Abstract
Fluconazole-entrapped multilamellar liposomes were prepared using the thin-film hydration method. The effects of cholesterol molar ratio, charge-inducing agents, and α-tocopherol acetate on encapsulation efficiency values and in vitro drug release of multilamellar liposomes were studied. Freeze-dried liposomal products were prepared with or without cryoprotectants. Results showed that incorporation of stearylamine resulted in an increased entrapment of fluconazole, whereas incorporation of dicetyl phosphate decreased the drug entrapment efficiency. The incorporation of α-tocopherol acetate into fluconazole multilamellar liposomes resulted in the increase of entrapment efficiency of fluconazole liposomes. In vitro release studies revealed that incorporation of cholesterol into multilamellar liposomal formulations decreased drug permeability from formulations. Positively charged fluconazole multilamellar liposomes gave rise to a slow release rate compared to neutral liposomes whereas negatively charged fluconazole liposomes showed a rapid release rate. Physical stability studies showed that lyophilized cake of liposomes without cryoprotectants was compact and difficult to reconstitute compared to fluffy easily reconstituted cakes upon using cryoprotectants. Fluconazole retained in freeze-dried liposomes without cryoprotectants was 63.452% compared to 91.877% using three grams of trehalose as a cryoprotectant per gram lipid in positively charged multilamellar liposomes. Physical stability studies showed superior potentials of the lyophilized product after reconstitution in comparison with those of a solution product.
Keywords: Fluconazole, Multilamellar liposomes, Ophthalmic, Freeze-drying, Stability
1. Introduction
In recent years, increasing attention is being paid to the development of controlled drug delivery system (CDDS). The advantages of CDDS over conventional therapy are numerous, including better plasma level profile, increased patient compliance, lower dosage and toxicity, possibility of targeting and more efficient utilization of active agent (Singla et al., 2000). Liposomes are one of the most suitable drug delivery systems to deliver the drug to the target organ and minimize the distribution of the drug to non-target tissues (Mezei, 1994; Fresta et al., 1993). The extent of absorption of an ophthalmic drug is limited by physiological factors such as reflex tearing and reflex blinking that exist in the precorneal area, resulting in the loss of an important drug (Lee, 1993). Liposomes can enhance corneal drug absorption achieved through their ability to come into intimate contact with the corneal and conjunctival surfaces. Earlier studies showed that liposome-associated idoxuridine is superior to the solution form of the drug in the treatment of Herpes simplex keratitis in rabbits (Dharma et al., 1986).
Fungal infections of the cornea, especially Candida, are considered a major problem in ophthalmology because of diagnostic and therapeutic difficulties (Narendran et al., 2008). Candida was the most common cause of fungal endophthalmitis. Fungal keratitis can impair vision or lead to total blindness. So various specific antifungal agents have been used (Klotz et al., 2000).
Fluconazole is a bis-triazole compound that exhibits a broad spectrum of antifungal activity belonging to the group of triazoles. It is effective against many fungal species including Candida (Price et al., 1994). It acts as a fungiostatic agent. The high penetration into the aqueous humor and low toxicity of fluconazole make it a good candidate for consideration as a topical ocular antifungal agent (Abbasoglu et al., 2001). The azole antifungal drugs inhibit the biosynthesis of ergosterol, the major sterol found in the fungal cell membrane, which is essential for the regulation of membrane fluidity and integrity and for fungal growth and proliferation (Ambrosini et al., 1998). Although topical fluconazole solutions in experimental Candida keratitis have proved to be effective, with good penetration into the cornea and aqueous humor, liposomal penetrations were still thought to provide potentially longer contact time, allowing greater penetration of the fluconazole (Behrens-Baumann et al., 1990; Yee et al., 1997; Yilmaz and Maden, 2005). Previous studies on liposomal formulations of fluconazole revealed that it was successful in eliminating experimental Candida albicans infection of the rabbit cornea and it was superior over fluconazole solution (Habib et al., 2009; Singh et al., 1993).
In general, the proposed types of liposome dispersions prepared from commercially available lipids do not meet the required standards for long-term stability of pharmaceutical preparations if they are stored as aqueous dispersions. The encapsulated drugs tend to leak out of the bilayer structure and the liposomes might aggregate or fuse on storage and it is generally necessary to use them within the first few months of preparation. As an alternative for storing aqueous liposomal dispersions, freeze-dried liposomes are proposed (Assadullahi et al., 1992; Madden and Boman, 1999).
Lyophilization increases the shelf-life of the finished product by preserving it in a relatively more stable dry state especially if the drug is not stable in aqueous systems. Some liposomal products in the market or in clinical trials are provided as a lyophilized powder (Sharma and Sharma, 1997). The leakage problem is one of the limiting factors in the commercial development of liposome products (Venuri et al., 1991). A variety of sugars, including sucrose, glucose, fructose, maltose, arabinose and trehalose, have been shown to act as protectants during dehydration/rehydration of liposomes (Crowe et al., 1985). Although other types of excipients have also been reported to exert cryoprotective effects (Li et al., 1992).
In the present study, fluconazole-entrapped multilamellar liposomes were prepared using the thin-film hydration method. The effects of cholesterol molar ratio, α-tocopherol acetate and charge-inducing agents on encapsulation efficiency values of multilamellar liposomes were studied. Characterization of the prepared multilamellar liposomes regarding particle size and in vitro drug release was preformed. Physical stability of liposomal formulations was done to investigate the stability of fluconazole liposomes at 4 and 25 °C. Also freeze-drying of liposomes was performed to study the effect of various cryoprotectants for their ability to protect liposomes against fusion and leakage during lyophilization processes to increase the stability of the stored liposomes.
