Abstract
Utilizing liquid chromatography–electro spray ionization-mass spectrometry (LC–(+,−)-ESI-MS) and liquid chromatography-photodiode array detection (LC-PDA) techniques, a dereplication strategy for the analysis of the secondary metabolites constituents of the genus Hypericum has been developed. From the crude methanolic extract of the aerial parts of H. triquetrifolium (leaves, stems, and flowers) and on the basis of their UV-profiles, chromatographic retention times and (+,−)-ESI-MS (TIC and SIM) mass spectral data, seven known (1–7) compounds were dereplicated fairly rapidly. The compounds were classified into three structural classes: phloroglucinols: hyperfirin and adhyperfirin; naphthodianthrones: hypericin, pseudo-hypericin, proto-hypericin, and protopseudo-hypericin; and the flavonoid rutin.
Keywords: Hypericum triquetrifolium, Clusiaceae, Dereplication, Phloroglucinols, Naphthodianthrones, Hyphenated techniques
1. Introduction
Nature is still a key player in drug discovery and development programs as a source for novel structures or lead compound. It represents a wealth and indispensable source for structural diversity. In the area of cancer, for example, of the 155 small molecules that were introduced over the time frame from 1940s to date; 73% are other than synthetic, with 47% actually being either natural products or directly derived therefrom (Newman and Cragg, 2007). Moreover, 61% of the new registered drugs are natural products or compounds derived from or inspired by natural products (Newman et al., 2003).
Conventional natural products discovery programs rely on bioactivity-directed fractionation methodology for the isolation, purification and structural elucidation of bioactive lead compounds from crude extracts, in which the bioassay results guide the purification processes. This process is often tedious, expensive, time consuming; moreover it may end up with disappointing outputs when isolating well known previously characterized bioactive compounds (Ghisalberti, 1993; Kingston, 1996). Hence there is currently great pressure on natural products lead discovery programs to develop their procedures to discriminate between previously known nuisance compounds and potential novel chemical entities more rapidly and efficiently at the level of the crude extract. This process which is termed dereplication is of great importance as huge amount of work can be circumvented when identifying previously known compounds at an early stage of the natural products drug discovery process before setting out large scale isolation project to re-isolate and re-characterize previously known compounds. Thus efforts will beoriented toward targeted isolation of compounds presenting novel spectroscopic features. By adopting this approach one can be assured that resources are spent on the most promising bioactive samples, hence increasing the potential for the isolation of structurally novel bioactive secondary metabolites (Cordell and Shin, 1999; Hostettmann et al., 2001; Wolfender et al., 2006). Thus, quick and easy methods for dereplication of natural product extracts are essential (Ackermann et al., 1996; Constant and Beecher, 1995).
Dereplication approaches make use of hyphenated techniques and search through natural products databases to determine the identity of an active compound at the earliest possible stage in the discovery process. UV spectroscopy and mass spectrometry are of prime importance in the field of dereplication procedures. When combined with HPLC, they create hyphenated techniques, of which LC-UV/PDA and LC–MS are the most popular. The UV profile of compounds in an extract, obtained using LC-UV/PDA, serves as a fingerprint that is characteristic of certain structural classes (Su et al., 2002; Alvi et al., 1995; Sedlock et al., 1992; Sun et al., 2002; Tsao and Yang, 2003). LC–MS is considered a selective, sensitive, and powerful tool (Niessen, 1999; Sarker and Nahar, 2005), as the molecular weight provides an entry point into the molecular formula of compounds.
In the current research project we have developed a simple, reliable, and cost effective dereplication strategy for the analyses of the secondary metabolite constituents of the genus Hypericum using Liquid Chromatography–Electro Spray Ionization-Mass Spectrometry (LC–ESI-MS) and Liquid Chromatography-Photodiode Array Detection (LC-PDA). In particular, phloroglucinols and naphthodianthrones which are considered the major active constituents of Hypericum species. The Hypericum genus is known to contain wealth of secondary metabolites, many of which are biologically active. Of the main constituents are naphthodianthrones (hypericin, pseudo-hypericin, proto-hypericin, and protopseudo-hypericin), phloroglucinols (hyperforin, adhyperforin, hyperfirin, and adhyperfirin), and a broad range of flavonoids (hyperoside and rutin) (Nahrstedt and Butterweck, 1997).
