Abstract
In the last few years, cancer chemotherapy has been successfully employed in the treatment of different types of human tumours. Unfortunately, the optimal clinical usefulness of this important treatment modality is usually limited secondary to the development of life-threatening multiple organ toxicity. Cancer chemotherapy may cause these toxic effects by mechanisms not involved in their anticancer activity that can severely affect the life of patients and represent a direct cause of death. Several experimental and clinical studies have demonstrated that some important anticancer drugs interfere with the absorption, synthesis, and excretion of carnitine in non-tumour tissues, resulting in a secondary carnitine deficiency which is reversed by carnitine treatment without affecting anticancer therapeutic efficacy. Prototypes of anticancer drugs that alter carnitine system are doxorubicin, cisplatin, carboplatin, oxaliplatin, cyclophosphamide and ifosfamide. Furthermore, cachectic cancer patients are especially at risk for carnitine deficiency due to decreased oral intake and/or increased renal losses. Altered serum and urine carnitine levels have been reported in cancer patients with various forms of malignant diseases. Recent studies in our laboratory have demonstrated that carnitine deficiency constitute a risk factor and should be viewed as a mechanism during development of oxazaphosphorines-induced cardiotoxicity in rats. Similarly, inhibition of gene expression of heart fatty acid-binding protein and organic cation/carnitine transporter in doxorubicin cardiomyopathic rat model has been reported. In view of these facts and in view of irreplaceability of these important anticancer drugs, this review aimed to highlight the role of carnitine depletion and supplementation during development of chemotherapy-induced multiple organ toxicity.
1. Introduction
1.1. L-carnitine
1.1.1. Historical background
Carnitine, a naturally occurring quaternary ammonium compound, was discovered in muscle extracts in the beginning of twentieth century by Gulewitsch and Krimberg in Moscow, Russia (Gulewitsch and Krimberg, 1905) and by Kutscher in Marburg, Germany (Kutscher, 1905). At that time carnitine was named from the Latin word carnis which means flesh. In 1927, the chemical structure of carnitine was identified as β-hydroxy-γ-trimethyl-amino-butyric acid (Tomita and Senju, 1927). In 1955, Fritz reported that muscle extracts added by means of rat liver homogenates effected an increase in palmitate oxidation rate (Fritz, 1955). In the same year, Friedman and Fraenkel (1955) found the reversible transfer of acetyl groups between carnitine and CoA-SH. In 1962, Bremer observed that the formed acetyl-carnitine was an effective supplier of acetyl groups for beta-oxidation in the mitochondria (Bremer, 1962a). Later this year, Bremer (1962b) was the first researcher who described the main physiological function of carnitine as carrier for the translocation of long-chain fatty acid from cytoplasmic compartment into mitochondria, where beta-oxidation enzymes are located for energy production. The physiological form of carnitine was identified in 1962 as the L (−) isomer, or levocarnitine (Kaneko and Yoshida, 1962). Since then, the term carnitine has always been referred to as L-carnitine. One year later, Bremer (1963) discovered the outer carnitine palmitoyl transferase enzyme (CPT I), an essential enzyme involved in long-chain fatty acid oxidation (Fig. 1).
Figure 1.
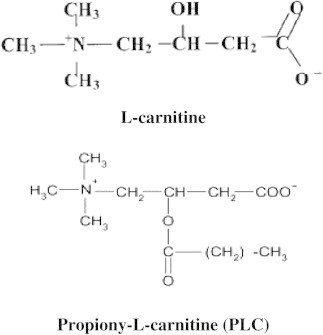
Chemical structure of L-carnitine and propionyl-L-carnitine.
1.1.2. Carnitine sources and pharmacokinetics
In humans, 75% of the total body carnitine originates from dietary sources and the remaining 25% from endogenous synthesis (Tein et al., 1996). Carnitine is synthesized from the amino acids lysine and methionine. The rate limiting step in carnitine biosynthesis is the availability of trimethyllysine at the intramitochondrial site of trimethyllysine hydroxylase activity (Rebouche et al., 1986).
L-carnitine is absorbed in the intestine by a combination of active transport and passive diffusion (Rebouche, 2004). Mucosal absorption of carnitine is saturated at 2 g (Harper et al., 1988). Following oral ingestion of 2 g carnitine, the maximum concentration is reached at about 3.5 h and then slowly decreases (Bach et al., 1983). Rebouche and Chenard (1991) suggested an oral bioavailability of 54–87% depending upon the quantity ingested. Muscle concentrations of carnitine are as much as 100 times higher than plasma indicating active uptake of carnitine, but at vastly different rates (Brass, 1992). Normally, 80% of the total myocardium carnitine uptake through the circulation and taken up by other tissues through active sodium dependent organic cation/carnitine transporter (OCTN2) (Tamai et al., 1998). Carnitine is excreted mainly in urine; however, a little is lost through the bile. L-carnitine is highly conservative since 95% of the filtered free carnitine (FC) is reabsorbed by the renal tubules, and the majority of the acyl-carnitine (AC) is excreted in urine, thus permitting excretion of the abnormal metabolites (Tanphaichitr and Leelahagul, 1993).
1.1.3. Normal carnitine levels
The total content of carnitine in the human body is about (16 g) 100 mmol (Bremer, 1983). Muscles contain 98% of that total amount, while 2% of carnitine is found in the liver and other tissues, respectively (Engel and Rebouche, 1984). Carnitine concentration in the heart is about 4.2 mmol/g tissue, which is over three times higher than that in the striated muscles (1.26 mmol/g), four times higher than that in the liver (0.94 mmol/g), and eight times higher than that in the kidney (0.52 mmol/g) (Steiber et al., 2004). The total carnitine concentration in plasma is usually in the range of 42–85 μmol/L and that of FC in the range of 35–70 μmol/L (Zdrojkowska-Krol et al., 1994). Under normal physiological conditions, 80% of total serum carnitine is FC and 20% is AC with normal AC/FC ratio of 0.25 (Campos et al., 1993; Bohles et al., 1994). A free carnitine level in the blood of <20 μmol/L is considered evidence of carnitine deficiency. A ratio of AC/FC more than 0.4 is considered abnormal and represents carnitine insufficiency in which there is insufficient FC relative to increased metabolic needs (Siliprandi et al., 1990).
1.1.4. Carnitine and propionyl-L-carnitine: application to therapy
L-carnitine and its short-chain derivatives, PLC, have been successfully used in the treatments and prevention of many diseases including ischemic heart disease (Spagnoli et al., 1982; Debska-Slizien et al., 1997; Arsenian et al., 1996), arrhythmia (Cacciatore et al., 1991; Rizzon et al., 1989), peripheral blood vessel diseases (Pola et al., 1992; Cipolla et al., 1999), primary carnitine deficiencies (Siliprandi et al., 1990), secondary carnitine deficiencies (Krähenbühl and Reichen, 2003; Brass, 2002), essential treatment for patients undergoing hemodialysis (Mir et al., 2002; Ahmad et al., 2001), treatment of male infertility (Matalliotakis et al., 2002), and treatment of beta-thalassemia (El-Beshlawy et al., 2004, 2005, 2007, 2008).
1.1.5. Physiological and biochemical alteration caused by cancer: role of carnitine
Anorexia, cachexia and malnutrition occur in many chronic disease states including cancer, heart failure, renal and liver failure, and autoimmune deficiency disease (AIDS). Cachexia occurs in up to one half of all patients diagnosed with cancer (Dewys et al., 1980; Toomey et al., 1995). Metabolic alterations caused by cancer cachexia have been attributed to a variety of interactions between the host and the tumour (Stewart et al., 1995). One of these metabolic changes that takes place throughout the body is the reduction in endogenous synthesis of carnitine. Low serum levels of carnitine in terminal neoplastic patients are decreased largely due to the decreased dietary intake and impaired endogenous synthesis of this substance (Hoang et al., 2007). These low serum carnitine levels also contribute to the progression of cachexia in cancer patients (Vinci et al., 2005; Malaguarnera et al., 2006).
1.2. Carnitine and cancer chemotherapy-induced multiple organ toxicity
1.2.1. Carnitine and doxorubicin-induced cardiomyopathy
Doxorubicin (DOX) was among the first anthracycline antibiotics to be in clinical use in cancer chemotherapy (Arcamone et al., 1969). It has a broad-spectrum antitumour activity against a variety of hematological and solid tumours (Carter, 1975). Unfortunately, the chronic administration of DOX is associated with the development of dose-dependent and irreversible cardiomyopathy, which restricts its usefulness in cancer chemotherapy (Goormaghtih and Ruysschaert, 1984; Kantrowitz and Bristow, 1984; Buzadar et al., 1985). Cardiomyopathy is the major limiting complication of DOX and affects 30–40% of the patients who receive a cumulative dose more than 500 mg/m2 (Van Vleet et al., 1980; Lefrak et al., 1973). Hence, even in responding tumours, it is necessary to discontinue DOX administration once a cumulative dose of 500 mg/m2 is reached to prevent the onset of irreversible cardiac damage (Buzadar et al., 1985; Lefrak et al., 1973). In view of the irreplaceability of DOX in cancer chemotherapy, one of the research aims being pursued most intensively is the possibility of eliminating its cardiotoxicity or reducing it to an acceptable level. In this regard, various strategies have been tried, exemplified by the use of free radical scavengers (Venkatesan, 1998; Al-Shabanah et al., 1998), iron chelators (Al-Harbi et al., 1992; Yeung et al., 1992), calcium antagonists (Rossi et al., 1994], histamine and adrenergic receptor blockers (Myers and Chabner, 1990).
