Abstract
A new series of 1-adamantyl derivatives was designed, synthesized and evaluated for their antimicrobial and antiviral activities. Representative derivatives of the newly synthesized compounds were tested. Ampicillin, clotimazole and the antiviral antibiotic aphidicolin were used as positive controls. Compound 18 proved to be the most active member of this series as antimicrobial against Staphylococcus aureus and Candida albicans and as antiviral with IC50 value of 0.21 mg/ml and CD50 value of 0.02 mg/ml while compound 19 proved to be the most active member of this series as antiviral with IC50 value of 0.21 mg/ml and CD50 value of 0.01 mg/ml.
Keywords: Synthesis, 1-Adamantyl, Antimicrobial testing, Antiviral screening
1. Introduction
Search for new antiviral agents is becoming the major interest in many academic and industrial research laboratories all over the world. This is due to the urge to discover new antiviral agents with more specificity and less toxicity, in addition to the various types of new viruses that are discovered nowadays and which is becoming a great challenge for the scientists. The adamantane nucleus was found to be important constituent in many antiviral drugs. This is because of its various mechanisms of action towards viruses. The incorporation of an adamantyl moiety into a pharmacologically-active molecule – in many cases – resulted in improving the therapeutic profile of the parent drug (Spano et al., 1970). Since the discovery of amantadine (I) (Stetter, 1962; Gerzon et al., 1963; Lee et al., 1966; Kirschbaum, 1983) as the first antiviral therapy for systemic use, several hundreds or even thousands of 1-adamantyl and 2-adamantyl derivatives were synthesized and tested for various biological activities. Rimantadine (II) and its biologically-active metabolites (III–V) (Manchand et al., 1990) were tested and some of them were proved to have antiviral activity. Antimicrobial and antiviral properties were also observed as a major biological effect of several fused adamantyl heterocycles systems such as oxadiazole (El-Emam et al., 2004), isoxazole (Makarova et al., 2002) and thiadiazole (Kritsanida et al., 2002). Moreover, several thiazolidinone derivatives were reported to display antimicrobial activity (Mehta et al., 2008; Subudhi et al., 2007; Sattigeri et al., 2005; Hirapara et al., 2003). Also thiazolidinone nucleus represented the major nucleus in several derivatives possessing hypnotic (Ergenc et al., 1999), anticonvulsant (Verma and Saraf, 2008), analgesic (Taranalli et al., 2007), antitumor (Guezel and Salman, 2009) and anthelmintic (Khan and Yusuf, 2009) activities. Furthermore, some oxadiazoline derivatives were proved to have anti-HIV activity (Chimirri et al., 1994). In addition, some other oxadiazoline derivatives showed other pharmacological activities such as anti-inflammatory (Tiperciuc et al., 1999), antitumor (Abadi et al., 2003), and antibacterial activity (Li et al., 2008). Accordingly, in the present investigation, some new heterocyclic derivatives carrying 1-adamantyl moiety were prepared as hybrid compounds which might possess antiviral and/or antimicrobial activity, and these were tested for such activities.
2. Experimental
2.1. General
Melting points (°C) were determined on Fischer–Johon melting point apparatus and are uncorrected. Microanalyses were performed in the microanalytical unit, Faculty of Sciences, Cairo University and the found values were within ±0.4% of the theoretical values. Reaction times were determined using TLC silica gel plates 60 F254“E. Merck” the spots were visualized by UV (366, 254 nm). 1H NMR spectra were recorded on a Bruker AC 250 FT NMR spectrometer at 250 MHz using CDCl3 or DMSO-d6 as solvent and TMS as internal standard. The chemical shifts were expressed in δ ppm. Splitting patterns were designated as follows: s, singlet; brs, broad singlet; d, doublet and m, multiplet. Mass spectra were recorded on a Shimadzu GC MS 1000 EX at 70 eV. The in vitro antimicrobial testing was performed at Department of Microbiology, Faculty of Pharmacy, Mansoura University, Egypt. The agar disc-diffusion method and a panel of standard strains (Staphylococcus aureus IFO 3060, Escherichia coli IFO 3301, and Candida albicans IFO 0583) were employed. Compounds 2 (Kolocouris et al., 2007), 3 (Bormasheva et al., 2008) and 4–17 (Henry and Colwell Jr., 1973) were prepared according to the literature procedures.