H. triquetrifolium Turra (Peter’s wort, wavyleaf St. John’s wort or Tangled Hypericum), is a wild growing weed in the northern part of Jordan; locally known as Roja. In their book Medicinal Plants of Jordan, Karim and Quraan reported that H. triquetrifolium is used traditionally as sedative, astringent, anti-spasmodic, for intestine and bile disorders and poisonous (Karim and Quraan, 1986). Anti-inflammatory, antioxidant and antinociceptive activities have also been reported in the literature for H. triquetrifolium (Apaydin et al., 1999; Tawaha et al., 2007; Couladis et al., 2002; Suzuki et al., 1984; Alali et al., 2007). H. triquetrifolium occurs as perennial herb with stiff patent decussate branches, and hence the plant has a more or less pyramidal aspect. Flowers 1–5 together in the summit of leafy branches and appear in summer (Al-Eisawi, 1998).
In a study carried out by Alali et al. (2007), hypericin content of methanolic extracts dried flowers, leaves, stems, and roots of H. triquetrifolium were determined by HPLC. Leaves showed the highest hypericin content of 0.36% w/w, while total aerial parts contained 0.11% w/w.
From the crude methanolic extract of the aerial parts of H. triquetrifolium (leaves, stems, and flowers) and on the basis of their UV-profiles, retention time and (+,−)-ESI-MS (TIC and SIM) mass spectral data, seven known (Newman and Cragg, 2007; Newman et al., 2003; Ghisalberti, 1993; Kingston, 1996; Cordell and Shin, 1999; Hostettmann et al., 2001; Wolfender et al., 2006) compounds were identified.
2. Materials and methods
2.1. General experimental procedures
LC–MS data were utilized using an Agilent® (Palo Alto, CA, USA) ion-trap mass spectrometer equipped with electrospray ionization source and an Agilent® 100 series HPLC. The separation was achieved using a Hypersil ODS (5 μm; 125 mm × 4 mm) column (Thermo Electron, Auchtermuchty, UK). The mobile phase used was: (A) 20 mM ammonium acetate; (B) acetonitrile. The flow rate was 1 ml min−1 in the following gradient system: 0–10 min, 50% B; 10–25 min, 90% B; 30–35 min, 50% B. The injection volume was 20 μl and the total run time was 35 min. The mass detector conditions were set as follows: ESI positive and negative ionization modes, full scan mode from 50 to 1000 m/z, capillary voltage set at –4000 V, ESI temperature 325 °C, gas flow rate 5 l min−1.
LC-UV/Vis PDA spectra were obtained on a Lachrom® Merck-Hitachi (Tokyo, Japan) HPLC, equipped with quaternary gradient L-7150 pump, L-7455 Diode-Array Detector, L-7200 auto-sampler, and D-7000 Interface in the range between 250 and 650 nm. Mobile phase, flow rate, analytical column, injection volume, and run times were identical to those used for LC–MS.
Ammonium acetate (extra pure) was obtained from Scharlau Chemie S.A. (Barcelona, Spain), HPLC-grade methanol was obtained from Tedia Company Inc. (Ohio, USA), HPLC-grade acetonitrile was obtained from LEDA, Scharlau Chemie S.A. (Barcelona, Spain), and (–)-hypericin standard was purchased from Sigma–Aldrich (Buchs, Switzerland).
2.2. Plant material
H. triquetrifolium were collected from Al-Mafraq, Northern part of Jordan during their flowering stage in July 2007. The collected material was identified by Professor Khalid Tawaha. A voucher specimen (PHC #104) was deposited in the herbarium of the Faculty of Pharmacy, Jordan University of Science and Technology (JUST), Irbid, Jordan. The plants raw material was cleaned and air-dried at room temperature and then grounded to fine powder using a blender, Moulinex® (Caen, France), passed through a 24 mesh sieve to generate homogeneous powder, stored at room temperature (22–23 °C), and protected from light until required for analyses.