2. Materials and methods
2.1. Chemicals
Fluconazole was obtained from Medicorp (city, India) as a gift sample. l-α-Phosphatidylcholine (PC), type X-E from dried egg yolk, cholesterol (Chol), stearylamine (SA), dicetyl phosphate (DP), β-glucose, d-trehalose and β-lactose were purchased from Sigma Chemical Co. (St. Louis, USA). α-Tocopherol acetate was purchased from La Roche (France). Chloroform, absolute ethyl alcohol, potassium dihydrogen phosphate and disodium hydrogen phosphate were purchased from El-Nasr Pharmaceutical Co. (Egypt). Diethyl ether was purchased from Merck (Germany). Spectra/Por dialysis membrane, 12,000–14,000 molecular weight cut off was purchased from Spectrum Laboratories Inc. (USA). All chemicals and solvents were of analytical grade.
2.2. Methods
2.2.1. Preparation of fluconazole multilamellar liposomes by thin-film hydration method
Fluconazole multilamellar liposomal formulations with different molar ratios were prepared using the thin-film hydration technique. The lipid components 200 mg [egg phosphatidylcholine and cholesterol either alone or mixed with charge-inducing agent and α-tocopherol acetate] with different molar ratios were dissolved in chloroform:methanol mixture [2:1, v/v] in a round-bottomed flask. Then 30 mg fluconazole, dissolved in 3 ml of the same solvent mixture, was added to the lipid solution. The organic solvents were slowly removed under reduced pressure, using a rotary evaporator, at 40 °C such that a very thin film of dry lipids was formed on the inner surface of the flask. The dry lipid film was slowly hydrated with 10 ml of phosphate buffered saline [pH 7.4]. The resulting suspension was mechanically shaken for 1 h at room temperature using mechanical shaker leading to the formation of multilamellar liposomes. The liposomal dispersion was left to mature over night at 4 °C to ensure full lipid hydration. All the above-mentioned steps were performed under aseptic conditions. All glasswares were sterilized by autoclaving. Phosphate buffered saline was passed through a 0.22 μm membrane filter, and the entire procedure was performed in a laminar flow hood (Esco. Singapore). Fluconazole liposomes were separated from unentrapped drug by centrifugation at 13,000 rpm for 1 h and at [−2 °C] using cooling ultracentrifuge. The cake, thus formed, was washed twice each with 10 ml phosphate buffered saline and recentrifuged again for 1 h.
2.2.2. Determination of fluconazole entrapment efficiency in liposomes
The entrapped drug concentrations were determined after the lysis of prepared liposomes with absolute alcohol and sonication for 10 min (Law and Shih, 2001). The concentration of fluconazole in absolute alcohol was determined spectrophotometrically at 261 nm. The entrapment efficiency expressed as entrapment percentage was calculated through the following relationship (Gulati et al., 1998).
Different factors influencing the encapsulation of fluconazole into multilamellar liposomes prepared by thin-film hydration method such as (cholesterol molar ratio, effect of charge and effect of α-tocopherol acetate) were studied to find out the conditions for optimal drug entrapment.
2.2.3. Particle size analysis
The mean particle size of the liposomes was determined by light scattering based on laser diffraction using the laser diffraction particle size analyzer [Malvern Master Sizer, Malvern Instruments Limited, Worcestershire, UK].
2.2.4. In vitro release of fluconazole from multilamellar liposomes
The release of fluconazole from all multilamellar liposomal formulations with different compositions was determined using the membrane diffusion technique. In brief, an accurately measured amount of fluconazole solution or fluconazole liposomal formulations, equivalent to 20 mg fluconazole, was suspended in 1 ml of phosphate buffered saline [pH 7.4] in a glass cylinder having a length of 20 cm and a diameter of 2.5 cm. This cylinder was fitted, before addition of liposomal suspension, with a presoaked membrane [Spectra/Por membrane] and was placed in a beaker (100 ml) containing 35 ml phosphate buffered saline [pH 7.4]. The whole set was placed on a magnetic stirrer adjusted to a constant speed [150 rpm]. Samples were collected every 1 h over a period of 10 h and assayed spectrophotometrically for drug content at 261 nm. The results were the mean values of three runs. The obtained results were subjected to kinetic treatment to determine the order of release pattern and release rate constant.
2.2.5. Stability study of liposomal formulations
Physical stability study of fluconazole liposomes was carried out to determine the comparative leakage of the drug from liposomes stored at different conditions compared to each other. After washing and removal of the free drug, each liposomal formulation was stored either at 4 °C or at 25 °C. At predetermined time intervals of 15, 30, 60 and 90 days (Vemuri and Rhodes, 1995). The entrapment efficiency of fluconazole liposomes were determined using stability indicating HPLC method (Xu and Lawrence, 1999). In brief, the stationary phase was at hypersil C18 (4.6 mm × 25 cm), the mobile phase was a mixture of 450 ml methanol and 550 ml 0.025 M sodium phosphate buffer with pH adjusted to 7 with phosphoric acid and was delivered at a flow rate of 1 ml/min. An aliquot of the stored samples of each formulation was diluted with phosphate buffered saline and centrifuged in a cooling ultracentrifuge at 13,000 rpm for 1 h to separate the free drug that leaked out of liposomes during the storage period. One hundred microliters of liposomal suspension were completely lysed by 0.1% (w/v) triton X-100 then transferred to 50 ml volumetric flask and the volume was completed with the mobile phase filtered through microfilter. Standard fluconazole solution were prepared by diluting specific volume of the stock standard concentration of (3 mg/ml) to get several concentrations (0.05, 0.075, 0.1, 0.125.0.15, and 0175 mg/ml). Fluconazole was detected at wave length 261 nm; the injection volume was 20 μl under these conditions; the retention time for fluconazole was 6.1 min.