2.2. Chemistry
(±) 3-(1-Adamantylcarbonylamino)-2-aryl-4-thiazolidinones (18–27): A mixture of the appropriate arylideneamino derivative 4–17 (0.004 mol) and mercaptoacetic acid (1.0 ml) in benzene or xylene (10 ml) was heated under reflux for 4 h. The solvent was then evaporated under reduced pressure and the resulted residue was washed several times with 10% sodium bicarbonate solution and finally with water, dried and crystallized from pet. ether to yield the products 18–27 in 80–90% yields. 18: 1.62–1.99 (m, 15H, Adamantyl-H), 3.66–3.81 (d, 2H, CH2, J = 16), 5.91 (s, 1H, CH), 7.08–7.39 (m, 5H, Ar-H, NH). 19: m/z (% Rel. Int.); 392 (0.06, M+ +1), 391 (0.16, M+). 1.60–2.0 (m, 15H, Adamantyl-H), 3.67–3.81 (d, 2H, CH2, J = 15.9), 5.89 (s, 1H, CH), 7.15 (s, 1H, NH), 7.33–7.37 (d, 4H, Ar-H, J = 8.6). 20: 1.63–1.99 (m, 15H, Adamantyl-H), 3.70–3.80 (d, 2H, CH2, J = 15.9), 6.39 (s, 1H, CH), 7.30–7.51 (m, 5H, Ar-H, NH). 21: m/z (% Rel. Int.); 437 (0.25, M+ +2), 435 (0.26, M+). 1.63–2.02(m, 15H, Adamantyl-H), 3.68–3.81 (d, 2H, CH2, J = 15.9), 5.88 (s, 1H, CH), 7.56 (s, 1H, NH), 7.29–7.52 (d, 4H, Ar-H, J = 7.9). 22: m/z (% Rel. Int.); 370 (0.03, M+). 23: 1.62–1.98 (m, 15H, Adamantyl-H), 2.98 (s, 6H, N(CH3)2), 3.64–3.79 (dd, 2H, CH2, J = 15.9), 5.83 (s, 1H, CH), 6.70–7.25 (m, 5H, Ar-H, NH). 24: 1.63–1.99 (m, 15H, Adamantyl-H), 3.75–3.80 (d, 2H, CH2, J = 15.3), 6.59 (d, 1H, J = 1.83, CH), 7.17 (s, 1H, NH), 7.24–7.36 (m, 3H, Ar-H). 25: 1.62–1.99 (m, 15H, Adamantyl-H), 3.66–3.81 (d, 2H, CH2, J = 15.8), 3.90 (s, 6H, OCH3), 5.87 (s, 1H, CH), 6.83–6.96 (m, 3H, Ar-H), 7.13 (s, 1H, NH). 26: 1.63–1.99 (m, 15H, Adamantyl-H), 3.68 (m, 2H, CH2), 6.62 (s, 1H, CH), 7.03–7.39 (m, 4H, NH, Ar-H). 27: 1.56–1.90 (m, 15H, Adamantyl-H), 3.69–3.82 (m, 2H, CH2), 5.71 (s, 1H, CH), 7.08–7.38 (m, 3H, Ar-H), 9.68 (s, 1H, NH).
(±) 2-(1-Adamantyl)-4-acetyl-5-aryl-1,3,4-oxadiazolines (28–33): A mixture of the appropriate arylideneamino derivative 4–17 (0.004 mol) and acetic anhydride (10 ml) was heated under reflux for 2 h. The excess acetic anhydride was then evaporated under reduced pressure and ice-water (50 ml) was added to the resulted oily or sticky residue and refrigerated for 2 h. The separated solid was filtered, washed with water, dried and crystallized from pet. ether to yield the products 28–33 in 35–50% yields. 28: 1.58–2.13 (m, 15H, Adamantyl-H), 2.23 (s, 3H, COCH3), 6.84 (s, 1H, CH), 7.06–7.39 (m, 4H, Ar-H). 29: 1.69–2.13 (m, 15H, Adamantyl-H), 2.39 (s, 3H, COCH3), 3.87 (s, 3H, OCH3), 7.69 (s, 1H, CH), 6.99–7.97 (m, 4H, Ar-H). 30: 1.59–2.07 (m, 15H, Adamantyl-H), 2.29 (s, 3H, COCH3), 7.59 (s, 1H, CH), 7.41–8.02 (m, 4H, Ar-H). 31: 1.84–2.16 (m, 18H, Adamantyl-H, COCH3), 7.26 (s, 1H, CH), 7.72–8.84 (m, 4H, Ar-H). 32: 1.60–2.08 (m, 15H, Adamantyl-H), 2.25 (s, 3H, COCH3), 6.94 (s, 1H, CH), 7.60–8.24 (dd, 4H, J = 1.83). 33: 1.73–2.50 (m, 18H, Adamantyl-H, COCH3), 7.75–8.86 (m, 4H, CH, Ar-H) (Scheme 1).
