Abstract
Toxic heavy metals in water, air and soil are global problems that are a growing threat to humanity. Heavy metals are widely distributed in the environment and some of them occur in food, water, air and tissues even in the absence of occupational exposure. The antioxidant and protective influences of vitamin E on a mixture of some heavy metals (Pb, Hg, Cd and Cu)-induced oxidative stress and renal and testicular injuries were evaluated in male mice. Exposure of mice to these heavy metals in drinking water for seven weeks resulted in statistical increases of plasma creatinine, urea and uric acid concentrations. The levels of glutathione (GSH) and superoxide dismutases (SOD) in kidney and testis tissues were significantly declined. Moreover, the histopathological evaluation of kidney and testis showed severe changes in mice treated with these heavy metals. Administration of vitamin E protected the kidney and testis of mice exposed to heavy metals as evidenced by appearance of normal histological structures, insignificant changes in the values of plasma creatinine, urea and uric acid, and the levels of kidney GSH and SOD, while the levels of testis GSH and SOD were notably decreased. These data suggest that the administration of vitamin E protects against heavy metals-induced renal and testicular oxidative stress and injuries.
Keywords: Antioxidant, Vitamin E, Heavy metals, Kidney, Testis, Mice
1. Introduction
Environmental pollution is the contamination of the ecosystem that causes instability, disorder, harm or discomfort to the physical systems or living organisms. Environmental factors have important links with infectious as well as non-infectious diseases of both acute and chronic nature. Global burden of disease attributable to selected sources of environment like water sanitation and hygiene, urban outdoor and indoor pollution, occupational carcinogens, noise and airborne particulates has been assessed to be 8–9%, measured either in terms of mortality or disability adjusted life years (DALYs). DALYs incorporates number of years lived with a disability due to disease or injury, weighted according to its severity (Ezzati et al., 2002). The increase in pollution is a major and global problem. This is due to the use of toxic chemicals or xenobiotic substances or by certain synthetic compounds such as heavy metallic compounds (Foulkes, 1990; Jagadeesan and Pillai, 2007). Of these heavy metallic compounds few reveal potential effects. They reach the environment after their liberation through industries (Migliore et al., 1999). Metallic compounds on land and water pose potential health hazard not only to livestock and wild life but also to fishes, birds, mammals and even to human beings. Heavy metallic compounds have emerged as a major class of industrial waste product (Budavari, 1996). They can be produced synthetically in laboratories from their derivatives. Heavy metals belong to the micropollutants. They are one of the main pollutants of the environment related to the biological activity most of them (Alekin, 1970). The physiological influence of metals on the organisms of human and animals is conditioned by the nature of metal, by the type of compounds and by the amount of them (Danielyan, 2010). Heavy metals are persistent environmental contaminants since they cannot be degraded or destroyed. Heavy metals are chemical elements capable of spreading in the environmental compartments and circulating between them. Indeed, heavy metals emitted to the atmosphere with the composition of fine particles or in the gaseous form are transported by atmospheric fluxes to considerable distances and enter ecosystems of remote regions. Many heavy metals are urgently necessary for functioning of the body of humans and other living organisms in small amounts and belong to the range of nutrients. Others, when passed on to the living organisms cause poisoning or death (Danielyan, 2010). According to the toxicity for humans and animals, the metals are divided into groups, in which, today, besides the heavy metals are included also other metals, which have a toxic influence on living organisms. Of the heavy metals in this list are included cadmium (Cd), copper (Cu), arsenic (As), nickel (Ni), mercury (Hg), lead (Pb), zinc (Zn), chromium (Cr) and from other toxic metals aluminum (Al) and etc. (Sullivan et al., 2002). The sources of pollution with heavy metals in the environment can be natural and anthropogenic. The natural sources include mother rocks and minerals of the metals. The main anthropogenic sources are agriculture, black and colored metallurgy, transport, and mining and related operations (Malaev et al., 2004; Vaněk et al., 2005; Vanderlinden et al., 2006; Conesa et al., 2007). Continuous environmental and occupational heavy metals exposure can lead to chronic nephropathy. However, many experimental studies showed that several heavy metals caused renal failure associated with severe histopatholgical and physiological alterations (Abdel-Moneim and Said, 2007; Kutlubay and Oğuz, 2007; Massanyi et al., 2007; Obianime and Roberts, 2009; Suradkar et al., 2009; Soudani et al., 2010). Due to the rapid industrialization and overgrowing urbanization, the toxic effects of heavy metals on male reproduction system have become a major health concern in the globe (Waldron, 1980; Waldron and Ediing, 1997). The evidence of the past twenty years have shown a disturbing trend in male reproductive health hazards due to careless use of these chemicals which causes detrimental effects on different organs. Therefore, broad-spectrum irreversible toxic actions at cellular and molecular level were observed mainly on the reproductive system of humans and experimental animals (Roy Chowdhury, 1992; Batra et al., 2001; Roy Chowdhury, 2004; Massanyi et al., 2007; Burukoğlu and Bayçu, 2008; Yasmina and Abdennour, 2008; Almansour, 2009; Obianime and Roberts, 2009).
Vitamin E is an important component in human diet and considered the most effective liposolouble antioxidant found in the biological system. It is composed of various subfamilies of which tocopherols and tocotrienols are the most studied. The structural difference between the two subfamilies is that tocotrienols possess three double bonds in their isoprenoid side chain and this structural difference results in differences in their efficacy and potency as antioxidants (Musalmah et al., 2002). Vitamin E is known to have been proven beneficial in some disease processes. It protects the body’s biological systems (Packer, 1991) by preventing lipid peroxidation (Evstigneeva et al., 1998). Tocotrienols are effective in preventing breast cancer growth (Rahmat et al., 1993) and reducing blood cholesterol levels (Qureshi et al., 1995). Vitamin E also affects bone by increasing bone trabecular formation (Xu et al., 1995), preventing bone calcium loss due to an oxidizing agent, ferric nitrilotriacetate (Yee and Ima-Nirwana, 1998) and preventing bone calcium loss in ovariectomised rats (Norazlina et al., 2000). Because of the health problems induced by many environmental pollutants, much effort has been expended in evaluating the relative antioxidant potency of vitamin E (Beytut et al., 2003; Pillai and Gupta, 2005; Jalili et al., 2007; El-Gharieb et al., 2010). Till today, there is no information regarding the effect of vitamin E on the renal and testicular toxicities induced by a mixture of heavy metals (Pb, Hg, Cd and Cu) exposure in mice or other mammals. Furthermore, precise action of vitamin E is not fully elucidated and the interaction between vitamin E and renal and testicular cells still requires further study. Therefore, the present study is designed to investigate the possibility that the administration of vitamin E would have a beneficial effect on these heavy metals-induced renal and testicular injuries.
2. Materials and methods
2.1. Animals
Sexually mature MFI male albino mice weighing 37.6–39.3 g were utilized in the present study. Mice were purchased from the Experimental Animal Unit of King Fahd Medical Research Center, King Abdul Aziz University, Jeddah, Saudi Arabia. The experimental animals were acclimatized to the laboratory conditions for one week before the start of experiments and caged in a quite temperature controlled room (24 ± 1 °C). Mice had free access to water and standard diet. The experiments were conducted in accordance with ethical guidelines of the Animal Care and Use Committee of King Abdul Aziz University.