2.3. Sample preparation and analysis
From the finely ground plant material, 1 g was accurately weighed and placed into a 250-ml round bottom flask fitted with a reflux condenser, and was refluxed for 20 min using 80 ml of HPLC methanol. The sample was then filtered, saving the filtrate. The herb material was then re-extracted twice with 60 ml HPLC methanol followed each time by filtration. The collected filtrates and washes were combined. The volume was then reduced to a final volume of about 3 ml using rotary evaporator (RE 200, Bibby Steriline Ltd., UK). The concentrated solution was transferred to a 25-ml volumetric flask and diluted to volume using methanol. Aliquots were removed and centrifuged at 4500 rpm for 5 min using EBA 20 centrifuge (Hettich-Zentrifugen GmbH & Co. KG, Tuttlingen, Germany), supernatants were transferred into glass vial, and stored in refrigerator until required for analysis (INA Methods Validation Program, 2000).
For the LC–MS and LC-UV/PDA studies, an aliquot of the supernatant of the methanolic extract of the plant was filtered through a 0.45 μm Teflon filter and then transferred into 2 ml amber HPLC vial. A 20 μl aliquot was injected. Hypericin standard was used for retention time matching.
3. Results and discussion
The general dereplication strategy used a three-step approach. First, LC–MS full scan total ion chromatograms (TIC), scanning m/z 50–1000, was firstly acquired in both positive and negative modes. This was followed by positive and negative selected ion monitoring (SIM) analysis to acquire the compounds that may not otherwise be detected in TIC. These data were searched across natural product databases. These information were cross referenced with the available published literature on spectral data and chromatographic elution pattern for phloroglucinols, naphthodianthrones, and flavonoids that were reported from the genus Hypericum (Tolonen et al., 2002; Fuzzati et al., 2001; Wolfender et al., 2003). Next, LC-UV/PDA was used to acquire the UV/Vis spectra of the compounds, and this fingerprint was used to group each compound into one of three structural classes that are typical constituents from the genus Hypericum. Finally, the chromatographic retention times (and hence, relative polarity) were used to further identify the structures of the compounds.
(−)-Hypericin reference standard was used to develop the optimum chromatographic separation conditions. A neutral mobile phase was used to able the switch between the positive and negative ionization modes simultaneously. The conditions were optimized for resolution and response as outlined in the experimental section. The total run time was 35 min; with a retention time of 16.1 min for hypericin.
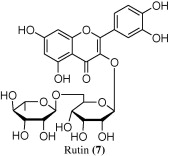
By utilizing the above described strategy and integrating the spectral data for each peak: retention time (polarity), UV profile, and mass spectral data, we were able to identify the chemical structures of seven compounds. Of these, two (5 and 6) were phloroglucinols, four were naphthodianthrones (1–4), and one flavonoid (7). UV/PDA spectra were very informative in determining the general structural class for each compound where the general patterns are quite characteristic. The following naphthodianthrones molecular ions, [M − 1]−, were detected in the negative mode: hypericin (m/z 504), pseudo-hypericin (m/z 520), proto-hypericin (m/z 506), and protopseudo-hypericin (m/z 522). The negative ionization mode was found to be highly sensitive for this class of phenolic compounds. While in the positive ionization mode the following phloroglucinols molecular ions, [M + 1]+, were detected: hyperfirin (m/z 468), and adhyperfirin (m/z 482). Finally, the flavonoid rutin was detected in both the positive (m/z 611) and negative (m/z 609) ionization modes. Comparing to TIC and as expected, SIM sensitivity was significantly higher for the three investigated chemical classes. Figs. 1 and 2 show the positive and TIC of the methanolic extracts of the aerial parts of H. triquetrifolium. Table 1 summarizes the retention times, UV max (nm), and the molecular ions of the identified compounds.
