Abstract
We studied the effect of gold nanoparticles (NPs) on oxidative stress markers including reduced glutathione (GSH) and malondialdehyde (MDA) in different organs of rats. Adult male Wistar-Kyoto rats were randomly divided into 3 groups of 5 animals each. One group served as control and received vehicle only. The remaining two groups were treated with 50 μl of 10 nm sized gold NPs, daily for 3 and 7 days, respectively. The rats were sacrificed 24 h after the last injection of NPs. Administration of gold NPs did not cause any significant change in GSH levels in liver, lung and heart on day 3 or day 7. There was no significant effect of gold NPs on MDA levels in lung and heart whereas significant increases in MDA levels were found in the liver of rats treated with gold nanoparticles on both 3 and 7 days post-dosing (ANOVA F = 7.113, P = 0.010). In conclusion, the findings of this preliminary study suggest that gold NPs of 10 nm diameter produce significant lipid peroxidation in rat liver however lungs and heart do not show any oxidative stress. Further studies are warranted to examine the effects of a broader dose range of gold NPs on the levels of free radical indices in different organs of rats.
Keywords: Gold nanoparticles, Oxidative stress, Glutathione, Malondialdehyde, Rats
1. Introduction
Although the biosafety of metallic gold is well recognized as the toxicological behavior of gold nanoparticles is not clearly understood. The cytotoxicity of gold nanoparticles has been studied in human cells and the results have shown that gold nanoparticles are nontoxic up to 250 mM, while ionic gold exhibits obvious cytotoxicity at 25 mM (Connor et al., 2005).
In general, most studies have excluded the toxicity of gold nanoparticles (4–18 nm in diameter) (Connor et al., 2005; Khan et al., 2007; Shukla et al., 2005) studying cell viability, pro-apoptotic effects, oxidative stress and inflammatory response. Brandenberger et al. (2010) concluded that non functionalized gold particles with a size of 13–20 nm in diameter do not cause acute adverse effects. In contrast, cytotoxic and pro-apoptotic effects of gold particles ⩽2 nm have been reported (Pan et al., 2007; Tsoli et al., 2005), as well as the impact of the charge of surface coatings on 2 nm particles on the reduction of cell viability (Goodman et al., 2004). This size effect could be explained by an increased catalytic activity for particles with less than 3–5 nm as described by Hvolbaek et al. (2007) or by potential interaction with the DNA for particles equal or smaller than 1.4 nm (Liu et al., 2003). Actually, in vitro cultures cannot replicate the complexity of an in vivo system or provide meaningful data about the response of a physiologic system to an agent (Zhang et al., 2010).
There are few toxicologic reports of gold nanoparticles in animal model, which is the preferred system for toxicologic evaluation of a novel agent and should be used to characterize the toxicity of gold nanoparticles. This investigation was therefore aimed to study the effect of gold nanoparticles (10 nm) on oxidative stress markers including glutathione (GSH) and malondialdehyde (MDA) in different organs of rats.
2. Materials and methods
2.1. Animals and treatment groups
Adult male Wistar-Kyoto rats, weighing 230 ± 20 g, were obtained from the Laboratory Animal Centre. The animals were housed in humidity- and temperature-controlled ventilated cages on a 12 h day/night cycle, with free access to standard laboratory rats’ diet and tap water. The animals were randomly divided into 3 groups of 5 animals each. One group served as control and received vehicle only. The remaining two groups were treated with gold nanoparticles for 3 and 7 days, respectively.
2.2. Gold nanoparticles (NPs) and dosing
Gold nanoparticles of 10 nm (MKN-Au-010 of concentration 0.01%) were purchased from MK Impex Corp., Ontario, Canada. GNPs demonstrate high electron density and are highly homogeneous in shape and size. Doses of 50 μl of 10 nm NPs in aqueous solution were administered to animals via intraperitoneal injection daily for 3 or 7 days. The rats were sacrificed 24 h after the last injection of NPs. The specimens of liver, lung and heart were collected for biochemical analyses. All experiments were conducted in accordance with guidelines approved by the Local Animal Care and Use Committee.