Scheme 1.
3. Biological screening
3.1. Antimicrobial screening of the tested compounds
Nutrient agar plates were seeded separately with 0.1 ml of 24 h diluted culture of S. aureus, E. coli and C. albicans. Cylindrical plugs were removed from the agar plate using a sterile cork borer. Five cups of 10 mm diameter were made in the seeded agar by the aid of sterile Weatherman tube. Twenty microliter of the solution of tested compounds in dimethylformamide (10 mg/ml) were added into the corresponding cups in different seeded strain agar, and 100 μl DMF were added in the fifth cup as a negative control, allowed to diffuse and incubated at 37 °C for 24 h. The zones of inhibition around each cup were measured using a caliper to the nearest 0.5 mm (Ronsted, 1972; Blain et al., 1970).
3.2. Antiviral and cytotoxicity screening
The compound samples were prepared for assay by dissolving in 50 ml of DMSO and diluting aliquots into sterile culture media at 0.4 mg/ml. These solutions were subdiluted to 0.02 mg/ml in sterile media and the two solutions used as stocks to test samples at 100, 50, 20, 10, 5, 2, and 1 μg/ml in triplicates in the wells of microtiter plates. The compounds were tested for antiviral activity against Herpes simplex type 1 (HSV-1) grown on Vero African green monkey kidney cells. Virus stocks were prepared as aliquots of culture medium from Vero cells infected at multiplicity of one virion per 10 cells and cultured 3 days. They were stored at −80 °C. Working stocks were prepared by titering virus by serial dilution in culture medium and assayed in triplicate on Vero monolayers in the wells of microtiter trays. Virus suspensions that gave about 30 plaques per well were stored at 4 °C until used. Vero African green monkey kidney cells were purchased from Viromed Laboratories, Minnetonka, MN, USA and grown in Dulbeccos modified Eagle’s medium supplemented with 10% (v/v) calf serum (HyClone Laboratories, Ogden, UT, USA), 60 μg/ml Penicillin G and 100 μg/ml Streptomycin sulfate maintained at 37 °C in a humidified atmosphere containing about 15% (v/v) CO2 in air. All medium components were obtained from Sigma Chemical Co., St. Louis, MO, USA unless otherwise indicated. Vero stocks were maintained at 34 °C in culture flasks filled with medium supplemented with 1% (v/v) calf serum. Subcultures for virus titration or antiviral screening were grown in the wells of microtiter trays (Falcon Microtest III 96-wells trays, Becton Dickinson Labware, Lincolin Park, NJ, USA) by suspending Vero cells in medium following trypsin–EDTA treatment, counting the suspension with a hemocytometer, diluting in medium containing 10% calf serum to 2 × 104 cells per 200 ml culture, aliquoting into each well of a tray and culturing until confluent (Hufford et al., 1991; El-Sherbeny et al., 1995; El-Subbagh et al., 2000). Microtiter trays with confluent monolayer cultures of Vero cells were inverted, the medium shaken out, and replaced with serial dilutions of sterile extracts in triplicate in 100 μl medium in each well. Trays wells were then inoculated with 30 plaque forming units of HSV-1 virus in 100 μl medium containing 10% (v/v) calf serum. In each tray, the last row of wells was reserved for controls that were not treated with compounds or not treated with virus. The trays were cultured for 6 h. The trays were inverted onto a pad of paper towels, the remaining cells rinsed carefully with medium, and fixed with 3.7% (v/v) formaldehyde in saline for at least 20 min. The fine cells were rinsed with water, and examined visually. Antiviral activity is identified as confluent, relatively unaltered monolayers of stained Vero cells treated with HSV-l. Cytotoxicity was estimated as the concentration that caused approximately 50% loss of the monolayer present around the plaques caused by HSV-l 126–128. The synthesized compounds were tested at the University of Minnesota for their possible antiviral and cytotoxicity activity. Aphidicolin (0.005 μg/ml) was used as a positive control. The compounds were tested against (HSV-1) grown on Vero African green monkey kidney cells (Hufford et al., 1991; El-Sherbeny et al., 1995; El-Subbagh et al., 2000).
