Abstract
It has been observed cannabinoid CB1 receptor signalling and the levels of endocannabinoid ligands significantly increased in the basal ganglia and cerebrospinal fluids of Parkinson’s disease (PD) patients. These evidences suggest that the blocking of cannabinoid CB1 receptors might be beneficial to improve movement disorders as a sign of PD. In this study, a dose–response study of the effects of intrastriatal injection of a cannabinoid CB1 receptor antagonist, AM251 and agonist, ACPA, on movement activity was performed by measuring the catalepsy of reserpinized and non-PD (normal) rats with bar test. Also the effect of co-administration the most effective dose of AM251 and several doses of ACPA were assessed. AM251 decreases the reserpine induced catalepsy in dose dependent manner and ACPA causes catalepsy in normal rats in dose dependant manner as well. AM251 significantly reverse the cataleptic effect in all three groups (1, 10, 100 ng/rat) that received ACPA. These results support this theory that cannabinoid CB1 receptor antagonists might be useful to alleviate movement disorder in PD. Also continuance of ACPA induced catalepsy in rats after AM251 injection can indicate that other neurotransmitters or receptors interfere in ACPA induced catalepsy. Based on the present finding there is an incomplete overlapping between cannabinoid CB1 receptor agonist and antagonist effects.
Keywords: Parkinson’s disease, Cannabinoid CB1 receptor, Striatum, Reserpine, Catalepsy, AM251, ACPA
1. Introduction
Parkinson’s disease (PD) is a degenerative disorder of the central nervous system that often impairs the sufferer’s motor skills, speech, and other functions. Parkinson’s disease belongs to a group of conditions called movement disorders. It is characterized by muscle rigidity, tremor, a slowing of physical movement (bradykinesia) and a loss of physical movement (akinesia) in extreme cases. The primary symptoms are the results of decreased stimulation of the motor cortex by the basal ganglia, normally caused by the insufficient formation and action of dopamine, which is produced in the dopaminergic neurons of the brain. Secondary symptoms may include high level cognitive dysfunction and subtle language problems. PD is both chronic and progressive (Blandini et al., 2000).
In recent studies, several reports has been presented about the role of cannabinoids in movement disorders such as PD. Cannabinoids are a group of compounds which receive their name after the identification of active constituent of the marijuana plant (Cannabis sativa). The effective principle in this plant was identified as Δ9-tetrahydrocannabinol (Δ9-THC) (Mechoulam et al., 1970; Fox, 2010). Two cannabinoid receptors have been identified: CB1 (with it’s isoform CB1a resulting from alternative splicing) and CB2. CB1 receptor is mainly placed in the nervous system, although is also expressed in other organs (Rinaldi-Carmona et al., 1994; Facci et al., 1995; Shire et al., 1995; Pertwee and Fernando, 1996; Pertwee et al., 1996a,b; Tsou et al., 1998; Fox, 2010). The CB2 receptor is mainly expressed in the immune system and is not associated with neurons (Facci et al., 1995; Galiegue et al., 1995; Schatz et al., 1997). The brain has a distinct distribution of expression of cannabinoid CB1 receptors that have high density in areas which control motor behavior such as the basal ganglia and cerebellum. But, cannabinoid CB1 receptors have low levels in brainstem that may explain the cannabinoid receptor agonists low toxicity, an attractive quality for putative therapeutic uses (Herkenham et al., 1991a,b; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998). Several recent reports suggest that the basal ganglia is involved in the motor effects of cannabinoids (Pertwee and Wickens, 1991; Romero et al., 1995, 1996; Garcia et al., 1996; Miller et al., 1998; Sanũdo-Penã and Walker, 1998a,b; Sanũdo-Penã et al., 1999; Sanũdo-Penã et al., 1996, 1998a,b; Corchero et al., 1999; Ferrari et al., 1999). Cannabinoids cause motor effects which seem to be mediated by the cannabinoid CB1 receptor (Rinaldi-Carmona et al., 1994). In general, activation of cannabinoid CB1 receptors by cannabinoid CB1 receptor agonists inhibit neurotransmission (Mackie and Hille, 1992; Mackie et al., 1995; Deadwyler et al., 1993; Howlett, 1995). Major effect of cannabinoids on movement is hypoactivity and catalepsy (Dewey, 1986; Hollister, 1986; Romero et al., 1996; Ferrari et al., 1999).
Nevertheless, also cannabinoid receptor agonists induce biphasic effects on movement that are time- and dose-dependent. An increase in motor activity has been observed with relatively low doses or immediately after administration of higher doses of cannabinoid receptor agonists. Later after administration, high doses of cannabinoid receptor agonists inhibit movement and produce catalepsy (Carlini et al., 1970; Davis et al., 1972; Dewey, 1986; Hollister, 1986). A biphasic effect on movement has also been reported for the endogenous ligand of the cannabinoid receptor anandamide (Sulcova et al., 1998). A more recent study of knockout mice for the cannabinoid CB1 receptor presented a reduction in the activity of these animals that suggests an activational role of CB1 receptors on movement (Zimmer et al., 1999). However, another study with knockout animals for the CB1 receptors failed to observe any basal effects on motor behavior (Ledent et al., 1999).
Also it has been found that the level of endocannabinoids and the activation of G proteins by cannabinoid agonists significantly increases in the postmortem basal ganglia of humans affected by PD (Lastres-Becker et al., 2001). The same stimulatory effect has been reported by measuring the levels of endocannabinoid ligands in cerebrospinal fluids of PD patients. Interestingly, these patients were untreated (Pisani et al., 2005). The increase in CB1 receptors was also seen in MPTP-treated marmosets, a primate PD model, and this disappeared after chronic levodopa administration to these animals (Lastres-Becker et al., 2001; Zhao et al., 2010). The same pattern was observed by Maccarrone et al. (2003) for the increase in endocannabinoid levels reported by these authors in a rat model of PD (Gubellini et al., 2002), which was also reversed by levodopa. This point suggests an unbalance between dopamine and endocannabinoids at the basal ganglia in PD (Fernández-Ruiz and González, 2005).
