Abstract
Synthesis and hardening of a new exoskeleton are essential to the arthropod molting process. The present study emphasizes the variations in the levels of hemolymph total free sugars, hepatopancreas glycogen and cuticular proteins during the molting stages of Portunus pelagicus. It also reports the effect of short-term starvation conditions on the biochemical constituents of the hemolymph. Intermolt crabs were subjected to 6 days of starvation and hemolymph samples were taken. Standard biochemical procedures were followed toward the quantification of total proteins, total free sugars and total lipids. The total free sugar level in the hemolymph of P. pelagicus was observed to increase during early premolt D0 (3.108 ± 0.032 g/ml) and a gradual decrease till late postmolt B stage (0.552 ± 0.124 g/ml), suggesting the need for total free sugars to provide energy for the apolysis process. Increase in the levels of hepatopancreas glycogen was observed from 1225 ± 0.04 μg/mg in early premolt D0 to 1700 ± 0.3 μg/mg in late premolt D2–3. This is in correlation with the decreased levels of free sugars during premolt stages, suggesting an increase in the storage of glycogen reserves in the hepatopancreas. Cuticular proteins increased during stage B (2.702 ± 0.093 g/ml) and stage C (3.065 ± 0.012 g/ml), indicating exoskeleton hardening and mineralization. Results of the starvation studies clearly showed a steady decline in the level of total free sugars till day 6 (0.099 ± 0.00 g/ml) when compared to the control (8.646 ± 0.08 g/ml). Gradual decrease of total lipids was also observed from the first day of the experiment (6.088 ± 2.44 g/ml) to the last day of the study (0.401 ± 0.20 g/ml) which was 85% lesser than the control (8.450 ± 0.49 g/ml)suggesting the efficient usage of total sugars to consolidate the loss of energy reserves during starvation. The knowledge of Molt-cycle events can be used as a tool for the evaluation of the developmental state providing a morphological reference system for physiological and biochemical studies related to crab aquaculture. Starvation studies enlightens that increasing carbohydrate levels in crab feed together with good protein content could alleviate the natural effects of starvation, improve farm productivity and reduce the deleterious impact of nitrogen pollution generated by rich-protein feeds used in crab farming.
Keywords: Molting, Cuticular proteins, Hepatopancreas, Hemolymph, Biochemical constituents
1. Introduction
Comparative biochemistry in crustaceans has developed in recent years due to the interest in aquaculture, but has taken many initial hypotheses from the insect biochemistry field. Insects are and will remain as a very useful model for biochemical research and precisely due to its evolutionary closeness, crustacean biochemistry can take advantage of the available insect knowledge (Law and Wells, 1989). The biochemical changes occurring in crustaceans during molting, feeding and starvation are indicators of their nutritional requirements and are an important basis for determining suitable diets.
Crustaceans differ from insects in that they combine to molt and grow even after the attainment of sexual maturity. In large decapod crustaceans such as crabs and lobsters, the female reproductive cycle is completed within the protracted intermolt period and molting is initiated only after reproductive arrest (quiescent period). Conversely, in many soft shelled shrimps and prawns, molting is permitted to occur during the course of oogenic cycle, thus revealing a close synchronization between molting and the reproductive process (Subramoniam, 2000). The pattern of changes, an increase in blood glucose and protein levels during premolt, a decrease following the molt and more or less rapid return to the intermolt level, have been reported (Telford, 1974). Renaud (1949) described the cycling of carbohydrates and lipids through the molt cycle of Cancer pagurus and an apparent increase in blood reducing substances during premolt. In a study of spiny lobster, Panulirus argus, Travis (1955, 1957) has shown a detailed pattern of the use of glycogen during the molt cycle, from which the elevated level of blood glucose in premolt and postmolt decreases could be predicted. Telford (1968) has cited several sugars including mannose, galactose, fructose, maltose and trehalose in marine decapods. Presumably, they play a less prominent role in the metabolism of freshwater crayfishes. McWhinnie and Saller (1960) found glucose to makeup 25% of the total reducing substances in the blood of Orconectes virilis, which is in close agreement with the observation of Telford (1974). There have also been studies which sought to determine relatedness among crustacean and insect exoskeletal proteins. Dennell (1947) showed similarities between the crustacean exoskeleton and the insect exoskeleton with respect to composition, deposition and hardening. Many insect and crustacean exoskeletal proteins have also been shown to share characteristics such as acidic isoelectric points and molecular weights of 31,000 daltons or smaller (O’Brien et al., 1991). More recently, immunological crossreactivity between crustacean exoskeletal proteins and insect storage hexamerins has been demonstrated (Stringfellow and Skinner, 1988; Kumari and Skinner, 1993).
Soluble glycoproteins from the organic matrix of the cuticle of brachyurans have been studied at all stages of the molt cycle. Electrophoretic patterns of extracts of lectin-binding proteins from the calcified exoskeleton layers of the Bermuda land crab Gecarcinus lateralis change dramatically during and after apolysis (Kumari and Skinner, 1995). These pre-molt changes in the existing cuticle relate to mineral dissolution and resorption rather than mineral deposition. Various mild aqueous solvents were used to extract glycoproteins from the anecdysial (intermolt) cuticle of the Atlantic shore crab Carcinus maenas, and the complex array of electrophoretic bands described from these extracts contained both O-linked and N-linked glycans (Compere et al., 2002).
Studies on the starvation of crustaceans in biochemical composition are intended to yield information that remains useful in understanding the ecophysiology of a population (Lehtonen, 1996). Artificially induced fasting and starvation may enlighten the metabolic routes used in hierarchical order and may describe novel biochemical and physiological adaptation mechanisms (Barclay et al., 1983). The ability of an organism to survive and recover from long periods of starvation is vital. Starvation can lead to a severe deficiency of nutrients. Therefore, starvation studies may be useful predictors to determine energetic and metabolic requirements (Guderley et al., 2003). Furthermore, the knowledge derived from the understanding of their biochemical processes may be the basis to optimize crustacean pond rearing efforts.