2.3. Analysis of glutathione (GSH)
The measurement of reduced glutathione (GSH) in different organ tissues (liver, lung, heart) was performed according to the procedure reported by Owen (1980). The tissue was homogenized in ice-cold perchloric acid (0.2 M) containing 0.01% of ethylenediaminetetraacetic acid (EDTA). The homogenate was centrifuged at 9,000g for 5 min, in a refrigerated centrifuge (4 °C). The enzymatic reaction was started by adding 100 μl of clear supernatant in a spectrophotometric cuvette containing 800 μl of 0.3 mM reduced nicotinamide adenine dinucleotide phosphate (NADPH), 100 μl of 6 mM 5,5-dithiobis-2-nitrobenzoic acid (DTNB) and 10 μl of 50 units/ml glutathione reductase (all these reagents were freshly prepared in a phosphate buffer at pH 7.5). The absorbance was measured over a period of 120 s at 412 nm at 30 °C. The GSH level was determined by comparing the rate of change of absorbance of the test solution with that of standard GSH.
2.4. Analysis of malondialdehyde (MDA)
The level of MDA in different organs of rats was analyzed spectrophotometrically as described earlier (Utley et al. 1967). Tissues were weighed and homogenized (10% w/v) in ice-cold 0.15 M potassium chloride in an ultraturax homogenizer. Tissue homogenate (1 mL) was incubated at 37 °C in a metabolic shaker for 2 h. One milliliter of 10% (w/v) trichloroacetic acid was mixed with homogenate followed by centrifugation at 3000 rpm for 10 min. Aliquots (1 mL) of the clear supernatant were mixed with 1 mL of 0.67% (w/v) 2-thiobarbituric acid and placed in a boiling water bath for 10 min, cooled and diluted with 1 mL distilled water. The absorbance of the solution was recorded at 535 nm, and the concentration of MDA was calculated using tetraethoxypropane as an external standard.
3. Results
Administration of gold nanoparticles did not cause any significant change in GSH levels in liver (ANOVA F2,12 = 1.007, P = 0.397), lung (ANOVA F2,12 = 3.306, P = 0.075) and heart (ANOVA F2,12 = 3.245, P = 0.078) on day 3 or day 7 (Table 1). The levels of GSH slightly increased on day 3 following the exposure to gold nanoparticles and returned to near normal levels on day 7 in liver, lung and heart. The percent change in GSH as compared to control is shown in Fig. 1 for quick view.
Table 1.
Effect of gold NPs on GSH levels in different organs of rats.
| Treatment | Liver | Lung | Heart |
|---|---|---|---|
| Control | 976.78 ± 97.72 | 153.34 ± 35.81 | 681.89 ± 129.19 |
| NP 3 days | 1154.23 ± 191.68 | 260.22 ± 45.03 | 1093.43 ± 126.57 |
| NP 7 days | 830.39 ± 171.27 | 145.19 ± 23.07 | 897.90 ± 76.84 |
Values are means ± SEM, nmoles/g tissue.
Figure 1.
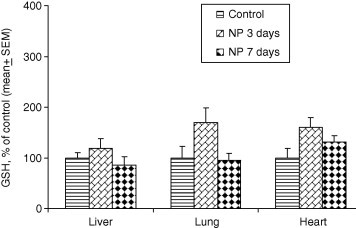
Percent change in the levels of GSH following the exposure to gold NPs for 3 and 7 days.
There was no significant effect of gold nanoparticles on MDA levels in lung (ANOVA F2,12 = 0.262, P = 0.774) and heart (ANOVA F2,12 = 0.867, P = 0.447). On the other hand, significant increases in MDA levels were found in the liver of rats treated with gold nanoparticles on both 3 and 7 days post-dosing (ANOVA F2,12 = 7.113, P = 0.010) (Table 2). The percent change in MDA as compared to control is shown in Fig. 2.