In this context, the present study was designed to explore the motor effects of a cannabinoid receptor antagonist, AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) and agonist, ACPA (N-(2-cyclopropyl)-5Z,8Z,11Z,14Z-eicosatetraenamide), intrastriatal injection in rats ± subjected to subcutaneous injection of reserpine.
2. Materials and methods
2.1. Animals
Adult male albino wistar rats (305 ± 35 g) from Ahvaz Jondishapur University of Medical Science (AJUMS) animal facility were used. Procedures involving animals and their care were conducted in compliance with Committee of Ethics in Research of the AJUMS. Animals were housed in cages (five per cage before stereotaxic surgery and after that one per cage) at 23 ± 2 °C and 12/12 light/dark cycle and were allowed free access to food and water.
2.2. Stereotaxic surgery, reserpine treatment and intrastriatal injection
2.2.1. Notice
Many reports indicate SNc damages are induced by several agents such as free radicals, 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), reserpine, phenithiazine and butyrophenones. SNc lesion in animals like rat leads to rigidity due to decreasing the inhibitory dopamine effects on the caudate nucleus and putamen, as the main rigidity-inductor neurotransmitter-releasing areas, located in the striatum. Because of these changes in the brain of the lesioned rat, rigidity was occurred on the limbs on both the sides. Chemical neurotoxins such as 6-OHDA or MPTP are the most commonly and the main used methods for creating the PD animal models. The use of MPTP is highly dangerous. The compound is able to be absorbed from the skin, gastrointestinal tract and blood brain barrier. Furthermore, MPTP as well as 6-OHDA destroys any catecholaminergic parts in the brain; which leads to complications (Fathi-Moghaddam and Shafiee Ardestani, 2008).
Reserpine was used in the present study because this is a safe to use rat model of PD that has been obviously demonstrated reserpine causes an increase in basal ganglia endocannabinoid level and shows symptoms that are similar to those seen in Parkinson’s disease (Di Marzo et al., 2000).
Anesthetized rats were placed in Narshige stereotaxic apparatus. A hole was drilled over the injection site, and a 22 gauge stainless steel guide cannula (NRK, IRIran) was aimed 2 mm above the corresponding infusion site: left dorsal striatum, AP = +0.5, L = −3, and V = −5.5 vs. bregma (Paxinos and Watson, 1998). The guide cannula was fastened to the skull with stainless steel screws and dental cement. Seven to ten days after stereotaxic surgery animals were treated with reserpine subcutaneously 3 mg kg−1 (dissolved in 1% glacial acetic acid in distilled water) or vehicle 1 ml kg−1. Eighteen hour later intrastriatal injections were performed in the home cage by putting a 30-gauge stainless steel internal cannula (SUPA, IRIran) connected to a Hamilton syringe and a delivery pump (Stoelting, Germany). Solutions were slowly injected over 5 min, and 1 min after the conclusion of injection, the internal cannula was carefully removed and catalepsy was measured.
After completion of all the experiments, half of the experimental rats, were injected intrastriatal 1 μl of methylene blue. Then were anesthetized with diethyl ether and humanly killed, brains were removed, and stored on 10% paraformaldehyde solution. Brains were sectioned. Cannula placements were mapped onto a stereotaxic atlas (Paxinos and Watson, 1998) and confirmed to be in the left dorsal striatum.
2.3. Chemicals and doses
AM251 and ACPA were bought from Tocris (England) and were dissolved in 30% DMSO/70% water for injection. Reserpine powder was bought from Fluka (Swiss) and was dissolved in 1% glacial acetic acid (Merck, Germany) in distilled water. For systemic injections, reserpine was injected at 3 mg kg−1 SC. For intracerebral infusions, AM251 was injected at 1, 10, 100 and 200 ng/μl and ACPA was injected at 1, 10 and 100 ng/μl. The most efficient AM251 dose was selected for cotreatment with CB1 receptor agonist. The corresponding vehicle was used for the control group (dose 0) in every treatment. All drugs were injected at a volume of 1 ml kg−1 body weight or at volumes of 1 μl (intrastriatal).
2.4. Measurement of catalepsy
Reserpine induced catalepsy and the effect of CB1 receptor agonist or antagonist was measured with the standard bar test (Sanberg et al., 1996), in a wooden chamber (length, 23 cm; width, 10.5 cm; height, 9 cm) with a horizontal metal bar (diameter, 0.4 cm; length, 10.5 cm) fixed at 9 cm above the floor, and at 4 cm from the back of the box. All experiments were carried out between 8:00 and 14:00. Animals were used only once. After a 30–60 min habituation period to the testing room, reserpinized and normal rats received one of the following intrastriatal treatments: (1) vehicle, (2) AM251 at four doses (1, 10, 100 and 200 ng/rat), (3) ACPA at three doses (1, 10 and 100 ng/rat), or (4) ACPA (1, 10 and 100 ng/rat) plus most efficient dose of AM251 (100 ng/rat). From that moment on, catalepsy was measured every 15 min during the whole session that lasted 2 h.
To measure catalepsy, the rat was gently lifted until its forepaws firmly grasped the metal bar. Then, the rat body was released and simultaneously a stopwatch was started. The time elapsed until the animal released both forepaws from the bar, up to a maximum of 300 s, was defined as the catalepsy time.