2.2.6. Freeze-drying and physical stability of liposomal formulations
The cryoprotectant (glucose, lactose or trehalose) was dissolved in phosphate buffered saline at different concentrations of 1, 2, 3 and 4 g/g dry phospholipids. Liposomal suspensions in buffer alone, or after mixing with equal volume of each cryoprotectant buffered solution, were freeze-dried where the liquid was frozen to −30 °C and then freeze-dried for 24 h under vacuum at −10 °C. The resulting lyophilization cakes were rehydrated to its original volume at room temperature with phosphate buffered saline [pH 7.4] and subjected to the following investigations.
2.2.7. Measurement of drug leakage after freeze-drying
After reconstitution, an aliquot of each formulation was centrifuged in an ultracentrifuge at 13,000 rpm for 1 h at [−2 °C] to separate the free drug which leaked out of liposomes in the supernatant during freeze-drying. The amount of drug in the supernatant was measured by using HPLC method.
2.2.8. Particle size analysis
The vesicle size range and mean diameter of the liposomes were determined after freeze-drying-rehydration cycle using the laser diffraction particle size analyzer.
3. Results and discussion
3.1. Drug entrapment efficiency
By inspection of Table 1, it is obvious that fluconazole encapsulation efficiency varied with the lipid composition and the type of charge inducer used in the prepared liposomes. Concerning the effect of cholesterol content on encapsulation efficiency, results showed that the percentage entrapment efficiency of fluconazole increased by increasing the cholesterol content. The percentage entrapment efficiency of fluconazole in multilamellar liposomes composed of egg phosphatidylcholine alone was 23.104%, whereas the percentage was increased to 44.675% in liposomes prepared with phosphatidylcholine:cholesterol in the ratio 1:0.6. Further increase in the entrapment efficiency to 50.236% was recorded for liposomes composed of phosphatidylcholine:cholesterol in the ratio 1:0.8. The one-way analysis of variance ANOVA showed a significant difference between the different concentrations of cholesterol used in the preparation of multilamellar liposomal formulations at P < 0.05. This increase in entrapment efficiency is explained by the fact that by increasing the cholesterol concentration in the lipidic bilayer, the rigidity of the latter increases steadily, resulting in a higher stability and reduced permeability of the liposomal membrane (Du Plessis et al., 1996), and hence greater drug retention (Perugini and Pavanetto, 1998).
Table 1.
The effect of cholesterol molar ratio and charge inducers on the encapsulation efficiency of fluconazole into multilamellar liposomes.
| Liposome composition (molar ratio) | Charge | Encapsulation efficiency (% ±S.D.) |
|---|---|---|
| Phosphatidylcholine | Neutral | 23.104 ± 0.197 |
| Phosphatidylcholine:cholesterol (1:0.6) | Neutral | 44.675 ± 0.324 |
| Phosphatidylcholine:cholesterol:stearylamine (1:0.6:0.1) | Positive | 52.657 ± 0.231 |
| Phosphatidylcholine:cholesterol:dicetyl phosphate (1:0.6:0.1) | Negative | 38.877 ± 0.164 |
| Phosphatidylcholine:cholesterol (1:0.8) | Neutral | 50.236 ± 0.335 |
| Phosphatidylcholine:cholesterol:stearylamine (1:0.8:0.1) | Positive | 59.998 ± 0.194 |
| Phosphatidylcholine:cholesterol:dicetyl phosphate(1:0.8:0.1) | Negative | 43.533 ± 0.244 |
Concerning the effect of charge-inducing agents on the encapsulation efficiency of fluconazole in the prepared multilamellar liposomes, the results showed that positively charged liposomes exhibited the highest encapsulation efficiency, followed by neutral ones and then the negatively charged liposomes using the same phosphatidylcholine:cholesterol molar ratio. The higher fluconazole encapsulation efficiency of positively charged liposomes compared to that of the negatively charged liposomes may be due to the electrostatic attraction force that occurred between the partial negative centers, available in the molecules of the drug and stearylamine. This attraction would account for the higher encapsulation efficiency values when compared to those of the negatively charged liposomes, where such electrostatic attraction would not occur (Law and Hung, 1998).
The effect of incorporation of α-tocopherol acetate (vitamin-E) on the encapsulation efficiency of fluconazole into multilamellar liposomes prepared by thin-film hydration method was investigated. Table 2 shows the result of the effect of incorporation of α-tocopherol acetate (vitamin-E) on encapsulation efficiency of fluconazole into multilamellar vesicles liposomes prepared by thin-film hydration method. Results showed that the percentage encapsulation efficiencies of fluconazole were increased, after the incorporation of α-tocopherol acetate (vitamin-E) into liposomes from 44.675% in the molar ratio 1:0.6 in neutral liposomes composed of (egg phosphatidylcholine:cholesterol) to 47.235% in the molar ratio 1:0.6:0.1 composed of (phosphatidylcholine:cholesterol:α-tocopherol acetate), from 52.657% to 56.341% in positively charged liposomes and from 38.877% to 41.998% in negatively charged liposomes of the same molar ratio. Student t-test shows that there is a significant difference between liposomal formulations of the same molar ratio after and before incorporation of α-tocopherol acetate at α = 0.05. The increase in the entrapment efficiency is explained by the fact that incorporation of α-tocopherol acetate into liposomal preparations improves the membrane rigidity and makes it more hydrophobic and less permeable (Clares et al., 2009). Moreover a reduction in the lipid peroxidation and decrease in the formation of lysophospholipid occur when tocopherol is added.
