Abstract
Traditional diets of people living in the Mediterranean basin are, among other components, very rich in extra-virgin olive oil, the most typical source of visible fat. Olive is a priceless source of monounsaturated and di-unsaturated fatty acids, polyphenolic antioxidants and vitamins. Oleuropein is the main glycoside in olives and is responsible for the bitter taste of immature and unprocessed olives. Chemically, oleuropein is the ester of elenolic acid and 3,4-dihydroxyphenyl ethanol, which possesses beneficial effects on human health, such as antioxidant, antiatherogenic, anti-cancer, anti-inflammatory and antimicrobial properties. The phenolic fraction extracted from the leaves of the olive tree, which contains significant amounts of oleuropein, prevents lipoprotein oxidation. In addition, oleuropein has shown cardioprotective effect against acute adriamycin cardiotoxicity and an anti-ischemic and hypolipidemic activities. Recently, oleuropein has shown neuroprotection by forming a non-covalent complex with the Aβ peptide, which is a key hallmark of several degenerative diseases like Alzheimer and Parkinson. Thus, a large mass of research has been accumulating in the area of olive oil, in the attempt to provide evidence for the health benefits of olive oil consumption and to scientifically support the widespread adoption of traditional Mediterranean diet as a model of healthy eating. These results provide a molecular basis for some of the benefits potentially coming from oleuropein consumption and pave the way to further studies on the possible pharmacological use of oleuropein to prevent or to slow down the cardiovascular and neurodegenerative diseases.
Keywords: Mediterranean diet, Olive, Oleuropein, Cardioprotective, Neuroprotective
1. Introduction
A relation between diet and heart health had been shown by experimental studies over 100 years ago. However, it was not until the epidemic rise of cardiovascular diseases in many industrialized countries during the middle of the 20th century that the identification of risk factors and the need to put in place preventive measures became crucial. The field of epidemiology provided the first leads to explain such risk factors. Epidemiology studies demonstrated that dietary saturated fat intake was significantly associated with serum cholesterol and the risk of coronary heart disease, and that serum cholesterol relates to coronary heart disease risk. The outcome of Keys et al. (1986) study brought up the concept of the cardioprotective properties of the dietary habits of Mediterranean populations, which appeared to have as the most common element the consumption of virgin olive oil. For the last few decades, several population studies aimed to solidify the initial observation from the Seven Country Study and demonstrate the relevance of virgin olive oil as a key cardioprotective component of the Mediterranean diet (Trichopoulou et al., 2003).
Ageing also represents a great concern in developed countries because of the increasing number of persons reaching advanced age and the number of related pathologies, such as Alzheimer’s disease, vascular dementia, Morbus Parkinson, diabetes or cancer. A typical Mediterranean diet, the high monounsaturated fat energy intake appeared to be associated with a reduced risk of age-related cognitive decline (Solfrizzi et al., 1999). This effect could be related to the role of monounsaturated fatty acids in maintaining the structural integrity of neuronal membranes (Solfrizzi et al., 1999; Panza et al., 2004). Moreover, high intake of monounsaturated fat showed protective effect against Alzheimer’s disease, whereas intake of saturated or trans-unsaturated fats leads to detrimental (Panza et al., 2004).
Several experimental data supported that virgin olive oil contains a higher concentration of three phenolic antioxidants classes, simple phenols, secoiridoids and lignans, and squalene than refined virgin oil and seed oils. For these reasons, in the Mediterranean region, where olive oil is an essential constituent of the diet, there is a lower incidence of cancer and heart disease (Gerber, 1994; Keys, 1995).
Oleuropein is the major bioactive compound of Olea europaea, widely known as the olive tree and present in high amount in unprocessed olive fruit and leaves. During maturation of fruit or as a results of olive processing (such as oil production), chemical and enzyme reactions occur which reduce the concentration of oleuropein and raise the concentration of hydroxytyrosol which is the principal degradation product of oleuropein. The oleuropein molecule consists of three structural subunits: a polyphenol, namely 4-(2-hydroxyethyl) benzene-1,2-diol which is also known as hydroxytyrosol (HT), a secoiridoid called elenolic acid and a glucose molecule (Fig. 1).
Figure 1.
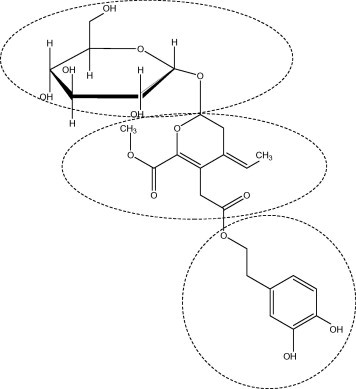
Molecular structure of oleuropein molecule.