Table 2.
Effect of gold NPs on MDA levels in different organs of rats.
| Treatment | Liver | Lung | Heart |
|---|---|---|---|
| Control | 0.186 ± 0.013 | 1.137 ± 0.149 | 0.628 ± 0.066 |
| NP 3 days | 0.286 ± 0.024⁎ | 1.152 ± 0.097 | 0.733 ± 0.061 |
| NP 7 days | 0.275 ± 0.017⁎ | 1.057 ± 0.105 | 0.706 ± 0.042 |
P < 0.05 versus control group (Dunnett’s test). Values are means ± SEM, nmoles/g tissue.
Figure 2.
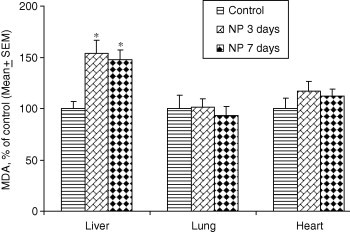
Percent change in the levels of MDA following exposure to gold NPs for 3 and 7 days. ∗P < 0.05 versus respective control group using Dunnett’s test.
4. Discussion
The results of this study showed that intraperitoneal injection of gold nanoparticles of 10 nm diameter do not cause any oxidative stress in lung and heart of rats up to 1 week post dosing. Recently, intraperitoneal injection of gold nanoparticles has been investigated by Lasagna-Reeves et al. (2010) and the results showed a low level of toxicity at the dose range 320–3200 μg/kg/day. Zhang et al. (2010) have noticed that gold nanoparticles by intraperitoneal injection are less toxic than oral administration at the dose of 1100 μg/kg (Zhang et al., 2010). Nanoparticles size is a key factor in biological responses to nanoparticles; smaller particles tend to be more toxic than larger ones. Exposure of gold nanoparticles (average diameter 5.3 ± 1 nm) produced oxidative stress within 24 h in Mytilus edulis (Tedesco et al., 2010). At sizes larger than 5 nm, the general assumption is that gold is chemically inert like the bulk. However, the chemical reactivity of gold particles for diameters less than 3 nm is most likely different than larger gold nanoparticles (Tsoli et al., 2005).
Exposure of three gold nanoparticle reference materials (10, 30 and 60 nm), as recommended by the National Institute of Standards and Technology Nelson, did not result any free radicals generation as measured by electron paramagnetic resonance spectroscopy (Nelson et al., 2011). Modified gold nanoparticles (10 nm size) have been shown to dismutate superoxide radicals (Cao et al., 2011). The gold coating supported on diamond nanoparticles exhibits about a twofold higher antioxidant activity than glutathione, one of the reference antioxidant systems (Martín et al., 2010). Gold nanoparticles have also attenuated lipopolysaccharide-induced nitric oxide production by inhibiting proinflammatory cascade (Ma et al., 2010). Zidki et al. (2000) have reported potential radical scavenging property of gold nanoparticles against alkyl radicals. Zhang et al. (2003) have also observed reduction in EPR signal (indication of radical scavenging) following the interaction of free radicals with 15 nm gold nanoparticles. Barathmanikanth et al. (2010) have described the effectual role of gold nanoparticles as an anti-oxidative agent, by inhibiting the formation of reactive oxygen species, scavenging free radicals and creating a sustained control over hyperglycemic conditions suggesting the potential of gold nanoparticles as an economic therapeutic remedy in diabetic complications. Recently, the protective effect of 13 and 50 nm gold NPs on joint swelling in a rat arthritis model has been attributed to increased level of antioxidant enzyme catalase by gold NPs (Leonavičienė et al., 2012). Moreover, there is a plea for developing new artificial antioxidant based on enhanced radical-scavenging activity by antioxidant-functionalized gold nanoparticles (Yin, 2007).