Table 2.
The effect of incorporation of α-tocopherol acetate (vitamin-E) on encapsulation efficiency of fluconazole into multilamellar liposomes.
| Liposome composition (molar ratio) | Charge | Encapsulation efficiency (% ±S.D.) |
|---|---|---|
| Phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.6:0.1) | Neutral | 47.235 ± 0.223 |
| Phosphatidylcholine:cholesterol:stearylamine:α-tocopherol acetate (1:0.6:0.1:0.1) | Positive | 56.341 ± 0.255 |
| Phosphatidylcholine:cholesterol:dicetyl phosphate:α-tocopherol acetate (1:0.6:0.1:0.1) | Negative | 41.998 ± 0.316 |
3.2. Particle size analysis
The results of particle size measurements for freshly prepared neutral, positively charged and negatively charged multilamellar liposomal dispersions with lipid component and α-tocopherol acetate in the molar ratio 1:0.8 are presented in Table 3. The particle size distribution of the tested liposomal formulations showed unimodal normal symmetrical frequency distribution patterns. The mean particle diameter of neutral multilamellar vesicles was found to be around 7.20 μm. The mean particle diameter of negatively charged vesicles was found to be 6.00 μm and positively charged was around 10.79 μm. These results can be attributed to the inclusion of a charge inducer in liposomes, which increased the spacing between the adjacent bilayers, resulting in the formation of liposomes larger in size compared with the neutral ones (Nagarsenker et al., 2002). This increase in particle size would account, as mentioned previously, for the higher encapsulation efficiency of the positive liposomes compared with that of the neutral.
Table 3.
Particle size distribution of fluconazole multilamellar liposomes (phosphatidylcholine:cholesterol 1:0.8 M ratio) of different charges.
| Cumulative distribution percentile | Particle volume (μm) of liposomal formulations |
||
|---|---|---|---|
| Neutrala | Negatively-chargedb | Positively-chargedc | |
| 10.0% | 2.62 | 1.48 | 4.49 |
| 20.0% | 3.36 | 2.63 | 5.92 |
| 50.0% | 4.93 | 4.11 | 8.73 |
| 80.0% | 6.69 | 5.86 | 10.78 |
| 90.0% | 7.38 | 5.24 | 11.35 |
| Distribution modal sizes (μm) | 7.20 | 6.00 | 10.79 |
Phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.8:0.1) molar ratio.
Phosphatidylcholine:cholesterol:dicetyl phosphate:α-tocopherol acetate (1:0.8:0.1:0.1) molar ratio.
Phosphatidylcholine:cholesterol:stearylamine:α-tocopherol acetate (1:0.8:0.1:0.1) molar ratio.
3.3. In vitro release studies
Release profiles of fluconazole from neutral, negatively charged and positively charged multilamellar liposomes prepared by thin-film hydration method and composed of phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.6:0.1) and (1:0.8:0.1) molar ratios are shown in Figs. 1 and 2, respectively. From the release profiles, it is obvious that the increase of cholesterol molar ratio in the prepared liposomal formulations progressively decreased the release of fluconazole from the vesicles. The percentage of drug released from the liposomal formulations after 10 h of the experiment from neutral liposomes (1:0.6:0.1) reached about 56.885%. For positively charged (1:0.6:0.1:0.1) liposomes, the release rate was slower than that of neutral liposomes and the percentage of drug released after 10 h was 51.847%. In contrast, negatively charged liposomes showed a rapid release rate where the amount of drug released after 10 h was 62.441% for the same molar ratio.
Figure 1.
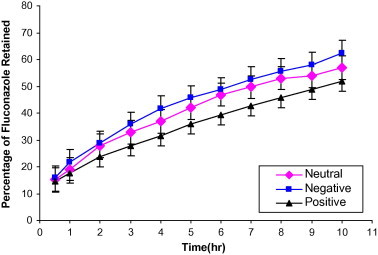
In vitro release of fluconazole from multilamellar liposomes composed of Phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.6:0.1) in phosphate buffered saline.
Figure 2.
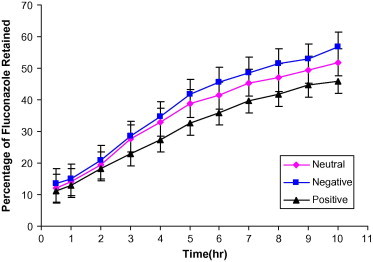
In vitro release of fluconazole from multilamellar liposomes composed of phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.8:0.1) in phosphate buffered saline.
Fig. 2 shows the release profile of fluconazole from neutral, positively charged and negatively charged multilamellar liposomal formulations composed of phosphatidylcholine:cholesterol of 1:0.8 M ratios in phosphate buffered saline. The results showed that increasing the molar ratio to cholesterol 80% decreased the release rate of the drug from liposomes. Positively charged liposomes had the slowest release rate and the highest encapsulation efficiency, relative to neutral and negatively charged liposomes. The significance of difference between the dissolution of different studied formulations was evaluated using one-way analysis of variance ANOVA followed by Dunnett Multiple comparison test. The differences were considered significant at P < 0.01. The order of release and encapsulation characteristics of fluconazole-containing liposomes may be due to the electrostatic attraction involved between the drug and the positively charged liposomes.