Proteins are critical for artificially reared crustaceans and are an expensive component of feeds for decapod crustaceans (Kureshy and Davis, 2000). Therefore, the dietary protein quantity and composition should be optimized to grant maximal growth (Shiau, 1998). Feed protein contents between 30% and 57% (w/w) are recommended for suitable growth of different species of the penaeid shrimp (Cordova-Murueta and Garcia-Carreno, 2002; Kureshy and Davis, 2000; Shiau, 1998). During a 28-day starvation study, in the hepatopancreas of the shrimp Penaeus japonicus, the glycogen stores were rapidly depleted, presumably being converted to glucose and are used as an energy source (Cuzon et al., 1980). Tail muscle lipids diminished progressively and proteins were next mobilized, but more slowly, eventually accompanied by muscular atrophy. Similar results were obtained for the shrimp Penaeus duorarum (Schafer, 1968) and for the Crangon crangon (Cuzon and Ceccaldi, 1973). However, the purple shore crab Hemigrapsus nudus during a 23-day starvation period used preferentially proteins (Neiland and Scheer, 1953) as reported for other decapods more recently (Anger, 2001). Moreover, crustacean responses to starvation appear to be influenced by the developmental stage. Spiny lobster Jasus edwardsii, phyllosoma larvae during a 6–11-day starvation catabolized more lipids than carbohydrates and proteins in stages II, IV and VI. These larvae were 14–40% lighter than their fed counterparts (Ritar et al., 2003). The main lipid storage organ in crustaceans is hepatopancreas. Lipids are mobilized to and from this organ through lipoproteins that bind and carry these hydrophobic molecules in the aqueous hemolymph environment. High density lipoproteins (HDL) and very high density lipoproteins (VHDL) are the main lipoproteins found in crustacean species (Lee and Puppione, 1978; Yepiz-Plascencia et al., 2000, 2002).
Reports about the metabolic requirements of protein and lipids under starvation in crustaceans are very contrasting. As mentioned before, several authors report protein as the main source of energy for starved crustaceans. During the starvation of crustaceans, there are three distinct phases of biomass degradation (Anger, 2001). Initially, energy-rich lipid reserves are preferentially mobilized, reflected in decreasing lipid:protein ratios, which is typical of short term food deprivation. When much of the accessible lipid pool has been depleted, proteins are increasingly utilized. A significant part of the lipid pool is bound in crucial cell structures such as membranes and hence is normally unavailable for energy metabolism. In the final phase of starvation prior to death, structural lipids may also be degraded so that the lipid:protein ratio decreases again (Mikami et al., 1995; Abrunhosa and Kittaka, 1997).
P. pelagicus (Linnaeus, 1758), the blue swimmer crab found in the intertidal estuaries of the Indian and Pacific Oceans, forms the important source of commercial fishery in the Thondi Coast. They are exported to South East Asian countries under live conditions. Because of their delicacy and larger size, the live mud crabs are always in greater demand and fetch a higher price in both national and international markets (Kathirvel, 1993). Swimming crabs, both P. pelagicus and P. sanguinolentus are being exported mostly in frozen and canned forms. The males are bright blue in color with white spots and long chelipeds. And the females are duller green or brown in color. Male and female P. pelagicus generally reach sexual maturity at a size of 70–90 mm in carapace width, when they are approximately one year old.
Besides seasonal changes in food availability, a common denominator in crustaceans and P. pelagicus in particular, is their constant feeding activity. Furthermore, they alternate episodes of feeding and fasting during development, which occurs through Molting and results in growing by sequential steps. Increase in body size at each ecdysis is non-linear; this is a hormonally controlled process which might last days or weeks, is continuous and accompanied by morphological, physiological and behavioral alterations occurring almost daily (Dall et al., 1990). This process requires a high amount of energy. Molting involves a series of stages with different feeding behavior. During intermolt, they feed actively, prior to molting, feeding declines until it stops completely during ecdysis. Finally feeding begins again in postmolt (Phlippen et al., 2000). Starvation can lead to a severe deficiency of nutrients. Starvation induction of crustaceans in the intermolt stage has been suggested to be a good model to try to understand the molecular and enzymatic changes that occur naturally during their growth process, although the effect of hormones must not be forgotten (Sanchez-Paz et al., 2003). Therefore, starvation studies may be useful predictors to determine energetic and metabolic requirements (Guderley et al., 2003).
The effects of starvation on the blue swimmer crab, P. pelagicus have not been examined in terms of accumulation and loss of the major body components viz., protein, lipid and carbohydrate. The findings of starvation studies can be used to determine the nutrients most critical as energy reserves and those catabolized or conserved in the face of increasing food deprivation. In view of the afore mentioned information, the present study was aimed primarily toward the examination of carbohydrate metabolism and cuticular proteins in P. pelagicus with reference to the molting cycle and secondly to study the effect of short-term starvation on biochemical constituents in adult males of P. pelagicus in the intermolt stage to gain insights on the connection between episodes of food shortage, metabolic preferences and sequence of the use of energy reserves.
2. Materials and methods
2.1. Collection & maintenance
Adult blue swimmer crabs, Portunus pelagicus, were caught from the Thondi Coast, Thondi (9o45’N 79o04’E). The crabs were transported to the laboratory in aerated plastic troughs. They were weighed and acclimatized for a week in tanks containing 10–15 cm of sand at the bottom at about 34 ± 2 ppt salinity and at room temperature (30 ± 2 °C). During the period, the crabs were fed with oyster (Crassostrea madrasensus) meat twice a day. The unconsumed meat and other debris particles were removed by siphoning. The water was removed and fresh sea water was introduced daily.
2.2. Analysis of Molt stages
Setal development of P. pelagicus was observed on the basis of the epidermal retraction observed at the posterior median part of the swimmeret. Molt stages were determined using morphological changes of the seta as described by Drach and Tchernigovtzeff (1967) using a light microscope (Optika B-350, Italy).
2.3. Extraction of cuticular proteins
Cuticular proteins were extracted from the exoskeleton following the method of Otoshi (1994). Briefly, the dorsal carapace portion of the exoskeleton of P. pelagicus was always used so that contaminants such as hair bristles could be excluded. The exoskeleton was brushed clean and rinsed of any visible cellular material with deionized water. It was then dried at approximately 60 °C until constant weight was achieved. The dried exoskeleton was then ground with mortar and pestle at room temperature until it was a fine powder. The proteins from the ground exoskeleton were then extracted with a 1% KCl solution, pH 7.5, using 12 ml solution per gram of ground exoskeleton. The extraction mixture was incubated overnight at 4 °C. The mixture was then centrifuged, for 5 min at 13,000 rpm (Remi C-24 BL Cooling Centrifuge, India). The supernatant was stored at 4 °C until further protein analysis.