2.2. Experimentation
The experimental mice were divided into four groups (n = 10). Mice of the first group served as controls and received normal drinking water without any heavy metals. Mice of the second group received a mixture of heavy metals (Pb, Hg, Cd and Cu) for 7 weeks in their drinking water as follows: 30 ppm Pb, 10 ppm Hg, 30 ppm Cd and 30 ppm Cu. Animals of the third group were exposed to the same drinking solution given to the second group and intraperitoneally injected with vitamin E at a dose of 50 IU/kg body weight, BW, five times weekly for 7 weeks. Mice of the fourth group were received normal drinking water and treated with vitamin E at the same doses given to the third group. After 7 weeks, blood samples were taken from orbital venous plexus under total anesthesia with diethyl ether. These blood samples were collected in the lithium heparin coated tubes. Plasma specimens were obtained and used for determination of creatinine, urea and uric acid using an automatic analyzer (Reflotron® Plus System, Roche, Germany). Kidney and testis homogenates were obtained using a tissue homogenizer. The homogenates (1:10 w/v) were prepared using a 100 mM KCl buffer (7:00 pH) containing EDTA 0.3 mM. All homogenates were centrifuged at 1500 rpm for 30 min at 4 °C and the supernatants were used for the biochemical assays of glutathione (GSH) and superoxide dismutases (SOD) levels using GSH and SOD assay kits (Sigma–Aldrich Com.) according to the manufacturer’s instruction with some modifications. Also, kidneys and testes were quickly removed, immersed in 10% formalin, dehydrated and embedded in paraffin, sectioned at 4 μm, stained with hematoxylin and eosin (H&E) and evaluated by light microscopy. Images representative of typical histological profile in control and all treated groups were captured with the aid of Motic imaging software.
2.3. Statistical analysis
The calculations and statistical analysis were carried out using the Statistical Package for Social Sciences (SPSS) for Windows version 12.0 software. All data were represented as mean ± standard deviation (SD). Data were subjected to one-way analysis of variance (ANOVA) followed by Student’s t-test. Statistical probability of P < 0.05 was considered to be significant.
3. Results
Data presented in Table 1 represent the levels of plasma creatinine, urea and uric acid concentrations and the kidney and testis values of GSH and SOD of the different experimental groups. There was significant alteration in renal function in mice exposed to heavy metals (group 2) in comparison to control and other treated groups as indicated by significant increases of creatinine (+151.4%), urea (+82.4%) and uric acid (+64.4%) concentrations. Also, the levels of GSH in kidney (−27.8%) and testis (−24.0%) were statistically decreased. In addition, significant decreases in the level of SOD in kidney (−40.0%) and testis (−27.0%) were noted. Insignificant changes in the levels of plasma creatinine, urea and uric acid, and kidney GSH and SOD were observed in mice treated with heavy metals plus vitamin E (group3), while the values of testis GSH (−14.9%) and SOD (−11.4%) were notably declined compared to controls and vitamin E treated mice (group 4). The levels of all studied parameters were not significantly altered in mice treated with only vitamin E (group 4) compared to control mice (group 1). Histopathological examination of the kidney and testis specimens showed severe alterations in mice exposed to heavy metals (group 2). Renal tubular dilatations with congestion of blood vessels with hemorrhage and degeneration of renal corpuscles were noted (Fig. 1B–F) compared to the normal structure of control group (Fig. 1A). Also, most of the seminiferous tubules of testes in this group showed complete absence of spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and spermatozoa and loss of spermatogenesis process (Fig. 2B–D) in comparison with normal structure of seminiferous tubules in control mice (Fig. 2A). Administration of vitamin E protected the kidney and testis of mice exposed to heavy metals as evidenced by appearance of normal structures of kidney, specially renal corpuscles, (Fig. 1G) and seminiferous tubule of testis (Fig. 2E). Additionally, the histopathological evaluation of only vitamin E treated mice (group 4) showed normal renal corpuscle (Fig. 1H) and seminiferous tubule (Fig. 2F) structures.
Table 1.
Levels of plasma creatinine, urea and uric acid, kidney GSH, kidney SOD, testis GSH and testis SOD (mean ± SD) in controls, heavy metals, heavy metals plus vitamin E and vitamin E treated mice (n = 6). Percentage changes are included in parentheses.
| Treatments |
||||
|---|---|---|---|---|
| Parameters | Control | Heavy metals | Heavy metals + vitamin E | Vitamin E |
| Plasma creatinine (mg/dL) | 0.37 ± 0.03 | 0.93 ± 0.05⁎,⁎⁎,⁎⁎⁎ (+151.4%) | 0.46 ± 0.05 (+24.3%) | 0.37 ± 0.02 (0.0%) |
| Plasma urea (mg/dL) | 13.95 ± 1.38 | 25.45 ± 3.09⁎,⁎⁎,⁎⁎⁎ (+82.4%) | 14.88 ± 2.46 (6.7%) | 14.70 ± 1.43 (+5.4%) |
| Plasma uric acid (mg/dL) | 1.88 ± 0.13 | 3.09 ± 0.31⁎,⁎⁎,⁎⁎⁎ (+64.4%) | 1.83 ± 0.23 (−2.7%) | 1.74 ± 0.27 (−7.5%) |
| Kidney GSH (μmol/g tissue) | 3.42 ± 0.50 | 2.47 ± 0.48⁎,⁎⁎,⁎⁎⁎ (−27.8%) | 3.30 ± 0.42 (−3.5%) | 3.27± ± 0.24 (−4.4%) |
| Kidney SOD (U/mg tissue) | 4.58 ± 0.38 | 2.75 ± 0.66⁎,⁎⁎,⁎⁎⁎ (−40.0%) | 4.41 ± 0.51 (−3.7%) | 4.80 ± 0.50 (+4.8%) |
| Testis GSH (μmol/g tissue) | 9.73 ± 0.94 | 7.40 ± 1.01⁎,⁎⁎⁎ (−24.0%) | 8.28 ± 0.861⁎,⁎⁎⁎⁎ (−14.9%) | 9.88 ± 1.39 (+1.5%) |
| Testis SOD (U/mg tissue) | 142.33± 11.33 | 103.97 ± 5.79⁎,⁎⁎,⁎⁎⁎ (−27.0%) | 126.17 ± 10.44⁎,⁎⁎⁎⁎ (−11.4%) | 147.15 ± 8.82 (+3.4%) |
P < 0.05: Student’s t-test (significance levels shown for difference between control and treated groups).
P < 0.05: Student’s t-test (significance levels shown for difference between mice exposed to heavy metals and heavy metals plus vitamin E).
P < 0.05: Student’s t-test (significance levels shown for difference between mice exposed to heavy metals and vitamin E).
P < 0.05: Student’s t-test (significance levels shown for difference between mice exposed to heavy metals plus vitamin E and vitamin E).
Figure 1.