The effects of L-carnitine and PLC on DOX-induced cardiomyopathy have extensively been investigated. Several experimental and clinical studies have reported that DOX induces its cardiomyopathy by inhibition of beta-oxidation of long-chain fatty acids with the consequent depletion of cardiac ATP and that L-carnitine supplementation protects the myocardium against this toxicity (De Leonardis et al., 1987; Abdel-aleem et al., 1997; Sayed-Ahmed et al., 1999a, 2000a; Strauss et al., 1998; Waldner et al., 2006). In early experiments, Alberts et al. (1978) investigated the protective effects of carnitine against acute (high-dose) and chronic (intermittent, low dose) DOX toxicity in rat and mice. They found that carnitine was able to decrease both acute and chronic DOX-linked lethality in normal rats without decreasing its antineoplastic activity or raising its bone marrow toxicity. Paterna et al. (1984) investigated whether L-carnitine could prevent DOX-induced cardiomyopathies in rabbits by promoting free fatty acid utilization for energy production and neutralizing long-chain free fatty acid toxicity occurring after ischemia. The results showed that L-carnitine reduced the frequency of cardiomyopathies and increased survival rate. Histopathological examination of myocardial tissues under light and electron microscopes revealed a marked decrease in mitochondrial lesions. Also, the possible protective effects of L-carnitine against DOX-induced cardiotoxicity were studied by Neri et al. (1986). Rat heart slices were incubated with DOX (14 mg/ml), L-carnitine (600 mg/ml), and DOX plus L-carnitine for 60 min. They measured cellular oxygen uptake, ATP, intracellular Ca2+ concentration and 14C-leucine incorporation (an index of protein synthesis). L-carnitine significantly reduced DOX-induced cardiac metabolic damage. The oxygen uptake inhibition decreased from 38% to 7%, the decrease in ATP concentration was reduced from 78% to 27% and the inhibition of protein synthesis from 11% to 6%. Therefore, according to Neri et al. (1986), L-carnitine appears to be a useful drug in the prevention of DOX-induced cardiotoxicity. The protective effects of L-carnitine against DOX-induced cardiotoxicity in rats were also studied by McFalls et al. (1986). Cardiomyopathy was induced by weekly intravenous injection of DOX (2 mg/kg) over a period of 6–7 weeks. Myocardial Carnitine concentration was not altered by DOX, but plasma carnitine concentration was increased resembling those of cardiomyopathies in adults.
Using DOX cardiomyopathic rat model, Shug (1987) designed an experiment to find an explanation for the protective effect of L-carnitine against DOX-induced cardiotoxicity. After 6 weeks of intravenous administration of DOX, rats treated without carnitine had a lower ejection fraction and left ventricular pressure than control or rats treated with DOX plus L-carnitine. Total carnitine in plasma was significantly higher in rats treated with DOX than control and DOX plus carnitine. They suggested that the increase in carnitine ester in plasma of DOX-treated rats was due to DOX-induced inhibition of long-chain fatty acid oxidation.
On the same line, Brady and Brady (1987) reported that DOX interacts with mitochondrial lipids as a part of its toxicity mechanism and thus inhibits CPT I, an essential enzyme involved in long-chain fatty acid oxidation, in isolated rat liver mitochondria in the presence of increasing concentrations of DOX. Based on these observations these authors suggested that DOX-induced cardiotoxicity may be, at least in part, due to the inhibition of CPT I. However, this hypothesis was challenged by Kashfi et al. (1990) who reported that the inhibition of CPT I by DOX was unrelated to its cardiotoxicity because less toxic analogs produced more potent inhibition of this enzyme. Finally in this regard, Beanlands et al. (1994) have shown an initial depression of fatty acid oxidation in a working heart preparation from rats injected chronically with DOX but again no mechanism for this effect has been ascertained.
Using a neonatal rat model, Strauss et al. (1998) evaluated the effect of DOX and DOX plus L-carnitine on the induction of de-novo heat shock protein 25 synthesis. DOX was injected intravenously at a dose of 9 mg/kg every three days to have a total dose of 27 mg/kg. L-carnitine was administered intravenously (20 mg/kg) before each subdose of DOX and then in drinking water (180 mg/kg) daily for 12 weeks. Heat shock protein of the homogenized heart tissue was determined by Western blot analysis. Results showed that de-novo synthesis of heat shock protein was three times more induced in carnitine-DOX rats than in DOX ones. The authors suggested that carnitine may enhance the cell-protecting mechanism based on an induction of shock protein, and this first cellular response could reduce the severity of DOX-induced-cardiomiopathy.
In isolated cardiac myocytes and mitochondria, Sayed-Ahmed et al. (1999a) studied the influence of L-carnitine on DOX-induced cardiac metabolic damage. The results showed that, after perfusion of the hearts with 0.5 mM DOX, they observed a significant 70% reduction of palmitate oxidation, whereas perfusion with L-carnitine increased palmitate oxidation by 37%. Addition of L-carnitine to the perfusate 10 min after perfusion with DOX, reversed 88% of DOX-induced inhibition of palmitate oxidation. In isolated mitochondria, DOX had no effect on acyl-CoA synthetase (an enzyme located on the outer mitochondrial membrane and responsible for the activation of long-chain fatty acids into their corresponding acyl-CoA forms) suggesting that DOX has no effect on the activation step of long-chain fatty acids. However, the oxidation of palmitoyl-CoA was significantly inhibited suggesting that DOX may inhibit CPT I or interfere with L-carnitine on binding site on this enzyme. The authors concluded that the addition of L-carnitine completely reversed the inhibition of palmitate and palmitoyl-CoA oxidation to control values. They added that L-carnitine did not interfere with the antitumour activity of DOX against the growth of Ehrlich ascites carcinoma cells.
In isolated rat heart myocytes and mitochondria, Sayed-Ahmed et al. (2000a) studied the effects of different concentrations of DOX in absence and presence of PLC on palmitoyl-CoA and palmitoyl-carnitine oxidation and on the activity of CPT 1. The results showed that DOX-induced concentration-dependent inhibition of substrates oxidation and inhibition of CPT I and that PLC completely reversed this inhibition to the control values. They concluded that DOX induced its cardiotoxicity by inhibition of CPT I and beta-oxidation of long-chain fatty acids with the consequent depletion of ATP in cardiac tissues, and that PLC can be used as a protective agent against DOX-induced cardiotoxicity (Fig. 2).
Figure 2.
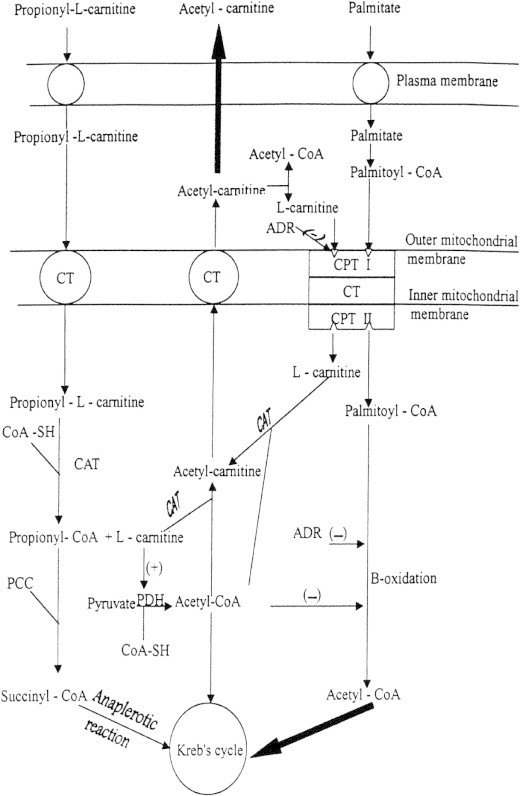
Proposed protective mechanism by PLC against DOX-induced cardiomyopathy. CT, carnitin acyl-carnitine translocase; CAT, carnitine acetyl transferase; CPT I, outer carnitine palmitoyltransferase; CPT II, inner carnitine palmitoyltransferase; PCC, propionyl-CoA carboxylase; ADR, adriamycin (trade name of DOX); (−) and (+) indicate inhibition and stimulation, respectively.
(Cited from Sayed-Ahmed et al., 2000a.)
In DOX cardiomyopathic rat model, Sayed-Ahmed et al. (2001) have initiated another study to confirm their in vitro findings and investigated the protective effects of PLC against DOX-induced cardiotoxicity. Chronic administration of DOX (3 mg/kg, I.P) significantly increased CK-MB, LDH, GOT and malondialdehyde and decreased reduced glutathione. Treatment with PLC induced complete reversal of these effects without decreasing the antitumour activity of DOX. In another study, Sayed-Ahmed et al. (2000b) investigated the effects of DOX on mRNA expression of heart fatty acid binding protein (H-FABP) using Northern blot analysis. Results showed that chronic administration of DOX caused dose-dependent and cumulative inhibition of H-FABP gene expression and that daily administration of L-carnitine protected against this effect in cardiac tissues. Authors concluded that DOX-induces its cardiotoxicity by inhibition of gene expression of H-FABP (Fig. 3).
Figure 3.
