Abstract
Investigation was carried out to find whether enhanced ultraviolet radiation influences the Malva parviflora L., Plantago major L., Rumex vesicarius L. and Sismbrium erysimoids Desf. of some annual desert plants. The seeds were grown in plastic pots equally filled with a pre-sieved normal sandy soil for 1 month. The planted pots from each species were randomly divided into equal groups (three groups). Plants of the first group exposed to white-light tubes (400–700 nm) 60 w and UV (365 nm) 8 w tubes. The second group was exposed to white-light tubes (400–700 nm) 60 w and UV (302 nm) 8 w tubes. The third group was exposed to white-light tubes (400–700 nm) 60 w and UV (254 nm) 8 w tubes, respectively, for six days. The results indicated that the chlorophyll contents were affected by enhanced UV radiation. The chlorophyll a, b, and total contents were decreased compared with the control values and reduced with the enhanced UV radiation, but the carotenoid was increased compared with the control and also reduced with the enhanced UV radiation. So, the contents of chlorophylls varied considerably. M. parviflora showed the highest constitutive levels of accumulated chlorophyll a, b, and total chlorophyll (0.463, 0.307 and 0.774 mg g−1 f w) among the investigated plant species. P. major showed the lowest constitutive levels of the chloroplast pigments, 0.0036, 0.0038 and 0.0075 mg g−1 f w for chlorophyll a, b, and total chlorophyll at UV-365 nm, respectively. The protein content was decreased significantly in both root and shoot systems compared with the control values but, it was increased with increasing wave lengths of UV-radiation of all tested plants. R. vesicarius showed the highest protein contents among the investigated plants; its content was 3.8 mg g−1 f w at UV-365 nm in shoot system. On the other hand, decreasing ultraviolet wave length induced a highly significant increase in the level of proline in both root and shoot of all tested plants. From the results obtained, it is suggested that proline can protect cells against damage induced by ultraviolet radiation. Statistically, the variations of the studied metabolic activities were significant due to UV radiation treatment in shoot and root system of all investigated plant species.
Keywords: UV-radiation, Photosynthetic pigments, Protein, Proline, Malva parviflora, Desert plants
1. Introduction
The fact that man-made chlorofluorocarbons have the potential to cause a depletion of ozone layers, which is responsible for the attenuation of solar UV-B radiation reaching the earth’s surface, has been proposed more than 30 years ago (Molina and Rowland, 1974). The depletion of the ozone layer is closely related to an increase in UV-B radiation on the earth’s surface (Kerr and McElroy, 1993). Solar radiation in the UV-B range (280–320 nm) corresponds to a minor percentage of the total solar energy but it is potentially harmful because these short wave lengths are capable of causing deleterious effect in cells. Plants are vulnerable to increased UV-B radiation because many cellular components, such as nucleic acids, proteins, lipids and quinones can absorb UV-B radiation directly (Jordan, 1996).
Due to damage of the stratospheric ozone layer the level of ultraviolet radiation reaching the biosphere, especially in the range of UV-B (280–320 nm), is increasing. Strong absorption of UV-B photons by biologically important macromolecules i.e. proteins and nucleic acids has a large effect on plant and animals metabolisms (Caldwell et al., 2003 Heisler et al., 2003).
The effects of UV-B on plants include inhibited growth, morphological changes and increase in the level of phenolic pigments (Sharma et al., 1998; Mackerness and Thomas, 1999; Hollosy, 2002; Brzezinska et al., 2006). Inhibition of photosynthesis belongs to the key factors responsible for physiological disorders and a decrease in the biomass of crop plants (Vass, 1997; Agrawal et al., 2004; Ines et al., 2007). The deleterious effect of UV-B on the efficiency of this process can be attributed to specific reductions in expression of important photosynthetic genes (Mackerness et al., 1997), a reduction in Rubisco activity (Vu et al., 1984), changes in ion permeability of thylakoid membranes (Doughty and Hope, 1973), and in the level of chlorophyll and carotenoids (Sharma et al., 1998; Gaberscik et al., 2002).
Simultaneously, many anatomical and morphological changes were observed, such as the reduction of plant height and leaf length/area (Deckmyn and Impens, 1998; Pukacki, 2000), leaf bronze, glazing, chlorosis and necrotic spots (Kakani et al., 2003). Thus, the effect of increased UV-B radiation on growth and physiology of many plants, including crop and terrestrial plant species, under both greenhouse and field conditions, has become one of the most important subjects of investigation in the last decades. Studies about the effect of natural, actual day UV-B radiation on five tropical species by excluding the UV-B radiation in natural sunlight provided evidence that tropical vegetation respects to actual level of natural solar UV-B radiation (Searles et al., 1995; Liu et al., 2005).