2.4. Starvation experiments
Only intermolt crabs were chosen for starvation experiments. After acclimation, the crabs were measured (carapace length, carapace width) and weighed (wet weight). Subgroups of 6 crabs each, were maintained for 0 (control group), 1, 2, 3, 4, 5 and 6 starvation days. The samples for biochemical analysis were obtained on every starvation day from the member of the subgroup.
2.5. Hemolymph sampling
Hemolymph samples were drawn from the crabs of various molting stages as well as from control and starved crabs through the arthrodial membrane of the pereiopods by using disposable syringes. Approximately 10 ml was obtained from each crab. The collected hemolymph was stored in separate vials under −20°C until further use.
2.6. Quantification of biochemical constituents
The biochemical composition of cuticular proteins, total free sugars in the hemolymph and hepatopancreas glycogen during the molt cycle and the level of total proteins, total free sugars and total lipids in the hemolymph of control and starved crabs was estimated following standard procedures. Estimation of total proteins and cuticular proteins was done as per the methodology of Bradford (1976). Estimation of total free sugars was done according to the methodology of Roe (1955). Estimation of glycogen in the hepatopancreas was done according to the methodology of Carroll et al. (1956). Estimation of total lipids was done according to the methodology of Barnes and Blockstock (1973). Extraction of lipids from sample was done following the procedure of Folch et al. (1957).
2.7. Data analysis
The results of the study were subjected to Two-way ANOVA to test whether the variations in the biochemical constituents among the various stages of molting and between the starvation periods are significant.
3. Results
3.1. Variations in the biochemical composition during the different molting stages
Results of the biochemical analysis when subjected to Two-way ANOVA clearly enumerated variations among the biochemical parameters during the different stages of molting (F < 0.05).
3.1.1. Total free sugars in hemolymph
Variations were observed in the levels of total free sugars in the hemolymph of P. pelagicus during the different stages of molting. Greater level of 3.108 ± 0.03 g/ml was observed during premolt D0 stage, following which a steady decline was noticed thereafter till postmolt B stage (0.552 ± 0.12 g/ml). Intermolt C stage had a significant increase in the free sugar titer of 1.318 ± 0.15 g/ml) (Fig. 1).
Figure 1.
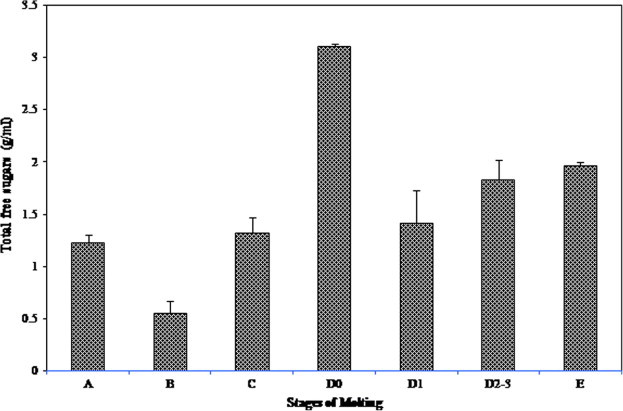
Variations in the total free sugars in the hemolymph during molting.
3.1.2. Hepatopancreas glycogen
Depletion of glycogen in the hepatopancreas was observed after the late premolt stages D2–3 (1700 ± 0.30 μg/mg). Postmolt B stage had a minimal level of 425 ± 0.02 μg/mg followed by postmolt A (837 ± 0.23 μg/mg). An increase in the glycogen level was observed thereafter in intermolt C (1012 ± 0.43 μg/mg) till late premolt stage (Fig. 2).
Figure 2.
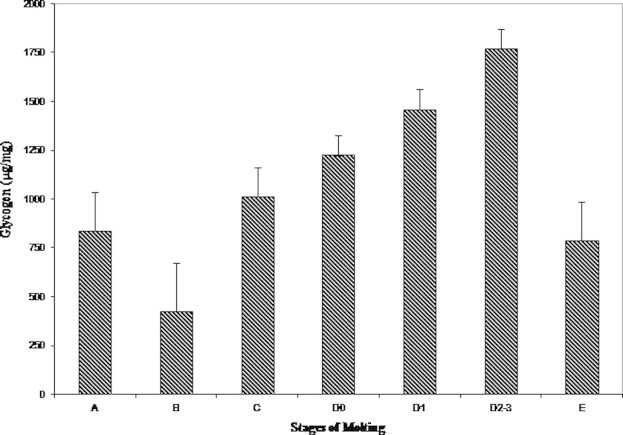
Variations in the hepatopancreas glycogen during molting.
3.1.3. Cuticular proteins
A steady decline in the level of cuticular proteins was observed right from the early premolt stage D0 (1.469 ± 0.32 mg/ml) in correlation with the onset of setogenesis. The protein level dropped to 0.688 ± 0.476 mg/ml during late premolt stage D2–3 and a significant increase in glycogen was noticed in postmolt A (0.854 ± 0.08 mg/ml) and postmolt B (2.702 ± 0.09 mg/ml). Cuticular proteins were found to be highly concentrated in the exoskeleton in the intermolt stage C (3.065 ± 0.02 mg/ml) (Fig. 3).
Figure 3.
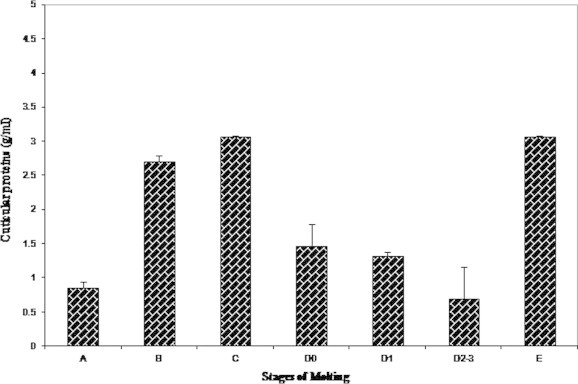
Variations in the cuticular proteins during molting.