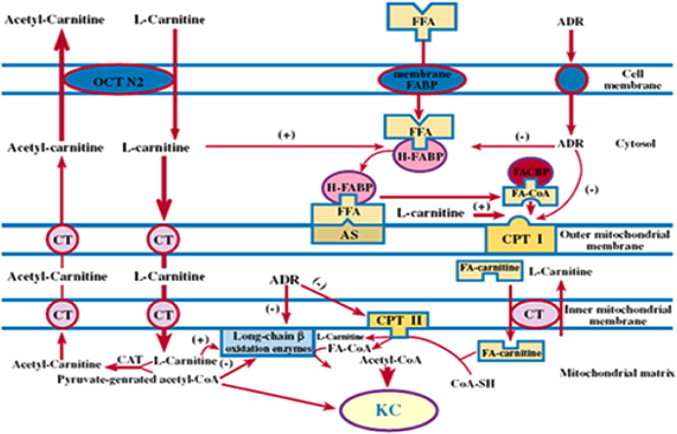
Inhibition of H-FABP mRNA expression in the heart as a novel mechanism for DOX-induced cardiotoxicity and for L-carnitine mediated protection against this effect. FFA: free fatty acid; CT: carnitine/acyl-carnitine translocase; FA-CoA: fatty acyl-CoA; CAT: carnitine acetyl transferase; FA-Carnitine: fatty acyl-carnitine; H-FABP: heart-type fatty acid binding protein; CPT I: outer carnitine palmitoyl transferase; CPT II: inner carnitine palmitoyl transferase; OCTN2: organic cation/carnitine transporter; (−) and (+) indicate inhibition and stimulation, respectively.
(Cited from Sayed-Ahmed et al. (2000b).)
More recently, Sayed-Ahmed et al. (2010) reported that chronic DOX therapy decreased the expression of H-FABP and OCTN2 mRNA expression and increased the expression of apoptotic genes in cardiac tissues that should be viewed as a mechanism during development of DOX-induced cardiomyopathy.
On the clinical level, De Leonardis et al. (1985) reported that DOX therapy is associated with chronic, dose-dependent and irreversible cardiomyopathy. In their study which involved nine patients, cardiotoxicity occurred in one third of all patients after cumulative dose of 300 mg/m2. Also, they investigated whether both acute and chronic DOX cardiotoxicity can be reversed by L-carnitine. They recommended systemic application of L-carnitine as adjuvant therapy during DOX treatment to prevent the development of DOX related cardiomyopathy. In another study, De Leonardis et al. (1987) examined the effects of L-carnitine on DOX and daunorubicin cardiotoxicity in 15 patients with breast and lung cancer. Their left ventricular performance was assessed by echocardiographical determination of the maximum velocities of fibre shortening and lengthening (VCFmax) as index for the systolic and diastolic function of the heart. They concluded that L-carnitine completely reversed DOX and daunorubicin induced decrease in VCF to the control values even after six therapeutic cycles.
The role of L-carnitine in DOX-induced cardiotoxicity was also studied by Mortensen et al. (1986) who examined 38 patients with solid tumours and hematological cancer by means of ECG, chest X-ray and echocardiography. They suggested the application of cardioprotective substances such as L-carnitine in order to reduce the severe DOX cardiotoxicity, pointing out that their use might be the beginning of a new era of prevention. Waldner et al. (2006) examined the effects of L-carnitine with a view to reducing cardiotoxicity of DOX-containing chemotherapy in 20 patients with Non-Hodgkin lymphoma. Patients were scheduled to receive 3 g L-carnitine before each chemotherapy cycle, followed by 1 g L-carnitine/day during the following 21 days. Carnitine-treated patients showed a rise in plasma carnitine which led to an increase of relative mRNA levels from CPT 1 and OCTN2. They concluded that biochemical and molecular analyses indicated a stimulation of oxidative metabolism in white blood cells through carnitine uptake.
Yaris et al. (2002) examined relationship between serum carnitine levels and cardiac dysfunction during treatment with DOX. Fifteen patients were evaluated prospectively. Measurement of carnitine levels and evaluation of cardiac function were performed prior to treatment, and after cumulative doses of 180 and 300 mg/m2 in 15 patients and 20 healthy children served as control group to obtain reference values. They concluded that there was a trend towards decreasing serum carnitine levels and increasing cardiac dysfunction with increasing cumulative doses of DOX. In contrast, Yoon et al. (2003) have reported that DOX caused significant elevations of plasma free acyl and total carnitine.
1.2.2. Carnitine and doxorubicin-induced hepatorenal dysfunction
Doxorubicin-induced hyperlipemia in rats was investigated by Bizzi et al. (1983). Results showed that massive hyperlipemia occurred 14–21 days after a single dose of DOX (7.5 mg/kg intravenous). Lipid synthesis and secretion were decreased. Mitochondrial oxidation of long-chain fatty acids was markedly reduced in kidney. Lipoprotein lipase activity was reduced in adipose tissue. They suggested that DOX-related hyperlipemia is due to reduced lipid storage and utilization and that Carnitine supplementation did not counteract hyperlipemia and proteinuria after DOX. Moreover, nephrotoxicity and oxidative stress induced by DOX in rats were investigated by Boonsanit et al. (2006) who found that L-carnitine can prevent renal impairment functionally, biochemically and histopathologically with a corresponding reduction of oxidative stress. Zeidan et al. (2002) evaluated the heart and liver responses after DOX toxic aggression, with and without exogenous L-carnitine protection. Results showed that DOX-induced long-term cardiac subcellular pathology included loss, disruption and disassembly of myofibrils, and mitochondrial swelling and condensation. On the other hand, the DOX-induced subcellular hepatic alterations consisted of polymorphic mitochondria, cytoplasmic vacuolization and accumulation of lipid droplets. However, these alterations were of less severity in carnitine-treated group in both heart and liver, suggesting that carnitine could be used as a possible hepatoprotector agent against DOX-induced toxicity.
1.2.3. Carnitine and cisplatin-induced nephrotoxicity
Cisplatin, cis-diamminedichloroplatinum II (CDDP), is an inorganic platinum compound with a broad-spectrum antineoplastic activity against various types of animal and human tumours (Loehrer and Einhom, 1984). Unfortunately, the optimal usefulness of CDDP as an important anticancer drug is usually limited secondary to its dose related nephrotoxicity (Lieberthal et al., 1996). It is well known that CDDP-induced nephrotoxicity is the most important dose-limiting factor in cancer chemotherapy (Lieberthal et al., 1996). Several mechanisms have been suggested, among which oxidative stress is the best studied mechanism. Oxidative stress is caused mainly by increasing lipid peroxidation and depletion of glutathione, which, in turn, induces apoptosis of renal proximal tubule cells and consequent kidney dysfunction (Zhang and Lindup, 1993; Kuhlmann et al., 1997). Furthermore, owing to the toxic effect of CDDP on renal tubules, CDDP inhibits specific membrane transport systems, with a consequent increase in the excretion of a number of essential endogenous substances, including L-carnitine and amino acids (Seguro et al., 1989; Heuberger et al., 1998; Chang et al., 2002).
Kidney plays an important role in keeping the homeostasis of carnitine by conserving 95% of the filtered carnitine (Mancinelli et al., 1995). In kidney, carnitine is reabsorbed efficiently by a specific sodium-dependent transport system located in the proximal tubule (Stieger et al., 1995). It is well documented that renal carnitine excretion increases dramatically in patients with proximal tubular damage such as Fanconi Syndrome (Bernardini et al., 1985; Steinmann et al., 1987). Heuberger et al. (1998) examined the effect of CDDP on plasma concentrations and urinary excretion of carnitine in ten patients with different malignancies treated with CDDP-based chemotherapy. The results showed that during treatment with CDDP, the total plasma carnitine concentration increased by approximately 30% and normalized 7 days after stopping therapy. Urinary excretion of total carnitine increased by a factor of 10 during CDDP administration and also normalized 7 days after cessation of chemotherapy. This increase was due to excretion of both free carnitine and acyl-carnitine that averaged approximately 1 mmol carnitine per day. Similarly, urinary clearance of total carnitine was increased during therapy with CDDP by a factor of approximately 8 and returned to normal 7 days after chemotherapy. They concluded that treatment with CDDP is associated with a tenfold increase in renal carnitine excretion, most likely due to inhibition of carnitine reabsorption by the proximal tubule of the nephron. Well-nourished patients support this loss of carnitine even after repeated cycles of chemotherapy without developing hypocarnitinaemia. However, cachectic patients with decreased dietary carnitine uptake may develop carnitine deficiency when treated repeatedly with chemotherapies including CDDP.
Mancinelli et al. (2007) evaluated the effect of carboplatin-based chemotherapy on plasma concentrations and urinary excretion of L-carnitine and its main ester, ALC, in 11 cancer patients on carboplatin therapy (1 h intravenous infusion for 1 h) in combination with docetaxel, paclitaxel or vinorelbine. Before carboplatin therapy, the mean ± SD plasma concentrations of L-carnitine and ALC were 47.8 ± 10.9 and 7.04 ± 1.04 nmol/ml, respectively, and remained constant throughout the entire study period. In contrast, urinary excretion of L-carnitine and ALC increased significantly during the chemotherapy from 115 ± 105 to 480 ± 348 μmol/day and from 41 ± 41 to 89 ± 52 μmol/day (P < 0.05) for L-carnitine and ALC, respectively, subsequently reverting to normal 6 days after the end of chemotherapy. Similarly, the renal clearance of L-carnitine and ALC increased substantially during chemotherapy from 1.67 ± 1.43 to 9.05 ± 9.52 ml/min and from 4.02 ± 4.51 to 7.97 ± 5.05 ml/min for LC and ALC, respectively, reverting to normal 6 days after the end of chemotherapy. Plasma concentrations and urinary excretion of glucose, phosphate and urea nitrogen and creatinine clearance were not affected by carboplatin therapy, indicating no impaired kidney function. They concluded that treatment with carboplatin was associated with a marked urinary loss of LC and ALC, most likely due to the inhibition of carnitine reabsorption in the kidney.