Table 4 shows linear regression analysis for the release data of neutral, positively charged and negatively charged liposomes. It reveals that the drug was released from liposomes by a diffusion-controlled mechanism and a linear correlation occurs when the percent of drug released was plotted against the square root of time according to Higuchi equation. These results are in agreement with many researchers who found that the drugs were released from liposomes by a diffusion-controlled mechanism (Vemuri and Rhodes, 1995).
Table 4.
Diffusional order of release of fluconazole from different liposomal formulations using the correlation coefficient parameter.
| Liposomal formulation composition (molar ratio) | Liposomal formulation charge | Kinetic analysis using correlation coefficient (r) according to |
||
|---|---|---|---|---|
| Zero order | First order | Diffusion | ||
| Phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.6:0.1) | Neutral | 0.9789 | 0.9925 | 0.9981 |
| Phosphatidylcholine:cholesterol:stearylamine:α-tocopherol acetate (1:0.6:0.1:0.1) | Positive | 0.9795 | 0.9949 | 0.9989 |
| Phosphatidylcholine:cholesterol:dicetyl phosphate:α-tocopherol acetate (1:0.6:0.1:0.1) | Negative | 0.9936 | 0.9962 | 0.9978 |
| Phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.8:0.1) | Neutral | 0.9783 | 0.9904 | 0.9943 |
| Phosphatidylcholine:cholesterol:stearylamine:α-tocopherol acetate (1:0.8:0.1:0.1) | Positive | 0.9895 | 0.9949 | 0.9952 |
| Phosphatidylcholine:cholesterol:dicetyl phosphate:α-tocopherol acetate (1:0.8:0.1:0.1) | Negative | 0.9815 | 0.9928 | 0.9933 |
3.4. Physical stability studies
Physical stability study of fluconazole liposomes was conducted at refrigeration temperature [4 °C] and at room temperature [25 ± 2 °C] for a period of 3 months. Drug leakage from the liposomes was evaluated at definite time intervals (Patel and Misra, 1999). The results are demonstrated in Table 5 in terms of percentage fluconazole retained in the liposomes. Fluconazole liposomal formulations investigated were: neutral (phosphatidylcholine:cholesterol:α-tocopherol acetate; 1:0.8:0.1 M ratio), positively charged (phosphatidylcholine:cholesterol:α-tocopherol acetate:stearylamine 1:0.8:0.1:0.1 M ratio) and negatively charged (phosphatidylcholine:cholesterol:α-tocopherol acetate:dicetyl phosphate; 1:0.8:0.1:0.1 M ratio) liposomes prepared by the thin-film hydration method. The percent fluconazole retained in liposomal formulations after 90 days were 88.853%, 87.721% and 86.439% for positively charged, negatively charged and neutral multilamellar liposomes, respectively. Positively charged liposomes showed better stability, as indicated by higher drug retention followed by the negatively charged liposomes then, the neutral liposomes.
Table 5.
Stability of multilamellar fluconazole liposomes (1:0.8) molar ratio of different charges stored at 4 °C and 25 °C.
| Time (days) | Fluconazole retained in multilamellar liposomes (% ±S.D.) |
|||||
|---|---|---|---|---|---|---|
| Stored at 25 °C |
Stored at 4 °C |
|||||
| Neutrala | Negatively-chargedb | Positively-chargedc | Neutrala | Negatively-chargedb | Positively-chargedc | |
| Zero | 100 ± 000 | 100 ± 000 | 100 ± 000 | 100 ± 000 | 100 ± 000 | 100 ± 000 |
| 15 | 85.439 ± 0.314 | 89.453 ± 0.521 | 92.652 ± 0.313 | 89.556 ± 0.244 | 93.386 ± 0.175 | 95.742 ± 0.426 |
| 30 | 79.986 ± 0.436 | 86.639 ± 0.421 | 89.864 ± 0.362 | 87.653 ± 0.177 | 92.476 ± 0.355 | 94.833 ± 0.217 |
| 60 | 73.974 ± 0.328 | 83.748 ± 0.352 | 88.649 ± 0.279 | 87.843 ± 0.466 | 88.638 ± 0.351 | 91.438 ± 0.531 |
| 90 | 72.785 ± 0.364 | 81.698 ± 0.522 | 84.548 ± 0.467 | 86.439 ± 0.288 | 87.721 ± 0.411 | 88.853 ± 0.253 |
Phosphatidylcholine:cholesterol:α-tocopherol acetate (1:0.8:0.1) molar ratio.
Phosphatidylcholine:cholesterol:dicetyl phosphate:α-tocopherol acetate (1:0.8:0.1:0.1) molar ratio.
Phosphatidylcholine:cholesterol:stearylamine:α-tocopherol acetate (1:0.8:0.1:0.1) molar ratio.
After 90 days storage at 25 °C, the retained drug was 84.548%, 81.698% and 72.785% for positively charged, negatively charged and neutral multilamellar liposomes, respectively. One-way ANOVA revealed that differences are significant at P < 0.05 between different formulations at refrigeration temperature [4 °C] and at room temperature [25 ± 2 °C].
