., Olinescu R., Bădescu I., Simionescu L., Popovici D. The influence of “selenium organicum” upon the hepatic function of carbon tetrachloride poisoned rats. J. Int. Med. 1995;33:113–120. [PubMed] [Google Scholar]
- Jenness R. Proceedings: bio-synthesis and composition of milk. J. Invest. Dermatol. 1974;63:109–118. doi: 10.1111/1523-1747.ep12678111. [DOI] [PubMed] [Google Scholar]
- Jirsová K., Mandys V. Induction of micronuclei and granular chromatin condensation in human skin fibroblasts influenced by cisplatin (cis-DDP) in vitro. Mutat. Res. 1994;310:37–44. doi: 10.1016/0027-5107(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Khanna N.D. Camel as milk animal. Indian Farming. 1986;36:37–40. [Google Scholar]
- Khynriam D., Prasad S.B. Cisplatin-induced genotoxic effects and endogenous glutathione levels in mice bearing ascites Dalton’s lymphoma. Mutat. Res. 2003;526:9–18. doi: 10.1016/s0027-5107(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Kliesch U., Adler I.D. Micronucleus test in bone marrow of mice treated with 1-nitropropane, 2-nitro-propane and cisplatin. Mutat. Res. 1987;192:181–184. doi: 10.1016/0165-7992(87)90053-4. [DOI] [PubMed] [Google Scholar]
- Knoess K.H. The camel as meat and milk animal. World Anim. Rev. 1977;22:39–44. [Google Scholar]
- Kohonen H., Pihlanto A. Milk protein-derived bioactive peptides-novel opportunities for health promotion. IDF Bull. 2003;363:17–26. [Google Scholar]
- Kon, S.K., 1972. Milk and Milk products. In: Human Nutrition. FAO Nutrition Studies No. 27. FAO, Rome, Italy. [PubMed]
- Konopacka M., Widel M., Rzeszowska W.J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mutat. Res. 1998;417:85–94. doi: 10.1016/s1383-5718(98)00095-3. [DOI] [PubMed] [Google Scholar]
- Konuspayeva G., Serikbayeva A., Loiseau G., Narmuratova M., Faye B. In: Desertification Combat and Food Safety: The Added Value of Camel Producers. Bernard Faye, Palmated Esenov., editors. IOS Press; Amisterdam, Ashgabad, Turkmenistan: 2004. pp. 158–167. [Google Scholar]
- Korhonen H., Pihlanto A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm. Des. 2001;9:1297–1308. doi: 10.2174/1381612033454892. [DOI] [PubMed] [Google Scholar]
- Krishna G., Nath J., Ong T. Inhibition of cyclophosphamide and mitomycin-C induced sister chromatid exchanges in mice by vitamin C. Cancer Res. 1986;46:2670–2674. [PubMed] [Google Scholar]
- Krishnaswamy G., Dewey W.C. Cisplatin induced cell killing and chromosomal aberrations in CHO cells: treated during G1 or S phase. Mutat. Res. 1993;293:161–172. doi: 10.1016/0921-8777(93)90067-q. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D. Antimutagenic and some other effects of conjugated linoleic acid. Br. J. Nutr. 2000;83:459–465. [PubMed] [Google Scholar]
- Liew C., Schut H.A., Chin S.F., Pariza M.W., Dashwood R.H. Protection of conjugated linoleic acids against 2-amino-3-methyl-imidazo [4,5-f] quinoline-induced colon carcinogenesis in the F344 rat: a study of inhibitory mechanisms. Carcinogenesis. 1995;16:3037–3043. doi: 10.1093/carcin/16.12.3037. [DOI] [PubMed] [Google Scholar]
- Masuda H., Tanaka T., Takahama U. Cisplatin generates superoxide anion by interaction with DNA in a cell-free system. Biochem. Biophys. Res. Commun. 1994;203:1175–1180. doi: 10.1006/bbrc.1994.2306. [DOI] [PubMed] [Google Scholar]
- Mehaia M.A. Vitamin C and riboflavin content in camel milk: effect of heat treatment. Food Chem. 1994;50:153–155. [Google Scholar]