4. Results and discussion
4.1. Chemistry
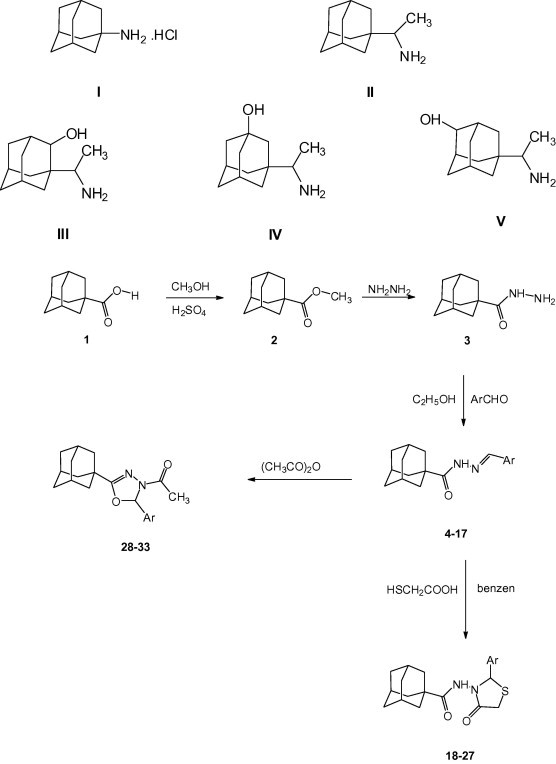
The starting material adamantane-1-carboxylic acid 1 is commercially-available; it was early prepared by Nomura et al. via cyanation of 1-bromoadamantane with Cuperous cyanide followed by hydrolysis with 60% sulphuric acid (Nomura et al., 1994). So, methyl adamantane-1-carboxylate 2 was easily prepared following the classical esterification method by heating adamantane-1-carboxylic acid with pure methanol in the presence of sulphuric acid as dehydrating agent to yield the target ester in 98% yield. Several methods were reported for the preparation of carboxylic acid hydrazides. These methods mainly involve the reaction of the carboxylic acid ester (mainly the methyl or the ethyl ester) in ethanol or the acid halides with hydrazine in the presence of triethylamine (Ficarra et al., 1983, 1984; Guzhova et al., 1986). Adamantane-1-carboxylic acid hydrazide 3 was successfully prepared in 95% yield by prolonged heating of methyl adamantane-1-carboxylate with excess hydrazine hydrate for 15 h in the absence of solvent.
The free primary amino group of carboxylic acid hydrazides readily reacts with carbonyl compounds to yield the corresponding anils (Schiff’s bases). The reaction is usually carried out in ethanol, acetic acid or in dimethylformamide according to the solubility of the reactants. Accordingly, adamantane-1-carboxylic acid hydrazide 3 was allowed to react with some substituted benzaldehydes, 2-thenaldehyde or 5-nitro-2-thenaldehyde in ethanol for one hour to yield the corresponding N-arylidene-1-adamantylcarboxhydrazides (4–17) in 85–90% yields. The reaction proceeded rapidly and the products were precipitated immediately during the reaction or on cooling (Ficarra et al., 1983; Guzhova et al., 1986; Fenech et al., 1979).
Mercaptoacetic acid was reported to react readily with compounds containing an arylideneamino function group in benzene or xylene to yield the corresponding 2-aryl-4-thiazolidinone derivatives (Krimmel, 1968; Moustafa et al., 1987; El-Subbagh et al., 1990). Accordingly, some of the arylidene derivatives 4–17 were reacted with mercaptoacetic acid in xylene to afford the corresponding racemates (±) 3-(1-adamantylcarbonylamino)-2-aryl-4-thiazolidinones (18–27). N-Arylidenecarbox-hydrazides were reported to undergo acetylative cyclization upon heating with acetic anhydride to yield the corresponding 4-acetyl-5-aryl-1,3,4-oxadiazolines (El-Gendy and Ismail, 1989). As a result, some of the arylidene derivatives 4–17 were heated with acetic anhydride to afford the corresponding racemates (±) 2-(1-adamantyl)-4-acetyl-5-aryl-1,3,4-oxadiazolines (28–33) (Table 1).
Table 1.