These results can be explained by the fact that charge on lipids is an important parameter influencing liposomal behavior. In this way, high surface potential might contribute to liposome physical stability by reducing the rate of aggregation and fusion of liposomes during storage (Armengol and Estelrich, 1995). The visual appearance observations revealed that the aqueous liposomal formulations stored at 4 °C showed no signs of separation or change in color up to 6 months. On the other hand, the liposomal formulations stored at room temperature showed slight separation and yellow discoloration after 90 days. These results were in agreement with what has been previously recommended that liposomal suspensions should be kept refrigerated to achieve the best stability.
3.5. Freeze-drying of fluconazole liposomes
Freeze-drying increases the shelf-life of liposomal formulations and preserves it in dried form as a lyophilized cake to be reconstituted with water for injection prior to administration. To maintain the same particle size distribution after freeze-drying-rehydration cycle, a cryoprotectant needs to be added. Various sugars were investigated for their ability to protect liposomes against fusion and leakage during lyophilization processes (Venuri et al., 1991). This protective ability can extend to both prevention of vesicle fusion and retention of encapsulated compound within the liposome (Madden and Boman, 1999).
Table 6 shows percent fluconazole retained in freeze-dried multilamellar liposomes, respectively, as a function of cryoprotectants/lipid mass ratio. It is clear that only 63.452% fluconazole is retained for positively charged multilamellar liposomes in the absence of cryoprotectants. The leakage of entrapped fluconazole is explained by the fact that in the absence of any protective agents; lyophilization and rehydration of a liposomal suspension can result in fusion and leakage of internal aqueous contents. The percentage of fluconazole retained in liposomes increased using increasing amounts of glucose for freeze-dried positively charged multilamellar liposomes. Whereas using lactose as a cryoprotectant, the amounts of fluconazole retained in liposomes were 72.735%, 81.821%, 86.249% and 89.952% for positively charged multilamellar liposomal formulations freeze-dried with 1, 2, 3 and 4 g lactose/g lipid, respectively. ANOVA test showed a significant difference between all parts at P < 0.05. Trehalose was found to be more effective than other sugars at lower concentration where, the amounts of fluconazole retained in liposomes were found to be 78.654%, 90.634%, 91.877% and 92.855% for positively charged multilamellar liposomes freeze-dried with 1, 2, 3 and 4 g trehalose/g lipid, respectively. ANOVA followed by Dunnett Multiple comparison test showed a significant difference between all parts at P < 0.05 except between the positive liposomes and the concentration of trehalose above 3 g. It is obvious that all the three sugars used, either monosaccharide (glucose) or disaccharide (lactose or trehalose), stabilized the vesicles in the freeze-dried state and protected the liposomes against fusion and leakage. However, lactose and glucose can achieve nearly similar protection. Also, with increasing concentrations of added cryoprotectants, retention of fluconazole during lyophilization increases steadily up to 3 g trehalose/g lipid. Previous studies also demonstrated the superiority of trehalose compared to other sugars in preventing the fusion and leakage of entrapped drug where trehalose has the ability to preserve biological membranes in the absence of water. It has been suggested that the presence of trehalose during the drying process preserves the structure of drug-loaded liposomes in the anhydrous state by replacing the water molecules normally bound to the lipid head groups. It was reported that almost 100% of the entrapped solute was retained in hydrated vesicles previously freeze-dried with trehalose (Sun et al., 1996).
Table 6.
Retention of fluconazole in freeze-dried multilamellar liposomes as a function of cryoprotectants/lipid mass ratio.
| g Cryoprotectant/g lipid | Fluconazole retained in liposomal formulations (% ±S.D.) |
||
|---|---|---|---|
| Neutrala | Negatively-chargedb | Positively-chargedc | |
| None | 61.544 ± 0.212 | 62.221 ± 0.456 | 63.452 ± 0.218 |
| 1 g glucose/g lipid | 64.254 ± 0.423 | 65.877 ± 0.327 | 66.454 ± 0.447 |
| 2 g glucose/g lipid | 67.329 ± 0.531 | 68.965 ± 0.463 | 69.789 ± 0.376 |
| 3 g glucose/g lipid | 81.705 ± 0.638 | 82.543 ± 0.237 | 82.844 ± 0.374 |
| 4 g glucose/g lipid | 81.644 ± 0.538 | 82.237 ± 0.448 | 83.994 ± 0.472 |
| 1 g lactose/g lipid | 70.784 ± 0.365 | 71.662 ± 0.463 | 72.735 ± 0.518 |
| 2 g lactose/g lipid | 83.133 ± 0.432 | 83.244 ± 0.165 | 81.821 ± 0.341 |
| 3 g lactose/g lipid | 85.174 ± 0.136 | 85.867 ± 0.581 | 86.249 ± 0.361 |
| 4 g lactose/g lipid | 88.328 ± 0.462 | 88.655 ± 0.418 | 89.952 ± 0.419 |
| 1 g trehalose/g lipid | 77.167 ± 0.376 | 77.871 ± 0.365 | 78.654 ± 0.356 |
| 2 g trehalose/g lipid | 86.932 ± 0.368 | 87.429 ± 0.384 | 90.634 ± 0.163 |
| 3 g trehalose/g lipid | 89.622 ± 0.451 | 90.633 ± 0.473 | 91.877 ± 0.278 |
| 4 g trehalose/g lipid | 91.659 ± 0.428 | 92.741 ± 0.276 | 92.855 ± 0.481 |
Phosphatidylcholine:cholesterol:α-tocopherol (1:0.8:0.1) molar ratio.