3.2. Variation in the survival, weight and body measurements of P. pelagicus on starvation
During the experiment no mortalities were recorded. Relatively significant reduction in body weight on day 6 (53.04 ± 2.34 g) when compared to the control (77.05 ± 3.03 g). Insignificant variations were observed in carapace length and carapace width of the starved crabs when compared to the control (Fig. 4).
Figure 4.
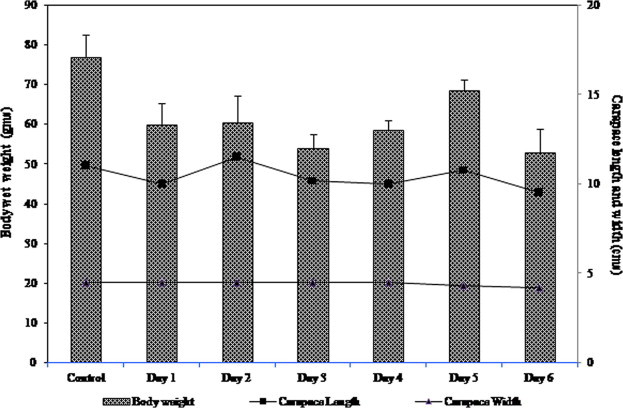
Weight and body measurements of P. pelagicus during starvation.
3.3. Biochemical composition of hemolymph on starvation
Along the starving periods, significant differences were observed in the relative biochemical composition of the hemolymph of the experimental crabs. A drastic decrease was observed in the levels of the constituents from day 2 of the starvation period except the total protein level. Two way–Analysis of Variance showed significant variations within the days of starvation and between all the biochemical constituents studied (F < 0.01).
3.3.1. Total proteins
Significant decrease in the level of total protein was observed on day 1 (2.670 ± 1.11 g/ml) when compared to the control (6.259 ± 0.01 g/ml). Thereafter, protein concentration peaked more than the control on day 5 (6.597 ± 2.35 g/ml), about 0.5% increase. An abrupt decline in the level of total proteins was observed (2.636 ± 1.52 g/ml) on day 6 which was approximately 30% of the control group (Fig. 5).
Figure 5.
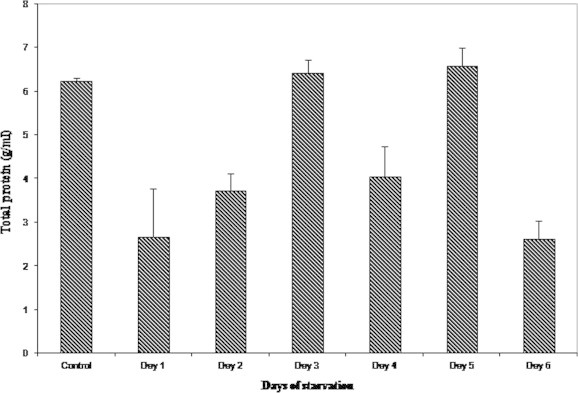
Variations in the hemolymph total protein on starvation.
3.3.2. Total free sugars
A gradual but a sharp decline was observed in the level of free sugars on starvation from control to day 6. The level of free sugars decreased to almost 50% that of the control group (8.646 ± 0.08 g/ml) on the very first day of starvation (4.409 ± 0.00 g/ml). Reduction in the level of free sugars from 4.123 ± 0.02 g/ml on day 2–0.099 ± 0.00 g/ml on day 6 was recorded (Fig. 6).
Figure 6.
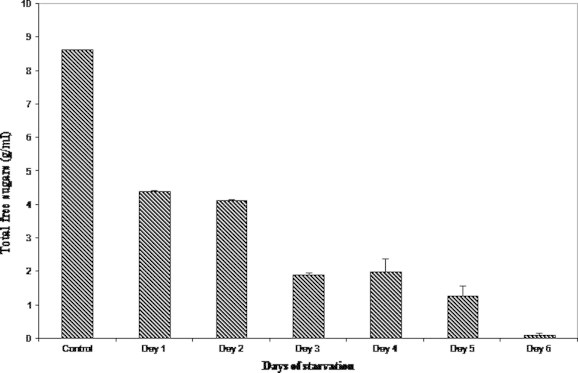
Variations in the hemolymph total free sugars on starvation.
3.3.3. Total lipids
The total lipid concentration of the hemolymph of the experimental crabs steadily decreased from day 1 till the termination of the experiment on day 2. Experimental crabs of day 3 showed a significant decrease in the level of total lipids (1.794 ± 0.088 g/ml) which was approximately 75% less than the control group (8.450 ± 0.49 g/ml). The final concentration on day 6 of starvation was 0.401 ± 0.20 g/ml, a 95% reduction in the total lipid level when compared to the control (Fig. 7).
Figure 7.
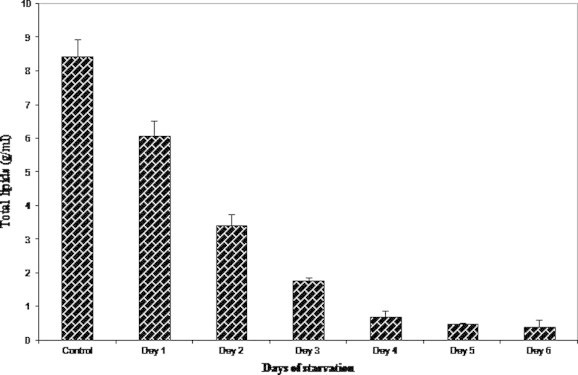
Variations in the hemolymph total lipids on starvation.
4. Discussion
Arthropod growth patterns characterized by molt cycles present some trade-offs such as the need for variable muscle atrophy and restoration to accommodate the body inside the new slightly bigger exoskeleton. Various environmental conditions such as temperature, light (Bermudes and Ritar, 2008; Bermudes et al., 2008), salinity (Romano and Zeng, 2006), or feeding treatments (Minagawa and Murano, 1993) can modify the molting cycle, which implies changes in numerous physiological, biochemical, and behavioral parameters (Chang, 1995; Anger, 2001).