The melting points, yield percentages, molecular formulae of compounds 4–33.
| Comp. No. | Ar | Melting point (°C) | Yield (%) | Mol. form. (Mol. wt.) |
|---|---|---|---|---|
| 4 | 4-FC6H4 | 203–205 | 92 | C18H21FN2O (300.37) |
| 5 | 4-ClC6H4 | 211–212 | 90 | C18H21ClN2O (316.82) |
| 6 | 2-ClC6H4 | 262–265 | 90 | C18H21ClN2O (316.82) |
| 7 | 4-BrC6H4 | 234–235 | 95 | C18H21BrN2O (361.28) |
| 8 | 3-CH3C6H4 | 241–243 | 86 | C19H24N2O (296.41) |
| 9 | 2-NO2C6H4 | 217–219 | 89 | C18H21N3O3 (327.38) |
| 10 | 3-NO2C6H4 | 248–249 | 90 | C18H21N3O3 (327.38) |
| 11 | 4-NO2C6H4 | 231–234 | 94 | C18H21N3O3 (327.38) |
| 12 | 4-(Me2N)C6H4 | 152–156 | 87 | C20H27N3O (325.45) |
| 13 | 2,6-Cl2C6H3 | 256–259 | 87 | C18H20Cl2N2O (351.27) |
| 14 | 3,4-(CH3O)2C6H3 | 232–234 | 85 | C20H26N2O3 (342.43) |
| 15 | 2-Cl,5-FC6H3 | 262–265 | 90 | C18H20ClFN2O (334.82) |
| 16 | 2-Cl,5-NO2C6H3 | 223–225 | 85 | C18H20ClN3O3 (361.82) |
| 17 | 2-Thienyl | 270–272 | 90 | C16H20N2OS (288.41) |
| 18 | 4-FC6H4 | 149–151 | 90 | C20H23FN2O2S (374.47) |
| 19 | 4-ClC6H4 | 182–184 | 87 | C20H23ClN2O2S (390.93) |
| 20 | 2-ClC6H4 | 210–212 | 85 | C20H23ClN2O2S (390.93) |
| 21 | 4-BrC6H4 | 177–179 | 89 | C20H23BrN2O2S (435.38) |
| 22 | 3-CH3C6H4 | 186–188 | 86 | C21H26N2O2S (370.51) |
| 23 | 4-(Me2N)C6H4 | 112–114 | 84 | C22H29N3O2S (399.55) |
| 24 | 2,6-Cl2C6H3 | 195–197 | 85 | C20H22Cl2N2O2S (425.37) |
| 25 | 3,4-(CH3O)2C6H3 | 175–177 | 89 | C22H28N2O4S (416.53) |
| 26 | 2-Cl,5-FC6H3 | 185–187 | 82 | C20H22ClFN2O2S (408.92) |
| 27 | 2-Thienyl | 230–232 | 82 | C18H22N2O2S (362.51) |
| 28 | 4-FC6H4 | 85–89 | 49 | C20H23FN2O2 (342.41) |
| 29 | 2-ClC6H4 | 110–112 | 50 | C20H23ClN2O2 (358.86) |
| 30 | 2-NO2C6H4 | 128–131 | 39 | C20H23N3O4 (369.41) |
| 31 | 3-NO2C6H4 | 165–169 | 42 | C20H23N3O4 (369.41) |
| 32 | 4-NO2C6H4 | 195–197 | 47 | C20H23N3O4 (369.41) |
| 33 | 2-Cl,5-NO2C6H3 | 159–163 | 45 | C20H22ClN3O4 (403.86) |
4.2. Biological screening
Representative derivatives of the newly synthesized compounds were tested for their antimicrobial activity against certain strains of pathogenic bacteria and the pathogenic fungi C. albicans. In addition, their cytotoxic activity using Vero-cell culture and their antiviral activity against Herpes Simplex Virus type 1 (HSV-1) were also determined.
4.2.1. Antimicrobial screening
Compounds were tested for their antimicrobial activity against the gram positive bacteria S. aureus (SA), the gram negative bacteria E. coli (EC), and the pathogenic fungi C. albicans (CA) using the agar diffusion method (Ronsted, 1972; Blain et al., 1970) The broad spectrum antibiotic Ampicillin and the antifungal drug Clotrimazole (Canasten) were used as positive controls. The zones of inhibition were measured using a caliper to the nearest 0.5 mm. The results of the antimicrobial screening of the tested compounds showed variable degree of activity (Table 2). The results showed that the compounds 22, 24, 26 and 32 are devoid of any inhibitory effect against the tested organisms. All the tested compounds except compounds 22, 24, 26 and 32 showed variable activity against the gram positive bacteria S. aureus. All the tested compounds showed variable antifungal activity against C. albicans except compounds 22–24, 26, 29 and 32.