Oleuropein possesses beneficial pharmacological effects such as cardiprotective effect antioxidant (Andreadou et al., 2006), anti-inflammatory (Visioli et al., 1998), inhibition of platelet aggregation (Petroni et al., 1995), anti-atherogenic (Manna et al., 2004; Visioli and Galli, 2001) activity, anti-cancer (Hamdi and Castellon, 2005), anti-microbial properties (Bisignano et al., 1999) and neuroprotective effect (Bazoti et al., 2006). This review summarizes the health benefits of oleuropein from virgin olive oil and leaf extract and its cardiprotective and neuroprotective effect as tools for future drug research.
2. Occurrence
Phenolic compounds are found in all parts of the plant but their nature and concentration varies greatly between the various tissues. In O. europaea, oleuropein, demethyloleuropein, ligstroside and oleoside represent the predominant phenolic oleosides (Soler-Rivas et al., 2000), whereas verbascoside (Ryan et al., 1999) is the main hydroxycinnamic derivative of olive fruit (Servili et al., 1999). Oleuropein is generally the most prominent phenolic compound in olive cultivars and may reach concentrations of up to 140 mg g−1 on a dry matter basis in young olives (Amiot et al., 1986) and 60–90 mg g−1 of dry matter in the leaves (Le Tutour and Guedon, 1992).
Various methods have been developed for qualitative and quantitative occurrence of phenolic and secoiridoid compounds analysis, from the most simple techniques to the more sophisticated such as TLC (Capasso et al., 1992), reversed phase HPLC (Ficarra et al., 1991; De Laurentis et al., 1997), GC–MS, FAMS or TMS (Baracco et al., 1995). In the fruits, phenyl acids, flavonoids and secoiridoids have been reported, the phenolic compounds representing 1–3% (w/v) Brenes et al., 1993. In the leaves, 19% (w/w) is oleuropein and 1.8% (w/w) flavonoids, of which 0.8% is luteolin 7-glucoside (Le Tutour and Guedon, 1992).
Oleuropein occurs not only in the Olea genus but also in many other genera belonging to the Oleaceae family and has been described in Fraximus excelsior, Fraximus angustifolia, Fraximus chinensis, Syringa josikaea, Syringa vulgaris, Philyrea latifolia, Ligustrum ovalifolium, Ligustrum vulgare and many others (Soler-Rivas et al., 2000).
3. Chemistry, synthesis and fate of oleuropein
3.1. Chemistry and synthesis
In olive, two main groups of compounds are present: fats, as glycerides and lipids, and phenols and related substances. Oleuropein is the first secoiridoid that structure was recognised in 1958–1965, but only several years later it was classified as secoiridoid, when this class of monoterpenoids was constituted (Fig. 2).
Figure 2.
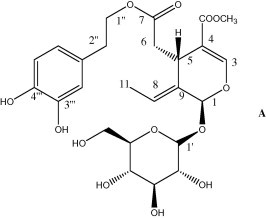
Structure of oleuropein.
Oleuropein structure was determined by Panizzi et al. (1958) and Panizzi and Scarpati (1960) from fresh leaves in amount from 1.5% to 2%. Only the absolute configurations of C-l and C-5 remained undetermined, as the cis/trans configuration of C-8/C-9 double bond. Inouye et al. (1970) determined some years later (Panza et al., 2004), the absolute configuration of chiral centres of the secoiridoid oleuropein A, relating A to the iridoid asperuloside B shown in Fig. 3. Bisdeoxy-acetyl asperuloside C, prepared by hydrogenolysis from asperuloside B, was opened by a series of oxidative steps to the two secoiridoid dimethyl esters D1 and D2, having racemic C-8 centre with hydroxyl in both R (D1) and S (D2) configurations. Dehydration of these two secondary alcohols afforded two possible olefins: S-alcohol D2 afforded the E-olefin E2, while R alcohol D1 gave the Z-olefin E1 shown in Fig. 3.
Figure 3.
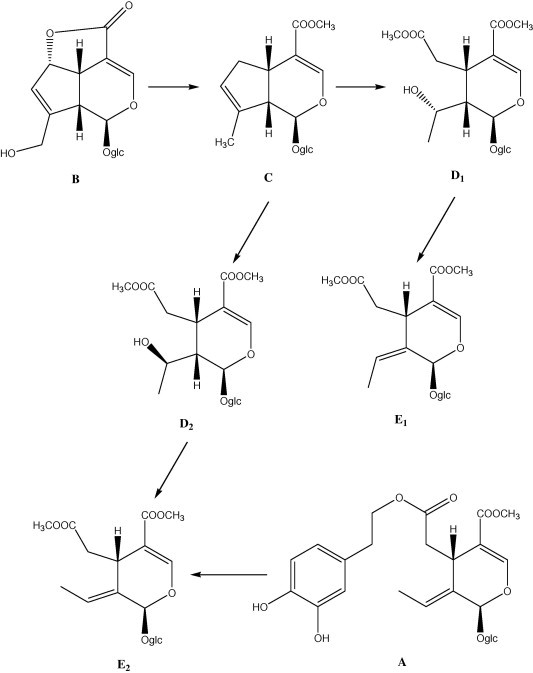
Absolute configuration of oleuropein ‘A’.