Alvarez-Fernadez et al. (2005) have studied the role of lipid, protein, carbohydrate and nucleic acid in the molt cycle of Norway lobster, Nephrops norvegicus. In their study, the lipid content in hepatopancreas has been found to increase along the premolt period (stages D0 and D3) to cover the increase in energetic requirements in later stages. This increase in requirements results from starvation from stage D3 until the end of the postmolt period, altogether with the formation of the new exoskeleton (Mayrand et al., 2000).
In the present study, the level of total free sugars in the hemolymph was assayed during the molt stages, revealing increase in its level during the premolt stages. A gradual decline was observed thereafter on the onset of postmolt A and B, and a sharp increase in intermolt C was seen and a higher level during early premolt D0. The results of the present study resemble those of Telford (1968), who has studied the changes in blood sugar composition during the molt cycle of the lobster Homarus americanus. Glucose levels were 35 percent higher in premolt than in intermolt and were 30 percent lower in postmolt. Besides its role in digestion, the digestive gland or hepatopancreas actively participates in the molt cycle, being the major site for storage glycogen, fats, and calcium during premolt and thus, in the mobilization of these reserves when needed in subsequent molt stages. When under stress (e.g., molting), the metabolic activities of the crabs increase rapidly, causing the hepatopancreas to release higher levels of metabolites into the hemolymph. Depletion of hepatopancreas glycogen in P. pelagicus was observed in the present study during postmolt A and B which arose to greater levels during intermolt C and early premolt D0. This runs in line with the observed levels of total free sugars in the hemolymph observed in the present study, thus envisaging the mobilization of glycogen reserves from the hepatopancreas during the molt cycle to provide energy in the form of sugars.
Cuticle proteins are suggested to be involved in the calcification process (Andersen, 1999; Kragh et al., 1997) and in chitin binding. In the present study, the concentration of cuticular proteins was studied during the molting stages of P. pelagicus revealing its increase from postmolt B to intermolt C stage. This provides a positive correlation between the thickening of the epidermis and cuticle during postmolt B and intermolt C and the increase in the cuticular protein level. Hemolymph proteins have been found to increase during intermolt C because the internal tissue growth takes place during this stage (Passano, 1960a,b), and is considerably low in postmolt, premolt and molt. Because the growth starts to occur during the stages A–B (postmolt), and in the premolt stage D the rate of growth decreases and the rate of feeding also decreases (Freeman and Perry, 1985).
Starvation studies give indications of the energy resources utilized by crustaceans and provide clues to the biochemical pathways. The 100% survival of P. pelagicus in the present study showed the capacity of tolerance at starving conditions for 6 days which lies in coincidence with the observations of Comoglio et al. (2004, 2005, 2008) for Litopenaeus vannamei and the southern king crab Lithodes santolla, exposed to starvation for 12 days. P. pelagicus in the present study has suffered considerable weight loss during the period of starvation. Steffens (1989) has suggested that starvation affects metabolic activities and during this period essential processes are maintained at the expense of accumulated endogenous energy reserves, which sometimes result in the loss of weight. However, some authors have detected that some crustaceans such as shrimps and lobsters compensate the weight of organic matter that they use in starving conditions with water uptake so that no loss of weight is detected (Dall, 1974; Wilcox and Jeffries, 1976).
P. pelagicus presents a significant variation in its biochemical composition under starvation. In the present study, total protein in the hemolymph remained constant during the experiment with only a slight decrease in its content on day 1 and day 6 of the fasting period. These results disagree with many reports that maintain that protein is the main energy source for most crustaceans but may explain previous findings. Mayzaud and Conover (1988) have also reported an increase of ammonia excretion and low values of O:N during the starvation period. Muhlia-Almazan and Garcia-Carreno (2002) showed that in L. vannamei, hepatopancreatic trypsin activity was significantly affected by food shortage (differences of 35% between 2 and 120 h of starvation), while chymotrypsin activity decreased 40% at the same starvation level. Protein changes may occur in which the crustacean switches to the use of one energy reserve to another, depending on the developmental stage. In the copepod Calanus finmarchicus, the use of energy changed: during the first 10 days of starvation and the protein content showed a moderate decline, suggesting that this organism copes with starvation utilizing endogenous reserves different than protein; however, during the next 21 days, total protein content was drastically reduced (Helland et al., 2003). In subterranean aquatic crustaceans changes have also been found. After 28 days of starvation, the isopod Asellus aquaticus responded with an immediate, linear and large decrease of all the energy reserves, most of which were fully recovered after a 7-day refeeding period. In contrast, prolonged fasting (180 days) in the isopod Stenasellus virei was characterized by three successive phases: (1) an immediate, but low, depletion of both glycogen and arginine phosphate, followed by (2) the utilization of triacylglycerides associated with glycogen resynthesis and finally (3) a slow depletion of both proteins (demonstrated by a slight increase in ammonia excretion rate) and lipids, always associated with a glycogen resynthesis. As in A. aquaticus, S. virei energy reserves were fully recovered after a 15-day refeeding period (Hervant and Renault, 2002). Strategies of fuel reserves usage may change depending on the species and the larval stage (Le Vay et al., 2001).
Carbohydrates and lipids showed significant variations in the present study indicating both rapid accumulation and depletion. Azeiteiro et al. (2003) have reported that the carbohydrates and lipids were the most affected during the refeeding period, when the accumulation of these constituents did not reach the starting levels in Mesopodopsis slabberi. From the entire set of metabolites, studied, total free sugars was the most drastically affected by starvation, dropping constantly from the beginning of the study and stabilizing to approximately 5% of its initial value after 6 days of food deprivation. The results of the present study indicate that glucose is the first source used by the crab for dealing with the lack of food. While total free sugars were rapidly consumed, protein concentration decreased slightly. Although it cannot be ruled out that proteins are used as an energy source, the rapid decrease of glucose indicates that it is the first fuel utilized. This response may be an adaptative strategy to avoid usage of high cost energy macromolecules, at the beginning of a food shortage episode. Prudent utilization of protein in very short starvation periods could represent energy protection in the case of prolonged food scarcity intervals.