Reduction in biomass accumulation due to UV-B exposure was found in several trees (Searles et al., 1995; Liu et al., 2005) and crop species (Kakani et al., 2003). Negative impact of enhanced UV-B radiation on cotton growth included reduction in height, leaf area, total biomass and fiber quality (Gao et al., 2003). Growth reduction is mediated through leaf expansion (Pinto et al., 1999), which is a consequence of the UV-B radiation effects on the rate and duration of both cell division and elongation (Hopkins et al., 2002). Increased UV-B radiation exposure reduced the photosynthetic rate of many species and in general, the reduction was more pronounced under growth chamber or greenhouse conditions than under field conditions (Kakani et al., 2003). Reduction in photosynthesis can be a consequence of damage to various molecular mechanisms of the photosynthetic machinery (Jansen et al., 1998). However, photosynthesis can also be depleted by stomatal density and opening as well as by reduction in stomatal conductance or by reduction in the chlorophyll content (Dai et al., 1995; Feng et al., 2003).
There have been well-documented effects of UV-B radiation and crop plants: barley (Schmitz and Weissenbock, 2003), wheat (Demir, 2000; Zheng et al., 2003; Zu et al., 2004), oats (Zuk-Golaszewska et al., 2003), maize (Barsig and maiz, 2000), soybean (Yuan et al., 2002) and cotton (Gao et al., 2003). These studies were conducted under field conductions, in greenhouses and closed chambers, with varying sources of light radiation.
UV-B radiations have an indirect damaging effect on plants. It is found that both chlorophyll a and b contents of leaves dropped in Phaseolus vulgaris leaves grown under UV-B stress (Michaela et al., 2000). Under UV-B exclusion, chlorophyll content of Fagus sylvestris leaves was higher, the chlorophyll a/b ratio and carotenoids content were lower than leaves under the ambient level of UV-B radiation (Laposi et al., 2002). The proline accumulated in the seedling shoots of rice (Oryza sativa), mustard (Brassica juncea) and mung bean (Vigna radiate) exposed to UV-B radiation was studied by Saradhi et al. (1995). They concluded that the level of proline in the seedling increased significantly with increase in UV-B exposure time. In addition, it has been suggested that exposure to ultraviolet radiation reduces plant growth vigor, chlorophyll contents, carotenoids, amino acids, proteins, total sugars and starch (Musil, 1996). UV radiation induced the accumulation of flavonoids, proline, copherol and ascorbate contents (Carlettia et al., 2003). Metabolic responses of soybean (Gylcine max) plant to increasing UV (A + B) radiation (Amal et al., 2006). Physiological and biochemical studies on the effects of UV (A + B) radiation and heavy metals on pea (Pisum sativum) plant (Amal, 2007).
The objective of this study was to investigate the effect of elevated ultraviolet radiation on photosynthesis and some metabolic activities (chlorophyll a, b, carotenoids, protein and proline contents). Four annual plant species were used in this study (Malva parviflora L., Plantago major L., Rumex vesicarius L. and Sismbrium erysimoides Desf.). It was also aimed to determine the extent of sensitivity of selected species to elevated ultraviolet radiation.
2. Material and methods
2.1. Plant material and plantation
The experiments were conducted using the seedling of M. parviflora L., P. major L., R. vesicarius L. and Sisymbrium erysimoides Desf. Seeds were sterilized with 2.5% sodium hypochlorite for 15 min and washed with distilled water. Seeds were sown in plastic pots (30 cm in height and 25 cm in diameter), equally filled with normal sand from field. All pots were watered regularly every two days with constant amounts until UV-treatment.
2.2. Irradiation system and growth conditions
After 15 days from seed soaking, the planted pots (each pot contains one plant) from each species were randomly divided into equal groups (three groups). Plants of the first group exposed to white-light tubes (400–700 nm), 60 w and UV (365 nm) 8 w tubes were used at 65 cm high from the base of a wooden table. The second group was exposed to white tubes and UV-B (302 nm) 8 w tubes. The third group was exposed to white tubes (400–700 nm) 60 w and UV (254 nm) 8 w tubes for 6 h in 6 days. After 6 days of continuous irradiation the plants including roots were taken out.
2.3. Chlorophyll determination
Pigments were extracted according to Hiscox and Israelstam (1979). Chlorophyll a, b, total and carotenoids were estimated in fresh plants leaf tissues. One gram of fresh leaf tissues were homogenized in 20 ml methanol–chloroform–water (MCW) in the proportions 12:5:3. The concentration of pigments was determined as mg g−1 using spectrophotometer.
2.4. Total protein measurements
Total protein content was extracted with trichloroacetic acid and NaOH. Total protein contents were estimated by using spectrophotometrical analysis (Lowry et al., 1951).
2.5. Estimation of proline
Proline contents in fresh shoot and root systems were extracted with sulphosalycilic acid. The liquids were used for estimating proline contents with the spectrophotometrical analysis (Bates et al., 1973).
2.6. Statistical analysis
Experimental data were subjected to a one-way analysis of variance (ANOVA) and significant differences between means were determined through the use of multiple range test.