Figure 1.
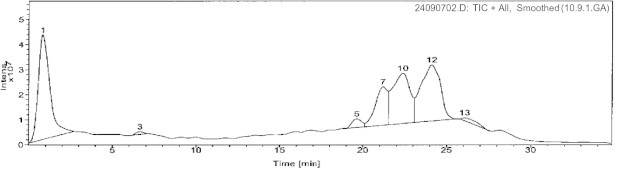
(+)-ESI TIC chromatogram of H. triqutrifolium; (1): Rutin, (10): Hyperfirin, and (12): Adhyperfirin.
Figure 2.
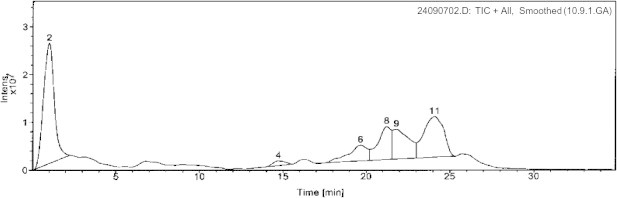
(−)-ESI TIC chromatogram of H. triqutrifolium; (2): Rutin.
Table 1.
Retention times, UV max, mass spectral data of the (+)- and (−)-ESI TIC and (+)- and (−)-SIM chromatographic peaks.
| tR (min) | UV max (nm) | Molecular ion | Compound |
|---|---|---|---|
| 16.1 | 288, 325, 465, 580 | 503 [M − 1]− | 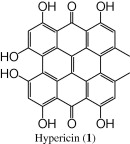 |
| 4.0 | 288, 325, 465, 580 | 519 [M − 1]− | 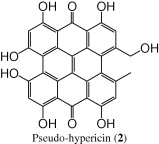 |
| 14.1 | 285, 375, 530, 590 | 505 [M − 1]− | 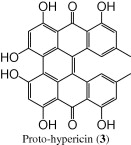 |
| 3.1 | 285, 375, 550 | 521 [M − 1]− | 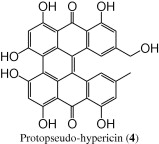 |
| 22.6 | 285 | 469 [M + 1]+ | 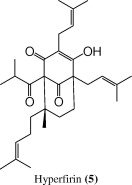 |
| 23.9 | 285 | 483 [M + 1]+ | 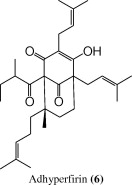 |
| 1.0 | 257, 360 | 611 [M + 1]+ 609 [M − 1]− |
 |
The (−)-ESI mass spectrum of hypericin (1) (tR = 16.1 min) showed a parent molecular ion at m/z 503 [M − 1]−. The UV/PDA spectrum of hypericin showed four absorption maxima at 288 nm, 325 nm, 465 nm and 580 nm, typical values for hypericin, provide additional proof for the identity of this compound (Tolonen et al., 2002, 2003; Liu et al., 2000; Mauri and Pietta, 2000; Brolis et al., 1998). The identity of this compound was verified by comparison of the ESI mass spectrum, UV/PDA spectrum, and the HPLC retention time with an authentic standard of hypericin, where complete matching was observed.
For the peak at tR = 4.0 min, the (−)-ESI mass spectrum showed parent molecular ion at m/z 519 [M − 1]−, and an identical UV/PDA spectra of hypericin; but eluted at an earlier time, more polar. These spectral data suggest the identity of this compound as pseudo-hypericin (2) (Tolonen et al., 2003; Liu et al., 2000; Mauri and Pietta, 2000; Brolis et al., 1998).
The (−)-ESI mass spectrum of the peak at tR = 14.1 min, showed a parent molecular ion at m/z 505 [M − 1]−, 2 Da less than the analogous peak in hypericin; it was also eluted at an earlier retention time (more polar) than hypericin. The UV/PDA spectrum of the compound showed the following absorption maxima 285 nm, 375 nm, 530 nm, and 590 nm. These data suggested that this compound was proto-hypericin (3) (Tolonen et al., 2003).