Recently, Haschke et al. (2010) investigated whether oxaliplatin alters plasma and urine carnitine level as the case with CDDP and carboplatin, and if so, what are the possible mechanisms. A total of 22 patients treated either with a single dose of cisplatin, carboplatin or oxaliplatin. Carnitine and kidney function parameters were determined in plasma and urine. Also, inhibition of OCTN2 in L6 cells (overexpressing OCTN2) and mRNA expression of OCTN2 in 293-EBNA cells were assessed. Results showed that renal excretion of free and short-chain acyl-carnitine increased 4–10 times during treatment and normalized 1 week after administration of cisplatin, carboplatin or oxaliplatin. Renal excretions of alpha1-microglobulin and other proximal tubular markers were also increased, compatible with a proximal tubular defect. Direct inhibition of OCTN2 expressed in L6 cells by cisplatin, oxaliplatin or platinum could not be demonstrated, and experiments using urine from patients treated with CDDP inhibited OCTN2 activity not more than the expected from the carnitine content in the respective urine sample. CDDP was associated with a time-and concentration-dependent decrease of OCTN2 mRNA and protein expression in 293-EBNA cells. They concluded that all platinum derivatives investigated are associated with renal tubular damage in humans without significantly affecting glomerular function. The rapid onset and complete reversibility of this effect favor a functional mechanism such as impaired expression of OCTN2 in proximal tubular cells.
One mechanism contributing to cancer-related fatigue involves the decrease in ATP synthesis secondary to carnitine deficiency. Hockenberry et al. (2009) examined fatigue and carnitine levels in 76 patients before and 1 week after ifosfamide (IFO), CDDP, or DOX chemotherapy. Results showed that there was a significant increase in free and total carnitine levels after treatment for patients receiving doxorubicin than patients receiving cisplatin or ifosfamide. Increased fatigue and decreased carnitine were significantly correlated a week after chemotherapy in children/adolescents who had received prior chemotherapy. They concluded that decreased carnitine and increased fatigue occurred after 1–2 courses of chemotherapy which provides support for an inverse relationship between carnitine and fatigue in children/adolescents with cancer.
On the experimental level, Sayed-Ahmed et al. (1999b) were the first to report that L-carnitine has a considerable protective effect against CDDP-induced nephrotoxicity in rats. Chang et al. (2002) demonstrated that L-carnitine strongly inhibited CDDP-induced mitochondrial dysfunction and DNA injury, lipid peroxidation, and apoptosis of epithelial cells in the kidney and small intestine without interfering with the tumoricidal action of CDDP against cancer cells inoculated in the peritoneal cavity. They concluded that L-carnitine may have therapeutic potential for inhibiting the side effects of CDDP and other anticancer agents in the kidney and small intestine. Two years later, Sayed-Ahmed et al. (2004) reported the progression of CDDP-induced nephrotoxicity in carnitine-depleted rat model. They concluded that carnitine deficiency is a risk factor and should be viewed as a mechanism in CDDP-related kidney dysfunction and that carnitine supplementation attenuates CDDP-induced nephrotoxicity. Moreover, Aleisa et al. (2007) reported that carnitine supplementation, using PLC, showed complete reversal of cisplatin-induced carnitine deficiency and energy starvation in rat kidney tissues. Consistent with these findings, moreover, Arafa (2008) reported that carnitine deficiency, whether being a causative clue or a sequela, might represent a risk factor in carboplatin nephropathy.
Renal interstitial fibrosis is a major complication of CDDP treatment, due to the increased accumulation of extracellular matrix (ECM) proteins that remodeling is important for the development of normal tissues; indeed, its malfunction might play a role in the etiology of various diseases. In this regard and recently, Martinez et al. (2009) examined the localization and expression of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor metalloproteinase-3 (TIMP-3) in rat kidney, 7, 10 and 15 days after treatment with CDDP, with and without L-carnitine. Results showed that CDDP increased expression of MMP-9 and decreased expression of TIMP-3 in glomerular and tubular sections. Pre-treatment with L-carnitine induced an important reduction in MMP-9 expression and increase in TIMP-3. They concluded that matrix remodeling by MMP-9 and TIMP-3, in the later stages, can play an important role in the development of glomerular sclerosis and interstitial fibrosis after CDDP treatment. Also, they postulated that L-carnitine protects from CDDP injury, by modulating the relationship between MMP-9 and TIMP-3.
Tufekci et al. (2009) investigated, on mechanism-based, the protective effects of ALC on CDDP-induced nephrotoxicity in rats. They concluded that antioxidative, antiapoptotic and anti-inflammatory properties of ALC were supported by the findings that this agent improves kidney function tests and has the effects of tissue protection and inhibition of apoptosis in cisplatin-induced nephrotoxicity. Altun et al. (2010) examined the effects of ALC on CDDP-induced cytotoxicity and oxidative stress in neuroblastoma cells. Results showed that ALC effectively inhibited the increase in CDDP-induced oxidized glutathione and lipid peroxidation formation in neuroblastoma cells.
1.2.4. Carnitine and cisplatin-induced neurotoxicity
In addition to bone marrow suppression and renal toxicity, neurotoxicity is a commonly occurring side effect of widely used chemotherapeutic agents like taxanes, cisplatin and vinca alkaloids. Neurotoxicity can cause antitumor therapy discontinuation or dose regimen modification. Pisano et al. (2003) tested the hypothesis that ALC may have a protective role on cisplatin and paclitaxel-induced neuropathy in rats. Their results indicated that ALC is a specific protective agent for chemotherapy-induced neuropathy after cisplatin or paclitaxel treatment without showing any interference with the antitumor activity of the drugs. Bianchi et al. (2005) investigated the effect of oral ALC (1 g two times daily) for 8 weeks in 25 patients with neuropathy grade 3 during paclitaxel or CDDP therapy, or grade 2 persisting for at least three months after discontinuing the drugs. They strongly recommend ALC testing in preventing progression or reverting symptoms during CDDP, paclitaxel and neurotoxic chemotherapy. Maestri et al. (2005) examined the effect of ALC in reversing peripheral neuropathy in patients with chemotherapy-induced peripheral neuropathy. A total of 27 patients received at least one cisplatin (n = 5) or one paclitaxel (n = 11)-based regimen, or a combination of both (n = 11). Patients with chemotherapy-induced peripheral neuropathy were treated with ALC 1 g by intravenous infusion over 1–2 h for at least 10 days. They concluded that ALC seems to be an effective and well-tolerated agent for the treatment of chemotherapy-induced peripheral neuropathy. Graziano et al. (2002) have demonstrated that oral carnitine supplementation (4 gm/day) for 7 days to cancer patients receiving either CDDP-based in 44 patients or IFo-based in 6 patients was effective in alleviating chemotherapy-induced fatigue and normalizing serum carnitine levels.
1.2.5. Carnitine and cisplatin-induced cardiomyopathy
Using an animal model of carnitine deficiency, Al-Majed et al. (2006) examined whether carnitine deficiency, secondary to CDDP-induced nephrotoxicity, could contribute or enhance CDDP-induced cardiac dysfunction. They concluded, for the first time, that carnitine deficiency and oxidative stress are risk factors and should be viewed as mechanisms during development of CDDP-related cardiomyopathy and that carnitine supplementation, using PLC, prevents the progression of CDDP-induced cardiotoxicity.
1.2.6. Carnitine and cisplatin-induced hepatotoxicity
In carnitine-depleted rat model, Al-Majed (2007) investigated whether or not carnitine deficiency is a risk factor and could contribute to CDDP-induced liver toxicity, and if so, whether carnitine supplementation using PLC could offer protection against this toxicity. The author concluded that oxidative stress is not the main cause of CDDP-related hepatotoxicity but rather carnitine deficiency provokes CDDP-induced hepatotoxicity and that carnitine supplementation prevents the development of CDDP-induced liver injury.
1.2.7. Carnitine and carboplatin-induced bone marrow toxicity
Abd-Allah et al. (2005) have initiated an in vitro study utilizing bone marrow cell cultures and reported that L-carnitine is effective not only in protecting against carboplatin-induced kidney damage but it is also effective in protecting against carboplatin-induced myelosuppression.
1.2.8. Carnitine and oxazaphosphorines-induced organs toxicity
Cyclophosphamide (CP) and ifosfamide (IFO) are alkylating oxazaphosphorine agents that are commonly used in most cancer chemotherapy and immunosuppressive protocols (Murphy et al., 1986; Preiss and Baumann, 2001). Unfortunately, the optimal clinical usefulness of these drugs is severely limited by a high incidence of urotoxicity, cardiotoxicity and nephrotoxicity (Brock et al., 1982; Colvin, 1999; Ludeman, 1999; Luo et al., 2008). The metabolic pathway of oxazaphosphorines leads to formation of chloroacetyl-CoA, with subsequent depletion of the CoA-SH levels. Carnitine is known to detoxify excess amounts of CoA-bound moieties with formation of acylcarnitines, which are excreted and detected in urine after IFO therapy resulting in secondary carnitine deficiency (Visarius et al., 1999; Dubourg et al., 2001). Another major metabolite of oxazaphosphorines is thiodiglycolic which when administered to rats, inhibits the carnitine-dependent oxidation of palmitic acid by 55%, but does not affect the oxidation of carnitine-independent substrates. Additionally, thiodiglycolic acid inhibited oxidation of palmitic acid but not palmitoyl-L-carnitine in isolated rat liver mitochondria, indicating that it either sequesters carnitine or inhibits CPT I (Hofmann et al., 1991).