3. Results
3.1. Chlorophyll changes
Malva parviflora showed the highest constitutive levels of accumulated chlorophyll a, b, and total chlorophyll among the investigated plant species. The highest values were 0.463, 0.307 and 0.774 mg g−1 f w for chlorophyll a, b, and total chlorophyll at UV-254 nm, respectively (Table 1, Fig. 1). P. major showed the lowest constitutive levels of the chloroplast pigments, 0.0036, 0.0038 and 0.0074 mg g−1 f w for chlorophyll a, b, and total chlorophyll at UV-365 nm, respectively (Table 1, Fig. 1). The contents of chlorophyll a, b, and total chlorophyll were decreased comparing with the control values and with increasing of UV radiation levels. Elevated UV radiation level in all investigated plant species stimulated chlorophyll synthesis, to a limited decrease at UV-254 nm radiation and the level of pigments was about 6-fold lower than in the control level. Similarity to chlorophyll contents, S. erysimoides showed the highest constitutive level of the carotenoids content, 0.443 mg g−1 f w at UV-254 nm, while the lowest level 0.041 mg g−1 f w at UV-365 nm recorded in R. vesicarius (Table 1, Fig. 1). Carotenoids content showed an increase of this pigment compared with the control level of all investigated plant species except P. major.
Table 1.
Effect of UV radiation on chlorophylls and carotenoid of four studied plant species (mg g−1 f w).
| Plant species | UVR | Chloro. a | Chloro. b | Chloro. T | Carotenoid |
|---|---|---|---|---|---|
| Malva parviflora | Control | 2.238 ± 0.00057 | 0.539 ± 0.311 | 2.954 ± 0.004 | 0.217 ± 0.0062 |
| UV-254 | 0.463 ± 0.0005 (1.775)∗ | 0.307 ± 0.0005 (0.232) | 0.774 ± 0.0045 (2.18)∗ | 0.415 ± 0.0051 (0.198)∗ | |
| UV-302 | 0.208 ± 0.00057 (2.029)∗ | 0.141 ± 0.001 (0.398)∗ | 0.352 ± 0.0028 (2.603)∗ | 0.172 ± 0.0025 (0.044)∗ | |
| UV-365 | 0.238 ± 0.0005 (1.99)∗ | 0.153 ± 0.0005 (0.386)∗ | 0.392 ± 0.0025 (2.562)∗ | 0.192 ± 0.0025 (0.024)∗ | |
| Plantago major | Control | 0.652 ± 0.0025 | 0.325 ± 0.005 | 0.976 ± 0.0052 | 0.133 ± 0.231 |
| UV-254 | 0.274 ± 0.0045 (0.378)∗ | 0.149 ± 0.001 (0.175)∗ | 0.142 ± 0.247 (0.833)∗ | 0.069 ± 0.120 (0.064)∗ | |
| UV-302 | 0.212 ± 0.0025 (0.440)∗ | 0.121 ± 0.001 (0.204)∗ | 0.1108 ± 0.191 (0.865)∗ | 0.059 ± 0.102 (0.074)∗ | |
| UV- 365 | 0.0036 ± 0.00015 (0.649)∗ | 0.0038 ± 0.0001 (0.321)∗ | 0.0074 ± 0.0001 (0.968)∗ | 0.112 ± 0.194 (0.021)∗ | |
| Rumex vesicarius | Control | 0.210 ± 0.0005 | 0.034 ± 0.0005 | 0.246 ± 0.0005 | 0.105 ± 0.0005 |
| UV-254 | 0.072 ± 0.00005 (0.138)∗ | 0.055 ± 0.0002 (0.0204)∗ | 0.126 ± 0.00011 (0.119)∗ | 0.158 ± 0.0103 (0.0523)∗ | |
| UV-302 | 0.071 ± 0.00005 (0.138)∗ | 0.053 ± 0.00005 (0.0185)∗ | 0.124 ± 0.00004 (0.121)∗ | 0.128 ± 0.0011 (0.0229)∗ | |
| UV-365 | 0.104 ± 0.0005 (0.106)∗ | 0.056 ± 0.0005 (0.022)∗ | 0.162 ± 0.0001 (0.083)∗ | 0.041 ± 0.0002 (0.064)∗ | |
| Sisymbrium erysimoides | Control | 0.753 ± 0.0005 | 0.263 ± 0.0005 | 1.018 ± 0.0023 | 0.187 ± 0.0026 |
| UV-254 | 0.284 ± 0.0046 (0.468)∗ | 0.079 ± 0.001 (0.184)∗ | 0.294 ± 0.005 (0.724)∗ | 0.443 ± 0.0015 (0.256)∗ | |
| UV-302 | 0.410 ± 0.0005 (0.342)∗ | 0.206 ± 0.0032 (0.057)∗ | 0.617 ± 0.0023 (0.401)∗ | 0.275 ± 0.058 (0.088)∗ | |
| UV-365 | 0.208 ± 0.0017 (0.545)∗ | 0.079 ± 0.001 (0.184)∗ | 0.288 ± 0.0104 (0.730) | 0.233 ± 0.0005 (0.046)∗ | |
1 - 2.238 ± 0.00057, The mean values of three replications in duplicated ± SD.