The (−)-ESI mass spectrum of the peak at tR = 3.1 min, showed a parent molecular ion at m/z 521 for [M − 1]−, 2 Da less than the analogous peak in pseudo-hypericin; it was also eluted at an earlier retention time than pseudo-hypericin. The UV/PDA spectrum of the compound showed absorption maxima at 285 nm, 375 nm, and 550 nm. These data suggested that this compound was protopseudo-hypericin (4) (Tolonen et al., 2003).
The (+)-ESI mass spectrum of the peak at tR = 23.9 min, showed a parent molecular ion at m/z 483 for [M + 1]+, while the UV/PDA spectrum showed an absorption maximum at 285 nm suggesting that compound is adhyperfirin (6) (Tatsis et al., 2007).
For the peak at tR = 22.6 min, the (+)-ESI mass spectrum showed parent molecular ion at m/z 469 for [M + 1]+, 14 Da less than the analogous peak in adhyperfirin suggesting a difference by only a methylene group (CH2, 14) with an identical UV/PDA spectrum; but eluted at an earlier time. These spectral data suggest the identity of this compound as hyperfirin (2) (Tatsis et al., 2007).
Finally, the (−)- and (+)-ESI mass spectra of peaks at tR = 1.0 min, showed parent molecular ions of 611 and 609 for [M + 1]+ and [M − 1]−, in the positive and negative ionization modes, respectively. The UV/PDA spectrum showed an absorption maximum at 257 nm and 360 nm, characteristic of flavonoids. These mass spectral data suggested that this compound is rutin (5) (Liu et al., 2000; Exarchou et al., 2006).
Worthy to be mentioned is that there were several peaks in the (−)- and (+)-ESI mass spectra of H. triquetrifolium that could not be identified or dereplicated to any obvious structural class reported previously from the genus Hypericum. These compounds could be potentially new, thus our future efforts will be focused toward their isolation and identification.
In summary, we have adapted a dereplication strategy in the current work for the analysis of the secondary metabolites (phloroglucinols, naphthodianthrones and flavonoids) in H. triqutrifolium based on integrating data from LC-UV/PDA (distinctive UV-spectra for different classes), LC–ESI-MS (TIC and SIM) and chromatographic elution pattern. We were able to tentatively identify seven major compounds; hypericin (1), pseudo-hypericin (2), proto-hypericin (3), protopseudo-hypericin (4), hyperfirin (5), adhyperfirin (6) and rutin (7).
Acknowledgment
The authors acknowledge the financial support from the Deanship of Scientific Research, JUST; Irbid, Jordan and the technical help of Mr. Munther Tahtamoni, Princess Haya Biotechnology Center, and Ms. Tamam El-Elimat, Pharmaceutical Research Center, JUST, Jordan.
References
- Ackermann B.L., Regg B.T., Colombo L., Stella S., Coutant J.E. Rapid analysis of antibiotic-containing mixtures from fermentation broths by using liquid chromatography-electrospray ionization-mass spectrometry and matrix-assisted laser desorption ionization-time-of-flight-mass spectrometry. J. Am. Soc. Mass Spectrom. 1996;7:1227–1237. doi: 10.1016/S1044-0305(96)00104-3. [DOI] [PubMed] [Google Scholar]
- Alali F.Q., Tawaha K., El-Elimat T., Syouf M., El-Fayad M., Abulaila K., Nielsen S.J., Wheaton W.D., Falkinham J.O., III, Oberlies N.H. Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: an ICBG project. Nat. Prod. Res. 2007;21:1121–1131. doi: 10.1080/14786410701590285. [DOI] [PubMed] [Google Scholar]
- Al-Eisawi, D.M., 1998. Field guide to wild flowers of Jordan and neighbouring countries, first ed., Jordan Press Foundation Al-Rai, Amman.