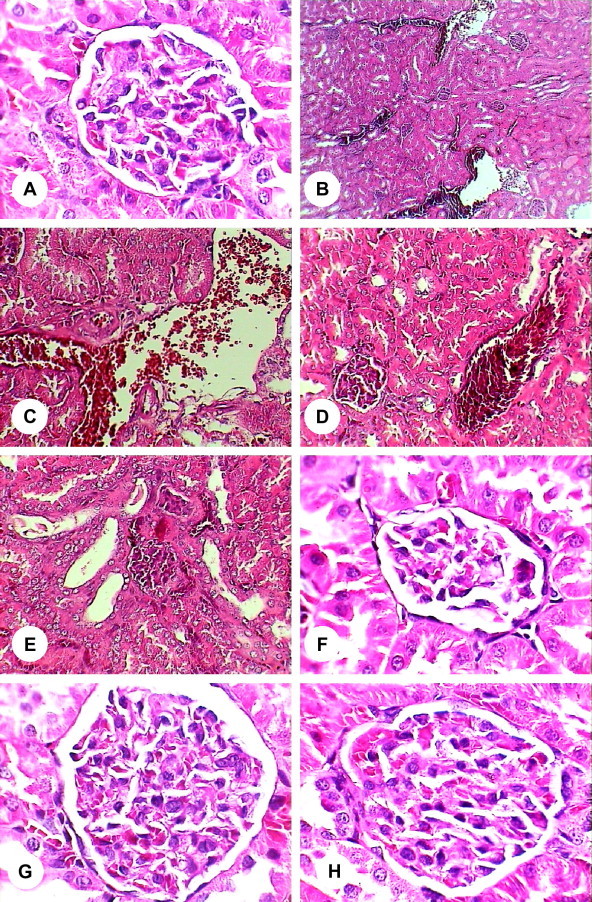
(A–H) Histological changes of kidney in each group. (A) Normal structure of renal corpuscle in control mice (1000×). (B) Renal cortex and medulla structure of heavy metals related mice (100×). (C–E) Renal cortex structures of heavy metals treated mice (400×). (F) Renal corpuscle structure of heavy metals treated mice (1000×). (G) Renal corpuscle structure of heavy metals plus vitamin E treated mice (1000×). (H) Renal corpuscle structure of vitamin E treated mice (1000×).
Figure 2.
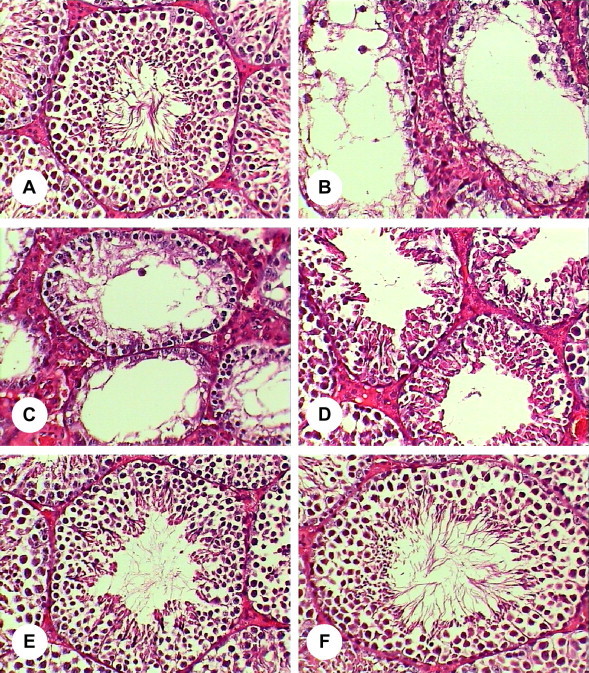
(A–F) Histological changes of testis in each group. (A) Normal structure of seminiferous tubule in control mice (400×). (B–D) Seminiferous tubules structure of heavy metals treated mice (400×). (E) Seminiferous tubule structure of heavy metals plus vitamin E treated mice (400×). (F) Seminiferous tubule structure of vitamin E treated mice (400×).
4. Discussion
It is well known that heavy metals are widely distributed in environment and some of them can cause physiological, biochemical and histological disorders. Humans are exposed to these metals from numerous sources, including contaminated air, water, soil and food. Therefore, the evaluation of toxic potentials of metals is important for the risk assessment of human beings ordinarily exposed to these substances. The physiological influence of metals on organisms, humans and animals is conditioned by the nature of metal, by the type of compounds and by their amount. Moreover, different scientific studies indicated that the degree of toxic manifestation of different metals depends on dose, duration, route of administration and other physiological factors, especially nutrition (Roy Chowdhury, 2009). The present work demonstrates that mice chronically intoxicated with a mixture of some heavy metals display a pronounced impairment in kidney function which is confirmed by the enhancement of plasma creatinine, urea and uric acid levels, and histopathological alterations. The results of the present work showed that the cortex is more affected than the medulla due to long-term treatment with heavy metals. This could be partly due to uneven distribution of heavy metals in the tissue of the kidney where about 90% of the total renal blood flow enters the cortex via the bloodstream. Accordingly, a relatively high concentration of these metals might reach the cortex via the bloodstream than that would enter the medulla. Several studies demonstrated a significant enhancement of blood creatinine, urea and uric acid concentrations, and renal histological alterations in experimental animals intoxicated with Pb, Hg, Cd, Cu and other heavy metals (Johnson and Kleinman, 1979; Moshtaghie et al., 1991; Brzóska et al., 2003; Odigie et al., 2004; Chen et al., 2006; Goran et al., 2008; Al-Madani et al., 2009; Saxena et al., 2009; Missoun et al., 2010).