Our results showed significant lipid peroxidation in liver of rats treated with gold nanoparticles. The liver and spleen are considered two dominant organs for biodistribution and metabolism of gold nanoparticles (de Jong et al., 2008; Semmler-Behnke et al., 2008; Chen et al., 2009; Balasubramanian et al., 2010). It is thought that nanoparticles should have final hydrodynamic diameters ⩽5.5 nm to be excreted from the rat body through kidneys (Choi et al., 2007). If gold nanoparticles are larger than this renal filtration cutoff, they are not excreted in urine; instead they are eliminated from the blood by the reticuloendothelial system and thus tend to accumulate in the spleen and liver (de Jong et al., 2008; von Maltzahn et al., 2009). Aggregation of nanoparticles could influence their ability to interact with or enter cells, and thus adds complexity to the system. If the in situ aggregation state of the nanoparticles is not considered, difficulties arise in the interpretation of data about nanoparticle biodistribution or uptake (Alkilany and Murphy, 2010). The organ distributions of gold nanoparticles are size-dependent while small gold nanoparticles of 5–15 nm have wider organ distribution than that of large gold nanoparticles of 50–100 nm (de Jong et al., 2008; Semmler-Behnke et al., 2008; Chen et al., 2009). It has been found that gold nanoparticles with a long blood circulation time can accumulate in the liver and spleen, and significantly affect the gene expression (Balasubramanian et al., 2010; Cho et al., 2009). Thus the hepatotoxicity of gold nanoparticles may be attributed to accumulation of nanoparticles in liver.
In conclusion, the findings of this preliminary study suggest that gold nanoparticles of 10 nm diameter produce significant lipid peroxidation in liver however lungs and heart do not show any oxidative stress. Further studies are warranted to examine the effects of a broader dose range of gold nanoparticles on the time course levels of free radical indices in different organs of rats, for a more clear understanding of the biosafety of gold nanoparticles.
Acknowledgments
We thank Raja Abbas Manthiri, Adnan Ali Khan and Vali Mohammad Mansoor for technical assistance. This research was financially supported by the National Science and Technology Innovation Plan (NSTIP), Research No. 08-ADV206-02 and Research No. 09-NAN670-02, College of Science, King Saud University, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alkilany A.M., Murphy C.J. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J. Nanopart. Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S.K., Jittiwat J., Manikandan J., Ong C.N., Yu L.E., Ong W.Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- Barathmanikanth S., Kalishwaralal K., Sriram M., Pandian S.R., Youn H.S., Eom S., Gurunathan S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010;8:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger C., Rothen-Rutishauser B., Mühlfeld C., Schmid O., Ferron G.A., Maier K.L., Gehr P., Lenz A.G. Effects and uptake of gold nanoparticles deposited at the air–liquid interface of a human epithelial airway model. Toxicol. Appl. Pharmacol. 2010;242:56–65. doi: 10.1016/j.taap.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Cao R., Jr., Villalonga R., Díaz-García A.M., Cao R., Rojo T., Rodríguez-Argüelles M.C. Gold nanoparticles enhancing dismutation of superoxide radical by its bis(dithiocarbamato) copper(II) shell. Inorg. Chem. 2011;50:4705–4712. doi: 10.1021/ic101770h. [DOI] [PubMed] [Google Scholar]
- Chen Y.S., Hung Y.C., Liau I., Huang G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009;4:858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W.S., Cho M., Jeong J. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Choi H.S., Liu W., Misra P. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E.E., Mwamuka J., Gole A., Murphy C.J., Wyatt M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- de Jong W.H., Hagens W.I., Krystek P., Burger M.C., Sips A.J.A.M., Geertsma R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Goodman C.M., McCusker C.D., Yilmaz T., Rotello V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- Hvolbaek B., Janssens T.V.W., Clausen B.S., Falsig H., Christensen C.H., Norskov J.K. Catalytic activity of Au nanoparticles. Nano Today. 2007;2:14–18. [Google Scholar]