2 - (0.545)∗, The significantly different at P < 0.05.
Figure 1.
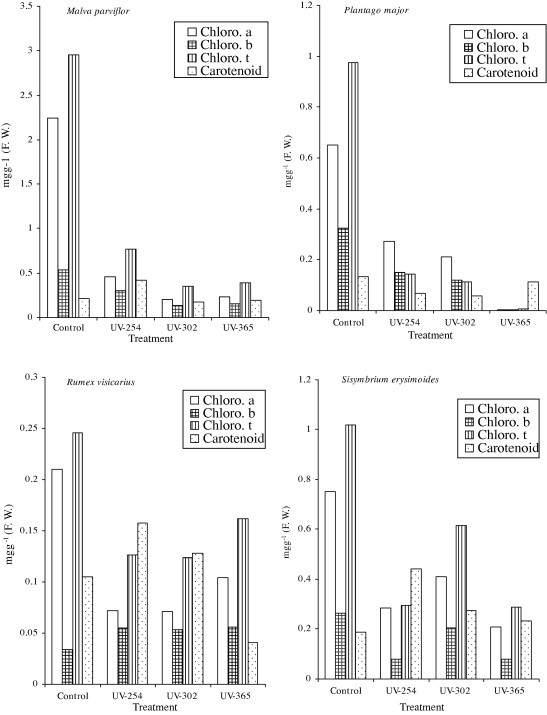
Effect of different doses of UV (254, 302 and 365 nm) radiation on chlorophyll of four studied plant species (mg g−1 f w).
3.2. Total protein contents
R. vesicarius showed the highest constitutive levels of accumulated protein contents among the investigated plant species, its content was 3.8 mg g−1 d w at UV-365 nm in shoot system (Table 2, Fig. 2). While, the highest constitutive level was recorded in root system in M. parviflora 2.08 mg g−1 d w at UV-365 nm (Table 2, Fig. 2), the lowest level of protein contents was 0.095 mg g−1 d w at UV-254 nm in shoot system of M. parviflora species. The root system of P. major species characterized by the lowest level of protein content was 0.144 mg g−1 d w at UV-254 nm (Table 2, Fig. 2). Generally, the protein contents were decreased compared with the control level and also increased with increasing of level UV of radiation.
Table 2.
Effect of UV radiation on protein and proline of shoot and root systems of four studied plant species (mg g−1 f & d w).
| Plant species | UVR | Shoot |
Root |
||
|---|---|---|---|---|---|
| Protein | Proline | Protein | Proline | ||
| Malva parviflora | Control | 2.34 ± 0.01 | 0.68 ± 0.0.01 | 2.074 ± 0.065 | 0.36 ± 0.01 |
| UV-254 | 0.095 ± 0.005 (2.245)∗ | 1.93 ± 0.06 (1.24)∗ | 1.35 ± 0.05 (0.724)∗ | 1.93 ± 0.06 (0.076)∗ | |
| UV-302 | 0.754 ± 0.0032 (1.586)∗ | 2.88 ± 0.03 (2.19)∗ | 1.816 ± 0.160 (0.258)∗ | 1.853 ± 0.015 (1.49)∗ | |
| UV-365 | 1.21 ± 0.01 (1.13)∗ | 1.65 ± 0.05 (0.966)∗ | 2.08 ± 0.104 (0.0086) | 0.38 ± 0.017 (1.47)∗ | |
| Plantago major | Control | 1.073 ± 0.064 | 0.64 ± 0.01 | 0.95 ± 0.05 | 0.643 ± 0.0057 |
| UV-254 | 0.31 ± 0.01 (0.763)∗ | 3.11 ± 0.01 (2.47)∗ | 0.144 ± 0.0052 (0.806)∗ | 2.953 ± 0.0503 (2.31)∗ | |
| UV-302 | 0.416 ± 0.015 (0.656)∗ | 2.89 ± 0.01 (2.24)∗ | 0.213 ± 0.015 (0.73)∗ | 1.66 ± 0.0529 (1.016) | |
| UV-365 | 0.92 ± 0.02 (0.153)∗ | 1.69 ± 0.01 (1.043)∗ | 0.35 ± 0.05 (0.6)∗ | 1.753 ± 0.0503 (1.11)∗ | |
| Rumex vesicarius | Control | 8.903 ± 0.1 | 0.65 ± 0.001 | 2.193 ± 0.268 | 0.646 ± 0.0057 |
| UV-254 | 1.714 ± 0.257 (7.189)∗ | 2.33 ± 0.001 (1.68)∗ | 0.17 ± 0.026 (2.023)∗ | 2.206 ± 0.179 (1.56)∗ | |
| UV-302 | 2.258 ± 0.035 (0.644)∗ | 2.31 ± 0.01 (1.66)∗ | 1.32 ± 0.026 (0.87)∗ | 1.86 ± 0.052 (1.21)∗ | |
| UV-365 | 3.80 ± 0.167 (5.096)∗ | 2.09 ± 0.01 (1.438)∗ | 1.93 ± 0.055 (0.256)∗ | 0.716 ± 0.0152 (0.07)∗ | |
| Sisymbrium erysimoides | Control | 0.816 ± 0.160 | 0.45 ± 0.05 | 3.08 ± 0.075 | 0.37 ± 0.0264 |
| UV-254 | 0.22 ± 0.026 (0.596)∗ | 5.19 ± 0.17 (4.74)∗ | 1.118 ± 0.027 (1.962)∗ | 1.94 ± 0.052 (1.57)∗ | |
| UV-302 | 0.356 ± 0.051 (0.46)∗ | 3.08 ± 0.07 (2.63)∗ | 1.88 ± 0.105 (1.2)∗ | 1.88 ± 0.1058 (1.51)∗ | |
| UV-365 | 0.588 ± 0.102 (0.228)∗ | 2.31 ± 0.01 (1.863)∗ | 2.89 ± 0.101 (0.19)∗ | 1.863 ± 0.0321 (1.49)∗ | |
1 - 2.34 ± 0.01, The mean values of three replications in duplicates ± SD.