Phosphatidylcholine:cholesterol:dicetyl phosphate:α-tocopherol (1:0.8:0.1:0.1) molar ratio.
Phosphatidylcholine:cholesterol:stearylamine:α-tocopherol (1:0.8:0.1:0.1) molar ratio.
Fusion or aggregation of liposomes during freeze-drying process can be monitored by measuring the mean particle diameter of liposomes after freeze-drying-rehydration cycle and compared with the results of freshly prepared liposomes of the same molar ratio. Results revealed that addition of cryoprotectants during freeze-drying of liposomes prevents aggregation of liposomes and maintains the same particle size distribution after freeze-drying-rehydration cycle. Mean particle diameters were found to be 7.44, 6.54 and 10.78 μm for neutral, negatively charged and positively charged multilamellar liposomes, respectively, freeze-dried with trehalose and rehydrated with water. These results are very close to those of freshly prepared liposomes where the mean particle diameters were found to be 7.20, 6.00 and 10.79 μm for freshly prepared neutral, negatively charged and positively charged multilamellar liposomes, respectively. Such results confirm the previously mentioned effects of cryoprotectants in maintaining the same particle distribution.
4. Conclusions
Fluconazole multilamellar liposomal formulations were prepared using the thin-film hydration method. Incorporation of cholesterol into fluconazole-entrapped multilamellar liposomes increased the rigidity or microviscosity of the bilayer, and lowered the permeability of fluconazole from liposomes resulting in greater drug retention. Incorporation of stearylamine resulted in an increased entrapment of fluconazole, whereas incorporation of dicetyl phosphate decreased the entrapment efficiency of fluconazole into liposomes. Incorporation of α-tocopherol acetate (vitamin-E) into fluconazole multilamellar liposomes resulted in the increase of entrapment efficiency of fluconazole liposomes. Positively charged fluconazole multilamellar liposomes gave rise to a slow release rate than neutral liposomes of the same molar ratios, whereas negatively charged fluconazole-entrapped multilamellar liposomes showed a faster release rate. Physico-chemical stability studies showed superior potentials of the lyophilized product after reconstitution in comparison with those of the solution product. We could conclude that all tested sugars have cryoprotectant effect that stabilized liposomes in the freeze-dried state, where trehalose offered the most superior cryoprotectant effect for freeze-dried fluconazole liposomes.
References
- Abbasoglu O.E., Hosal B.M., Sener B., Erdemoglu N., Gursel E. Penetration of topical fluconazole into human aqueous humor. Exp. Eye Res. 2001;72:147–151. doi: 10.1006/exer.2000.0936. [DOI] [PubMed] [Google Scholar]
- Ambrosini A., Bossi G., Dante S., Dubinib G., Leone L., Bossi M., Zolese G. Lipid–drug interaction: thermodynamic and structural effects of antimicotic fluconazole on DPPC liposomes. Chem. Phys. Lipids. 1998;95:37–47. [Google Scholar]
- Armengol X., Estelrich J. Physical stability of different liposome compositions obtained by extrusion method. J. Microencapsulation. 1995;12(5):525–535. doi: 10.3109/02652049509006783. [DOI] [PubMed] [Google Scholar]
- Assadullahi T.P., Hamid K., Hider R.C. In-vitro stability of liposomes in the presence of polymers, trehalose and sodium taurocholate. J. Microencapsulation. 1992;9:317–327. doi: 10.3109/02652049209021246. [DOI] [PubMed] [Google Scholar]
- Behrens-Baumann W., Klinge B., Rűchel R. Topical fluconazole for experimental candida keratits in rabbits. Br. J. Ophthalmol. 1990;74:40–42. doi: 10.1136/bjo.74.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clares B., Gallardo V., Medina M.M., Ruiz M.A. Multilamellar liposomes of triamcinolone acetonide: preparation, stability, and characterization. J. Lipos. Res. 2009;19(3):197–206. doi: 10.1080/08982100902736571. [DOI] [PubMed] [Google Scholar]
- Crowe L., Crowe J., Rudolph A., Womersley C., Appel L. Preservation of freeze-dried liposomes by trehalose. Arch. Biochem. Biophys. 1985;242:240–247. doi: 10.1016/0003-9861(85)90498-9. [DOI] [PubMed] [Google Scholar]
- Dharma S., Fishman P., Peyman G. A preliminary study of corneal penetration of 125I-labeled idoxuridine liposomes. Acta Ophthalmol. 1986;64:298–301. doi: 10.1111/j.1755-3768.1986.tb06923.x. [DOI] [PubMed] [Google Scholar]
- Du Plessis J., Ramachandran C., Weiner N., Muller D. The influence of lipid composition and lamellarity of liposomes on the physical stability of liposomes upon storage. Int. J. Pharm. 1996;127:273–278. [Google Scholar]
- Fresta M., Puglisi G., Panico A., Di Marco S., Mazzone G. CDP–choline entrapment and release from multilamellar and reverse-phase evaporation liposomes. Drug Dev. Ind. Pharm. 1993;19:559–585. [Google Scholar]