2.5. Statistical analysis
One Way Variance Analysis (ANOVA) with Tukey’s post hoc made comparison between groups and differences with p-values <0.05 were considered significant.
3. Results
3.1. Effect of ACPA on normal rats
ACPA caused cataleptic phenomenon in normal rats dose dependently. ACPA (1, 10 and 100 ng/rat) receiving groups were shown significant difference from the control group with p < 0.001 except at 120 in 1 ng/rat dose which was found p < 0.01. Also between 1 and 10 ng/rat doses always there was significant difference with at least p < 0.01 but there is no difference between 10 and 100 ng/rat doses except 105 (p < 0.05) and 120 (p < 0.01) (Figs. 1 and 2).
Figure 1.
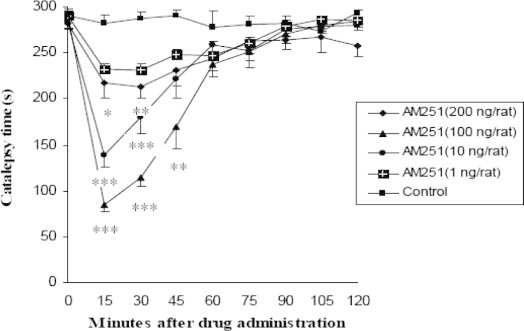
Dose–response and time dependent effect of intrastriatal injection of several doses of cannabinoid CB1 receptor antagonist, AM251, on catalepsy time in rats which received reserpine (n = 7, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Data are shown as the mean ± SEM followed by Tukey–Kramer as post-ANOVA test.
Figure 2.
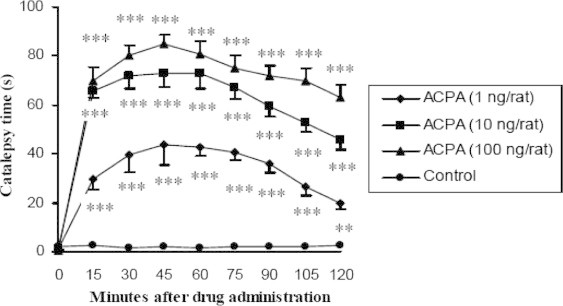
Dose–response and dose-dependent effect of intrastriatal injection of several doses of cannabinoid CB1 receptor agonist, ACPA, on catalepsy time in non-PD rats (n = 7, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Data are shown as the mean ± SEM followed by Tukey–Kramer as post-ANOVA test.
3.2. Effect of AM251 on the catalepsy of parkinsonian rats
AM251 decreased the reserpine induced catalepsy dose dependently. One hundred ng/rat injections showed a maximum of anti-cataleptic effect comparing to test groups (1, 10, 100 and 200 ng/rat). One hundred ng/rat significantly differed with control group at 15, 30 (p < 0.001) and 45 (p < 0.01). Ten ng/rat had significant difference with control group at 15, 30 (p < 0.001) and 200 ng/rat had significant difference with control group at 15 (p < 0.05) and 30 (p < 0.01). However, 1 ng/rat never showed a significant difference with control group. In all groups maximum anti-cataleptic effect was observed at 15 min after intrastriatal AM251 injection and about 1 h after injection it reversed. An interesting point was observed when the increase in AM251 doses up to 100 ng/rat caused to anti-cataleptic effects and the increase in the doses moreover 100 up to 200 ng/rat anti-cataleptic effects significantly decreased that may indicate a biphasic effect for AM251 (Figs. 2–4).
Figure 3.
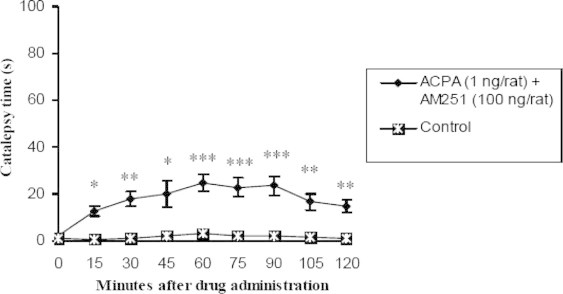
Comparison of effect of contemporary intrastriatal injection of cannabinoid receptor agonist, ACPA (1 ng/rat) and antagonist most effective dose (100 ng/rat) on catalepsy time with control group (n = 7, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Data are shown as the mean ± SEM followed by Tukey–Kramer as post-ANOVA test.
Figure 4.
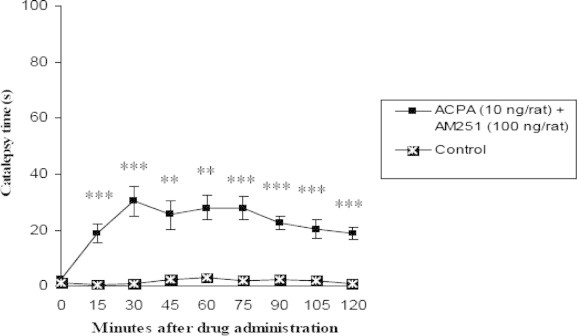
Comparison of effect of contemporary intrastriatal injection of cannabinoid receptor agonist, ACPA (10 ng/rat) and antagonist most effective dose (100 ng/rat) on catalepsy time with control group (n = 7, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Data are shown as the mean ± SEM followed by Tukey–Kramer as post-ANOVA test.
3.3. Effect of ACPA and AM251 contemporary injection on normal rats
Those groups whose received ACPA (1, 10 and 100 ng/rat) and AM251 most effective dose, in all times showed significant difference p < 0.05 in comparison with control group but there was not found any significant difference between test groups and the above at all (Figs. 4–6).