One of the most important roles of lipids in crustaceans is related to reproduction, since they are associated with the maturation of oocytes and the survival of the initial larval stages. Total lipids decreased sensibly (90%) reaching the lowest values after 6 days of starvation. Both, sterols and acylglycerides may be the main cause. It has been proposed that in crustaceans, neutral lipids are preferentially catabolized during starvation, while polar lipids (phospholipids and cholesterol) are conserved due to their role as structural components of cell membranes (Heath and Barnes, 1970; Bourdier and Amblard, 1989; Stuck et al., 1996). A large reduction in total lipids (particularly a total depletion of triacylglycerides stores) as a response to starvation for the lake dwelling copepod Acanthodiaptomus denticorni was reported (Bourdier and Amblard, 1989). Similar results were found for larvae, adult and sub-adult lobsters (Stuck et al., 1996). Ritar et al. (2003) reported that lipid dry weight in lobster larval stages II, IV and VI, declined during starvation to 81%, 41%, and 73%, respectively, compared to fed larvae. Additionally, polar lipids were the only lipid class significantly reduced during starvation (45%, 38%, and 70%) in stages II, IV and VI, respectively. The next most abundant lipid class in phyllosoma was sterol, and was the only lipid class conserved during starvation at all stages.
Studies about the metabolism of crustaceans and their ability to adapt to environmental variations contribute to the understanding and elucidation of perhaps new mechanisms. More research is necessary to understand the biochemical and physiological aspects of crustacean nutritional requirements, especially considering the high degree of flexibility in the digestive physiology of crustaceans, as an essential part of their ability to grow, survive, and reproduce when the food supply changes or depletes.
5. Conclusion
The results of the present study emphasizes the role of biochemical constituents in the molting process and thereby throwing light on the molting stages that could be accounted for commercial procurement. Furthermore, the quantification of cuticular proteins of P. pelagicus through the molt stages in the present study is an initial step toward discovering answers for the following questions in the near future. In P. pelagicus, feeding takes place throughout the year, except during a few weeks of the Molting–mating period, when feeding ceases or is at a minimum. In this context, the results of the present study give new and relevant biological information about the physiological and biochemical responses during starving conditions about an important commercial species inhabiting the Palk bay.
Acknowledgement
This research was partially financed by a project funded by the University Grants Commission (UGC/F.No.39-566/2010 SR), Government of India, New Delhi.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abrunhosa F.A., Kittaka J. Effect of starvation on the first larvae of Homarus americanus and phyllosomas of Jasus verreauxi and J. edwardsii. Bull. Mar. Sci. 1997;61:73–80. [Google Scholar]
- Alvarez-Fernadez, I., Sardà, F., Rotllant, G., Verísimo, P., Fernández, L., 2005. Role of lipid, protein, carbohydrate and nucleic acid as indicators of the Molting cycle of Norway lobster (Nephrops norvegicus). ASLO Summer meeting 2005, June 19-24, Santiago de Compostela, Spain.
- Andersen S.O. Exoskeletal proteins from the crab, Cancer pagurus. Comp. Biochem. Physiol. 1999;123A:203–211. doi: 10.1016/s1095-6433(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Anger, K., 2001. The biology of decapod crustacean larvae, in: Crustacean Issue 14. A.A. Balkema Publishers, Swets and Zeitlinger, Lisse, ISSN 0168-6456, pp. 1–420.
- Azeiteiro U.M., Fonseca J.C., Pastorinho R., Morgado F., Marques J.C. Patterns of variation in the biochemical composition of Mesopodopsis slabberi. Inst. Esp. Oceanogr. 2003;19(1–4):433–442. [Google Scholar]
- Barclay M.C., Dall W., Smith D.M. Changes in lipid and protein during starvation and the Molting cycle in the tiger prawn, Penaeus esculentus Haswell. J. Exp. Mar. Biol. Ecol. 1983;68:229–244. [Google Scholar]
- Barnes H., Blockstock J. Estimation of lipids in marine animals and tissues: detailed investigation of the sulpho phosphovanillin method for total lipids. J. Exp. Mar. Biol. Ecol. 1973;12:103–118. [Google Scholar]
- Bermudes M., Ritar A.J. Response of early stage spiny lobster Jasus edwarsii phyllosoma larvae to changes in temperature and photoperiod. Aquaculture. 2008;281:63–69. [Google Scholar]
- Bermudes M., Ritar A.J., Carter C.G. The ontogeny of physiological response to light intensity in early stage spiny lobster (Jasus edwarsii) larvae. Comp. Biochem. Physiol. 2008;150A:40–45. doi: 10.1016/j.cbpa.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Bourdier G.G., Amblard C.A. Lipids in Acanthodiaptomus denticornis during starvation and fed on three different algae. J. Plankton Res. 1989;11:1201–1212. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carroll N.V., Longley R.W., Roe J.H. The determination of glycogen in liver and muscle by the use of anthrone reagent. J. Biol. Chem. 1956;220:586–593. [PubMed] [Google Scholar]
- Chang E.S. Physiological and biochemical changes during the molt cycle in decapod crustaceans, an overview. J. Exp. Mar. Biol. Ecol. 1995;193:1–14. [Google Scholar]
- Comoglio L.I., Gaxiola G., Roque A., Cuzon G., Amin O. The effect of starvation on refeeding, digestive enzyme activity, oxygen consumption and ammonia excretion in juvenile white shrimp Litopenaeus vannamei. J. Shellfish Res. 2004;23(1):243–249. [Google Scholar]
- Comoglio L.I., Smolko L., Amin O. Effects of starvation on oxygen consumption, ammonia excretion and biochemical composition of the hepatopancreas on adult males of the false southern king crab Paralomis granulose. Comp. Biochem. Physiol. 2005;140B:411–416. doi: 10.1016/j.cbpc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Comoglio L.I., Goldsmit J., Amin O. Starvation effects on physiological parameters and biochemical composition of the hepatopancreas of the southern king crab Lithodes santolla. Rev. Biol. Mar. Oceanogr. 2008;43(2):345–353. [Google Scholar]
- Compere P., Jaspar-versali M.F., Goffinet G. Glycoproteins from the cuticle of the Atlantic shore crab Carcinus maenas. Biol. Bull. 2002;202:61–73. doi: 10.2307/1543223. [DOI] [PubMed] [Google Scholar]
- Cordova-Murueta J.H., Garcia-Carreno F.L. Nutritive value of squid and hydrolyzed protein supplement in shrimp feed. Aquaculture. 2002;210:371–384. [Google Scholar]
- Cuzon G., Ceccaldi H.J. Influence of the fasting stabulation on the metabolism of the shrimp Crangon crangon (L.) C. R. Soc. Biol. 1973;167:66–69. [PubMed] [Google Scholar]
- Cuzon G., Cahu C., Aldrin J.F., Messager J.L., Stephan G., Mevel M. Starvation effect on metabolism of Penaeus japonicus. Proc. World Maric. Soc. 1980;11:410–423. [Google Scholar]
- Dall W. Indices of nutritional state in the western rock lobster Panulirus longipes:1. Blood and tissue constituents and water content. J. Exp. Mar. Biol. Ecol. 1974;16:176–180. [Google Scholar]
- Dall W., Hill B.J., Rothlisberg P.C., Staples D.J. Academic Press; London: 1990. The Biology of the Penaeidae, Advances in Marine Biology. [Google Scholar]
- Dennell R. The occurrence and significance of phenolic hardening in the newly formed cuticle of Crustacea Decapoda. Proc. R. Soc. London. 1947;134B:485–503. doi: 10.1098/rspb.1947.0027. [DOI] [PubMed] [Google Scholar]
- Folch J., Lee M., Stanley G.H.S. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957;226:477–509. [PubMed] [Google Scholar]
- Freeman, J.A., Perry, H.M., 1985. The crustacean molt cycle and hormonal regulation: its importance in soft shell blue crab production, in: Perry, H.M., Malone, R.F. (Eds.), Proceedings of the National Symposium on the Soft-Shelled Blue Crab Fishery, pp. 23–39.