2 - (1.438)∗, The significantly different at P < 0.05.
Figure 2.
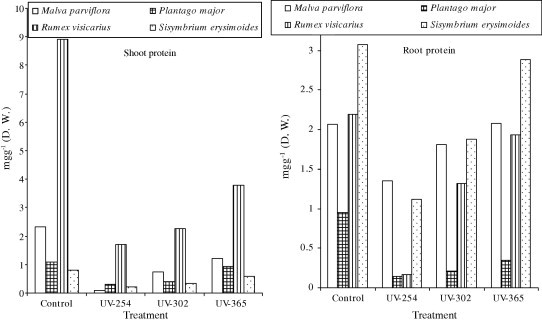
Effect of different doses of UV (254, 302 and 365 nm) radiation on shoot and root systems of protein contents of four studied plant species (mg g−1 d w).
3.3. Total proline content
S. erysimoides showed the highest constitutive levels of accumulated proline contents among the studied plant species in the shoot system (Table 2, Fig. 3). The highest proline content was 5.19 mg g−1 f w at UV-254 nm in shoot system, while, the highest proline content in root system was 2.953 mg g−1 f w at UV-254 nm was recorded in P. major (Table 2, Fig. 3). In M. parviflora, shoot and root systems were characterized by the lowest constitutive levels of proline contents were 1.65 mg g−1 f w (in shoot) and 0.38 mg g−1 f w (in root) at UV-365 nm (Table 2, Fig. 3). The proline contents in shoot and root systems were increased compared with the control values in all plant species under investigation (Table 2, Fig. 3). Also, proline contents in all studied plant species were decreased with increasing of the level of UV-radiation.
Figure 3.
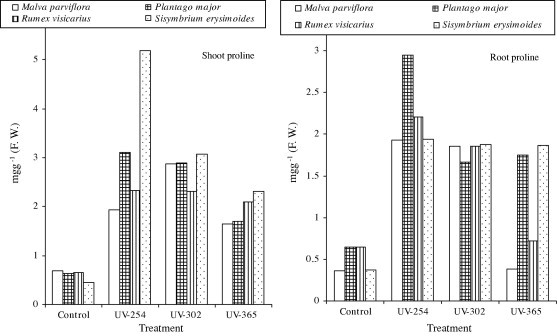
Effect of different doses of UV (254, 302 and 365 nm) radiation on shoot and root systems of proline contents of four studied plant species (mg g−1 f w).
4. Discussion
Over the last decades numerous studies have been published on the effect of elevated UV-B radiation on terrestrial plants. In a vast major majority, these studies concern cultivated plants, while only a few experiments have involved trees and several conifer species (Kakani et al., 2003; Zheng et al., 2003; Zu et al., 2004). Adverse environmental factors, including UV-B radiation, may affect metabolic and pathological changes in plants.
Changes in contents of chloroplast pigments are evidences of high UV radiation tolerance of analyzed plants. Photosynthetic pigments mainly constitute of chlorophyll a, b, total chlorophyll and carotenoids are of vital importance in photosynthesis changes. The contents of chloroplast pigments are evidences of high UV radiation tolerance of four analyzed plant species, M. parviflora, P. major, R. vesicarius and S. erysimoides (Table 1). Pigments of photosynthetic apparatus can be destroyed by UV radiation, with comparative loss of photosynthetic capacity (Jordan et al., 1994). Chlorophylls and carotenoids effected by different elevated UV radiation, where carotenoids are generally being less effected than chlorophylls (Pfundel et al., 1992). Also, chlorophyll b in R. vesicarius is less effected by elevated UV radiation compared with the other studied plant species. It has been reported that UV (254, 302 and 365 nm) radiation resulted in reduction in the amount of chlorophyll a as opposed to chlorophyll b and might point as more selective destruction of chlorophyll a biosynthesis or degradation of precursors (Marwood and Greenberg, 1996). The UV radiation stimulated the biosynthesis of UV- absorbing compounds and carotenoids, both of which perform a photoprotective function. The carotenoids are implicated in the direct protection of the photosystems against UV radiation (Middleton and Teramura, 1993). On the comparison with the control, carotenoid contents were reducted only in P. major, while were increased than the control in the other studied plant species (Table 1, Fig. 1). Statistically, the variation in all chlorophylls and carotenoids were significant due to elevated UV radiation except chlorophyll b in M. parviflora at UV-254 nm and total chlorophyll in S. erysimoids at UV-365 nm were non- significant (Table 1).