A simple work up on oleuropein A, transesterification with methanol of ester function at C-7 afforded the E-olefin E2, so demonstrating the absolute configuration of the C-8/C-9 double bond, as well as all chiral centres of oleuropein A. A similar approach was used for the partial synthesis of oleuropein A (Bianco et al., 1992) that was depicted in Fig. 4.
Figure 4.
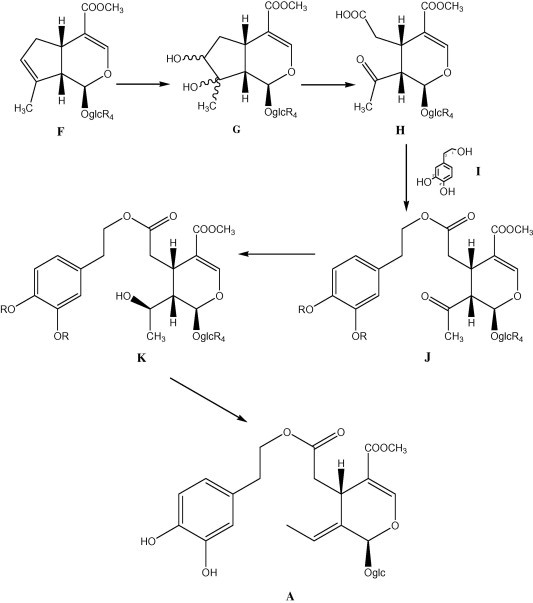
Partial synthesis of oleuropein.
The chiral starting product was a glucosidic iridoid, the bisdeoxy-asperuloside F. The partial synthesis starts with the protection of compound F with benzyl groups that can easily be eliminated at the end of the synthesis in mild hydrogenolytic conditions that do not interfere with ester functions of oleuropein. After protection, compound F is osmilated to diol G. This last compound was selectively oxidized at the vicinal diol function with sodium periodate and successively with Jones’s reagent, affording the desired acid H. Direct esterification of compound H with dioxy-phenyl-ethanol I, having phenolic functions protected with benzyl moieties, gave the key intermediate secoiridoid J. The last step of the synthesis is the stereoselective reduction of J that afforded the alcohol K as a single product, with the desired (S) absolute configuration at C-8 centre. The compound K was then dehydrated, giving the C-8/C-9 double bond with the correct configuration. The final removing of the protecting benzyl groups by hydrogenolysis afforded the oleuropein A.
Oleuropein A has been a recognised chemo-taxonomic marker of O. europaea; however its presence in olives is attributed to the ripening stage of fruits. The experimental data were achieved for some Spanish, Portuguese and Italian cultivars, which were examined in different stages of ripening (green, cherry and black) (Bianco and Uccella, 2000; Bastoni et al., 2001). These data confirmed the previously obtained results (Donaire et al., 1975; Amiot et al., 1989), with a decrease of oleuropein A during olive maturation and a contemporary increase of oleuropein derivatives.
3.2. Biosynthesis
The biosynthesis of oleuropein in the Oleaceae proceeds via a branching in the mevalonic acid pathway from the secondary metabolism resulting in the formation of oleosides (Damtoft et al., 1992). From these compounds secoiridoids are derived (Damtoft et al., 1993). The biosynthesis of oleosides is similar to that of secologanin-derived secoiridoids in Gentianales and Cornales. In these the carbon skeleton is derived from mevalonic acid. Geraniol, 10-hydroxygeranoil as well as 10-hydroxynerol and iridoidal are known to be precursors of loganin. Later deoxyloganic acid, 7-epiloganic acid and loganic acid are incorporated into ligustroside, a direct precursor of oleuropein, via 7-ketologanic acid as intermediate. The sequences of the steps between deoxyloganic acid and 7-ketologanin may differ with plant species and time of year (Damtoft et al., 1993). In O. europaea both possible epoxides of secologanin and secoxyloganin could be the precursors for oleuropein (Damtoft et al., 1995). A plausible biosynthetic route from deoxyloganic acid, 7-epiloganic acid, 7-ketologanic acid, 8-epikingisidic acid, oleoside 11-methyl ester, 7-β-1-d-glucopyranosyl 11-methyl oleoside and ligustroside to oleuropein was proposed by Damtoft et al. for Oleaceae (Fig. 5) (Damtoft et al., 1992).