Marthaler et al. (1999) were the first to measure urinary excretion of carnitine in 5 patients with advanced soft tissue sarcoma during a continuous infusion of IFO over 5 days at a dose of 2.8–3.2 g/m2 per day. The results showed that the excretion of both free and total carnitine significantly increased on the first day of chemotherapy and then gradually decreased. The average loss of carnitine during a chemotherapy cycle amounted to 8.5 mmol (10% of total carnitine stores). They concluded that formation and excretion of carnitine esters and the metabolites of IFO and/or a decreased renal tubular reabsorption could account for this marked loss, which might lead to symptomatic carnitine deficiency after several chemotherapy cycles.
Using carnitine-depleted rat model, recent studies (Darweesh, 2009; Fatani et al., 2010), investigated whether carnitine deficiency plays a role and should be viewed as a mechanism during development of CP-induced cardiomyopathy as well as exploring on mechanism-based if carnitine supplementation using PLC could offer protection against this toxicity. They concluded that carnitine deficiency is a risk factor and should be viewed as a mechanism in CP-related cardiomyopathy, serum and urine carnitine levels should be monitored and viewed as indices of CP-induced multiple organ toxicity and that carnitine supplementation, using PLC, prevents the development of CP-induced cardiotoxicity. On the same track, Darweesh (2009) investigated the effects of the standard IFO-induced Fanconi Syndrome regimen on serum, urine and cardiac carnitine levels in normal and carnitine-depleted rats and its relationship to IFO-induced cardiotoxicity. They concluded that using IFO at the standard doses that induce Fanconi Syndrome is associated with increased urinary losses of carnitine. The authors added that this carnitine deficiency, secondary to Fanconi Syndrome, provokes IFO-related cardiotoxicity and that carnitine supplementation, using PLC, ameliorates the severity of IFO-induced multiple organ toxicity.
1.2.9. Carnitine and bleomycin-induced pulmonary fibrosis
The optimal clinical usefulness of bleomycin as an important antineoplastic drug is usually limited due to the development of dose and time-dependent interstitial pneumonitis and pulmonary fibrosis. In bleomycin-induced lung fibrosis rat model, (Daba et al., 2002) have demonstrated that L-carnitine (500 mg/kg) decreased bleomycin-induced elevations of serum tumour necrosis-alpha (TNF-α), lipid peroxide level in lung tissues and enhanced responsiveness of pulmonary arterial rings to 5-HT. Moreover, Sayed-Ahmed et al. (2004) reported that acetyl-carnitine attenuated bleomycin-induced acute lung injury, which was attributed to its free radical scavenging properties with the consequent improvement in mitochondrial function and ATP production.
2. Conclusion
Depending on the data presented in this review on the experimental and clinical levels, L-carnitine should be viewed as a leading candidate and must be given along with doxorubicin, cisplatin, carboplatin, oxaliplatin, cyclophosphamide and ifosfamide to block their multiple organ toxicities and to permit larger doses of these anticancer drugs to be administered, thereby killing more cancer cells and increasing the chances of patient survival.
References
- Abd-Allah A.R., Al-Majed A.A., Al-Yahya A.A., Fouda S.I., Al-Shabanah O.A. L-Carnitine halts apoptosis and myelosuppression induced by carboplatin in rat bone marrow cell cultures (BMC) Arch. Toxicol. 2005;7:406–413. doi: 10.1007/s00204-004-0643-3. [DOI] [PubMed] [Google Scholar]
- Abdel-aleem S., El-Merzabani M.M., Sayed-Ahmed M., Taylor D.A., Lowe J.E. Acute and chronic effects of adriamycin on fatty acid oxidation in isolated cardiac myocytes. J. Mol. Cell. Cardiol. 1997;29:789–797. doi: 10.1006/jmcc.1996.0323. [DOI] [PubMed] [Google Scholar]
- Ahmad S., Robertson H.T., Golper T.A., Wolfson M., Kurtin P., Katz L.A., Hirschberg R., Nicora R., Ashbrook D.W., Kopple J.D. Multicenter trial of L-carnitine in maintenance hemodialysis patients II. Clin. Biochem. Effects Kid. Intern. 2001;38:912–918. doi: 10.1038/ki.1990.290. [DOI] [PubMed] [Google Scholar]
- Alberts D.S., Yel-Mei P., Moon T.E., Bressler R. Carnitine prevention of adriamycin toxicity in mice. Biomedicine. 1978;29:256–258. [PubMed] [Google Scholar]
- Aleisa A.M., Al-Majed A.A., Al-Yahya A.A., Al-Rejaie S.S., Bakheet S.A., Al-Shabanah O.A., Sayed-Ahmed M.M. Reversal of cisplatin-induced carnitine deficiency and energy starvation by propionyl-L-carnitine in rat kidney tissues. Clin. Exp. Pharmacol. Physiol. 2007;34:1252–1259. doi: 10.1111/j.1440-1681.2007.04714.x. [DOI] [PubMed] [Google Scholar]
- Al-Harbi M.M., Al-Gharably N.M., Al-Shabanah O.A., Al-Bekairi A.M., Osman A.M., Tawafik H.N. Prevention of doxorubicin-induced myocardial and haematological toxicities in rats by the iron chelator desferrioxamine. Cancer Chemother. Pharmacol. 1992;31:200–204. doi: 10.1007/BF00685548. [DOI] [PubMed] [Google Scholar]
- Al-Majed A.A., Sayed-Ahmed M.M., Al-Yahya A.A., Aleisa A.M., Al-Rejaie S.S., Al-Shabanah O.A. Propionyl-L-carnitine prevents the progression of cisplatin-induced cardiomyopathy in a carnitine-depleted rat model. Pharmacol. Res. 2006;53:278–286. doi: 10.1016/j.phrs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Al-Majed A.A. Carnitine deficiency provokes cisplatin-induced hepatotoxicity in rats. Basic Clin. Pharmacol. Toxicol. 2007;100:145–150. doi: 10.1111/j.1742-7843.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- Al-Shabanah O., Mansour M., El-Kashef H., Al-Bekairi A. Captopril ameliorates myocardial and hematological toxicities induced by adriamycin. Biochem. Mol. Biol. Int. 1998;17:419–427. doi: 10.1080/15216549800202802. [DOI] [PubMed] [Google Scholar]
- Altun Z.S., Güneş D., Aktaş S., Erbayrktar Z., Olgun N. Protective effects of acetyl-L-carnitine on cisplatin cytotoxicity and oxidative stress in neuroblastoma. Neurochem. Res. 2010;35(3):437–443. doi: 10.1007/s11064-009-0076-8. [DOI] [PubMed] [Google Scholar]
- Arafa H.M. Carnitine deficiency aggravates carboplatin nephropathy through deterioration of energy status, oxidant/anti-oxidant balance, and inflammatory endocoids. Toxicology. 2008;254:51–60. doi: 10.1016/j.tox.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Arcamone F., Franceschi G., Tenco S., Selva S. Adriamycin (14-hydroxy daunorubicin), a novel antitumour antibiotic. Tetrahedron Lett. 1969;13:1007–1010. doi: 10.1016/s0040-4039(01)97723-8. [DOI] [PubMed] [Google Scholar]
- Arsenian M.A., New P.S., Cafasso C.M. Safety, tolerability, and efficacy of a glucose–insulin–potassium–magnesium–carnitine solution in acute myocardial infarction. Am. J. Cardiol. 1996;78:477–479. doi: 10.1016/0002-9149(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Bach A.C., Schirardin H., Sihr M.O., Storck D. Free and total carnitine in human serum after oral ingestion of L-carnitine. Diabetes Metab. 1983;9:121–124. [PubMed] [Google Scholar]
- Beanlands R.S., Shaikh N.A., Wen W.H., Dawood F., Ugnat A.M., McLaughlin P.R., Carere R., Liu P.P. Alterations in fatty acid metabolism in adriamycin cardiomyopathy. J. Mol. Cell. Cardiol. 1994;26:109–119. doi: 10.1006/jmcc.1994.1012. [DOI] [PubMed] [Google Scholar]
- Bernardini I., Rizzo W.B., Dalakas M., Bernar J., Gahl W.A. Plasma and muscle free carnitine deficiency due to renal Fanconi Syndrome. J. Clin. Invest. 1985;75:1124–1130. doi: 10.1172/JCI111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi G., Vitali G., Caraceni A., Ravaglia S., Capri G., Cundari S., Zanna C., Gianni L. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur. J. Cancer. 2005;41:1746–1750. doi: 10.1016/j.ejca.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Bizzi A., Ceriani L., Gerundino M., Spina A., Tacconi M.T., Veneroni E. Adriamycin causes hyperlipemia as a consequence of nephrotoxicity. Toxicol. Lett. 1983;18:291–300. doi: 10.1016/0378-4274(83)90109-1. [DOI] [PubMed] [Google Scholar]
- Bohles H., Evangeliou A., Bervoets K., Eckert I., Sewell A. Carnitine esters in metabolic disease. Eur. J. Pediatr. 1994;153:S57–S61. doi: 10.1007/BF02138779. [DOI] [PubMed] [Google Scholar]