Under various stress condition, plants may reduce specific change in protein synthesis that enable them to cope with such stress (Santos et al., 1998). Different UV radiation reduces protein, nucleic acids and other macro molecules, which causes conformational changes in their structure (Bassman, 2004). The total protein content was decreased compared with the control level in shoot and root system of all studied plant species, but the decrease in protein was higher in shoot than root system for all investigated plant species (Table 2, Fig. 2). The protein content was increased with increasing of elevated UV radiation for all studied plant species. Statistically, variations in protein contents in shoot and root system of all studied plant species were significant due to elevated UV radiation treatment except protein content in root system of M. parviflora was non-significant at UV-365 nm (Table 2). The decrease in total protein contents of all plant species might be a concomitant of the retarded growth rate of the treated plants as a result of reduced photosynthetic performance, which would lead to a predicted decrease in the nitrogen pool. In addition, UV radiation damages lipids, nucleic acids and proteins in leaves of higher plants (Jordan, 1996; Vass, 1997).
The data of the present study showed that the different levels of UV radiation induced increases in shoot and root proline contents in all tested plant species (Table 2, Fig. 3). The present binding is in agreement with the results of Demir (2000) and Amal et al. (2006). They concluded that ultraviolet light caused an increase in the proline content of four cultivars of wheat. Similarly, seedling of rice and Mung bean has been reported to accumulate proline in the shoots when exposed to ultraviolet radiation. Alia et al., 1997 reported that UV radiation induced proline accumulation protects plants against UV promoted peroxidative process. Statistically, the increases in proline contents were significant due to UV radiation treatment in shoot and root of all studied plant species, except in root system of P. major was non-significant at UV-302 nm (Table 2). The significant increase in proline contents was an important factor for providing higher tolerance to UV radiation treated plant species. In addition increasing proline content is referred to as protective mechanism due to the generation of reactive oxygen species by UV radiation. There are three possible causes of the free proline accumulation under stress: first, stimulation of proline synthesis from glutamic acids, which has been found to be dependent on the abscisic acid concentration; second, inhibition of proline oxidation to other soluble compounds; and, third, inhibition of protein synthesis. From the results obtained, it is suggested that proline can protect cells against damage induced by ultraviolet radiation.
References
- Agrawal S.B., Dheera J.R., Anoop S. Effects of supplemental ultraviolet-B and mineral nutrients on growth, biomass allocation and yield of wheat (Triticum aestivum L.) Trop. Ecol. 2004;45(2):315–325. [Google Scholar]
- Alia P., Saradhi P., Prassana M., Mohanty P. Involvement of proline in protecting thylakoid membranes against free radical induced photodamage. J. Photochem. Photobiol-B, Biol. 1997;38:253–257. [Google Scholar]
- Amal A. Physiological and biochemical studies on the effects of UV A + B radiation and heavy metals on pea (Pisum sativum) plant. Assiut. Univ. J. Bot. 2007;36(1):1–21. [Google Scholar]
- Amal A., Dina Z., Abd Elghafar M. Metabolic responses of soybean (Glycine max) plant to in increasing UV (A + B) radiation. Assiut. Univ. J. Bot. 2006;35(2):107–125. [Google Scholar]
- Barsig M., Maiz R. Fine structure, carbohydrates and photosynthetic pigments of sugar maize leaves under UV-B radiation. Environ. Exp. Bot. 2000;43:121–134. [Google Scholar]
- Bassman J.H. Ecosystem consequences of enhanced solar ultraviolet radiation: secondary plant metabolites as mediators of multiple trophic interactions in terrestrial plant communities. J. Photochem. Photobiol. 2004;79(5):382–398. doi: 10.1562/si-03-24.1. [DOI] [PubMed] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–208. [Google Scholar]
- Brzezinska E., Kozlowska M., Stachowiak J. Response of three conifer species to enhanced UV-B radiation; consequences for photosynthesis. Polish. J. Environ. Stud. 2006;15(4):531–536. [Google Scholar]
- Caldwell M.M., Ballare C.L., Bornman J.F., Flint S.D., Djorn L.O., Teramura A.H., Kulandaivelu G., Tevini M. Terrestrial ecosystems, increased solar ultraviolet radiation and interaction with other climate change factors. Photochem. Photobiol. Sci. 2003;2:29–38. doi: 10.1039/b211159b. [DOI] [PubMed] [Google Scholar]
- Carlettia P., Masia A., Wonischb A., Grillb D., Tauszb M., Ferretti M. Changes in antioxidant and pigment pool dimensions in UV-B irradiated maize seedlings. Environ. Exp. Bot. 2003;50(2):149–157. [Google Scholar]
- Dai, T. A., Arnon, D. I. and Day, Q. 1995. Effects of UV-B radiation on stomatal density and opening in rice (Oryza sativa L.). Annals of botany 76, 65–70.