Table 2.
The inhibitory effect of the tested compounds against the gram positive bacteria Staphyloccous aureus (SA), the gram negative bacteria Escherichia coli (EC), and the pathogenic fungi Candida albicans (CA) using 20 μl concentration of the tested compounds. The cytotoxic concentration (CD50) and the antiviral activity against HSV-1 of the tested compounds and the antiviral antibiotic aphidicolin.
| Compound⁎ | The tested organism |
% Reduction in number in plaques | Minimum Antiviral Conc. (mg/ml) | Cytotoxicity (CD50) mg/ml | ||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | ||||
| 18 | S++ | R | S++ | 18 | 0.21 | 0.02 |
| 19 | S+ | R | S+ | 20 | 0.21 | 0.01 |
| 21 | S++ | R | S+ | 20 | 0.20 | 0.03 |
| 22 | R | R | R | – | – | – |
| 23 | S+ | R | R | 19 | 0.20 | 0.03 |
| 24 | R | R | R | – | – | – |
| 26 | R | R | R | – | – | – |
| 28 | S++ | R | S+ | – | – | – |
| 29 | S+ | R | R | – | – | – |
| 32 | R | R | R | – | – | – |
| Ampicillin | S++++ | S+++ | Not tested | – | – | – |
| Clotrimazole | Not tested | Not tested | S++++ | – | – | – |
| Aphidicolin | – | – | – | 100 | 0.005 | 0.20 |
R = resistant, S+ = weakly active, S++ = moderately active, S+++ = strongly active, S++++ = remarkably active.
Compounds not listed in the table are completely inactive.
4.2.2. Antiviral and cytotoxicity activity
The newly synthesized compounds 18, 19, 21–24, 26, 28, 29 and 32 were tested for their cytotoxic activity using Vero-cell culture and their antiviral activity was tested against Herpes simplex virus type 1 (HSV-1) using the antiviral antimitotic antibiotic aphidicolin as a positive control (Hufford et al., 1991; El-Sherbeny et al., 1995; El-Subbagh et al., 2000). The results of the cytotoxic and antiviral activity of the synthesized compounds and the antiviral antibiotic Aphidicolin are shown in (Table 2). The results showed that compounds 18, 19, 21 and 23 showed weak antiviral activity. The rest of tested compounds were found to be inactive.
5. Conclusion
The results of both antimicrobial and antiviral screening revealed that compounds 18, 21 and 28 are the most active agents against gram positive bacteria. Compounds 18 and 19 proved to be the most active members among the tested compounds as antifungal and antiviral agents respectively. Although compounds 19 and 21 displayed almost the same activity as antiviral agents, the later showed higher cytotoxicity (three times more toxic) than the former.
Structure activity correlation of the obtained results showed that the presence of the thiazolidinone ring favors both antimicrobial and antiviral activities than the oxadiazoline ring. Furthermore, in the thiazolidinone series, the presence of electron-withdrawing atom at the para position of the phenyl ring favors both activities rather than electron-donating groups which caused the loss of activity. In addition, the presence of 2,6-disubstituents of the phenyl ring resulted in loss of both the antiviral and the antimicrobial activities.