Figure 5.
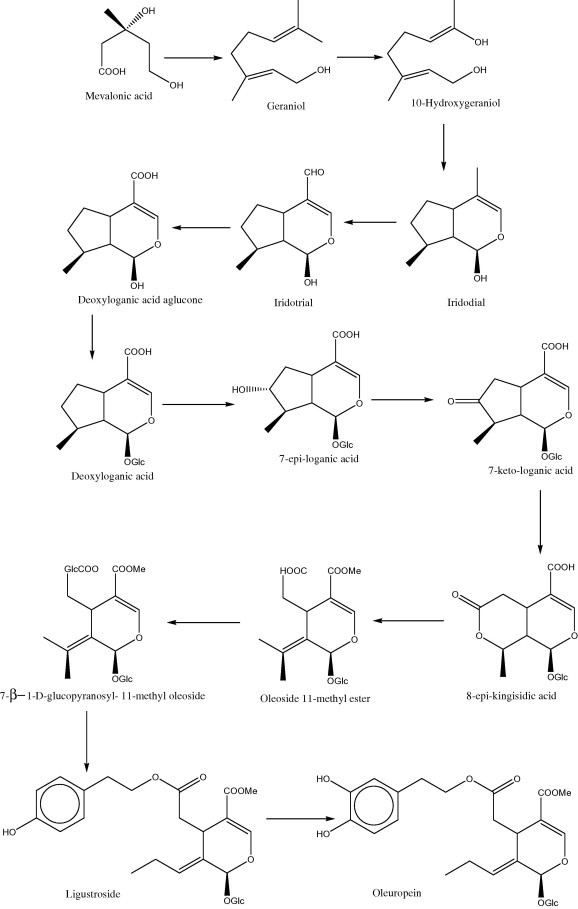
Proposed biosynthetic pathway for oleuropein in Oleaceae.
3.3. Fate of oleuropein
In the development of the olive fruit, three phases are usually distinguished: a growth phase during which accumulation of oleuropein occurs; a green maturation phase coinciding with a reduction in the levels of chlorophyll and oleuropein; and a black maturation phase characterised by the appearance of anthocyanins and during which the oleuropein level continues to fall (Amiot et al., 1989). Therefore oleuropein is very abundant in the early stages: in young fruits it could reach 14% of dry matter. Albeit lower, its level is still very important at harvest for green picked cultivars (Amiot et al., 1986). In the black cultivars its level declines rapidly during maturation (Limiroli et al., 1995); in some varieties (Oeuropaea var leccino) it can even fall to zero when they are completely black (Bianco et al., 1993). Elenolic acid glucoside and demethyloleuropein, glucosylated derivatives of oleuropein, appear at the beginning of green maturation as the oleuropein levels decline. Then they accumulate, reaching their maximum during black maturation, until demethyloleuropein becomes the major constituent of black olives (Bianco et al., 1993). It is possible that these two compounds are formed from oleuropein by the action of esterases, because esterase activity increased considerably during the first phase of maturation and reached a maximum during black maturation (Amiot et al., 1989). The fruit O. europaea appears to accumulate only glucosylated derivatives of oleuropein. In contrast, dihydroxytyrosol and non-glucosylated secoiridoids derived from oleuropein were found in the leaf (Amiot et al., 1989, 1990).
The decline in oleuropein also coincided with the decline in other quantitatively less important oleosides such as ligustroside and the increase in other phenolic compounds such as certain flavonoids and verbascoside (Amiot et al., 1989). In young small olives, verbascoside is present only in traces, while ligustroside and cornoside are relatively abundant. When green olives reach normal size, the ligustroside disappears and the cornoside follows the same trend as the other compounds, being easily transformed into halleridone (Bianco et al., 1993).
Considerable differences in the content of tyrosol, hydroxytyrosol and tyrosol glucoside were also found to occur in the fruits during growth and ripening of the drupe (Angerosa et al., 1995; Limiroli et al., 1996), the increase in their levels consistently correlating with the hydrolysis of the components with higher molecular weight (Climato et al., 1990). The elenolic acid glucoside and hydroxytyrosol contents could be considered to be indicators for the maturation of olives (Esti et al., 1998). Because of its interaction with a diphenol oxidase (PPO; EC 1.10.3.2), oleuropein is also involved in the browning that occurs in green table olives either after impact and wounding during harvesting or during subsequent technological treatments. Initially, this PPO is associated with the chloroplast membranes, becoming increasingly soluble during maturation.
Therefore the degree of browning varies considerably depending on the physiological stage of the fruit. The browning was found to be correlated to the oleuropein content and not with PPO activity, indicating that endogenous substrates are the main limiting factor (Goupy et al., 1991).