Figure 5.
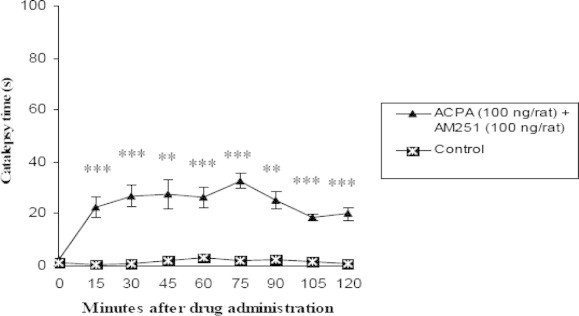
Comparison of effect of contemporary intrastriatal injection of cannabinoid receptor agonist, ACPA (100 ng/rat) and antagonist most effective dose (100 ng/rat) on catalepsy time with control group (n = 7, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Data are shown as the mean ± SEM followed by Tukey–Kramer as post-ANOVA test.
Figure 6.
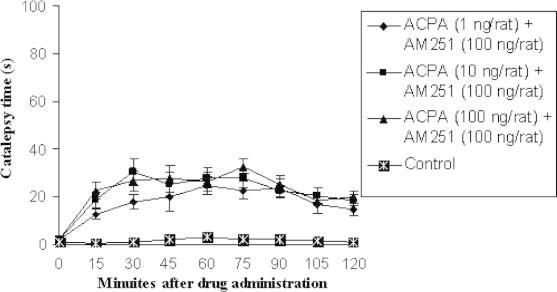
Comparison of effect of contemporary intrastriatal injection of several doses of cannabinoid receptor agonist, ACPA and antagonist most effective dose (100 ng/rat) on catalepsy time with control group (n = 7). Data are shown as the mean ± SEM followed by Tukey–Kramer as post-ANOVA test.
4. Discussion
Reserpine, an alkaloid extracted from the roots of an Indian plant, Rauwolfia serpentina, blocks the ability of aminergic transmitter vesicles to take up and store biogenic amines, probably by interfering with an uptake mechanism that depends on Mg2+ and ATP (Benowits, 2004).
In reserpine induced model of PD, catecholamine stores are depleted and a motor syndrome characterized by decreased initiation and speed of voluntary movements, rigidity, and a hunched posture is observed (Colpaert, 1987). These symptoms bear many similarities to those seen in Parkinson’s disease and are ameliorated by dopaminergic anti-parkinsonian drugs (Carlsson et al., 1957). Thus, the reserpine-treated rodent provides a useful model of Parkinson’s disease (Di Marzo et al., 2000).
Within the basal ganglia, cannabinoid receptors are assumed to be predominantly localized on presynaptic terminals of the GABAergic striatonigral and striatopallidal terminals (Ameri, 1999). This finding indicates that CB1 receptors are located presynaptically on the degenerating terminal of the striatal projection neuron. Cannabinoid receptors have been shown to coexist with both dopamine D1 and D2 receptors on striatonigral and striatopallidal terminals (Herkenham et al., 1991a; Mansour et al., 1992) and apparently produce opposite effects on second messengers and neurotransmitter release, that is, CB1 receptor activation is able to inhibit a D1-mediated increase in cAMP accumulation (Bidaut-Russell and Howlett, 1991; Zhao et al., 2010; Fox, 2010) and also able to inhibit the D2-mediated inhibition of cAMP accumulation (Glass and Felder, 1996).
In this study with intrastriatal injection of ACPA normal rats involved in catalepsy that increased dose dependently. It is interesting that there is a significant difference between 1 and 10 ng/rat test groups however in comparison of 10 and 100 ng/rat test groups catalepsy time has increased but there is not significant difference between them unless at 105 and 120 min after drug administration. Beside the present study have been reported that catalepsy is symmetrical to cannabinoid receptor agonist dose (Brotchie, 2003; Meschler et al., 2000; Järbe et al., 2002; Walton et al., 1938; Dewey, 1986) however Sulcova et al. (1998) has demonstrated that anandamide, an endogenous cannabinoid receptor agonist, has biphasic effect on catalepsy, first anandamide reduces catalepsy and in higher doses it causes catalepsy increasment. Also Souilhac et al. (1995) has reported that unilateral injection of a cannabinoid CB1 agonist in mice leads to contralateral turning behavior. Because CB1 cannabinoid receptors exist in dopaminergic terminals of nigrostriatal direct (Ameri, 1999), it is possible that intrastriatal injection of CB1 cannabinoid receptor agonist, ACPA, causes to dopamine release reduction and strengthening of GABAergic neurons in striatonigral and striatopallidal directs. These conditions approximately is similar to PD patients that inhibitory effect of indirect route in basal ganglia has increased and extraordinary inhibitory effect of internal globus pallidus (GPi) and reticular substantia nigra (SNr) on thalamus leads to reduction of thalamus excitatory effect on brain cortex by glutamatergic neurons. These events probably cause to catalepsy which is induced by cannabinoid CB1 receptor agonist. Although this theory is not certainly and presence of excitatory D1 receptors and inhibitory D2 receptors in the vicinity of another, and glutamate release in striatum are not ignorable. Therefore events which have been observed after cannabinoid CB1 receptor agonist injection probably are resultant of all of inhibitory and excitatory neurotransmitters.
Intrastriatal injection of cannabinoid CB1 receptor antagonist reversed reserpine induced catalepsy in dose and time dependent manner. Also in the time of drug injection and some minutes after that all of animals in all test groups involved in contralateral turning behavior and after that increasment of movement activity were clear. From 1 to 100 ng/rat induced anti-cataleptic effect, but in 200 ng/rat anti-cataleptic effect have reduced. This event can be explained by propounding a biphasic effect for AM251, cannabinoid CB1 receptor antagonist.