- Guderley H., Lapointe D., Bedard M., Dutil J.D. Metabolic priorities during starvation: enzyme sparing in liver and white muscle of Atlantic cod, Gadus morhua L. Comp. Biochem. Physiol. 2003;2A:347–356. doi: 10.1016/s1095-6433(03)00089-8. [DOI] [PubMed] [Google Scholar]
- Heath I.R., Barnes H. Some changes in biochemical composition with seasons and during molting cycle of the common shore crab Carcinus maenas. J. Exp. Mar. Biol. Ecol. 1970;5:199–233. [Google Scholar]
- Helland S., Nejstgaard J.C., Fyhn H.J., Egge J.K., Bamstedt U. Effects of starvation, season, and diet on the free amino acid and protein content of Calanus finmarchicus females. Mar. Biol. 2003;143:297–306. [Google Scholar]
- Hervant F., Renault D. Long-term fasting and realimentation in hypogean and epigean isopods: a proposed adaptive strategy for groundwater organisms. J. Exp. Biol. 2002;205:2079–2087. doi: 10.1242/jeb.205.14.2079. [DOI] [PubMed] [Google Scholar]
- Kathirvel M. Handbook of Aquafarming of Shrimp, Lobster and Mudcrabs. MPEDS Publication; Cochin: 1993. Mudcrab; p. 72. [Google Scholar]
- Kragh M., Mølbak L., Andersen S.O. Cuticular proteins from the lobster, Homarus americanus. Comp. Biochem. Physiol. 1997;118B:147–154. doi: 10.1016/s0305-0491(97)00055-2. [DOI] [PubMed] [Google Scholar]
- Kumari S.S., Skinner D.M. Proteins of crustacean exoskeleton II: immunological evidence for their relatedness to cuticular proteins of two insects. J. Exp. Zool. 1993;265:195–210. [Google Scholar]
- Kumari S.S., Skinner D.M. Proteins of the crustacean exoskeleton, III. Glycoproteins in the Bermuda land crab Gecarcinus lateralis. J. Exp. Zool. 1995;271:413–424. doi: 10.1002/jez.1402730504. [DOI] [PubMed] [Google Scholar]
- Kureshy, N. and Davis, D.A. (2000). Metabolic requirements for protein by pacific white shrimp, Litopenaeus vannamei, in: Cruz-Suarez, L.E., Ricque-Marie, D., Tapia-Salazar, M.A., Civera-Cerecedo, R. (Eds.), Avances en Nutricio´ n Acuıcola V. Memorias del V Simposium de Nutricio´ n Acuı´cola. 19–22 Noviembre, 2000. Me´ rida, Yucata´ n. pp. 161–180.
- Law J.H., Wells M.A. Insects as biochemical models. J. Biol. Chem. 1989;264:16335–16338. [PubMed] [Google Scholar]
- Le Vay L., Jones D.A., Puello-Cruz A.C., Sangha R.S., Ngamphongsai C. Digestion in relation to feeding strategies exhibited by crustacean larvae. Comp. Biochem. Physiol. 2001;128A:623–630. doi: 10.1016/s1095-6433(00)00339-1. [DOI] [PubMed] [Google Scholar]
- Lee R.F., Puppione D.L. Serum lipoproteins in the spiny lobster, Panulirus interruptus. Comp. Biochem. Physiol. 1978;59B:239–243. doi: 10.1016/0305-0491(78)90253-5. [DOI] [PubMed] [Google Scholar]
- Lehtonen K.K. Ecophysiology of the benthic amphipod Monoporeia affinis in an open-sea area of the northern Baltic Sea: seasonal variation in body composition, with bioenergetic considerations. Mar. Ecol. Prog. Ser. 1996;143:87–98. [Google Scholar]
- Mayrand E., Dutil J.D., Guderley H. Changes in muscle of postMolt snow crab (Chionoecetes opilio Fabricius) fed different rations. J. Exp. Mar. Biol. Ecol. 2000;243:95–113. doi: 10.1016/s0022-0981(00)00286-0. [DOI] [PubMed] [Google Scholar]
- Mayzaud P., Conover R.J. O:N atomic ratio as a tool to describe zooplankton metabolism. Mar. Ecol. Prog. Ser. 1988;45:289–302. [Google Scholar]
- McWhinnie M.A., Saller P.N. Analysis of blood sugars in the crayfish Orconectes virilis. Comp. Biochem. Physiol. 1960;1:110–112. [Google Scholar]
- Mikami S., Greenwood J.G., Gillespie N.C., Kittaka J. The effect of starvation and feeding regimes on survival, interMolt period and growth of cultured Panulirus japonicas and Thenus sp. Phyllosomas. Crustaceana. 1995;68:160–169. [Google Scholar]
- Minagawa M., Murano M. Larval feeding rhythms and food consumption by the red frog crab Ranina ranina (Decapoda, Raninidae) under laboratory conditions. Aquaculture. 1993;113(3):251–260. [Google Scholar]
- Muhlia-Almazan A., Garcia-Carreno F.L. Influence of molting and starvation on the synthesis of proteolytic enzymes in the midgut of the white shrimp Penaeus vannamei. Comp. Biochem. Physiol. 2002;133:383–394. doi: 10.1016/s1096-4959(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Neiland K.A., Scheer B.T. The influence of fasting and of sinus gland removal on body composition of Hemigrapsus nudus. Physiol. Comp. Oecologl. 1953;3:321. [Google Scholar]
- O’Brien J.J., Kumari S.S., Skinner D.M. Proteins of crustacean exoskeletons: I. Similarities and differences among proteins of the four exoskeletal layers of four brachyurans. Biol. Bull. 1991;181:427–441. doi: 10.2307/1542363. [DOI] [PubMed] [Google Scholar]
- Otoshi, A., 1994. Distribution and function of the hemolymph proteins, hemoecdysin and hemocyanin, in relation to the molt cycle of the juvenile Dungeness crab,Cancer magister and size-specific molting and reproductive capability of the adult female C. magister. Thesis, University of Oregon.