4. Bioavailability of oleuropein
Phenolic compounds from virgin olive oil have been demonstrated to be highly bioavailable. Vissers et al. found that absorption of administered ligistroside-aglycone, hydroxytyrosol, tyrosol and oleuropein-aglycone was 55–60% in human subjects (Vissers et al., 2002). They also suggested that an important step in the metabolism of olive oil phenolics oleuropein-glycoside ad oleuropein and ligistroside-aglycones is their formation into hydroxytyrosol or tyrosol (Vissers et al., 2002). It was supported by their finding that 15% of an oleuropein-glycoside supplement administered to healthy human subjects was excreted in urine as hydroxytyrosol and tyrosol (Vissers et al., 2002). Another two studies showed that oleuropein is rapidly absorbed after oral administration, with maximum plasma concentration occurring 2 h after administration. Hydroxytyrosol was its most important metabolite. Both compounds are rapidly distributed and excreted in urine mainly as glucoronides or in very low concentrations as free forms (Tan et al., 2003; Boccio et al., 2003). Furthermore, the mechanism of absorption of olive oil phenolics remains unclear.
5. Cardioprotective effect of oleuropein
Epidemiological studies have shown that regular consumption of phenol-rich foods is inversely associated with cardiovascular disease (Stoclet et al., 2004). Oleuropein has a beneficial effect on several aspects of cardiovascular disease via its vasodilatory, anti-platelet aggregation, anti-inflammatory and antioxidant properties. A graphic summary of the mechanisms of olive oil constituents is shown in Fig. 6.
Figure 6.
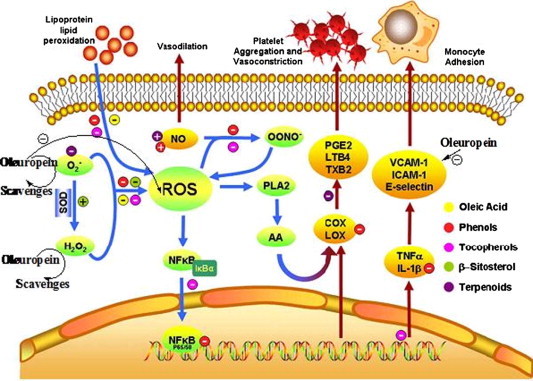
Proposed model for the action mechanisms of oleic acid and minor compounds from olive oil based on the literature gathered for the present review. Despite the number of studies contributing to this model, several gaps that should be filled with further investigation are still present. The main mechanism by which the components of olive oil influence endothelial activation involves inhibition and/or scavenging of ROS. Oleic acid, oleuropein and β-sitosterol may reduce intracellular ROS by creating a less-oxidant environment through inhibition of intracellular ROS production. β-Sitosterol may also enhance SOD activity, hence decreasing levels. This reduction has also been observed for the terpenoid oleanolic acid, although the mechanism is not presently known. Tocopherols and phenolic compounds are potent antioxidants that may help reduce lipid peroxidation and scavenge intracellular ROS and free NO•, reducing the formation of OONO−. ROS can activate the NFκB, which is then translocated into the nucleus, where it binds to recognition sequences in DNA to induce gene expression. This mobilization of NFκB is blocked by α-tocopheryl succinate but not by α-tocopherol. In contrast, phenolic compounds have been proposed to act blocking the formation of NFκB/DNA binding complexes. NFκB modulates the expression of cytokines, LOX and COX, thereby affecting the levels of adhesion molecules and eicosanoids. However, some of the minor compounds of olive oil may act directly on these enzymes and cytokines. LOX and COX activities are inhibited at different points by phenolics and triterpenoids whereas IL-1β expression is inhibited by phenolics and tocopherols, contributing to protect the endothelium against vasoconstriction, platelet aggregation and monocyte adhesion. Vasodilation is also suggested to be enhanced by oleuropein and oleanolic acid through an increase in the production of NO.
5.1. Antioxidant activity
Visioli and Galli reported that oleuropein effectively inhibited CuSO4-induced LDL oxidation and proposing a link between the Mediterranean diets correlated with a lower incidence of coronary heart disease (Visioli and Galli, 1994). In 2002 and 2006, Visioli et al. (2002, 2006) documented that the oleuropein exert potent antioxidant activities, such as inhibition of low density lipoproteins oxidation and free radical scavenging. It modifies pathophysiological processes at cellular level favorably, e.g. by inhibiting not only the production of superoxide anions and the respiratory burst of neutrophils, but also platelet aggregation and the production of thromboxane and leukotriene B4 by neutrophils. According to De la Puerta et al. (2001), oleuropein has both the ability to scavenge nitric oxide and to cause an increase in the inducible nitric oxide synthase (iNOS) expression in the cell. A scavenging effect of oleuropein was demonstrated with respect to hypochlorous acid (HOCl) (Visioli et al., 2002). HOCl is an oxidative substance produced in vivo by neutrophil myeloperoxidase at the site of inflammation and can cause damage to proteins including enzymes.