- Boonsanit D., Kanchanapangka S., Buranakarl C. L-carnitine ameliorates doxorubicin-induced nephritic syndrome in rats. Nephrology (Carlton) 2006;11(4):313–320. doi: 10.1111/j.1440-1797.2006.00592.x. [DOI] [PubMed] [Google Scholar]
- Brady L.J., Brady P.S. Hepatic and cardiac carnitine palmitoyltransferase activity. Effects of adriamycin and galactosamine. Biochem. Pharmacol. 1987;36:3419–3423. doi: 10.1016/0006-2952(87)90320-0. [DOI] [PubMed] [Google Scholar]
- Brass E.P. Interaction of carnitine and propionate with pyruvate oxidation by hepatocytes from clofibrate-treated rats: importance of coenzyme A availability. J. Nutr. 1992;122:234–240. doi: 10.1093/jn/122.2.234. [DOI] [PubMed] [Google Scholar]
- Brass E.P. Pivalate-generating prodrugs and carnitine homeostasis in man. Pharmacol. Rev. 2002;54:589–598. doi: 10.1124/pr.54.4.589. [DOI] [PubMed] [Google Scholar]
- Bremer J. Carnitine metabolism and function. Physiol. Rev. 1983;63:1420–1479. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Bremer J. Carnitine in intermediary metabolism. Reversible acetylation of carnitine by mitochondria. J. Biol. Chem. 1962;237:2228–2231. [PubMed] [Google Scholar]
- Bremer J. Carnitine in intermediary metabolism. The metabolism of fatty acid esters of carnitine by mitochondria. J. Biol. Chem. 1962;237:3628–3632. [PubMed] [Google Scholar]
- Bremer J. Carnitine in intermediary metabolism-the biosynthesis of palmitoyl-carnitine by cell sub-fractions. J. Biol. Chem. 1963;238:2774–2779. [PubMed] [Google Scholar]
- Brock N., Stekar P.J., Scheef W. Studies on the urotoxicity of oxazaphosphorine cytostatics and its prevention. III. Profile of action of sodium 2-mercaptoethane sulfonate (mesna) Eur. J. Cancer Clin. Oncol. 1982;18:1377–1387. doi: 10.1016/0277-5379(82)90143-2. [DOI] [PubMed] [Google Scholar]
- Buzadar A.V., Marcus C., Smith T.L., Blumenschein G.R. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55:2761–2765. doi: 10.1002/1097-0142(19850615)55:12<2761::aid-cncr2820551206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Campos Y., Huertas R., Lorenzo G., Bautista J., Gutierrez E., Aparicio M., Alesso L., Arenas J. Plasma carnitine insufficiency and effectiveness of L-carnitine therapy in patients with mitochondrial myopathy. Muscle Nerve. 1993;16:150–153. doi: 10.1002/mus.880160205. [DOI] [PubMed] [Google Scholar]
- Cacciatore L., Cerio R., Ciarimboli M., Cocozza M., Coto V., D’Alessandro A., D’Alessandro L., Grattarola G., Imparato L., Lingetti M. The therapeutic effect of L-carnitine in patients with exercise-induced stable angina: a controlled study. Drugs Exp. Clin. Res. 1991;17:225–235. [PubMed] [Google Scholar]
- Carter S.K. Adriamycin – a review. J. Natl. Cancer Inst. 1975;55:1265–1274. doi: 10.1093/jnci/55.6.1265. [DOI] [PubMed] [Google Scholar]
- Chang B., Nishikawa M., Sato E., Utsumi K., Inoue M. L-carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch. Biochem. Biophys. 2002;405:55–64. doi: 10.1016/s0003-9861(02)00342-9. [DOI] [PubMed] [Google Scholar]
- Cipolla M.J., Nicoloff A., Rebello T., Amato A., Porter J.M. Propionyl-L-carnitine dilates human subcutaneous arteries through an endothelium-dependent mechanism. J. Vasc. Surg. 1999;29:1097–1103. doi: 10.1016/s0741-5214(99)70251-x. [DOI] [PubMed] [Google Scholar]
- Colvin O.M. An overview of cyclophosphamide development and clinical applications. Curr. Pharm. 1999;5:555–560. [PubMed] [Google Scholar]
- Daba M.H., Abdel-Aziz A.A., Moustafa A.M., Al-Majed A.A., Al-Shabanah O.A., El-Kashef H.A. Effects of L-carnitine and ginkgo biloba extract (EG b 761) in experimental bleomycin-induced lung fibrosi. Pharmacol. Res. 2002;45:461–467. doi: 10.1006/phrs.2002.0985. [DOI] [PubMed] [Google Scholar]
- Darweesh, A.Q., 2009. Possible mechanisms of oxazaphosphorines-induced cardiotoxicity in normal and carnitine-depleted rats. M.Sc. Thesis, King Saud University.
- De Leonardis V., Neri B., Bacalli S., Cinelli P. Reduction of cardiac toxicity of anthracyclines by L-carnitine: preliminary overview of clinical data. Int. J. Clin. Pharmacol. Res. 1985;5:137–142. [PubMed] [Google Scholar]
- De Leonardis V., De Scalzi M., Neri B., Bartalucci S., Cinelli P. Echocardiographic assessment of anthracycline cardiotoxicity during different therapeutic regimens. Int. J. Clin. Pharmacol. Res. 1987;7:307–311. [PubMed] [Google Scholar]
- Debska-Slizien A., Owczarzak A., Król E. The influence of L-carnitine and simultaneous erythropoetin and L-carnitine administration on erythrocyte metabolism in hemodialysis patients. Adv. Ren. Nutr. Metab. Ed. Bios. 1997:137–144. [Google Scholar]
- Dewys W.D., Begg C., Lavin P.T., Band P.R., Bennett J.M., Bertino J.R., Cohen M.H., Douglass H.O., Jr., Engstrom P.F., Ezdinli E.Z., Horton J., Johnson G.J., Moertel C.G., Oken M.M., Perlia C., Rosenbaum C., Silverstein M.N., Skeel R.T., Sponzo R.W., Tormey D.C. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- Dubourg L., Michoudet C., Cochat P., Baverel G. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J. Am. Soc. Nephrol. 2001;12:1615–1623. doi: 10.1681/ASN.V1281615. [DOI] [PubMed] [Google Scholar]
- El-Beshlawy A., El Accaoui R., Abd El-Sattar M., Gamal El-Deen M.H., Youssry I., Shaheen N., Hamdy M., El-Ghamrawy M., Taher A. Effect of L-carnitine on the physical fitness of thalassemic patients. Ann. Hematol. 2007;86:31–34. doi: 10.1007/s00277-006-0181-6. [DOI] [PubMed] [Google Scholar]
- El-Beshlawy A., Ragab L., Fattah A.A., Ibrahim I.Y., Hamdy M., Makhlouf A., Aoun E., Hoffbrand V., Taher A. Improvement of cardiac function in thalassemia major treated with L-carnitine. Acta Haematol. 2004;111:143–148. doi: 10.1159/000076522. [DOI] [PubMed] [Google Scholar]
- El-Beshlawy A., Seoud H., Ibrahim A., Youssry I., Gabre H., Isma’eel H., Aoun E., Taher A. Apoptosis in thalassemia major reduced by a butyrate derivative. Acta Haematol. 2005;114:155–159. doi: 10.1159/000087890. [DOI] [PubMed] [Google Scholar]
- El-Beshlawy A., Youssry I., El-Saidi S., El Accaoui R., Mansi Y., Makhlouf A., Taher A. Pulmonary hypertension in beta-thalassemia major and the role of L-carnitine therapy. Pediatr. Hematol. Oncol. 2008;25:734–743. doi: 10.1080/08880010802244035. [DOI] [PubMed] [Google Scholar]
- Engel A.G., Rebouche C.J. Carnitine metabolism and inborn errors. J. Inher. Metabol. Dis. 1984;7:38–43. doi: 10.1007/BF03047372. [DOI] [PubMed] [Google Scholar]
- Fatani A.G., Darweesh A.Q., Rizwan L., Aleisa A.M., Al-Shabanah O.A., Sayed-Ahmed M.M. Carnitine deficiency aggravates cyclophosphamide-induced cardiotoxicity in rats. Chemotherapy. 2010;56:71–81. doi: 10.1159/000298822. [DOI] [PubMed] [Google Scholar]
- Friedman S., Fraenkel G. Reversible enzymatic acetylation of carnitine. Arch. Biochem. Biophys. 1955;51:491–501. doi: 10.1016/0003-9861(55)90515-4. [DOI] [PubMed] [Google Scholar]
- Fritz I.B. The effect of muscle extracts on the oxidation of palmitic acid by liver slices and homogenates. Acta Physiol. Scand. 1955;34:367–385. doi: 10.1111/j.1748-1716.1955.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Goormaghtih E., Ruysschaert J.M. Anthracycline glycoside–membrane interactions. Biochim. Biophys. Acta. 1984;779:271–288. doi: 10.1016/0304-4157(84)90013-3. [DOI] [PubMed] [Google Scholar]
- Graziano F., Bisonni R., Catalano V., Silva R., Rovidati S., Mencarini E., Ferraro B., Canestrari F., Baldelli A.M., De Gaetano A., Giordani P., Testa E., Lai V. Potential role of levocarnitine supplementation for the treatment of chemotherapy-induced fatigue in non-anaemic cancer patients. Br. J. Cancer. 2002;86:1854–1857. doi: 10.1038/sj.bjc.6600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulewitsch W., Krimberg R. Zur Kenntunis der extraktionsstoffe der muskeln. 2. Mittei-lung uber das carnitine. Hoppe-Seyler’s Z. Physiol. Chem. 1905;45:326–330. [Google Scholar]
- Harper P., Elwin C.E., Cederblad G. Pharmacokinetics of intravenous and oral bolus doses of L-carnitine in healthy subjects. Eur. J. Clin. Pharmacol. 1988;35:555–562. doi: 10.1007/BF00558253. [DOI] [PubMed] [Google Scholar]
- Haschke M., Vitins T., Lüde S., Todesco L., Novakova K., Herrmann R., Krähenbühl S. Urinary excretion of carnitine as a marker of proximal tubular damage associated with platin-based antineoplastic drugs. Nephrol. Dial. Transplant. 2010;25:426–433. doi: 10.1093/ndt/gfp456. [DOI] [PubMed] [Google Scholar]