All of animals which treated with agonist and antagonist co-administration involved in catalepsy. Although in these groups catalepsy intensity was less than groups which received agonist, all of three groups (1, 10 and 100 ng/rat) had significant difference comparing to control group. However in this section of study, test groups received 100 ng/rat of AM251 and several doses of ACPA (1, 10 and 100 ng/rat) no significant difference was observed between them. Järbe et al. (2002) have shown that systemic administration of rimonabant (a synthetic cannabinoid CB1 receptor) has reversed the effect of systemic administration of Δ9-THC completely (Järbe et al., 2002). As we know reserpine blocks the ability of aminergic transmitter vesicles to be reuptaked and restore dopamine, norepinephrine and serotonine, incomplete reverse of ACPA effects can be related to interfering of other neurotransmitters such as dopamine (Banoua et al., 2004) or serotonine (Pires et al., 2005). Piers et al. in 2005 has demonstrated a role for serotonine and it’s receptors in cannabinoids induced catalepsy. He has reported that serotonine selective reuptake inhibitors such as paroxetine and sertralin improve haloperidol induced catalepsy in mice.
Hyperactivity of the endocannabinoid transmission (recording CB1 receptors or endocannabinoid levels) has been also reported in the basal ganglia in different rat models of PD (Mailleux and Vanderhaeghen, 1993; Romero et al., 2000; Di Marzo et al., 2000; Gubellini et al., 2002). Most of data point that endocannabinoid transmission becomes overactive in the basal ganglia in PD. This occurred in the case of administration of reserpine (Di Marzo et al., 2000; Zhao et al., 2010) or dopaminergic antagonists (Mailleux and Vanderhaeghen, 1993) or during the degeneration of these neurons with local application of 6-hydroxydopamine (Mailleux and Vanderhaeghen, 1993; Romero et al., 2000; Gubellini et al., 2002) or MPTP (Lastres-Becker et al., 2001), and it is compatible with the hypokinesia that is a symptom of this disease. This would also support the suggestion that CB1 receptor antagonists might be useful to alleviate bradykinesia in PD, as well as to decrease the development of dyskinesia caused by prolonged replacement therapy with levodopa (Brotchie, 2000, 2003; Romero et al., 2000; Di Marzo et al., 2000; Lastres-Becker et al., 2001; Fox et al., 2002; Fernández-Ruiz and González, 2005; Zhao et al., 2010). In this theory, CB1 receptor blockade would inhibit the excessive inhibition of GABA uptake produced by the increased activation of CB1 receptors in striatal projection neurons (Maneuf et al., 1996; Romero et al., 1998), thus allowing a faster removal of this inhibitory neurotransmitter from the synaptic cleft, which would reduce hypokinesia. Recently several studies have presented the capability of rimonabant, a selective antagonist of CB1 receptors, to improve hypokinesia in animal models of PD, but other laboratories have shown opposite results (Di Marzo et al., 2000; Meschler and Howlett, 2001; El-Banoua et al., 2004; Casteels et al., 2010). In addition, no effects were found in the only clinical trial developed so far (Mesnage et al., 2004). It is possible that the blocking of CB1 receptors might be useful only at special phases of the disease. In this sense, Fernández-Espejo et al. (2005) have demonstrated that rimonabant alleviate motor inhibition in 6-hydroxydopamine-lesioned rats with extremely high degeneration of dopaminergic neurons (95% of neuronal loss) but not in rats with high dopaminergic degeneration (85–95%), which presents an additional advantage since it would allow for an anti-parkinsonism compound in a stage of the disease when the classic dopaminergic therapy is generally failed. On the other hand, rimonabant might provide improvement of symptoms through mechanisms without depending of recovering dopamine transmission, which might be particularly interesting for those patients that do not respond to classic therapy of dopaminergic replacement.
Also there are some other documents for the level increasment of COX-2 enzyme in PD basal ganglia especially in SNc. This increasment has reported in the brain of dead patients too and is independent to the kind of SNc lesion (McGeer and McGeer, 2002; Mladenovic et al., 2004; Fathi Moghaddam et al., 2008). Some other biochemicals such as 2-arachidonyl glycerol, endogenous cannabinoid CB1 receptor agonist, and prostaglandins which have common biochemical origin (Mladenovic et al., 2004), seems evaluating of prostaglandins role in cannabinoid effects in PD is essential.
These results support this theory that cannabinoid CB1 receptor antagonists might be useful to alleviate movement disorder in PD. Also continuance of ACPA induced catalepsy in rats after AM251 injection can indicate that other neurotransmitters or receptors interfere in ACPA induced catalepsy. Based on the present findings there is an incomplete overlapping between cannabinoid CB1 receptor agonist, ACPA, and antagonist, AM251, effects that consideration of the effects of ACPA and AM251 in other animal models of PD and contemporary study of cannabinoid CB1 receptor with other receptors which are presented in basal ganglia such as D1, D2 and glutamate can light up way for new treatments in PD.
Acknowledgments
This research was partially supported by Ahvaz Jondishapur University of Medical Sciences. All technicians who provided supports during the study are gratefully acknowledged. It should be finally stated that partial fulfillment of this research was accepted and will be presented at World-Pharma Congress 2010 as poster.
Footnotes
This study is a result of Pharmacy Doctorate Thesis by Seyed Mohammad Zarei Abarghouei.
Contributor Information
Hadi Fathi Moghaddam, Email: hfmoghaddam@yahoo.com.
Mehdi Shafiee Ardestani, Email: shafieeardestani@gmail.com.