Coni and his research team (Coni et al., 2000) who found that the addition of 10% (wt:wt) extra-virgin olive oil and 7 mg/kg oleuropein to the standard diet reduces plasma levels of total cholesterol and increases the ability of LDL to resist oxidation in the rabbits. The antioxidant potential of aqueous oleuropein has been investigated in the isolated rat heart by Manna et al. (2004). Male Sprague–Dawley (250–300 g) rats were heparinised and anesthetized with thiopental by intraperitoneal injection. The hearts were excised and perfused in the retrograde Langendorff mode under constant flow. The organs were subjected to 30 min of no-flow global ischemia and then reperfused. The protective effect of aqueous oleuropein (20 μg/g) against the postischemic oxidative burst was investigated by measuring the release, in the coronary effluent, of the oxidized glutathione, a sensitive marker of heart’s exposure to oxidative stress. Reflow in ischemic hearts was accompanied by a prompt release of oxidized glutathione; in ischemic hearts pretreated with oleuropein, this release was significantly reduced (Manna et al., 2004). De la Puerta et al. (1999) determined the anti-eicosanoid and antioxidant effects in leukocytes of the principal phenolic compounds (oleuropein, tyrosol, hydroxytyrosol and caffeic acid) from the polar fraction of non-refined olive oil.
Visioli et al. (2000) demonstrated that the administration of catecholic phenolic compounds from olive oil, oleuropein, decrease, in a dose-dependent manner, the urinary excretion of 8-iso-PGF2α, indicating lower in vivo lipid peroxidation processes in supplemented volunteers. In 2006, Andreadou et al. (2006) treatment with oleuropein for 6 wk reduced total cholesterol and triglyceride concentrations along with reduction of the infarct size, conferred strong antioxidant protection and reduced the circulating lipids. This is the first experimental study in vivo that suggests the possibility of using an olive constituent in the treatment of ischemia.
5.2. Anti-inflammatory effect
Visioli et al. (1998) showed that oleuropein increase nitric oxide (NO) production in macrophages challenged with lipopolysaccharide, through an induction of the inducible form of the enzyme nitric oxide synthase, thus increasing the functional activity of these immunocompetent cells. It is well known that oleuropein elicits anti-inflammatory effects by inhibiting the lipoxygenase activity and the production of leucotrien B4 (De la Puerta et al., 1999).
5.3. Anti-atherogenic effect
In terms of protection from atherosclerosis, the formation of chloramines via the myeloperoxidase-catalyzed formation of hypochloric acid and the subsequent chlorination of apolipoprotein (apo)B-100 has been identified as one of the initiating agents in LDL modification, which leads to uncontrolled uptake by macrophages. Visioli and Galli (2001) reported that oleuropein showed anti-atherogenic activity through the assessment of various markers, such as a reduced formation of short-chain aldehydes (evaluated as thiobarbituric acid-reacting substances [TBARS]) and of lipid peroxides, by a higher vitamin E content in the residual LDL (indicating sparing of endogenous antioxidants), and by a reduced formation of malondialdehydelysine and 4-hydroxynonenal-lysine adducts, indicating protection of the apoprotein layer. Carluccio et al. (2003) reported that oleuropein reduces monocytoid cell adhesion to stimulated endothelium, as well as vascular cell adhesion molecule-1 (VCAM-1) mRNA and protein, which is an essential early steps in atherogenesis. Manna et al. (2004) showed the potential protective effects of oleuropein, in the isolated rat heart pretreatment with 20 μg/g oleuropein against the post-ischemic oxidative burst in the coronary effluent and the release of oxidized glutathione, a sensitive marker of heart’s exposure to oxidative stress and a key factor in the pathogenesis of atherosclerosis. The extent of lipid peroxidation was evaluated by measuring thiobarbituric acid-reactive substance concentration in the muscle (Manna et al., 2004). The anti-atherosclerotic effect of aqueous olive leaf extract was also demonstrated in rabbits on a high-lipid diet. Twenty-four rabbits were assigned to control, high-lipid diet, or high-lipid diet supplemented with (OLE) aqueous olive leaves extract (contains oleuropein) for 6 weeks. Animals in the high-lipid diet group had higher levels of cholesterol, triglycerides and LDL cholesterol, as well as a thick layer of lipid disposition in the aortic intima compared to those in the OLE group. These results support olive leaf’s anti-atherosclerotic effect, most likely related to suppression of inflammation (Wang et al., 2008).