- Passano L.M. Molting and its control. In: Waterman T.H., editor. vol. I. Academic Press; New York: 1960. pp. 473–536. (The Physiology of Crustacea). [Google Scholar]
- Passano L.M. Metabolism and growth. In: Waterman T.H., editor. vol. 1. Academic Press; New York: 1960. pp. 507–509. (The Physiology of Crustacea, Molting and its control). [Google Scholar]
- Phlippen M.K., Webster S.G., Chung J.S., Dircksen H. Ecdysis of decapod crustaceans is associated with a dramatic release of crustacean cardioactive peptide into the haemolymph. J. Exp. Zool. 2000;203:521–536. doi: 10.1242/jeb.203.3.521. [DOI] [PubMed] [Google Scholar]
- Renaud L. Le cycle des reserves organiques chez les curstaces decapods. Ann. Inst. Oceanogr. Monaco. 1949;24:259–357. [Google Scholar]
- Ritar A.J., Dunstan G.A., Crear B.J., Brown M.R. Biochemical composition during growth and starvation of early larval stages of cultured spiny lobster (Jasus edwardsii) phyllosoma. Comp. Biochem. Physiol. 2003;136A:353–370. doi: 10.1016/s1095-6433(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Roe J.H. The determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955;212:35–343. [PubMed] [Google Scholar]
- Romano N., Zeng C. The effects of salinity on the survival, growth and haemolymph osmolality of early juvenile blue swimmer crabs, Portunus pelagicus. Aquaculture. 2006;260:151–162. [Google Scholar]
- Sanchez-Paz A., Garcia-Carreno L.F., Muhlia-Almazan A., Hernandez-Saavedra N., Yepiz-Plascencia G. Differential expression of trypsin mRNA in the white shrimp (Penaeus vannamei) midgut gland under starvation conditions. J. Exp. Mar. Biol. Ecol. 2003;292:1–7. [Google Scholar]
- Schafer H.J. Storage materials utilized by starved pink shrimp, Penaeus duorarum Burkenroad. FAO Fish Rep. 1968;57:393–403. [Google Scholar]
- Shiau S.Y. Nutrient requirements of penaeid shrimps. Aquaculture. 1998;164:77–93. [Google Scholar]
- Steffens W. Ellis Horwood; Chichester: 1989. Principles of fish nutrition. 384. [Google Scholar]
- Stringfellow L.A., Skinner D.M. Molt-cycle correlated patterns of synthesis of integumentary proteins in the land crab Gecarcinus lateralis. Dev. Biol. 1988;128:97–110. [Google Scholar]
- Stuck K.C., Watts S.A., Wang S.Y. Biochemical responses during starvation and subsequent recovery in postlarval white shrimp, Penaeus vannamei. Mar. Biol. 1996;125:33–45. [Google Scholar]
- Subramoniam T. Crustacean ecdysteriods in reproduction and embryogenesis. Comp. Biochem. Physiol. 2000;125:135–156. doi: 10.1016/s0742-8413(99)00098-5. [DOI] [PubMed] [Google Scholar]
- Telford M. Changes in blood sugar composition during the Molt cycle of the lobster, Homarus americanus. Comp. Biochem. Physiol. 1968;26:917–926. [Google Scholar]
- Telford M. Blood glucose in crayfish II. Variations induced by artificial stress. Comp. Biochem. Physiol. 1974;48A:555–560. doi: 10.1016/0300-9629(74)90738-5. [DOI] [PubMed] [Google Scholar]
- Travis D.F. The Molting cycle of the spiny lobster, Panulirs argus – III. Physiological changes which occur in the blood and urine during the normal Molting cycle. Biol. Bull. 1955;109:484–503. [Google Scholar]
- Travis D.F. The Molting cycle of the spiny lobster, Panulirus argus – IV. Post-ecdysial histological and histochemical changes in the hepatopancreas and integumental tissues. Biol. Bull. 1957;113:451–479. [Google Scholar]
- Wilcox J.R., Jeffries H.P. Hydration in the sand shrimp Crangon septemspinosa: relation to diet. Biol. Bull. 1976;150:522–530. doi: 10.2307/1540689. [DOI] [PubMed] [Google Scholar]
- Yepiz-Plascencia G., Vargas-Albores F., Higuera-Ciapara I. Penaeid shrimp hemolymph lipoproteins. Aquaculture. 2000;191:177–189. [Google Scholar]
- Yepiz-Plascencia G., Jimenez-Vega F., Romo-Figueroa M.G., Sotelo-Mundo R.R., Vargas-Albores F. Molecular characterization of the bifunctional VHDL-CP from the hemolymph of the white shrimp Penaeus vannamei. Comp. Biochem. Physiol. 2002;132B:585–592. doi: 10.1016/s1096-4959(02)00074-x. [DOI] [PubMed] [Google Scholar]