- Khan J.A., Pillai B., Das T.K., Singh Y., Maiti S. Molecular effects of uptake of gold nanoparticles in HeLa cells. ChemBioChem. 2007;8:1237–1240. doi: 10.1002/cbic.200700165. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves C., Gonzalez-Romero D., Barria M.A. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010;393:649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Leonavičienė L., Kirdaitė G., Bradūnaitė R. Effect of gold nanoparticles in the treatment of established collagen arthritis in rats. Medicina (Kaunas). 2012;48:91–101. [PubMed] [Google Scholar]
- Liu Y., Meyer-Zaika W., Franzka S., Schmid G., Tsoli M., Kuhn H. Gold-cluster degradation by the transition of B-DNA into A-DNA and the formation of nanowires. Angew. Chem. Int. Ed. 2003;42:2853–2857. doi: 10.1002/anie.200250235. [DOI] [PubMed] [Google Scholar]
- Ma J.S., Kim W.J., Kim J.J. Gold nanoparticles attenuate LPS-induced NO production through the inhibition of NF-kappaB and IFN-beta/STAT1 pathways in RAW264.7 cells. Nitric Oxide. 2010;23:214–219. doi: 10.1016/j.niox.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Martín R., Menchón C., Apostolova N., Victor V.M., Alvaro M., Herance J.R., García H. Nano-jewels in biology. Gold and platinum on diamond nanoparticles as antioxidant systems against cellular oxidative stress. ACS Nano. 2010;4:6957–6965. doi: 10.1021/nn1019412. [DOI] [PubMed] [Google Scholar]
- Nelson, B.C., Petersen, E.J., Marquis, B.J., et al., 2011. NIST gold nanoparticle reference materials do not induce oxidative DNA damage. Nanotoxicology. [Epub ahead of print]. [DOI] [PubMed]
- Owen O.G. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Pan Y., Neuss S., Leifert A. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- Semmler-Behnke M., Kreyling W.G., Lipka J. Biodistribution of 1.4- and 18-nm gold particles in rats. Small. 2008;4:2108–2111. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- Shukla R., Bansal V., Chaudhary M., Basu A., Bhonde R.R., Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir. 2005;21:10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- Tedesco S., Doyle H., Blasco J., Redmond G., Sheehan D. Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat. Toxicol. 2010;100:178–186. doi: 10.1016/j.aquatox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Tsoli M., Kuhn H., Brandau W., Esche H., Schmid G. Cellular uptake and toxicity of Au55 clusters. Small. 2005;1:841–844. doi: 10.1002/smll.200500104. [DOI] [PubMed] [Google Scholar]
- Utley H.G., Bernheim F., Hockstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967;118:29–32. [Google Scholar]
- Von Maltzahn G., Park J.H., Agrawal A. Computationally guided photothermal tumor therapy using longcirculating gold nanorod antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. Quest for better antioxidants: a commentary on “enhanced radical-scavenging activity by antioxidant-functionalized gold nanoparticles: a novel inspiration for development of new artificial antioxidant”. Free Radic. Biol. Med. 2007;43:1229–1230. doi: 10.1016/j.freeradbiomed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Zhang X.D., Wu H.Y., Wu D. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Berg A., Levanon H., Fessenden R.W., Meisel D. On the interactions of free radicals with gold nanoparticles. J. Am. Chem. Soc. 2003;125:7959–7963. doi: 10.1021/ja034830z. [DOI] [PubMed] [Google Scholar]
- Zidki T., Cohen H., Meyerstein D. Reactions of alkyl-radicals with gold and silver nanoparticles in aqueous solutions. Phys. Chem. Chem. Phys. 2000;8:3552–3556. doi: 10.1039/b604140j. [DOI] [PubMed] [Google Scholar]