- Alvi K.A., Peterson J., Hofmann B. Rapid identification of elaiophylin and geldanamycin in streptomyces fermentation broths using CPC coupled with a photodiode array detector and LC–MS methodologies. J. Ind. Microbiol. Biotechnol. 1995;15:80–84. doi: 10.1007/BF01569804. [DOI] [PubMed] [Google Scholar]
- Apaydin S., Zeybek U., Ince I., Elgin G., Karamenderes C., Ozturk B., Tuglular I. Hypericum triquterifolium Turra extract exhibits antinociceptive activity in the mouse. J. Ethnopharmacol. 1999;67:307–312. doi: 10.1016/s0378-8741(99)00071-9. [DOI] [PubMed] [Google Scholar]
- Brolis M., Gabetta B., Fuzzati N., Pace R., Panzeri F., Peterlongo F. Identification by high-performance liquid chromatography-diode array detection-mass spectrometry and quantification by high-performance liquid-chromatography-UV absorbance detection of active constituents of Hypericum perforatum. J. Chromatogr. A. 1998;825:9–16. [Google Scholar]
- Constant H.L., Beecher C.W.W. A method for the dereplication of natural product extracts using electrospray HPLC/MS. Nat. Prod. Lett. 1995;6:193–196. [Google Scholar]
- Cordell G.A., Shin Y.G. Finding the needle in the haystack. The dereplication of natural product extracts. Pure Appl. Chem. 1999;71:1089–1094. [Google Scholar]
- Couladis M., Baziou P., Verykokidou E., Loukis A. Antioxidant activity of polyphenols from Hypericum triquetrifolium Turra. Phytother. Res. 2002;16:769–770. doi: 10.1002/ptr.1062. [DOI] [PubMed] [Google Scholar]
- Exarchou V., Fiamegos Y.C., vanBeek TA,, Nanos C., Vervoort J. Hyphenated chromatographic techniques for the rapid screening and identification of antioxidants in methanolic extracts of pharmaceutically used plants. J. Chromatogr. A. 2006;1112:293–302. doi: 10.1016/j.chroma.2005.11.077. [DOI] [PubMed] [Google Scholar]
- Fuzzati N., Gabetta B., Streponi I., Villa F. High performance liquid chromatography-electrospray ionisation mass spectrometry and multiple mass spectrometry studies of hyperforin degradation products. J. Chromatogr. A. 2001;926:187–198. doi: 10.1016/s0021-9673(01)01000-7. [DOI] [PubMed] [Google Scholar]
- Ghisalberti, E.L., 1993. Detection and isolation of bioactive natural products. In: Colegate, S.M., Molyneux, R.J. (Eds.), Detection, Isolation and Structural Determination, CRC Press Inc., Florida, pp. 9–57.
- Hostettmann K., Wolfender J., Terreaux C. Modern screening techniques for plant extracts. Pharm. Biol. 2001;39:18–32. doi: 10.1076/phbi.39.s1.18.0008. [DOI] [PubMed] [Google Scholar]
- INA Methods Validation Program, 2000. Determination of Hypericin and Pseudohypericin in St. John’s Wort by High Performance Liquid Chromatography, 107.000. Institute for Nutraceutical Advancement, Denver, CO, pp. 1–4.
- Karim, F., Quraan, S., 1986. Medicinal plants of Jordan, Yarmouk University, Irbid, Jordan.
- Kingston, D.G.I., 1996. Natural products as pharmaceuticals and sources for lead structures. In: Meyer, P. (Ed.), The Practice of Medicinal Chemistry, Academic Press Limited, London, pp. 101–116.