- Deckmyn G., Impens I. Effects of solar UV-B irradiation on vegetative and generative growth of Bromus catharticus. Environ. Ex. Bot. 1998;40:179–191. [Google Scholar]
- Demir Y. Growth and proline content of germinating wheat genotypes under ultraviolet light. Turk. J. Bot. 2000;24:67–70. [Google Scholar]
- Doughty J.C., Hope A.B. Effects of ultraviolet radiation on the membranes of Chara coralline. J. Membr. Biol. 1973;13:185–197. [Google Scholar]
- Feng H., Allen D.J., Gitz D.C. The effect of enhanced ultraviolet-B radiation on growth, photosynthesis and stable carbon isotope composition (d13C) of two soybean cultivars (Glycine max) under field conditions. Environ. Exp. Bot. 2003;49:1–8. [Google Scholar]
- Gaberscik A., Voncina M., Trost T., Germ M., Bjorn L.O. Growth and production of buckwheat (Fagopyrum esculentum) treated with reduced, ambient and enhanced UV-B radiation. J. Photobiol. B: Biol. 2002;66:30–42. doi: 10.1016/s1011-1344(01)00272-x. [DOI] [PubMed] [Google Scholar]
- Gao W., Zheng Y., Slusser J.R., Heisler G.M. Impact of enhanced ultraviolet-B irradiance on cotton growth, development, yield, and qualities under field conditions. Agric. For. Meteorol. 2003;120:241–255. [Google Scholar]
- Heisler G.M., Grant R.H., Gao W., Slusser J.R. Ultraviolet radiation and its impacts on agriculture and forests. Agric. For. Meteorol. 2003;120(3):120–133. [Google Scholar]
- Hiscox J.D., Israelstam G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57:1332–1345. [Google Scholar]
- Hollosy F. Effects of ultraviolet radiation on plant cells. Micron. 2002;33:179–192. doi: 10.1016/s0968-4328(01)00011-7. [DOI] [PubMed] [Google Scholar]
- Hopkins L., Hewitt E.J., Mark U. Ultraviolet-B radiation reduces the rates of cell division and elongation in the primary leaf wheat (Triticum aestivum L. CV Maris Huntsman) Plant, Cell Environ. 2002;25:617–624. [Google Scholar]
- Ines C., Terezinha F.F., Anne L.D. Growth and physiological responses of sunflower plants exposed to ultraviolet-B radiation. Sci. Rural. 2007;37(1):85–90. [Google Scholar]
- Jansen M.A.K., Van Den R., Noort E. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- Jordan B.R. The effects of ultraviolet-B radiation on plants: a molecular perspective. Adv. Bot. Res. 1996;22:97–162. [Google Scholar]
- Jordan B.R., James P.E., Strid A., Anthony R.G. The effect of ultraviolet-b radiation on gene expression and pigment composition in etiolated and green pea leaf tissue UV-B induced changes are gene-specific and dependent upon the developmental stage. Plant, Cell Environ. 1994;17:45–54. [Google Scholar]
- Kakani V.G., Reddy K.R., Zhao D., Sailaja K. Field crop responses to ultraviolet-B radiation: a review. Agric. For. Meteorol. 2003;120:191–218. [Google Scholar]
- Kerr J.B., McElroy C.T. Evidence for large upward trend of ultraviolet-B radiation linked to ozone depletion. Science. 1993;262:1032–1034. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- Laposi R., Veres S.Z., Mile O., Meszaros I. Photosynthesis–ecophysiological properties of beech (fagus sylvestris L) under the exclusion of ambient UV-B radiation. Proceeding of 7th Hungarian congess on plant physiology. Acta Biol. Szegediensis. 2002;46:243–245. [Google Scholar]
- Liu L.X., Oha T.Y., Xewn N.O. Solar UV-B radiation on growth, photosynthesis and the xanthophyll cycle in tropical acacias and eucalyptus. Environ. Exp. Bot. 2005;54:121–130. [Google Scholar]
- Lowry O., Rosebrough N., Farr A., Randall R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:263–275. [PubMed] [Google Scholar]
- Mackerness S.A., Jordan B.R., Thomas B. UV-B effects on the expression of genes encoding proteins involved in photosynthesis. In: Lumsden P.J., editor. Plants and UV-B Responses to Environmental Changes. Cambridge University; 1997. p. 113. [Google Scholar]
- Mackerness S.A., Thomas B. Effects of UV-B radiation on plants: gene expression and signal transduction pathways. In: Smallwood M.F., Calvert C.M., Bowles D.J., editors. Plant Responses to Environmental Stress. Bios Scientific Publishers; Oxford: 1999. pp. 17–24. [Google Scholar]
- Marwood C.A., Greenberg B.M. Effect of supplementary UV-B radiation on chlorophyll systems during chloroplast development in Sspirodela oligarrhiza. J. Photochem. Photobiol. 1996;64:664–670. [Google Scholar]
- Michaela, A., Norbert, K., and George, N. 2000. Effect of cold and UV-B stress on scavenging systems of phaseolus vulgarius leaves poster. American society of plant biologist found online at http://www.uni-bonnide/obstbau.