The present study indicated that the exposure to heavy metals produced testicular damage, which led to spermatogenic arrest. Similar observations were noted in experimental animals exposed to heavy metals (Massanyi et al., 2007; Burukoğlu and Bayçu, 2008; Yasmina and Abdennour, 2008; Almansour, 2009; Obianime and Roberts, 2009). The potential toxicity of heavy metals caused alteration in sperm morphology, count, motility as well as biochemical disruptions of enzymes and hormones (Roy Chowdhury, 2009). Toxicity is manifest in male reproductive system by deposition of Pb in testes, epididymis, vas deferens, seminal vesicle and seminal ejaculate. Pb has an adverse effect on sperm count and retarded the activity of alive sperm. Moreover, motility as well as prolonged latency of sperm melting both in exposed person and experimental animals were observed after Pb exposure (Lancranjan et al., 1975; Roy Chowdhury et al., 1986). Study with male CF-1 mice indicated the significant decrease in epididymal sperm count at a low dose of Pb exposure (via drinking water). Moreover, the decreased motility and increased incidence of teratospermia at higher dose of Pb exposure along with inhibition of post-meiotic cells mainly pachytene spermatocyte were noted. In the same experiment the detachment of germinal cell layer from basal membrane, atrophy of Leydig cells plus interstitial edema and low density of seminal plasma were also observed. Additionally, Madhavi et al. (2007) showed that Pb induced cytogenetic damage in germ cells of mice. Hg is a spermato, steroido- and fetotoxic agent. Hg exhibited structural alteration of testicular tissue along with biochemical change. The control testis of albino rat showed sharp localization of ACPase, ATPpase and ALKPase in PTM, spermatogeic cell and Leydig cell membrane (Roy Chowdhury and Vachhrajani, 1997). When Hg and its compound, methyl mercury chloride, affected these membrane-bound hydrolytic enzymes in rats it resulted in sharp decrease of these enzymes, co-related with progressive degeneration of peritubular membrane. Hg also caused the structural and functional disintegration of these enzymes due to its high affinity towards the enzyme’s (SH) group (Roy Chowdhury and Vachhrajani, 1987). The prominent features of Hg induced toxicity are: (1) depletion and clogging of different spermatogenic cells, (2) presence of pyknotic or karyotectic pachytene nuclei, (3) absence of nuclear chromatin at stage XII in dividing cells, (4) absence of noticeable lumen and (5) presence of vacuolated early elongated spermatid along with dispositioning of acrosome. The intensity of damage is directly proportional to the duration of exposure (Vachhrajani et al., 1990). The action of Cd is spermatogenic stage specific. Testicular histopathological evaluation using light and electron microscopy showed that Cd produced an extensive germ cells apoptosis in Sprague–Dawley rats (Al-Azemi et al., 2010). El-Shahat et al. (2009) showed that the administration of Cd caused marked morphological changes in the form of swelling, congestion, hemorrhage and necrosis in testes of Sprague–Dawley rats. High dose of Cd exposure caused rapid testicular edema, hemorrhage and necrosis. Cd exerted deleterious effect on the vascular structure of testis that may be the result of varying degrees of Cd induced ischemia. Degeneration of testicular tissue after different doses of Cd exposure caused rupture of blood vessels (Kar and Das, 1960). Also, histologically testes showed dose dependent seminiferous epithelial necrosis, degeneration and loss of spermatozoa in albino rats exposed to Cd (Devy et al., 2006). Electron microscopic observation revealed that DNA fragmentation in mouse testicular tissue after Cd exposure showed a positive effect. Studies on workers exposed to electric welding reveal increased semen concentration of Cu along with lowered sperm count, sperm viability and semen volume (Wu et al., 1996). There are reports that long term ingestion of CuCl2 adversely affects sexual behavior, fertility and testicular and accessory sex organ weight in adult male rats (Bataineh et al., 1998). Chronic exposure of rats to CuCl2 fumes show decreased concentrations of plasma follicle stimulating hormone (FSH), luteinizing hormone (LH) and testosterone and dysfunction of virile gonads and disorders in spermatogenesis (Gabuchyan, 1987). In addition, Chattopadhyay et al. (2005) suggested that Cu has got a dose-dependent effect on testicular steroidogenesis and spermatogenesis and serum testosterone and LH level in maturing male rats.
In mice treated with heavy metals there were significant decreases in the levels of GSH and SOD in kidney and testis tissues. Glutathione, a tripeptide present in the majority of cells, is responsible for hydrophilic xenobiotics conjugation. GSH serves many vital physiological functions including protection of cells from reactive oxygen species (ROS), detoxification of exogenous compounds, and amino acid transport (Kojima-Yuasa et al., 2005; Mendoza-Cózatl et al., 2005). Sulphydryl group of glutathione is essential for its antioxidant activity against some forms of ROS in cells (Cnubben et al., 2001). Much of the pathology is associated with the decrease in intracellular GSH concentration (Rouach et al., 1997). Therefore, GSH concentration is important for survival of the cells. It is also a substrate for glutathione peroxidase. Probably the most important protective mechanism for free radical scavenging and inhibition of electrophilic xenobiotics attack on cellular macromolecules involves tripeptide glutathione (Cnubben et al., 2001). Due to nucleophilic thiol group, it can detoxify substances in one of three ways: (1) conjugation catalyzed by glutathione-S-transferases (GST), (2) chemical reaction with a reactive metabolite to form a conjugate and (3) donation of proton or hydrogen atom to reactive metabolites or free radicals. Reactive intermediates can react with GSH either by a direct chemical reaction or by a GST-mediated reaction preventing possible cell death. Regarding the role of glutathione in the protection against oxidative stress and detoxification of xenobiotics, its availability in the reduced form (GSH) may be a key factor in maintenance of health. It has been established in several different animal models, as well as in humans, that a decrease in GSH concentration may be associated with aging and pathogenesis of many diseases (Aruoma et al., 1989; Boehme et al., 1992; Smith et al., 1993; Lomaestro and Malone, 1995; Dröge et al., 1997). Superoxide dismutases (SODs) belong to a family of antioxidant enzymes that catalyze the dismutation of superoxide to yield hydrogen peroxide and oxygen (Johnson et al., 2005). SOD is essentially a protective enzyme which scavenges the superoxide ions produced as cellular by-products during oxidative stress (Pushpakiran et al., 2004). Its decreased activity can lead to adverse effects because superoxide anions are extremely toxic and may accumulate in the cells. Many studies indicate that heavy metals act as catalysts in the oxidative reactions of biological macromolecules therefore the toxicities with these metals might be due to oxidative tissue damage (Stohs and Bagchi, 1993; Hultberg et al., 1999, 2001; Cuypers et al., 1999; Leonard et al., 2004; Flora et al., 2008). Redox-active metals, such as iron (Fe), Cu and chromium (Cr), undergo redox cycling whereas redox-inactive metals, such as Pb, Cd, Hg and others deplete cells’ major antioxidants, particularly thiol-containing antioxidants and enzymes. Either redox-active or redox-inactive metals may cause an increase in production of ROS such as hydroxyl radical (HO•), superoxide radical () or hydrogen peroxide (H2O2). Enhanced generation of ROS can overwhelm cells’ intrinsic antioxidant defenses, and result in a condition known as “oxidative stress”. Cells under oxidative stress display various dysfunctions due to lesions caused by ROS to lipids, proteins and DNA. Consequently, it is suggested that metal-induced oxidative stress in cells can be partially responsible for the toxic effects of heavy metals (Ercal et al., 2001). In recent research papers it was determined that the effect of antioxidant supplementation followed heavy metals exposure. They suggest that antioxidants may play an important role in abating some health hazards of heavy metals in connection with an interaction of physiological free radicals (health effects). So, multiple mechanisms may be responsible for ROS production in toxic metal exposure. Among them, alterations in thiol status, increased lipid peroxidation, production of ROS, and damage to cell’s antioxidant defense systems are well known for all redox-active and inactive elements (Kamiński et al., 2007).