- Osanto S., Thijssen J.C., Woldering V.M., van Rijn J.L., Natarajan A.T., Tates A.D. Increased frequency of chromosomal damage in peripheral blood lymphocytes up to nine years following curative chemotherapy of patients with testicular carcinoma. Environ. Mol. Mutagen. 1991;17:71–78. doi: 10.1002/em.2850170202. [DOI] [PubMed] [Google Scholar]
- Paget G.E., Barnes J.M. vol. 1. Academic Press; 1964. pp. 135–136. (Evaluation of Drug Activities and Pharmacokinetics). [Google Scholar]
- Povirk L.F., Austin M.J.F. Genotoxicity of bleomycin. Mutat. Res. 1991;257:136–137. doi: 10.1016/0165-1110(91)90022-n. [DOI] [PubMed] [Google Scholar]
- Ramel C., Alekperov U.K., Ames B.N., Kada T., Wattenberg L.W. Inhibition of mutagenesis and their relevance to carcinogenesis. Mutat. Res. 1986;168:47–65. doi: 10.1016/0165-1110(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Rao M.V. Mercury and its effects on mammalian system – a critical review. Indian J. Environ. Toxicol. 1997;7:3–11. [Google Scholar]
- Rao M.B., Gupta R.C., Dastur N.N. Camel’s milk and milk products. Indian J. Dairy Sci. 1970;23:71–78. [Google Scholar]
- Rao M.V., Chinoy N.J., Suthar M.B., Rajvanshi M.I. Role of ascorbic acid on mercuric chloride-induced genotoxicity in human blood cultures. Toxicol. in Vitro. 2001;15:649–654. doi: 10.1016/s0887-2333(01)00081-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985;13:102–122. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Sato K., Kusaka Y., Zhang Q., Deguchi Y., Li B., Okada K., Nakakuki K., Muraoka R. Direct effect of inorganic mercury on citrate uptake by isolated rat renal brush border membrane vesicles. Ind. Health. 1997;35:456–460. doi: 10.2486/indhealth.35.456. [DOI] [PubMed] [Google Scholar]
- Sawaya W.N., Khalil J.K., Al-Shabat A., Al-Mohammad H. Chemical composition and nutritional quality of camel milk. J. Food Sci. 1984;49:744–747. [Google Scholar]
- Schmid W. Chemical mutagen on in vivo somatic mammalian cells. Agenths. Actions. 1973;3:77–85. doi: 10.1007/BF01986538. [DOI] [PubMed] [Google Scholar]
- Schmid W. The micronucleus test. Mutat. Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- Shukla Y., Taneja P. Antimutagenic effects of garlic extract on chromosomal aberrations. Cancer Lett. 2002;176:31–36. doi: 10.1016/s0304-3835(01)00774-1. [DOI] [PubMed] [Google Scholar]
- Sohail M.A. The role of the Arabian camel (Caamelus droedarius) in animal production. World Rev. Anim. Prod. 1983;19:37–40. [Google Scholar]
- Van Boekel M.A., Weerens C.N., Holstra A., Scheidtweiler C.E., Alink G.M. Antimutagenic effects of casein and its digestion products. Food-Chem. Toxicol. 1993;31:731–737. doi: 10.1016/0278-6915(93)90144-n. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi K.K., Venu R. In vivo anticlastogenic effects of l-ascorbic acid in mice. Mutat. Res. 1999;438:47–51. doi: 10.1016/s1383-5718(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Yagil, R., 1982. Camels and camel milk. Food and Agriculture Organization, Anim. Prod. Health Paper No. 26, Rome, Italy.
- Yagil R., Saran A., Etzion Z. Camel’s milk: for drinking only? Comp. Biochem. Physiol. 1984;78:263–266. doi: 10.1016/0300-9629(84)90143-9. [DOI] [PubMed] [Google Scholar]
- Yang H., Stuart G.R., Glickman B.W., de Boer J.G. Modulation of 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine-induced mutation in the cecum and colon of big blue rats by conjugated linoleic acid and 1,2-dithiole-3-thione. Nutr. Cancer. 2001;39:259–266. doi: 10.1207/S15327914nc392_16. [DOI] [PubMed] [Google Scholar]