References
- Abadi A., Abdel Haleem A., Hassan G. Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem. Pharm. Bull. 2003;51:838–844. doi: 10.1248/cpb.51.838. [DOI] [PubMed] [Google Scholar]
- Blain J., Linnest E., Traunat J. American Society of Microbiology; Bethesda, MS, USA: 1970. Manual of Clinical Microbiology. (pp. 300–303) [Google Scholar]
- Bormasheva K.M., Nechaeva O.N., Moiseev I.K. Reactions of adamantyl-substituted keto esters with hydrazine and phenylhydrazine. Russ. J. Org. Chem. 2008;44(12):1760–1764. [Google Scholar]
- Chimirri A., Grasso S., Monforte A.M., Monforte P., Zappala M., Carrotti A. Synthesis and in vitro anti HIV activity of novel Δ2-1,2,4-oxadiazoline derivatives. Farmaco. 1994;49:509–511. [PubMed] [Google Scholar]
- El-Emam A.A., Al-Deeb O.A., Al-Omar M., Lehmann J. Synthesis, antimicrobial and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminoethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- El-Gendy A.A., Ismail M.M. Indole derivatives. III. Synthesis of 1,3,4-oxadiazolyl- and thiazolylindole derivatives. Egypt. J. Pharm. Sci. 1989;30:35–42. [Google Scholar]
- El-Sherbeny M.A., El-Ashmawy M.B., El-Subbagh H.I., El-Emam A.A., Badria F.A. Synthesis, antimicrobial and antiviral evaluation of certain thienopyrimidine derivatives. Eur. J. Med. Chem. 1995;30:445–449. [Google Scholar]
- El-Subbagh H.I., El-Emam A.A., El-Ashmawy M.B., Shehata I.A. Thienobenzopyranones. III. New 4H-thieno[2,3-b][1]benzothiopyran-4-ones carrying different heterocyclic moieties of expected pharmacoclogical interest. Arch. Pharm. Res. 1990;13:24–27. [Google Scholar]
- El-Subbagh H.I., Abu-Zaid S.M., Mahran M.A., Badria F.A., El-Rahman M.A. Synthesis and biological evaluation of certain alpha, beta-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem. 2000;43:2915–2921. doi: 10.1021/jm000038m. [DOI] [PubMed] [Google Scholar]
- Ergenc N., Capan G., Guanay N.S., Ozkirimili N.S., Gungor M., Ozbey S., Kendi E. Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazo-lindione derivatives. Arch. Pharm. (Weinheim) 1999;332:343–347. doi: 10.1002/(sici)1521-4184(199910)332:10<343::aid-ardp343>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Fenech G., Monforte P., Chimirri A., Grasso S. Reaction of 1-formyl adamantine with heterocyclic compounds. Mass spectra and antibacterial and antifungal activity. J. Hetercycl. Chem. 1979;16:347–351. [Google Scholar]
- Ficarra P., Ficarra R., Tommasini A., Fenech G. Compounds with potential antitumor activity. II. N,N′-(1-diadamantane dicarboxamides) Farmaco Ed. Sci. 1983;38:418–424. [PubMed] [Google Scholar]
- Ficarra R., Ficarra P., Tommasini A., Fenech G., Pizzimenti F.C., Bisignano G. 1-Adamantanecarboxylic acid hydrazides with presumed antimicrobial activity. Boll. Chim. Farm. 1984;123:317–321. [PubMed] [Google Scholar]
- Gerzon K., Krumkalns E.V., Brindle R.L., Marshall F.J., Root M.A. The adamantly group in medicinal agents. I. Hypoglycemic N-arylsulsonyl-N′-adamantyl ureas. J. Med. Chem. 1963;6:760–763. doi: 10.1021/jm00342a029. [DOI] [PubMed] [Google Scholar]
- Guezel O., Salman A. Synthesis and biological evaluation of new 4-thiazolidinone derivatives. J. Enzyme Inhib. Med. Chem. 2009;24:1015–1023. doi: 10.1080/14756360802608021. [DOI] [PubMed] [Google Scholar]
- Guzhova S.V., Danilenko G.I., Korobchenko L.V., Denisova L.V., Andreeva O.T., Boreko E.I., Danilenko V.F., Baklan V.F. Adamantoyl-1-hydrazines as inhibitors of vaccine virus. Fiziol. Akt. Veshchestva. 1986;18:24–26. [Google Scholar]
- Henry, D.W., Colwell Jr., W.T., 1973. Nitrothiophenes. US 3733319 19730515.
- Hirapara K., Joshi A., Patel S., Parekh H. Some novel arylamides and thiazolidinones. J. Inst. Chem. (India) 2003;75:168–170. [Google Scholar]
- Hufford C.D., Badria F.A., Abou-Karam M., Shier W.T., Rogers R.D. Preparation, characterization and antiviral activity of microbial metabolites of stemodin. J. Nat. Prod. 1991;54:1534–1552. doi: 10.1021/np50078a008. [DOI] [PubMed] [Google Scholar]
- Khan S.A., Yusuf M. Synthesis and biological evaluation of some thiazolidinone derivatives of steroids as antibacterial agents. Eur. J. Med. Chem. 2009;44:2597–2600. doi: 10.1016/j.ejmech.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum, J., 1983. Analytical Profiles of Drug Substances. In: Florey, K. (Ed.), vol. 12. Academic Press, New York, pp. 1–36.