From the present results, it is obvious that treating heavy metals-intoxicated rats with vitamin E significantly protected the kidney and testis structures and functions as compared to the controls. These observations were confirmed by insignificant alterations in the levels of plasma creatinine, urea and uric acid, kidney GSH and SOD, and an appearance of normal structures of kidney, specially renal corpuscles, and seminiferous tubule of testis, while the values of testis GSH and SOD were statistically decreased. Vitamin E supplement may be beneficial in reducing and slowing progressive kidney diseases that are significantly accelerated by oxidative stress. Vitamin E therapy may also be effective in reducing cardiovascular disease associated with chronic renal failure and the uremia state. Vitamin E therapy is also considered as a mean of correcting plasma antioxidant status and attenuating the cardiovascular disease that accompanies kidney failure. Vitamin E allows free radicals to abstract a hydrogen atom from the antioxidant molecule rather than from polyunsaturated fatty acids, thus breaking the chain of free radical reactions, the resulting antioxidant radicals being a relatively unreactive species (Pascoe et al., 1987). In many studies vitamin E neutralizes lipid peroxidation and unsaturated membrane lipids because of its oxygen scavenging effect (Aldana et al., 2001; John et al., 2001). Therefore vitamin E supplementation sufficient to protect the organism from toxic agent and free radical damage is a time consuming process. It is concluded that vitamin E is an essential component of the kidney for protection of this tissue against peroxidative damage (Champe and Harvey, 1987). Hanafy and Soltan (2004) investigated the protective effect of vitamin E on cobalt (Co), Pb, or Hg nitrate and a mixture of them induced nephrotoxicity in Norway strain rats. They concluded that the combined exposure to a mixture of vitamin E and examined heavy metals can minimize the histological alteration and diminish the serum creatinine and blood urea level. Turguta et al. (2006) reported that the administration of the antioxidant agent vitamin E together with aluminum (Al) resulted in the recovery of malondialdehyde (MDA) and GSH levels in Al administered Balb-c mice. That is, a chronic high dose of Al can lead to tissue oxidative injury, and vitamin E is capable of preventing the deleterious effects of Al+3 ions. Kutlubay et al. (2007) reported that vitamin E antagonizes the toxic effects of Al at the testicular histological level of rats, thus potentially contributing to an amelioration of the testis histology in the Al-treated rats. Agarwal et al. (2010) examined the effect of both pre- and post-treatment of vitamin E on Hg induced acute toxicity in rats. Hg resulted in oxidative injury and metallothionein mRNA expression together with alterations in tissue histology and accumulation of Hg in the body organs. The ameliorating potential of vitamin E was observed in Hg administered rats. Moreover, they stated that their findings indicate that vitamin E provides complete protection from Hg toxicity in the liver with both pre- and post-treatments. As Hg is nephrotoxic and neurotoxic, it is interesting to note that post-treatment of vitamin E showed more protection in the kidney compared to pre-treatment. In brain tissue, partial protection was observed on oxidative stress parameters. Additionally, they suggested that post-treatment with vitamin E could be more beneficial than pre- treatment in Hg intoxication. Choi and Rhee (2003) showed that the renal morphological changes observed by both light and electron microscopy revealed mitochondria and tubule epithelial cell edema in Cd-exposed Sprague–Dawley rats, yet this was alleviated with the highest level of vitamin E supplementation. The urinary beta(2)-microglobulin levels indicated that glomerular injury was higher in the Cd-poisoned rats than in the control group, but were lowered by vitamin E supplementation. Although the glomerular filtration rate (GFR) of the Cd-treated rats was significantly lower than that of the control group, the vitamin E-supplemented rats exhibited a similar GFR to the control rats, suggesting that vitamin E protected the kidney from functional damage. Angiotensin converting enzyme activity, blood pressure and heart rate were all significantly higher in the Cd-poisoned rats, but each remained nearly normal with vitamin E supplementation. Accordingly, these results indicate that vitamin E supplementation in chronic Cd-poisoned rats normalized renal dysfunction and blood pressure regulation (Choi and Rhee, 2003). Yang et al. (2006) indicated that Cd can severely destroy testicular tissues and affect spermatogenesis in rats. α-Tocopherol (vitamin E) treatment can protect testicular tissue and preserve spermatogenesis from the detrimental effects of Cd but its effectiveness is dependent on the dose of Cd exposed. Osfor et al. (2010) reported that vitamin E could improve daily food intake, body weight gain and feed efficiency ratio; reduced Cu and Pb levels in serum and tissues as well as diminished urea and creatinine levels in Cu and Pb intoxicated rats. Treatment with vitamin E was very effective in the prevention of oxidative damage. Trivedi et al. (1998) demonstrated that Pb at both low and high doses induced lipid peroxidation in liver, and heart lipid peroxidation was observed in rats treated with a high dose of Pb while kidney showed no significant change in lipid peroxidation at both doses. Liver and heart lipid peroxidation decreased in vitamin E pretreated rats administered high dose of Pb as compared to rats treated with high dose of Pb without vitamin E. The SOD activity in heart increased in rats administered Pb at a high dose. However, vitamin E pretreatment showed a mild effect in lowering heart SOD in rats treated with high dose of Pb. Liver and kidney SOD activity were not significantly changed with Pb with or without vitamin E pretreatment as compared to controls. The treatment with Pb, both at low and high doses, decreased catalase (CAT) activity in liver and kidney tissues. However, vitamin E pretreatment, prior to low dose of Pb administration, increased liver and kidney CAT activities but not at high dose of Pb intoxication. Kidney GSH content increased with Pb treatment without any lowering in GSH in vitamin E-pretreated rats administered Pb, while liver and heart GSH were not significantly affected with administration of Pb with or without vitamin E pretreatment. Glutathione-S-transferase (GST) activity increased in liver and kidney at a high dose of Pb. However, vitamin E-pretreated rats administered Pb also showed increased GST activity in tissues. Moreover, they concluded that vitamin E pretreatment partially attenuates Pb-induced oxidative stress by altering antioxidant enzymes. Beytut et al. (2003) and Ognjanović et al. (2003) demonstrated the effectiveness of vitamin E in reducing oxidative stress in Cd-treated animals and suggested that reductions in increased lipid peroxidation due to Cd toxicity may be an important factor in the action of vitamin E. Hassan and Awad (2007) showed that the exposure to Cd caused marked elevation in the level of lipid peroxidation and a decline in SOD, glutathione peroxidase (GSH-Px) and CAT activities accompanied by an increase in the rate of hemoglobin autoxidation in Swiss albino rats. Additionally, they demonstrated that the treatment with vitamin E significantly reduced the changes caused by Cd exposure in all examined parameters. Moreover, they suggested that these results indicate that alterations caused by Cd are connected with free radicals generation and used antioxidants effectively to protect against Cd intoxication. In conclusion, the present study showed that vitamin E has protective effect on heavy metals-induced renal and testicular oxidative stress and injuries. This study therefore suggests that vitamin E may be a useful preventive agent against the effect of the studied heavy metals at least partly due to its antioxidant properties.
References
- Abdel-Moneim A.M., Said K.H. Acute effect of cadmium treatment on the kidney of rats: biochemical and ultrastructural studies. Pak. J. Biol. Sci. 2007;10:3497–3506. doi: 10.3923/pjbs.2007.3497.3506. [DOI] [PubMed] [Google Scholar]
- Agarwal R., Goel S.K., Chandra R., Behari J.R. Role of vitamin E in preventing acute mercury toxicity in rat. Environ. Toxicol. Pharmacol. 2010;29:70–80. doi: 10.1016/j.etap.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Al-Azemi M., Omu F.E., Kehinde E.O., Anim J.T., Oriowo M.A., Omu A.E. Lithium protects against toxic effects of cadmium in the rat testes. J. Assist. Reprod. Genet. 2010;27:469–476. doi: 10.1007/s10815-010-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldana L., Tsutsumi V., Craigmill A., Silveira M.I., De Mejia E.G. α-Tocopherol modulates liver toxicity of the prethroid cypermethrin. Toxicol. Lett. 2001;125:107–116. doi: 10.1016/s0378-4274(01)00427-1. [DOI] [PubMed] [Google Scholar]
- Alekin, O., 1970. Basic hydrochemistry. Leningrad.