- Liu F.F., Ang C.Y.W., Heinze T.M., Rankin J.D., Beger R.D., Freeman J.P., Lay J.O., Jr. Evaluation of major active components in St. John’s Wort dietary supplements by high performance liquid chromatography with photodiode array detection and electrospray mass spectrometric confirmation. J. Chromatogr. A. 2000;888:85–92. doi: 10.1016/s0021-9673(00)00555-0. [DOI] [PubMed] [Google Scholar]
- Mauri P., Pietta P. High performance liquid chromatography/electrospray mass spectrometry of Hypericum perforatum extracts. Rapid Commun. Mass Spectrom. 2000;14:95–99. doi: 10.1002/(SICI)1097-0231(20000130)14:2<95::AID-RCM843>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Nahrstedt A., Butterweck V. Biologically active and other chemical constituents of the herb Hypericum perforatum L. Pharmacopsychiatry. 1997;30(Suppl.):129–134. doi: 10.1055/s-2007-979533. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Newman D., Cragg G., Snader K. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Niessen W.M.A. State-of-the-art in liquid chromatography-mass spectrometry. J. Chromatogr. A. 1999;856:179–197. doi: 10.1016/s0021-9673(99)00480-x. [DOI] [PubMed] [Google Scholar]
- Sarker S., Nahar L. Hyphenated techniques. In: Sarker S., Latif Z., Gray A.I., editors. Natural Products Isolation. HUMANA Press; Totowa, NJ: 2005. pp. 233–268. [Google Scholar]
- Sedlock D.M., Sun H.H., Smith W.F., Kawaoka K., Gillum A.M., Cooper R. Rapid identification of teleocidins in fermentation broth using HPLC photodiode array and LC/MS methodology. J. Ind. Microbiol. Biotechnol. 1992;9:45–52. [Google Scholar]
- Su Q., Rowley K.G., Balazs N.D.H. Carotenoids: separation methods applicable to biological samples. J. Chromatogr. B. 2002;781:393–418. doi: 10.1016/s1570-0232(02)00502-0. [DOI] [PubMed] [Google Scholar]
- Sun L., Rezaei K.A., Temelli F., Ooraikul B. Supercritical fluid extraction of alkylamides from Echinacea angustifolia. J. Agric. Food Chem. 2002;50:3947–3953. doi: 10.1021/jf0200265. [DOI] [PubMed] [Google Scholar]
- Suzuki O., Katsumata Y., Oya M., Bladt S., Wagner H. Inhibition of monoamine oxidase by hypericin. Planta Med. 1984;50:272–274. doi: 10.1055/s-2007-969700. [DOI] [PubMed] [Google Scholar]
- Tatsis E.C., Boeren S., Exarchou V., Troganis A.N., Vervoort J., Gerothanassis I.P. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry. 2007;68:383–393. doi: 10.1016/j.phytochem.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Tawaha K., Alali F.Q., Gharaibeh M., Mohammad M., El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007;104:1372–1378. [Google Scholar]
- Tolonen A., Uusitalo J., Hohtola A., Jalonen J. Determination of naphthodianthrones and phloroglucinols from Hypericum perforatum extracts by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:396–402. doi: 10.1002/rcm.591. [DOI] [PubMed] [Google Scholar]
- Tolonen A., Hohtola A., Jalonen J. Fast HPLC/PDAanalysis of naphthodianthrones and phloroglucinols from Hypericum perforatum extracts. Phytochem. Anal. 2003;14:306–309. doi: 10.1002/pca.720. [DOI] [PubMed] [Google Scholar]
- Tsao R., Yang R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: towards a total phenolic index using high-performance liquid chromatography. J. Chromatogr. A. 2003;1018:29–40. doi: 10.1016/j.chroma.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Wolfender J.L., Verotta L., Belvisi L., Fuzzati N., Hostettmann K. Structural investigations of isomeric oxidised forms of hyperforin by HPLC-NMR and HPLC-MSn. Phytochem. Anal. 2003;14:290–297. doi: 10.1002/pca.718. [DOI] [PubMed] [Google Scholar]
- Wolfender J., Queiroz E.F., Hostettmann K. The importance of hyphenated techniques in discovery of new lead compounds from nature. Exp. Opin. Drug Discov. 2006;1:237–260. doi: 10.1517/17460441.1.3.237. [DOI] [PubMed] [Google Scholar]