References
- Ameri A. The effect of cannabinoids on the brain. Prog. Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Banoua F.E., Caraballo I., Flores J.A., Galan-Rodriguez B., Fernandez-Espejo E. Effects on turning of microinjections into basal ganglia of D1 and D2 dopamine receptors agonists and the cannabinoid CB1 antagonist SR141716A in a rat Parkinson’s model. Neurobiol. Dis. 2004;16:377–385. doi: 10.1016/j.nbd.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Benowits N.L. Antihypertensive agents. In: Katzung B.G., editor. Basic & Clinical Pharmacology. ninth ed. McGraw-Hill Press; New York: 2004. [Google Scholar]
- Bidaut-Russell M., Howlett A.C. Cannabinoid receptor-regulated cyclic AMP accumulation in the rat striatum. J. Neurochem. 1991;57:1769–1773. doi: 10.1111/j.1471-4159.1991.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Blandini F., Nappi G., Tassorelli C., Martignoni E. Functional changes in the basal ganglia circuitry in Parkinson’s disease. Prog. Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Brotchie J.M. The neural mechanisms underlying levodopa-induced dyskinesia in Parkinson’s disease. Ann. Neurol. 2000;47:S105–S114. [PubMed] [Google Scholar]
- Brotchie J.M. CB1 cannabinoid receptor signalling in Parkinson’s disease. Curr. Opin. Pharmacol. 2003;3(1):54–61. doi: 10.1016/s1471-4892(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Carlini E.A., Santos M., Claussen U., Bieniek D., Korte F. Structure activity relationship of four tetrahydrocannabinols and the pharmacological activity of five semi-purified extracts of Cannabissativa. Psychopharmacologia. 1970;18:82–93. doi: 10.1007/BF00402387. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Lindquist M., Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature (London) 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- Casteels C., Baitar A., Bormans G., Laere K.V. In vivo type 1 cannabinoid receptor mapping in the 6-hydroxydopamine lesion rat model of Parkinson’s disease. Brain Res. 2010;1316:153–162. doi: 10.1016/j.brainres.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Colpaert F.C. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology. 1987;26:1431–1440. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Corchero J., Romero J., Berrendero F., Fernandez-Ruiz J., Ramos J.A., Fuentes J.A., Manzanares J. Time-dependent differences of repeated administration with D9-tetrahydrocannabinol in proenkephalin and cannabinoid receptor gene expression and G-protein activation by mu-opioid and CB1-cannabinoid receptors in the caudate–putamen. Brain Res. Mol. Brain Res. 1999;67:148–157. doi: 10.1016/s0169-328x(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Davis W.M., Moreton J.E., King W.T., Pace H.B. Marihuana on locomotor activity: biphasic effect and tolerance development. Res. Commun. Chem. Pathol. Pharmacol. 1972;3:29–35. [PubMed] [Google Scholar]
- Deadwyler S.A., Hampson R.E., Bennett B.A., Edwards T.A., Mu J., Pacheco M.A., Ward S.J., Childers S.R. Cannabinoids modulate potassium current in culture hippocampal neurons. Recept. Channels. 1993;1:121–134. [PubMed] [Google Scholar]
- Dewey W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- Di Marzo V., Hill M.P., Bisogno T., Crossman A.R., Brotchie J.M. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- El-Banoua F., Caraballo I., Flores J.A., Galán-Rodriguez B., Fernández-Espejo E. Effects on turning of microinjections into basal ganglia of D1 and D2 dopamine receptors agonists and the cannabinoid CB1 antagonist SR141716A in a rat Parkinson’s model. Neurobiol. Dis. 2004;16:377–385. doi: 10.1016/j.nbd.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Facci L., Dal Toso R., Romanello S., Buriani A., Skaper S.D., Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi Moghaddam H., Shafiee Ardestani M., Saffari M., Navidpour L., Shafiee A., Rahmim A. Dopaminergic but not glutamatergic neurotransmission is increased in the striatum after selective COX-2 inhibition in hemiparkinsonian rats. Basic Clin. Pharmacol. Toxicol. 2008;103:293–296. doi: 10.1111/j.1742-7843.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- Fathi Moghaddam H., Shafiee Ardestani M. Electrical lesion of substantia nigra pars compacta: an alternative and convenient way to generate animal model of Parkinson’s disease. Irn. J. Pharmacol. Ther. 2008;7:53–56. [Google Scholar]
- Fernández-Ruiz J., González S. Cannabinoid control of motor function at the basal ganglia. In: Pertwee R.G., editor. Cannabinoids. vol. 168. Springer-Verlag; Heidelberg (Germany): 2005. pp. 479–507. (Handbook of Experimental Pharmacology). [DOI] [PubMed] [Google Scholar]
- Ferrari F., Ottani A., Giuliani D. Cannabimimetic activity in rats and pigeons of HU 210, a potent antiemetic drug. Pharmacol. Biochem. Behav. 1999;62:75–80. doi: 10.1016/s0091-3057(98)00114-2. [DOI] [PubMed] [Google Scholar]
- Fox S.H., Henry B., Hill M., Crossman A., Brotchie J. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov. Disord. 2002;17:1180–1187. doi: 10.1002/mds.10289. [DOI] [PubMed] [Google Scholar]
- Fox S.H. Cannabinoids. Encycloped. Mov. Dis. 2010;12:178–182. [Google Scholar]
- Garcia L., de Miguel R., Ramos J.A., Fernandez-Ruiz J.J. Perinatal D9-tetrahydrocannabinol exposure in rats modifies the responsiveness of midbrain dopaminergic neurons in adulthood to a variety of challenges with dopaminergic drugs. Drug Alcohol Depend. 1996;42:155–166. doi: 10.1016/s0376-8716(96)01276-8. [DOI] [PubMed] [Google Scholar]