- Al-Madani W.A., Siddiqi N.J., Alhomida A.S. Renal toxicity of mercuric chloride at different time intervals in rats. Biochem. Insights. 2009;2:37–45. [Google Scholar]
- Almansour M.I. Histological alterations induced by lead in the testes of the quail Coturnix coturnix. Res. J. Environ. Toxicol. 2009;3:24–30. [Google Scholar]
- Aruoma O.I., Halliwell B., Hoey B.M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Bataineh H., Al-Hamood M.H., Elbetieha A.M. Assessment of aggression, sexual behaviour and fertility in adult male rat following long term ingestion of four industrial metal salts. Hum. Exp. Toxicol. 1998;17:570–576. doi: 10.1177/096032719801701008. [DOI] [PubMed] [Google Scholar]
- Batra N., Nehru B., Bansal M.P. Influence of lead and zinc on rat male reproduction at ‘biochemical and histopathological levels’. J. Appl. Toxicol. 2001;21:507–512. doi: 10.1002/jat.796. [DOI] [PubMed] [Google Scholar]
- Beytut E., Yuce A., Kamiloglu N.N., Aksakal M. Role of dietary vitamin E in cadmium-induced oxidative damage in rabbit’s blood, liver and kidneys. Int. J. Vitam. Nutr. Res. 2003;73:351–355. doi: 10.1024/0300-9831.73.5.351. [DOI] [PubMed] [Google Scholar]
- Boehme D.S., Maples K.R., Henderson R.F. Glutathione released by pulmonary alveolar macrophages in response to particles in vitro. Toxicol. Lett. 1992;60:53–60. doi: 10.1016/0378-4274(92)90046-m. [DOI] [PubMed] [Google Scholar]
- Brzóska M.M., Moniuszko-Jakoniuk J., Piłat-Marcinkiewicz B., Sawicki B. Liver and kidney function and histology in rats exposed to cadmium and ethanol. Alcohol Alcohol. 2003;38:2–10. doi: 10.1093/alcalc/agg006. [DOI] [PubMed] [Google Scholar]
- Budavari S. Merck and Co., Inc.; White House Station, NJ: 1996. An encyclopedia of chemicals, drugs and biologicals. [Google Scholar]
- Burukoğlu D., Bayçu C. Protective effects of zinc on testes of cadmium-treated rats. Bull. Environ. Contam. 2008;81:521–524. doi: 10.1007/s00128-007-9211-x. [DOI] [PubMed] [Google Scholar]
- Champe P.C., Harvey R.A. Vitamin E. In: Barnes D., Robinson S., Hoeltze L.E., Baldwin T.J.B., editors. Lippincott’s Illustrated Review Biochemistry. Lippincott Com.; Philadelphia: 1987. pp. 311–331. (Chapter 27: Vitamins) [Google Scholar]
- Chattopadhyay A., Sarkar M., Biswas N.M. Dose-dependent effect of copper chloride on male reproductive function in immature rats. Kathmandu Univ. Med. J. 2005;3:392–400. [PubMed] [Google Scholar]
- Chen Z., Meng H., Xing G., Chen C., Zhao Y., Jia G., Wang T., Yuan H., Ye C., Zhao F., Chai Z., Zhu C., Fang X., Ma B., Wan L. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006;163:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Rhee S.J. Effects of vitamin E on renal dysfunction in chronic cadmium-poisoned rats. J. Med. Food. 2003;6:209–215. doi: 10.1089/10966200360716625. [DOI] [PubMed] [Google Scholar]
- Cnubben N.H.P., Rietjens I.M.C.M., Wortelboer H., van Zanden J., van Bladeren P.J. The interplay of glutathione-related processes in antioxidant defense. Environ. Toxicol. Pharmacol. 2001;10:141–152. doi: 10.1016/s1382-6689(01)00077-1. [DOI] [PubMed] [Google Scholar]
- Conesa H.M., Faz A., Arnaldos R. Initial studies for the phytostabilization of a mine tailing from the Cartagena – La Union Mining District (SE Spain) Chemosphere. 2007;66:38–44. doi: 10.1016/j.chemosphere.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Cuypers A., Vangronsveld J., Clijsters H. The chemical behaviors of heavy metals play a prominent role in the induction of oxidative stress. Free Radic. Res. 1999;31:39–43. doi: 10.1080/10715769900301301. [DOI] [PubMed] [Google Scholar]
- Danielyan, A., 2010. The problem of pollution with heavy metals and possible risks related to that in watersheds with the developed metallurgical industry. In: BALWOIS 2010 Conference. Ohrid, Republic of Macedonia, pp. 1–9.
- Devy A., Khan B.A., Kumar Cadmium chloride induced histopathological and biochemical changes in the testes of adult albino rats. Toxicol. Int. 2006;13:61–64. [Google Scholar]
- Dröge W., Gross A., Hack V. Role of cysteine and glutathione in HIV infection and cancer cachexia: therapeutic intervention with N-acetylcysteine. Adv. Pharmacol. 1997;38:581–600. doi: 10.1016/s1054-3589(08)61000-5. [DOI] [PubMed] [Google Scholar]
- El-Gharieb M.A., El-Masry T.A., Emara A.M., Hashem M.A. Potential hepatoprotective effects of vitamin E and Nigella sativa oil on hepatotoxicity induced by chronic exposure to malathion in human and male albino rats. Toxicol. Environ. Chem. 2010;92:391–407. [Google Scholar]
- El-Shahat A., Gabr A., Meki A., Mehana E. Altered testicular morphology and oxidative stress induced by cadmium in experimental rats and protective effect of simultaneous green tea extract. Int. J. Morphol. 2009;27:757–764. [Google Scholar]
- Ercal N., Gurer-Orhan H., Aykin-Burns N. Toxic metals and oxidative stress Part 1: mechanisms involved in metal induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Evstigneeva R.P., Volkov I.M., Chudinova V.V. Vitamin E as a universal antioxidant and stabilizer of biological membranes. Membr. Cell Biol. 1998;12:151–172. [PubMed] [Google Scholar]
- Ezzati M., Lopez A.D., Rodgers A., Vander Hoorn S., Murray C.J.L. The comparative risk assessment collaborative group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Flora S.J.S., Mittal M., Mehta A. Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian J. Med. Res. 2008;128:501–523. [PubMed] [Google Scholar]
- Foulkes E.C. vol. II. CRC Press; Florida, USA: 1990. (Biological Effects of Heavy Metals in Metal Carcinogenesis). [Google Scholar]
- Gabuchyan V.V. Impaired mechanism of the reproductive function in copper chloride exposed white male rats. Gig. Tr. Prof. Zabol. 1987;9:28–31. [PubMed] [Google Scholar]
- Goran G.V., Crivineanu V., Papuc C., Crivineanu C.D. Effect of sea-buckthorn alcoholic extracts (Hippophe fructus) on hepatic and renal functions in laboratory rat. Bull. Univ. Agric. Sci. Vet. Med. 2008;65:288–292. [Google Scholar]
- Hanafy S., Soltan M.E. Effects of vitamin E pretreatment on subacute toxicity of mixture of Co, Pb, and Hg nitrate-induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2004;17:159–167. doi: 10.1016/j.etap.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Hassan N.S., Awad S.M. Reverse effect of vitamin E on oxidative stress, derivatives and conductivity changes of hemoglobin induced by exposure to cadmium. J. Appl. Sci. Res. 2007;3:437–443. [Google Scholar]