- Heuberger W., Berardi S., Jacky E., Pey P., Krahenbuhl S. Increased urinary excretion of carnitine in patients treated with cisplatin. Eur. J. Clin. Pharmacol. 1998;54:503–508. doi: 10.1007/s002280050504. [DOI] [PubMed] [Google Scholar]
- Hoang B.X., Graeme Shaw D., Pham P., Levine S. Restoration of cellular energetic balance with L-carnitine in the neuro-bioenergetic approach for cancer prevention and treatment. Med. Hypotheses. 2007;69:262–272. doi: 10.1016/j.mehy.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Hockenberry M.J., Hooke M.C., Gregurich M., McCarthy K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J. Pediatr. Hematol. Oncol. 2009;31:664–669. doi: 10.1097/MPH.0b013e3181b259a7. [DOI] [PubMed] [Google Scholar]
- Hofmann U., Eichelbaum M., Seefried S., Meese C.O. Identification of thiodiglycolic acid, thiodiglycolic acid sulfoxide, and (3-carboxymethylthio)lactic acid as major human biotransformation products of S-carboxymethyl-L-cysteine. Drug Metab. Dispos. 1991;19:222–226. [PubMed] [Google Scholar]
- Kaneko T., Yoshida R. On the absolute configuration of L-carnitine (vitamin BT) Bull. Chem. Soc. Jpn. 1962;35:1153–1155. [Google Scholar]
- Kantrowitz N.E., Bristow M.R. Cardiotoxicity of antitumor agents. Prog. Cardiovasc. Dis. 1984;27:195–200. doi: 10.1016/0033-0620(84)90004-5. [DOI] [PubMed] [Google Scholar]
- Kashfi K., Israel M., Sweatman T.W., Seshadri R., Cook G.A. Inhibition of mitochondrial carnitine palmitoyltransferases by adriamycin and adriamycin analogues. Biochem. Pharmacol. 1990;40:1441–1448. doi: 10.1016/0006-2952(90)90438-q. [DOI] [PubMed] [Google Scholar]
- Krähenbühl S., Reichen J. Carnitine metabolism in patients with chronic liver disease. Hepatology. 2003;25:148–153. doi: 10.1053/jhep.1997.v25.pm0008985281. [DOI] [PubMed] [Google Scholar]
- Kuhlmann M.K., Burkhardt G., Kohler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol. Dial. Transplant. 1997;12:2478–2480. doi: 10.1093/ndt/12.12.2478. [DOI] [PubMed] [Google Scholar]
- Kutscher F. Uber liebigs fleischextrakt. Mitteilung I. Z. Unters Nahr. Genubm. 1905;10:528–537. [Google Scholar]
- Lefrak E.A., Pitha J., Rosenhiem J., Gotibieb J.H. A clinicopathological analysis of adriamycin cardiotoxicity. Cancer. 1973;32:303–309. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Lieberthal W., Triaca V., Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs necrosis. Am. J. Physiol. 1996;240:F700–F708. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- Loehrer P.J., Einhom L.H. Cisplatin. Ann. Intern. Med. 1984;100:704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Ludeman S.M. The chemistry of the metabolites of cyclophosphamide. Curr. Pharm. 1999;5:627–634. [PubMed] [Google Scholar]
- Luo X.O., Mo Y., Ke Z.Y., Xu L., Jiang X.Y., Zhang T.T., Chen S.M. High-dose chemotherapy without stem cell transplantation for refractory childhood systemic lupus erythematosus. Chemotherapy. 2008;54:331–335. doi: 10.1159/000151539. [DOI] [PubMed] [Google Scholar]
- Maestri A., De Pasquale Ceratti A., Cundari S., Zanna C., Cortesi E., Crinò L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005;91:135–138. doi: 10.1177/030089160509100206. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M., Risino C., Gargante M.P., Oreste G., Barone G., Tomasello A.V., Costanzo M., Cannizzaro M.A. Decrease of serum carnitine levels in patients with or without gastrointestinal cancer cachexia. World J. Gastroenterol. 2006;12:4541–4545. doi: 10.3748/wjg.v12.i28.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A., D’Iddio S., Bisonni R., Graziano F., Lippe P., Calvani M. Urinary excretion of L-carnitine and its short-chain acetyl-L-carnitine in patients undergoing carboplatin treatment. Cancer Chemother. Pharmacol. 2007;60:19–26. doi: 10.1007/s00280-006-0341-3. [DOI] [PubMed] [Google Scholar]
- Mancinelli A., Longo A., Shanahan K., Evans A.M. Disposition of L-carnitine and acetyl-L-carnitine in the isolated perfused rat kidney. J. Pharmacol. Exp. Ther. 1995;274:1122–1128. [PubMed] [Google Scholar]
- Marthaler N.P., Theresa Visarius T., Kupfer A., Lauterburg B.H. Increased urinary losses of carnitine during ifosfamide chemotherapy. Cancer Chemother. Pharmacol. 1999;44:170–172. doi: 10.1007/s002800050963. [DOI] [PubMed] [Google Scholar]
- Martinez G., Costantino G., Clementi A., Puglia M., Clementi S., Cantarella G., De Meo L., Matera M. Cisplatin-induced kidney injury in the rat: L-carnitine modulates the relationship between MMP-9 and TIMP-3. Exp. Toxicol. Pathol. 2009;61:183–188. doi: 10.1016/j.etp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Matalliotakis I., Koumantaki Y., Evageliou A., Matalliotakis G., Goumenou A., Koumantakis E. L-carnitine levels in the seminal plasma of fertile and infertile men: correlation with sperm quality. Int. J. Fertil. Womens Med. 2002;45:236–240. [PubMed] [Google Scholar]
- McFalls E.O., Paulson D.J., Gilbert E.F., Shug A.L. Carnitine protection against adriamycin-induced cardiomyopathy in rats. Life Sci. 1986;38:497–505. doi: 10.1016/0024-3205(86)90028-7. [DOI] [PubMed] [Google Scholar]
- Mir S., Kantar M., Yalaz M., Keskinoglu A., Coker I., Huseyinov A. Effect of hemodialysis on carnitine levels in children with chronic renal failure. Pediatr. Int. 2002;44:70–73. doi: 10.1046/j.1442-200x.2002.01498.x. [DOI] [PubMed] [Google Scholar]
- Mortensen S.A., Aabo K., Jonsson T., Baandrup U. Clinical and non-invasive assessment of anthracycline cardiotoxicity: perspectives on myocardial protection. Int. J. Clin. Pharmacol. Res. 1986;6:137–150. [PubMed] [Google Scholar]
- Murphy S.B., Bowman W.P., Abromowitch M., Mirro J., Ochs J., Rivera G., Pui C.H., Berard F.D. Results of treatment of advanced-stage Burkitt’s lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J. Clin. Oncol. 1986;4:1732–1739. doi: 10.1200/JCO.1986.4.12.1732. [DOI] [PubMed] [Google Scholar]
- Myers C.E., Chabner B.A. Anthracyclines. In: Chabner B.A., Collins J.M., editors. Cancer Chemotherapy: Principles and Practice. J.B. Lippincott Co.; Philadelphia: 1990. pp. 356–381. [Google Scholar]
- Neri B., Cini-Neri G., Bartalucci S., Bandinelli M. Protective effect of L-carnitine on cardiac metabolic damage induced by doxorubicin in vitro. Anticancer Res. 1986;6:659–662. [PubMed] [Google Scholar]
- Paterna S., Furitano G., Scaffidi L., Barbarino C., Campisi D., Parisi D., Carreca I. Effects of L-carnitine on adriamycin-induced cardiomyopath in rabbit. Int. J. Tissue React. 1984;6:91–95. [PubMed] [Google Scholar]
- Pisano C., Pratesi G., Laccabue D., Zunino F., Lo Giudice P., Bellucci A., Pacifici L., Camerini B., Vesci L., Castorina M., Cicuzza S., Tredici G., Marmiroli P., Nicolini G., Galbiati S., Calvani M., Carminati P., Cavaletti G. Paclitaxel and cisplatin-induced neurotoxicity: a protective role of acetyl-L-carnitine. Clin. Cancer Res. Clin. Cancer Res. 2003;9(15):5756–5767. [PubMed] [Google Scholar]
- Pola P., De Martini D., Gerardino L., De Rossi S., Tondi P. The action of propionyl-L-carnitine on the vasal endothelium: increased t-PA synthesis and a decrease in the activity of PAI-1. A preliminary study. Drugs Exp. Clin. Res. 1992;18:343–348. [PubMed] [Google Scholar]
- Preiss R., Baumann F. Cyclophosphamide and related anticancer drugs. J. Chromatogr. B Biomed. Sci. Appl. 2001;764:173–192. doi: 10.1016/s0378-4347(01)00279-1. [DOI] [PubMed] [Google Scholar]
- Rebouche C.J., Chenard C.A. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites. J. Nutr. 1991;121:539–546. doi: 10.1093/jn/121.4.539. [DOI] [PubMed] [Google Scholar]
- Rebouche C.J., Lehman L.J., Olson L. Epsilon-N-trimethyllysine availability regulates the rate of carnitine biosynthesis in the growing rat. J. Nutr. 1986;116:751–759. doi: 10.1093/jn/116.5.751. [DOI] [PubMed] [Google Scholar]
- Rebouche C.J. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann. NY Acad. Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- Rizzon P., Biasco G., Di Biase M., Boscia F., Rizzo U., Minafra F., Bortone A., Siliprandi N., Procopio A., Bagiella E. High doses of L-carnitine in acute myocardial infarction: metabolic and antiarrhythmic effects. Eur. Heart J. 1989;10:502–508. doi: 10.1093/oxfordjournals.eurheartj.a059519. [DOI] [PubMed] [Google Scholar]