5.4. Anti-hypertensive effect
Animal studies demonstrated that the aqueous and ethanolic olive leaves extract (OLE) given to hypertensive rats at dosages ranging from 100 to 1000 mg/kg for 2–6 weeks significantly lowered mean arterial pressure and heart rate (Osim et al., 1999; Khayyal et al., 2002). Another animal study showed methanolic OLE (consists oleuropein) given to salt-sensitive, genetically hypertensive rats at 60 mg/kg body weight for 6 weeks prevented the development of severe hypertension and atherosclerosis and improved insulin resistance (Somova et al., 2003).
In a human clinical trial ethanolic OLE reduced blood pressure in 40 borderline hypertensive pairs of monozygotic twins. Twins from each pair were assigned to control or two treatment groups receiving either 500 or 1000 mg OLE daily for 8 weeks. Body weight, heart rate, blood pressure, glucose and lipids were measured at 2-week intervals. Blood pressure values decreased within pairs, with an average difference in systolic pressure up to 6 mmHg between the 500-mg OLE group and control group and up to 13 mmHg difference between 500- and 1000-mg groups after 6 weeks; maximum differences in diastolic blood pressure in the same two groups were 5 mmHg in each. At the end of the study, mean blood pressure remained unchanged for those in the control and 500-mg groups, while those in the 1000-mg group reported a significant decrease in mean systolic blood pressure (137 ± 10 to 126 ± 6; p < 0.01). All subjects reported decreases in cholesterol, with no significant changes in other parameters (Perrinjaquet-Moccetti et al., 2008). Another clinical trial (n = 30) reported significant decreases in blood pressure in hypertensive patients given 400 mg aqueous OLE four times daily for 3 months. Full text of the study was unavailable and published in French and actual percentage decrease was not reported in the abstract (Cherif et al., 1996).
5.5. Anti-platelet aggregation
Petroni et al. (1995) reported that components of the phenolic fraction of olive oil in vitro inhibit platelet function and eicosanoid formation (thromboxane B2 and 12-hydroxyeicosatetraenoic acid (12-HETE). Olive leaf polyphenols have been shown to inhibit in vitro platelet function in blood obtained from 11 healthy, non-smoking males. Olive leaf polyphenols (at increasing concentrations of oleuropein) effected a significant dose-dependent suppression of platelet-ATP release and platelet aggregation (Singh et al., 2008). Recently Dell’Agli et al. (2008) reported that olive oil phenols (oleuropein-aglycone) inhibit platelet aggregation via cAMP-PDE inhibition mechanism in the venous blood sample of the healthy volunteers; one of the targets of the biological effect.
5.6. Oleuropein protectant activity against doxorubicin induced cardiotoxicity
Adriamycin (doxorubicin (DXR)) is a commonly used antineoplastic agent, but its use is mainly limited by the occurrence of dose-dependent cardiotoxicity Cole et al., 2006. It also induces hepatotoxic (Kwiecien et al., 2006) and neurotoxic (Joshi et al., 2007) effects. Andreadou et al. (2007) demonstrated that oleuropein successfully treats DXR-induced cardiotoxicity by inhibiting lipid peroxidation products, decreasing oxidative stress and reducing nitric oxide synthase (iNOS) in cardiomyocytes. Recently, Andreadou et al. (2009) showed that oleuropein completely restored the changes of metabolites to the normal levels. Acetate and succinate constitute novel biomarkers related to DXR, and oleuropein treatment aids the compensation of distressed energy metabolic pathways.
6. Neuroprotective effect
According to the free radical theory, ageing is the result of the oxidative injury, mainly to mitochondria, throughout the lifetime. Some of the oxidative damages can not entirely be counteracted and lead to cellular dysfunction. Mitochondrial membranes are very sensitive to free radical attack because of the presence of double bond carbon-carbon in the lipid tails of its phospholipids, which leads to the production of cognitive and neurodegenerative disease. In vitro (Moosmann and Behl, 1999) and epidemiological (German and Walzem, 2000) studies pointed out the positive impact of natural extracted polyphenols on the incidence of age-related disorders, such as dementia. In a study (Panza et al., 2004) reported that oleuropein, was evaluated towards the decrease or even prevention of Aβ aggregation, which is inherent to Alzheimer’s disease (AD). The potential effect of oleuropein on the brain function has been reported that AD is analogous to atherosclerosis, because they both are age-dependent diseases in which abnormal accumulation of a normal metabolite (cholesterol and Aβ, respectively) precedes clinical symptoms and leads to disease (Golde and Eckman, 2001; Hofman et al., 1997). The link between heart disease, hypercholesterolemia and AD (Refolo et al., 2000) involve similar mechanisms in the pathogenesis of these disorders. The circumstantial evidence that cholesterol-related interventions can alter Aβ deposition (Refolo et al., 2001; Jick et al., 2000), suggest that oleuropein could be promising in the management of AD. Furthermore, the importance of inflammatory processes in the clinical manifestation of AD (Markesbery and Carney, 1999; Heininger, 1999), combined with the epidemiological evidence of a protective effect of anti-inflammatory agents (Stewart et al., 1997) against AD, suggest that a polyphenolic natural extract, such as oleuropein, could prove effective against age dependent. The diagrammatic representation of the neuroprotective role of oleuropein is shown in Fig. 7.