- Hultberg B., Andersson A., Isaksson A. Thiol and redox reactive agents exert different effects on glutathione metabolism in Hela cell culture Clin. Chim. Acta. 1999;283:21–32. doi: 10.1016/s0009-8981(99)00028-5. [DOI] [PubMed] [Google Scholar]
- Hultberg B., Andersson A., Isaksson A. Interaction of metals and thiol in cell damage and glutathione distribution: potentiation of mercury toxicity by dithiothreitol. Toxicology. 2001;156:93–100. doi: 10.1016/s0300-483x(00)00331-0. [DOI] [PubMed] [Google Scholar]
- Jagadeesan G., Pillai S. Hepatoprotective effect of taurine against mercury induced toxicity in rat. J. Environ. Biol. 2007;28:753–756. [PubMed] [Google Scholar]
- Jalili Sh., Ilkhanipour M., Heydari R., Farshid A.A., Salehi S. The effects of vitamin E on endosulfan-induced oxidative stress in rat heart. Pak. J. Nutr. 2007;6:375–380. doi: 10.3923/pjbs.2007.1922.1925. [DOI] [PubMed] [Google Scholar]
- John S., Kale M., Rathore N., Bhatnagar D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 2001;12:500–504. doi: 10.1016/s0955-2863(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Johnson D.R., Kleinman L.I. Effects of lead exposure on renal function in young rats. Toxicol. Appl. Pharmacol. 1979;48:361–367. doi: 10.1016/0041-008x(79)90419-8. [DOI] [PubMed] [Google Scholar]
- Johnson W.T., Johnson L.A.K., Henry C., Lukaski H.C. Serum superoxide dismutase 3 (extracellular superoxide dismutase) activity is a sensitive indicator of Cu status in rats. J. Nutr. Biochem. 2005;16:682–692. doi: 10.1016/j.jnutbio.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Kamiński P., Kurhalyuk N., Szady-Grad M. Heavy metal-induced oxidative stress and changes in physiological process of free radicals in the blood of White Stork (Ciconia ciconia) chicks in polluted areas. Pol. J. Environ. Stud. 2007;16:555–562. [Google Scholar]
- Kar A.B., Das R.P. Testicular changes in rats after treatment with cadmium chloride. Acta Biol. Med. Ger. 1960;5:153–173. [PubMed] [Google Scholar]
- Kojima-Yuasa A., Umeda K., Ohkita T., Opare Kennedy D., Nishiguchi S., Matsui-Yuasa I. Role of reactive oxygen species in zinc deficiency-induced hepatic stellate cell activation. Free Radic. Biol. Med. 2005;39:631–640. doi: 10.1016/j.freeradbiomed.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Kutlubay R., Oğuz E.O. Histological and ultrastructural evidence for protective effects on aluminum-induced kidney damage by intraperitoneal administration of α-tocopherol. Int. J. Toxicol. 2007;26:95–101. doi: 10.1080/10915810701221173. [DOI] [PubMed] [Google Scholar]
- Kutlubay R., Oğuz E.O., Can B., Guven M.C., Sinik Z., Tuncay Ö.L. Vitamin E protection from testicular damage caused by intraperitoneal aluminium. Int. J. Toxicol. 2007;26:297–306. doi: 10.1080/10915810701470952. [DOI] [PubMed] [Google Scholar]
- Lancranjan I., Popescu H.I., GAvănescu O., Klepsch I., Serbănescu M. Reproductive ability of workmen occupationally exposed to lead. Arch. Environ. Health. 1975;30:396–401. doi: 10.1080/00039896.1975.10666733. [DOI] [PubMed] [Google Scholar]
- Leonard S.S., Harris G.K., Shi X.L. Metal-induced oxidative stress and signal transduction. Free Radic. Biol. Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Lomaestro B.M., Malone M. Glutathione in health and disease: pharmacotherapeutic issues. Ann. Pharmacother. 1995;29:1263–1273. doi: 10.1177/106002809502901213. [DOI] [PubMed] [Google Scholar]
- Madhavi D., Devi K.R., Rao K.K., Reddy P.P. Modulating effect of Phyllanthus fruit extract against lead genotoxicity in germ cells of mice. J. Environ. Biol. 2007;28:115–117. [PubMed] [Google Scholar]
- Malaev M., Nekrasova G., Bezel B. The reaction of hydrophytes to the pollution of environment with heavy metals. Ecology. 2004;4:230–235. [Google Scholar]
- Massanyi P., Lukac N., Slivkova J., Kovacik J., Makarevich A.V., Chrenek P., Toman R., Forgacs Z., Somosy Z., Stawarz R., Formicki G. Mercury-induced alterations in rat kidneys and testes in vivo. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2007;42:865–870. doi: 10.1080/10934520701370410. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl D., Loza-Tavera H., Hernández-Navarro A., Moreno-Sánchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Migliore L., Cocchi L., Nesti C., Sabbioni E. Micronuclei assay and FISH analysis in human lymphocyte treated with six metal salts. Environ. Mol. Mutagen. 1999;34:279–284. doi: 10.1002/(sici)1098-2280(1999)34:4<279::aid-em8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Missoun F., Slimani M., Aoues A. Toxic effect of lead on kidney function in rat Wistar. Afr. J. Biochem. Res. 2010;4:21–27. [Google Scholar]
- Moshtaghie A.A., Raisi A., Goodarzi H. A study of the effect of cadmium toxicity on serum proteins and it’s relation to proteinuria in male rats. J. Islamic Acad. Sci. 1991;4:192–195. [Google Scholar]
- Musalmah M., Fairuz A.H., Gapor M.T., Ngah W.Z.W. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac. J. Clin. Nutr. 2002;11:S448–S451. doi: 10.1046/j.1440-6047.11.s.7.6.x. [DOI] [PubMed] [Google Scholar]
- Norazlina M., Ima-Nirwana S., Gapor M.T., Khalid B.A.K. Palm vitamin E is comparable to α-tocopherol in maintaining bone mineral density in ovariectomised female rats. Exp. Clin. Endocrinol. Diabetes. 2000;108:305–310. doi: 10.1055/s-2000-7758. [DOI] [PubMed] [Google Scholar]
- Obianime A.W., Roberts I.I. Antioxidants, cadmium-induced toxicity, serum biochemical and the histological abnormalities of the kidney and testes of the male Wistar rats. Niger. J. Physiol. Sci. 2009;24:177–185. doi: 10.4314/njps.v24i2.52910. [DOI] [PubMed] [Google Scholar]
- Odigie I.P., Ladipo C.O., Ettarh R.R., Izegbu M.C. Effect of chronic exposure to low levels of lead on renal function and renal ultrastructure in SD rats. Niger. J. Physiol. Sci. 2004;19:27–32. [Google Scholar]
- Ognjanović B.I., Pavlović S.Z., Maletić S.D., Žikić R.V., Štajn A.S., Radojicić R.M., Saičić Z.S., Petrovic V.M. Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol. Res. 2003;52:563–570. [PubMed] [Google Scholar]