- Rossi F., Filippelli W., Russo S., Filippelli A., Berrino L. Cardiotoxicity of doxorubicin: effects of drugs inhibiting the release of vasoactive substances. Pharmacol. Toxicol. 1994;75:99–107. doi: 10.1111/j.1600-0773.1994.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed M.M., Salman T.M., Gaballah H.E., Abou-El-Naga S.A., Raffaella, Nicolai R., Calvani Propionyl-L-carnitine as protector against adriamycin-induced cardiomyopathy. Pharmacol. Res. 2001;43:513–520. doi: 10.1006/phrs.2000.0786. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed M.M., Al-Shabanah O.A., Hafez M.M., Aleisa A.M., Al-Rejaie S.S. Inhibition of gene expression of heart fatty acid binding protein and organic cation/carnitine transporter in doxorubicin cardiomyopathic rat model. Eur. J. Pharmacol. 2010;640:143–149. doi: 10.1016/j.ejphar.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed M.M., Mansour H.H., Gharib O.A., Hafez H.F. Acetyl-L-carnitine modulates bleomycin-induced oxidative stress and energy depletion in lung tissues. J. Egypt. Natl. Cancer Inst. 2004;16(4):237–243. [PubMed] [Google Scholar]
- Sayed-Ahmed M.M., Rishk A.M., Soloma S., Abdel-aleem S. Protection by L-carnitine against the inhibition of gene expression of heart fatty acid binding protein by chronic administration of doxorubicin. J. Egypt. Natl. Cancer Inst. 2000;12:275–281. [Google Scholar]
- Sayed-Ahmed M.M., Shaarawy S., Shouman S.A., Osman A.M. Reversal of doxorubicin-induced cardiac metabolic damage by L-carnitine. Pharmacol. Res. 1999;39:289–295. doi: 10.1006/phrs.1998.0438. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed M.M., Khattab M., Khalifa A., El-Khabany M. And Osman, A.M: Potenial promise of using L-carnitine and Coenzyme Q10 as protective agents against cisplatin-induced nephrotoxicity. J. Egypt. Natl. Cancer Inst. 1999;11:167–173. [Google Scholar]
- Sayed-Ahmed M.M., Shouman S.A., Rezk B.M., Khalifa M.H., Osman A.M., El-Merzabani M.M. Propionyl-L-carnitine as potential protective agent against adriamycin-induced impairment of fatty acid beta-oxidation in isolated heart mitochondria. Pharmacol. Res. 2000;41:143–150. doi: 10.1006/phrs.1999.0583. [DOI] [PubMed] [Google Scholar]
- Seguro A.C., Shimizu M.H., Kudo L.H., dos Santos Rocha A. Renal concentration defect induced by cisplatin. The role of thick ascending limb and papillary collecting duct. Am. J. Nephrol. 1989;9:59–65. doi: 10.1159/000167938. [DOI] [PubMed] [Google Scholar]
- Shug A.L. Protection from adriamycin-induced cardiomyopathy in rats. Z. Kardiol. 1987;76:46–52. [PubMed] [Google Scholar]
- Siliprandi N., Menabo Di Lisa R., Ciman M., Sartorelli L. Transport and function of carnitine in muscle. J. Clin. Chem. Clin. Biochem. 1990;28:303–306. [PubMed] [Google Scholar]
- Spagnoli L.G., Corsi M., Villaschi S., Palmieri G., Maccari F. Myocardial carnitine deficiency in acute myocardial infarction. Lancet. 1982;1:1419–1420. doi: 10.1016/s0140-6736(82)92540-5. [DOI] [PubMed] [Google Scholar]
- Steiber A., Kerner J., Hoppel C.L. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004;25:455–473. doi: 10.1016/j.mam.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Steinmann B., Bachmann C., Colombo J.P., Gitzelmann R. The renal handling of carnitine in patients with selective tubulopathy and with Fanconi Syndrome. Pediatr. Res. 1987;21:201–204. doi: 10.1203/00006450-198702000-00018. [DOI] [PubMed] [Google Scholar]
- Stewart D.J., Morgan L.R., Jr., Verma S., Maroun J.A., Thibault M. Pharmacology, relative bioavailability, and toxicity of three different oral cyclophosphamide preparations in a randomized, cross-over study. Invest. New Drugs. 1995;13:99–107. doi: 10.1007/BF02614228. [DOI] [PubMed] [Google Scholar]
- Stieger B., O’Neill B., Krahenbuhl S. Characterization of L-carnitine transport by rat kidney brush-border membrane vesicles. Biochem. J. 1995;309:393–402. doi: 10.1042/bj3090643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M., Anselmi G., Hermoso T., Tejero F. Carnitine promotes heat shock protein synthesis in adriamycin-induced cardiomyopathy in a neonatal rat experimental model. J. Mol. Cell. Cardiol. 1998;30:2319–2325. doi: 10.1006/jmcc.1998.0793. [DOI] [PubMed] [Google Scholar]
- Tamai I., Ohashi R., Nezu J., Yabuuchi H., Oku A., Shimane M., Sai Y., Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J. Biol. Chem. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- Tanphaichitr V., Leelahagul P. Carnitine metabolism and human carnitine deficiency. Nutrition. 1993;9:246–254. [PubMed] [Google Scholar]
- Tein I., Bukovac S.W., Xie Z.W. Characterization of the human plasmalemmal carnitine transporter in cultured skin fibroblasts. Arch. Biochem. Biophys. 1996;329:145–155. doi: 10.1006/abbi.1996.0203. [DOI] [PubMed] [Google Scholar]
- Tomita M., Senju Y. Uber die aminoverbindungen, welche die Biuretreaktion zeigen. III. Spaltungen der γ-Amino-β-oxybuttersaure in die optisch aktiven komponenten. Hoppe-Seyler’s Z. Physiol. Chem. 1927;169:263–277. [Google Scholar]
- Toomey D., Redmond H.P., Bouchier-Hayes D. Mechanisms mediating cancer cachexia. Cancer. 1995;76:2418–2426. doi: 10.1002/1097-0142(19951215)76:12<2418::aid-cncr2820761204>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Tufekci O., Gunes D., Ozoğul C., Kolatan E., Altun Z., Yilmaz O., Aktaş S., Erbayraktar Z., Kirkim G., Mutafoğlu K., Soylu A., Serbetçioğlu B., Güneri E.A., Olgun N. Evaluation of the effect of acetyl L-carnitine on experimental cisplatin nephrotoxicity. Chemotherapy. 2009;55:451–459. doi: 10.1159/000240020. [DOI] [PubMed] [Google Scholar]
- Van Vleet J., Ferrans V., Weririch W. Cardiac disease induced by chronic adriamycin administration in dogs and an evaluation of vitamin E and selenium as cardioprotectants. Am. J. Pathol. 1980;99:13–42. [PMC free article] [PubMed] [Google Scholar]
- Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br. J. Pharmacol. 1998;124:425–427. doi: 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci E., Rampello E., Zanoli L., Oreste G., Pistone G., Malaguarnera M. Serum carnitine levels in patients with tumoral cachexia. Eur. J. Intern. Med. 2005;16:419–423. doi: 10.1016/j.ejim.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Visarius T.M., Stucki J.W., Lauterburg B.H. Inhibition and stimulation of long-chain fatty acid oxidation by chloroacetaldehyde and methylene blue in rats. J. Pharmacol. Exp. Ther. 1999;289:820–824. [PubMed] [Google Scholar]
- Waldner R., Laschan C., Lohninger A., Gessner M., Tüchler H., Huemer M., Spiegel W., Karlic H. Effects of doxorubicin-containing chemotherapy and a combination with L-carnitine on oxidative metabolism in patients with non Hodgkin lymphoma. J. Cancer Res. Clin. Oncol. 2006;132:121–128. doi: 10.1007/s00432-005-0054-8. [DOI] [PubMed] [Google Scholar]
- Yaris N., Ceviz N., Coskun T., Akytuz C., Buyukpamukcu M. Serum carnitine levels during the doxorubicin therapy. Its role in cardiotoxicity. J. Exp. Clin. Cancer Res. 2002;21:165–170. [PubMed] [Google Scholar]
- Yeung T.K., Jaenke R.S., Wilding D., Creighton A.M., Hopewell J.W. The protective activity of ICRF-187 against doxorubicin-induced cardiotoxicity in the rat. Cancer Chemother. Pharmacol. 1992;30:58–64. doi: 10.1007/BF00686486. [DOI] [PubMed] [Google Scholar]
- Yoon H.R., Hong Y.M., Boriack R.L., Bennett M.J. Effect of L-carnitine supplementation on cardiac carnitine palmitoyltransferase activities and plasma carnitine concentrations in adriamycin-treated rats. Pediatr. Res. 2003;53:788–792. doi: 10.1203/01.PDR.0000057988.62605.13. [DOI] [PubMed] [Google Scholar]
- Zdrojkowska-Krol E., Karpowicz B., Rutkowski B. Reference values of carnitine in plasma and urine. Diag. Lab. 1994;30:413–420. [Google Scholar]
- Zeidan Q., Strauss M., Porras N., Anselmi G. Differential long-term subcellular responses in heart and liver to adriamycin stress. Exogenous L-carnitine cardiac and hepatic protection. J. Submicrosc. Cytol. Pathol. 2002;34:315–321. [PubMed] [Google Scholar]
- Zhang J.G., Lindup W.E. Role of mitochondria in cisplatin-induced oxidative damage exhibited by rat renal cortical slices. Biochem. Pharmacol. 1993;45:2215–2222. [PubMed] [Google Scholar]