- Gulati M., Grover M., Singh M., Singh S. Study of azathioprine encapsulation into liposomes. J. Microencapsulation. 1998;15:485–494. doi: 10.3109/02652049809006875. [DOI] [PubMed] [Google Scholar]
- Habib F.S., Fouad E.A., Abdel-Rhaman M.S., Fathalla D. Liposomes as an ocular delivery system of fluconazole: in-vitro studies. Acta opthalmol. J. 2009 doi: 10.1111/j.1755-3768.2009.01584.x. [DOI] [PubMed] [Google Scholar]
- Klotz S.A., Penn C.C., Negvesky G.J., Butrus S.I. Fungal and parasitic infections of the eye. Clin. Microbiol. Rev. 2000;13:662–685. doi: 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S.L., Hung H.Y. Properties of acyclovir-containing liposomes for potential ocular delivery. Int. J. Pharm. 1998;161:253–259. [Google Scholar]
- Law S.L., Shih C. Characterization of calcitonin-containing liposomes formulations for intranasal delivery. J. Microencapsulation. 2001;18:211–221. doi: 10.1080/02652040010000334. [DOI] [PubMed] [Google Scholar]
- Lee V.H.L. Precorneal, corneal and postcorneal factors. In: Mitra A.K., editor. Ophthalmic Drug Delivery Systems. Marcel Dekker; New York: 1993. pp. 59–82. [Google Scholar]
- Li Z., Gallet O., Gaillard C., Lemoine R., Delrot S. The sucrose carrier of the plant plasmalemma III. Partial purification and reconstitution of active sucrose transport in liposomes. Biochem. Biophys. Acta. 1992;1103:259–267. doi: 10.1016/0005-2736(92)90095-4. [DOI] [PubMed] [Google Scholar]
- Madden T.D., Boman N. Lyophilization of liposomes. In: Janoff A.S., editor. Marcel Dekker, Inc.; New York and Basel: 1999. pp. 261–307. (Liposomes Rational Design). [Google Scholar]
- Mezei M. Liposomes as penetration promoters and localizers of topically applied drugs. In: Hsieh D.S., editor. Marcel Dekker; New York: 1994. pp. 171–191. (Drug Permeation Enhancement, Theory and Applications). [Google Scholar]
- Nagarsenker M.S., londh V.Y., Nadkarni G.D. Preparation and evaluation of liposomes of indomethacin. Drug Dev. Ind. Pharm. 2002;26:313–321. doi: 10.1081/ddc-100100359. [DOI] [PubMed] [Google Scholar]
- Narendran N., Balasubramaniam B., Johnson E., Dick A., Mayer E. Five-year retrospective review of guideline-based management of fungal endophthalmitis. Acta Ophthalmol. 2008;86:525–532. doi: 10.1111/j.1600-0420.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Patel V., Misra A. Encapsulation and stability of clofazimine liposomes. J. Microencapsulation. 1999;16(3):357–367. doi: 10.1080/026520499289077. [DOI] [PubMed] [Google Scholar]
- Perugini P., Pavanetto F. Liposomes containing boronophenylalanine for boron neutron capture therapy. J. Microencapsulation. 1998;15:473–483. doi: 10.3109/02652049809006874. [DOI] [PubMed] [Google Scholar]
- Price M.F., LaRocco M.T., Gentry L.O. Fluconazole susceptibilities of Candida species and distribution of species recovered from blood cultures over a 5-year period. Antimicrob. Agents Chemother. 1994;38:1422–1424. doi: 10.1128/aac.38.6.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Sharma U. Liposomes in drug delivery: progress and limitations. Int. J. Pharm. 1997;154:123–140. [Google Scholar]
- Singh M., Singh M.P., Maiti S.N., Gandhi A., Micetich R.G., Atwal H. Preparations of liposomal fluconazole and their in vitro antifungal activity. J. Microencapsulation. 1993;10:229–236. doi: 10.3109/02652049309104389. [DOI] [PubMed] [Google Scholar]
- Singla A.K., Chawla M., Singh A. Potential application of carbomer in oral mucoadhesive controlled drug delivery system. Drug Dev. Ind. Pharm. 2000;26:913–924. doi: 10.1081/ddc-100101318. [DOI] [PubMed] [Google Scholar]
- Sun W., Leopold A., Crowe L., Crowe J. Stability of dry liposomes in sugar glasses. Biophys. J. 1996;70:1769–1776. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri S., Rhodes C. Encapsulation of a water-soluble drug in a liposome preparation: removal of free drug by washing. Drug Dev. Ind. Pharm. 1995;21(11):1329–1338. [Google Scholar]
- Vemuri S., Rhodes C. Development and validation of a drug release rate method for a water-soluble drug in a liposome preparation. Drug Dev. Ind. Pharm. 1995;21(11):1353–1364. [Google Scholar]
- Venuri S., Yu C., DeGroot J.S., Wangsatornthnakun V., Venkataram S. Effect of sugars on freeze-thaw and lyophilization of liposomes. Drug Dev. Ind. Pharm. 1991;17:327–348. [Google Scholar]
- Xu Quanyun A., Lawrence A. Pharmaceutical Press; London, England: 1999. Stability-indicating HPLC Methods for Drug Analysis. pp. 174–177. [Google Scholar]
- Yee R.W., Cheng C.J., Meenakshi S., Ludden T.M., Wallace J.E., Rinaldi M.G. Ocular penetration and pharmacokinetics of topical fluconazole. Cornea. 1997;16:64–71. [PubMed] [Google Scholar]
- Yilmaz S., Maden A. Severe fungal keratitis treated with subconjunctival fluconazole. Am. J. Ophthalmol. 2005;140:454–458. doi: 10.1016/j.ajo.2005.03.074. [DOI] [PubMed] [Google Scholar]