- Osfor M.M.H., Ibrahim H.S., Mohamed Y.A., Ahmed A.M., Abd El Azeem A.S., Hegazy A.M. Effect of alpha lipoic acid and vitamin E on heavy metals intoxication in male albino rats. J. Am. Sci. 2010;6:56–63. [Google Scholar]
- Packer L. Protective role of vitamin E in biological systems. Am. J. Clin. Nutr. 1991;53:1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- Pascoe G., Olafsdottier F., Read D. Vitamin E protection against chemical-induced cell injury. I. Maintenance of cellular protein thiols as a cytoprotective mechanism. Arch. Biochem. Biophys. 1987;256:150–158. doi: 10.1016/0003-9861(87)90433-4. [DOI] [PubMed] [Google Scholar]
- Pillai A., Gupta S. Antioxidant enzyme activity and lipid peroxidation in liver of female rats co-exposed to lead and cadmium: effects of vitamin E and Mn2+ Free Radic. Res. 2005;39:707–712. doi: 10.1080/10715760500092444. [DOI] [PubMed] [Google Scholar]
- Pushpakiran G., Mahalakshmi K., Anuradha C.V. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids. 2004;27:91–96. doi: 10.1007/s00726-004-0066-8. [DOI] [PubMed] [Google Scholar]
- Qureshi A.A., Bradlow B.A., Brace L., Manganello J., Peterson D.M., Pearce B.C., Wright J.J.K., Gapor A., Elson C.E. Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids. 1995;30:1171–1177. doi: 10.1007/BF02536620. [DOI] [PubMed] [Google Scholar]
- Rahmat A., Ngah W.Z., Shamaan N.A., Gapor A., Abdul Kadir K. Longterm administration of tocotrienols and tumor-marker enzyme activities during hepatocarcinogenesis in rats. Nutrition. 1993;9:229–232. [PubMed] [Google Scholar]
- Rouach H., Fataccioli V., Gentil M., French S.W., Morimoto M., Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury A. Toxicological assessment of reproductive health in occupation and community environment. Proc. Zool. Soc. 1992;45:103–114. [Google Scholar]
- Roy Chowdhury A. Male reproductive toxicity-new perspective in life science. In: Chatterjee P., Chanda A.K., editors. Life Science in Modern Perspective. University of Calcutta; 2004. pp. 97–105. [Google Scholar]
- Roy Chowdhury A. Recent advances in heavy metals induced effect on male reproductive function-A retrospective. Al Ameen J. Med. Sci. 2009;2:37–42. [Google Scholar]
- Roy Chowdhury A., Rao R.V., Gautam A.K. Histochemical changes in the testes of lead induced experimental rats. Folia Histochem. Cytobiol. 1986;24:233–238. [PubMed] [Google Scholar]
- Roy Chowdhury A., Vachhrajani K.D. Effect of mercuric chloride on hydrolytic enzymes of rat testicular tissues. Indian J. Exp. Biol. 1987;25:542–547. [PubMed] [Google Scholar]
- Roy Chowdhury A., Vachhrajani K.D. Methylmercury induced effect on seminiferous PTM in rats. Indian J. Physiol. Allied Sci. 1997;51:9–15. [Google Scholar]
- Saxena P.N., Anand S., Saxena N., Bajaj P. Effect of arsenic trioxide on renal functions and its modulation by Curcuma aromatica leaf extract in albino rat. J. Environ. Biol. 2009;30:527–531. [PubMed] [Google Scholar]
- Smith L.J., Houston M., Anderson J. Increased levels of glutathione in bronchoalveolar lavage from patients with asthma. Am. Rev. Respir. Dis. 1993;147:1461–1464. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- Soudani N., Sefi M., Ben Amara I., Boudawara T., Zeghal N. Protective effects of selenium (Se) on chromium (VI) induced nephrotoxicity in adult rats. Ecotoxicol. Environ. Saf. 2010;73:671–678. doi: 10.1016/j.ecoenv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of metals ions. Free Radic. Biol. Med. 1993;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Sullivan, J., Stoltenberg, D., Manoyan, S., Huang, J., Zdanowicz, R., Redmon, W., 2002. Upper Mississippi River Water Quality Assessment Report.
- Suradkar S.G., Ghodasara D.J., Vihol P., Patel J., Jaiswal V., Prajapati K.S. Haemato-biochemical alterations induced by lead acetate toxicity in Wistar rats. Vet. World. 2009;2:429–431. [Google Scholar]
- Trivedi R., John S., Rathore N., Kale M., Bhatnagar D. Effect of vitamin E on the changes in lead-induced lipid peroxidation and antioxidant enzymes in rat tissues. Trace Elem. Electrolytes. 1998;15:200–204. [Google Scholar]
- Turguta G., Enlib Y., Kaptanoğlub B., Turguta S., Gença O. Changes in the levels of MDA and GSH in mice serum, liver and spleen after aluminum administration. East. J. Med. 2006;11:7–12. [Google Scholar]
- Vachhrajani K.D., Roy Chowdhury A., Dutta K.K. Testicular toxicity of methylmercury. Reprod. Toxicol. 1990;6:355–361. doi: 10.1016/0890-6238(92)90199-4. [DOI] [PubMed] [Google Scholar]
- Vanderlinden K., Ordóñez R., Polo M.J., Giráldez J.V. Mapping residual pyrite after a mine spill using non co-located spatiotemporal observations. J. Environ. Qual. 2006;3:21–36. doi: 10.2134/jeq2004.0389. [DOI] [PubMed] [Google Scholar]
- Vaněk A., Borůvka L., Drábek O., Mihaljevič M., Komárek M. Mobility of lead, zinc and cadmium in alluvial soils heavily polluted by smelting industry. Plant Soil Environ. 2005;51:316–321. [Google Scholar]
- Waldron H.A. Academic Press; London: 1980. Metals in the Environment. [Google Scholar]
- Waldron H.A., Ediing C. fourth ed. Butterworth Heinemann; Oxford: 1997. Occupational Health Practice. [Google Scholar]
- Wu W., Zhang Y., Zhang F. Studies on semen quality in workers exposed to manganese and electric welding. Zhonghua Yu Fang Yi Xue Za Zhi. 1996;30:266–268. [PubMed] [Google Scholar]
- Xu H., Watkins B.A., Seifert M.F. Vitamin E stimulates trabecular bone formation and alters epiphyseal cartilage morphometry. Calcif. Tissue Int. 1995;57:293–300. doi: 10.1007/BF00298885. [DOI] [PubMed] [Google Scholar]
- Yang H.S., Han D.K., Kim J.R., Sim J.C. Effects of α-tocopherol on cadmium-induced toxicity in rat testis and spermatogenesis. J. Korean Med. Sci. 2006;21:445–451. doi: 10.3346/jkms.2006.21.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmina M., Abdennour C. Influence of vitamin C on testicular functions of domestic rabbit Oryctolagus cuniculus under mercury exposure. Eur. J. Sci. Res. 2008;22:197–204. [Google Scholar]
- Yee J.K., Ima-Nirwana S. Palm vitamin E protects against ferric nitrilotriacetate-induced impairment of bone calcification. Asia Pac. J. Pharmacol. 1998;13:1–7. [Google Scholar]