- Middleton E.H., Teramura A.H. The role of flavonol glycosides and carotenoids in protecting soy bean from ultraviolet-b damge. Plant Physiol. 1993;103:714–724. doi: 10.1104/pp.103.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M.J., Rowland F.S. Stratospheric sink for chlorofluoromethanes – chlorine atomic-catalysed destruction of ozone. Nature. 1974;249:810–812. [Google Scholar]
- Musil C.F. Accumulated effect of elevated ultraviolet-b radiation over multiple generations of the arid-environment annual dimorph the sinuate DC (Asteraceae) Plant Cell Environ. 1996;19(9):1017–1027. [Google Scholar]
- Pfundel E.E., Ppan R.S., Dilley R.A. Inhibition of violaxanthin deep oxidation by ultraviolet-b radiation in isolated chloroplasts and intact leaves. J. Plant Physiol. 1992;98:1372–1380. doi: 10.1104/pp.98.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M.E., Mazza C.A., Kraus G.H. Effects of UV-B radiation on growth, photosynthesis, UV-B absorbing compounds and NADP – amlic enzyme in bean (Phaseolus vulgaris L.) grown under different nitrogen conditions. J. Photochem. Photobiol. B. 1999;48:200–209. doi: 10.1016/s1011-1344(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Pukacki P.M. Effects of sulphur, fluoride and heavy metal pollution on the chlorophyll fluorescence of Scots pine (Pinus sylvestris L.) needles. Dendrobiology. 2000;45:83–96. [Google Scholar]
- Santos I., almeida J.M., Salema R. Plants of zea mays L.developed under enhanced UV- b radiation. I. Some ultrastructural and biochemical aspects. J. Plant Physiol. 1998;141:450–456. [Google Scholar]
- Saradhi P.P., Alia Sandeep A., Prasad K., Arora S. Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem. Biophys. Res. Commun. 1995;209(1):1–5. doi: 10.1006/bbrc.1995.1461. [DOI] [PubMed] [Google Scholar]
- Schmitz H.R., Weissenbock G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry. 2003;64:234–256. doi: 10.1016/s0031-9422(03)00203-6. [DOI] [PubMed] [Google Scholar]
- Searles P.S., Thomas M.J., Jane F.T. The response of five tropical dicotyledon species to solar ultraviolet-B radiation. Am. J. Bot. 1995;82:445–453. [Google Scholar]
- Sharma P.K., Amand P., Sankhalkar S., Shetye R. Photochemical and biochemical changes in wheat seedlings exposed to supplementary ultraviolet-B radiation. Plant Sci. 1998;21:132–145. [Google Scholar]
- Vass I. Marcel Dekker, Inc.; New York: 1997. Adverse effects of UV-B light on the structure and function of the photosynthetic apparatus. In: Handbook of photosynthesis pessarakli. pp. 931–949. [Google Scholar]
- Vu C.V., Allen L.H., Gaward L.H. Effects of enhanced UV-B radiation (280–320 nm) on ribulose-1.5-bisphosphate carboxylase in pea and soybean. Environ. Exp. Bot. 1984;24:131–144. [Google Scholar]
- Yuan L., Yanqun Z., Chen J., Chen H. Intra-specific responses in crop growth and yield of 20 Soybean cultivars to enhanced ultraviolet-B radiation under field conditions. Field Crops Res. 2002;78:1–16. [Google Scholar]
- Zheng Y., Gao W., Slusser J.R., Grant R.H., Wang C.H. Yield and yield formation of field winter wheat in response to supplemental solar ultraviolet-B radiation. Agric. For. Meteorol. 2003;120:279–293. [Google Scholar]
- Zu Y., Li Y., Chen J., Chen H. Intra-specific responses in grain quality of 10 wheat cultivars to enhanced UV-B radiation under field conditions. J. Photochem. Photobiol. 2004;74:95–117. doi: 10.1016/j.jphotobiol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Zuk-Golaszewska K., Upadhyaya M.K., Golaszewski J. The effect of UV-B radiation on plant growth and development. Plant Soil Environ. 2003;49(3):135–140. [Google Scholar]