- Galiegue S., Mary S., Marchand J., Dussossoy D., Carriere D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Glass M., Felder C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors result in an augmentation of forskolin-stimulated cAMP in striatal neurons. Soc. Neurosci. Abs. 1996;615:4. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P., Picconi B., Bari M., Battista N., Calabresi P., Centonze D., Bernardi G., Finazzi-Agro A., Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J. Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A.B., de Costa B.R., Richfield E.K. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lynn A.B., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Characterization and localization of a cannabinoid receptor in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister L.E. Health aspects of cannabis. Pharmacol. Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- Howlett A.C. Pharmacology of cannabinoid receptors. Annu. Rev. Pharmacol. Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- Järbe T.U.C., Andrzejewski M.E., DiPatrizio N.V. Interactions between the CB1 receptor agonist Δ9-THC and the CB1 receptor antagonist SR-141716 in rats: open-field revisited. Pharmacol. Biochem. Behav. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I., Cebeira M., de Ceballos M., Zeng B.-Y., Jenner P., Ramos J.A., Fernández-Ruiz J. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur. J. Neurosci. 2001;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- Ledent C., Valverde O., Cossu G., Petitet F., Aubert J.F., Beslot F., Bohme G.A., Imperato A., Pedrazzini T., Roques B.P., Vassart G., Fratta W., Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Maccarrone M., Gubellini P., Bari M., Picconi B., Battista N., Centonze D., Bernardi G., Finazzi-Agro A., Calabresi P. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J. Neurochem. 2003;85:1018–1025. doi: 10.1046/j.1471-4159.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- Mackie K., Hille B. Cannabinoid inhibit N-type calcium channels in neuroblastoma–glioma cells. Proc. Natl. Acad. Sci. USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K., Lai Y., Westenbroek R., Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. Neuroscience. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P., Vanderhaeghen J.J. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–688. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Mailleux P., Vanderhaeghen J.J. Dopaminergic regulation of cannabinoid receptor mRNA levels in the rat caudate-putamen: an in situ hybridization study. J. Neurochem. 1993;61:1705–1712. doi: 10.1111/j.1471-4159.1993.tb09807.x. [DOI] [PubMed] [Google Scholar]
- Maneuf Y.P., Nash J.E., Crossman A.R., Brotchie J.M. Activation of the cannabinoid receptor by Δ9-tetrahydrocannabinol reduces GABA uptake in the globus pallidus. Eur. J. Pharmacol. 1996;308:161–164. doi: 10.1016/0014-2999(96)00326-3. [DOI] [PubMed] [Google Scholar]
- Mansour A., Meador-Woodruff J.H., Zhou Q., Civelli O., Akil H., Watson S.J. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1992;46:959–971. doi: 10.1016/0306-4522(92)90197-a. [DOI] [PubMed] [Google Scholar]
- McGeer, P.L., McGeer. E.G., 2002. Innate immunity, local inflammation, and degenerative disease. Sci. Aging Knowledge Environ. 29, review 3. [DOI] [PubMed]
- Mechoulam R., Shani A., Edery H., Grundfeld Y. Chemical basis of hashish activity. Science. 1970;169:611–612. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]
- Meschler J.P., Conley T.J., Howlett A.C. Cannabinoid and dopamine interaction in rodent brain: effects on locomotor activity. Pharmacol. Biochem. Behav. 2000;67:567–573. doi: 10.1016/s0091-3057(00)00390-7. [DOI] [PubMed] [Google Scholar]
- Meschler J.P., Howlett A.C. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Mesnage V., Houeto J.L., Bonnet A.M., Clavier I., Arnulf I., Cattelin F., Le Fur G., Damier P., Welter M.L., Agid Y. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson’s disease. Clin. Neuropharmacol. 2004;27:108–110. doi: 10.1097/00002826-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Miller A., Sanũdo-Penã M.C., Walker J.M. Ipsilateral turning behavior induced by unilateral microinjections of a cannabinoid into the rat subthalamic nucleus. Brain Res. 1998;793:7–11. doi: 10.1016/s0006-8993(97)01475-3. [DOI] [PubMed] [Google Scholar]
- Mladenovic A., Provic M., Raicevic N. 6-Hydroxydopamine increases the level of TNF alpha and bax mRNA in the striatum and induces apoptosis of dopaminergic neurons in hemiparkinsonian rats. Brain Res. 2004;996:237–245. doi: 10.1016/j.brainres.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Academic Press; San Diego: 1998. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Pisani A., Fezza F., Galati S., Battista N., Napolitano S., Finazzi-Agro A., Bernardi G., Brusa L., Pierantozzi M., Stanzione P., Maccarrone M. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005;57:777–779. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- Pertwee R.G., Fernando S.R. Evidence for the presence of cannabinoid CB1 receptors in mouse urinary bladder. Br. J. Pharmacol. 1996;118:2053–2058. doi: 10.1111/j.1476-5381.1996.tb15643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R.G., Fernando S.R., Nash J.E., Coutts A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R.G., Gozie J.A., Hawksworth G.M. Further evidence for the presence of cannabinoid CB1 receptors in mouse vas deferens. Eur. J. Pharmacol. 1996;296:169–172. doi: 10.1016/0014-2999(95)00790-3. [DOI] [PubMed] [Google Scholar]