- Kolocouris N., Zoidis G., Foscolos G.B., Fytas G., Prathalingham S.R., Kelly J.M., Naesens L., De Clercq E. Design and synthesis of bioactive adamantane spiro heterocycles. Bioorg. Med. Chem. Lett. 2007;17(15):4358–4362. doi: 10.1016/j.bmcl.2007.04.108. [DOI] [PubMed] [Google Scholar]
- Krimmel, C.P., 1968. N-(Dialkylaminoalkyl)adamantanecarboxamides. US Patent 3374244 19680319.
- Kritsanida M., Mouroutsou A., Marakos P., Pouli N., Papakonstantinou-Garoufalias S., Pannecouque C., Witvrouw M., De Clerq E. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Farmaco. 2002;57:253–257. doi: 10.1016/s0014-827x(01)01189-2. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Dobson P.R., Van Rooyen C.E. Antiviral substances: 6-aminonicotinamideand 1-adamantanamine hydrochloride. Chemotherapy. 1966;11:163–177. doi: 10.1159/000220453. [DOI] [PubMed] [Google Scholar]
- Li D.-J., Dan F.-J., Fu H.-Q. Synthesis and antibacterial activities of bis-1,3,4-oxadiazoline derivatives. Heterocycl. Commun. 2008;14:465–468. [Google Scholar]
- Makarova N.V., Boreko E.I., Moiseev I.K., Pavlova N.I., Nikolaeva S.N., Zemtsova M.N., Vladyko G.V. Antiviral activity of adamantane-containing heterocycles. Pharm. Chem. J. 2002;36:3–6. [Google Scholar]
- Manchand P.S., Cerruti R.L., Martin J.A., Hill C.H., Merrett J.H., Keech E., Belshe R.B., Connell E.V., Sim I.S. Synthesis and antiviral activity of rimantadine. J. Med. Chem. 1990;33:1992–1995. doi: 10.1021/jm00169a029. [DOI] [PubMed] [Google Scholar]
- Mehta D., Sengar N., Pathak A. 4-Thiazolidinone, a new profile of various pharmacological activities. Orient. J. Chem. 2008;24:441–454. [Google Scholar]
- Moustafa M.A., Eisa H.M., El-Emam A.A., El-Kerdawy M.M. Synthesis and characterization of new tetrazole derivatives. J. Pharm. Belg. 1987;42:38–43. [PubMed] [Google Scholar]
- Nomura, M., Kyouda, M., Hirokawa, T., Fujihara, Y., Sugiura, M., 1994. Studies on the synthesis of physiologically active substances. VIII. Synthesis and physiological activity of acid amides with an adamantly group. Nippon Nogei Kagaku Kaishi 68, 973–977.
- Ronsted P. Disposable plastic tray for large assays of antibiotics. J. Antimicrob. Agents Chemother. 1972;2:49–50. doi: 10.1128/aac.2.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattigeri V.J., Soni A., Singhal S., Khan S., Pandya M., Bhateja P., Mathur T., Rattan A., Khanna J., Mehta A. Synthesis and antimicrobial activity of novel thiazolidinones. ARKIVOC (Gainesville, FL, US) 2005;2:46–59. [Google Scholar]
- Spano R., Linari G., Marri R. 1-Adamantanecarboxylic acid amide of aminoantipyrine. J. Med. Chem. 1970;13:554. doi: 10.1021/jm00297a053. [DOI] [PubMed] [Google Scholar]
- Stetter H. Advances in the chemistry of organic ring systems with urotropine (adamantine) structure. Angew. Chem. 1962;74:361–374. [Google Scholar]
- Subudhi B., Panda P., Kundu T., Sahoo S., Pradhan D. Synthesis and biological evaluation of some benzimidazole and thiazolidinone derivatives. J. Pharm. Res. 2007;6:114–118. [Google Scholar]
- Taranalli A.D., Bhat A.R., Srinivas S., Saravanan E. Evaluation of certain novel thiazolidinones for anti-inflammatory, analgesic, antipyretic and cyclooxygenase inhibitory activity in animals. J. Cell Tissue Res. 2007;7:1061–1066. [Google Scholar]
- Tiperciuc B., Parou A., Palag M., Oniga O., Chran D. Heterocycles 82. The synthesis and the study of the anti-inflammatory activity of some 3-N-acetyl-2-R-5-[2′-aryl-4′-methylthiazole-5′-yl]-Δ2-1,3,4-oxadiazoline. Farmacia. 1999;47:77–84. [Google Scholar]
- Verma A., Saraf S.K. 4-Thiazolidinone. A biologically active scaffold. Eur. J. Med. Chem. 2008;43:897–905. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]