- Pertwee R.G., Wickens A.P. Enhancement by chlordiazepoxide of catalepsy induced in rats by intravenous or intrapallidal injections of enantiomeric cannabinoids. Neuropharmacology. 1991;30:237–244. doi: 10.1016/0028-3908(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Pires J.G., Bonikovski V., Futuro-Neto H.A. Acute effects of selective serotonin reuptake inhibitors on neuroleptic-induced catalepsy in mice. Braz. J. Med. Biol. Res. 2005;38:1867–1872. doi: 10.1590/s0100-879x2005001200015. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M., Barth F., Heaulme M., Shire D., Calandra B., Congy C., Martinez S., Maruani J., Neliat G., Caput D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Romero J., Garc´ıa-Palomero E., Lin S.Y., Ramos J.A., Makriyannis A., Ferna´ndez-Ruiz J.J. Extrapyramidal effects of methanadamide, an analog of anandamide, the endogenous CB1 receptor ligand. Life Sci. 1996;15:1249–1257. doi: 10.1016/0024-3205(96)00086-0. [DOI] [PubMed] [Google Scholar]
- Romero J., Berrendero F., Pérez-Rosado A., Manzanares J., Rojo A., Fernández-Ruiz J., de Yébenes J.G., Ramos J.A. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000;66:485–494. doi: 10.1016/s0024-3205(99)00618-9. [DOI] [PubMed] [Google Scholar]
- Romero J., de Miguel R., Garc´ıa-Palomero E., Fernández-Ruiz J.J., Ramos J.A. Time-course of the effects of anandamide, the putative endogenous cannabinoid receptor ligand, on extrapyramidal function. Brain Res. 1995;694:223–232. doi: 10.1016/0006-8993(95)00835-e. [DOI] [PubMed] [Google Scholar]
- Romero J., de Miguel R., Ramos J.A., Fernández-Ruiz J. The activation of cannabinoid receptors in striatonigral neurons inhibited GABA uptake. Life Sci. 1998;62:351–363. doi: 10.1016/s0024-3205(97)01117-x. [DOI] [PubMed] [Google Scholar]
- Sanberg, P.R., Martinez, R., Shytle, R.D., Cahill, D.W., 1996. The catalepsy test: is a standardized method possible?. In: P.R. Sanberg, K.-P. Ossenkoop, M. Kavaliers, (Eds.), Motor Activity and Movement Disorders, Humana Press, Totava, 1996. pp. 197–211.
- Sanũdo-Penã M.C., Force M., Tsou K., Miller A.S., Walker J.M. Effects of intrastriatal cannabinoids on rotational behavior in rats: interactions with the dopaminergic system. Synapse. 1998;30:221–226. doi: 10.1002/(SICI)1098-2396(199810)30:2<221::AID-SYN12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sanũdo-Penã M.C., Patrick S.L., Patrick R.L., Walker J.M. Cannabinoid control of movement in the basal ganglia in an animal model of Parkinson’s disease. Neurosci. Lett. 1998;248:171–174. doi: 10.1016/s0304-3940(98)00368-1. [DOI] [PubMed] [Google Scholar]
- Sanũdo-Penã M.C., Patrick S.L., Patrick R.L., Walker J.M. Effects of intranigral cannabinoids on rotational behavior in rats: interactions with the dopaminergic system. Neurosci. Lett. 1996;206:21–24. doi: 10.1016/0304-3940(96)12436-8. [DOI] [PubMed] [Google Scholar]
- Sanũdo-Penã M.C., Tsou K., Walker J.M. Motor actions of cannabinoids in the basal ganglia output nuclei. Life Sci. 1999;65:703–713. doi: 10.1016/s0024-3205(99)00293-3. [DOI] [PubMed] [Google Scholar]
- Sanũdo-Penã M.C., Walker J.M. Effects of intrapallidal cannabinoids on rotational behavior in rats: interactions with the dopaminergic system. Synapse. 1998;28:27–32. doi: 10.1002/(SICI)1098-2396(199801)28:1<27::AID-SYN4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sanũdo-Penã M.C., Walker J.M. A novel neurotransmitter system involved in the control of motor behavior by the basal ganglia. Ann. N. Y. Acad. Sci. 1998;860:475–479. doi: 10.1111/j.1749-6632.1998.tb09081.x. [DOI] [PubMed] [Google Scholar]
- Schatz A.R., Lee M., Condie R.B., Pulaski J.T., Kaminski N.E. Cannabinoid receptors CB1and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol. Appl. Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Shire D., Carillon C., Kaghad M., Calandra B., Rinaldi-Carmona M., LeFur G., Caput D., Ferrara P. An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J. Biol. Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- Souilhac J., Poncelet M., Rinaldi-Carmona M., Le Fur G., Soubrie´ P. Intrastriatal injection of cannabinoid receptor agonists induced turning behavior in mice. Pharmacol. Biochem. Behav. 1995;51:3–7. doi: 10.1016/0091-3057(94)00396-z. [DOI] [PubMed] [Google Scholar]
- Sulcova E., Mechoulam R., Fride E. Biphasic effects of anandamide. Pharmacol. Biochem. Behav. 1998;59:347–352. doi: 10.1016/s0091-3057(97)00422-x. [DOI] [PubMed] [Google Scholar]
- Tsou K., Brown S., Mackie K., Sanũdo-Penã M.C., Walker J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Walton R.P., Martin L.F., Keller J.H. The relative activity of various purified products obtained from American grown hashish. J. Pharmacol. Exp. Ther. 1938;62:239–251. [Google Scholar]
- Zhao P., Leonoudakis D., Abood M.E., Beattie E.C. Cannabinoid receptor activation reduces TNFα-induced surface localization of AMPAR-type glutamate receptors and excitotoxicity. Neuropharmacology. 2010;58:551–558. doi: 10.1016/j.neuropharm.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A., Zimmer A.M., Hohmann A.G., Herkenham M., Bonner T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]