Figure 7.
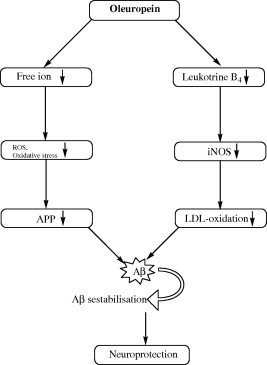
Diagramic representation of the neuroprotective role of oleuropein.
7. Conclusion
The traditional Mediterranean diet contains, unlike the Northern European and American diet, a considerable proportion of vegetables, cereals, fruit, fish, milk, wine and olive oil. The beneficial effects of olive oil are attributed to a favorable fatty acid profile and to the presence of some minor components that are also responsible for its unique flavour and taste. The major constituent of the leaves, virgin olive oil and unprocessed olive drupes of O. europaea is oleuropein. There are a number of researches documented on the cardioprotective role of oleuropein and their possible therapeutic tools for pharmacological treatment of CHD. But still very few studies reported that the relation of oleuropein and neuroprotection viz. anti-Parkinsonism’s action and dementia and schizophrenia. There is further need of research to investigate the neuroprotective role of oleuropein and its relation with cardiovascular disease.
References
- Amiot M.J., Fleuriet A., Macheix J.J. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 1986;34:823–826. [Google Scholar]
- Amiot M.J., Fleuriet A., Macheix J.J. Accumulation of oleuropein derivatives during olive maturation. Phytochemistry. 1989;28:67–69. [Google Scholar]
- Amiot M.J., Fleuriet A., Macheix J.J. Accumulation of oleuropein derivatives during olive maturation. Phytochemistry. 1989;28:67–70. [Google Scholar]
- Amiot M.J., Tacchini M., Fleuriet A., Macheix J.J. The technological debittering process of olives: characterization of fruits before and during alkaline treatment. Sci. Alim. 1990;10:619–632. [Google Scholar]
- Andreadou I., Iliodromitis E.K., Mikros E., Constantinou M., Agalias A., Magiatis P., Skaltsounis A.L., Kamber E., Tsantili-Kakoulidou A., Kremastinos D.T. The olive constituent oleuropein exhibits anti-ischemic, antioxidative and hypolipidemic effects in anesthetized rabbits. J. Nutr. 2006;136:2213–2219. doi: 10.1093/jn/136.8.2213. [DOI] [PubMed] [Google Scholar]
- Andreadou I., Sigala F., Iliodromitis E.K., Papaefthimiou M., Sigalas C., Aligiannis N., Savvari P., Gorgoulis V., Papalabros E., Kremastinos D.T. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J. Mol. Cell. Cardiol. 2007;42:549–558. doi: 10.1016/j.yjmcc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Andreadou I., Papaefthimiou M., Zira A., Constantinou M., Sigala F., Skaltsounis A.L., Tsantili-Kakoulidou A., Iliodromitis E.K., Kremastinos D.T., Mikros E., Tsantili-Kakoulidou Anna. Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR Biomed. 2009;22:585–592. doi: 10.1002/nbm.1370. [DOI] [PubMed] [Google Scholar]
- Angerosa F., D’Alessandro N., Konstantinou P., Di Giacinto L. GC–MS evaluation of phenolic compounds in virgin olive oil. J. Agric. Food Chem. 1995;43:1802–1807. [Google Scholar]
- Baracco A., Bertin G., Gnocco E., Legorati M., Sedocco S., Catinella S., Favretto D., Traldi P. A comparison of the combination of fast-atom bombardment with tandem mass spectrometry and of gas chromatography with mass spectrometry in the analysis of a mixture of kaempferol, kaempferide, luteolin and oleuropein. Rapid Commun. Mass Spectrom. 1995;9:427–436. [Google Scholar]
- Bastoni L., Bianco A., Piccioni F., Uccella N. Biophenolic profile in olives by nuclear magnetic resonance. Food Chemistry. 2001;73:145–151. [Google Scholar]
- Bazoti F.N., Bergquist J., Markides K., Tsarbopoulos A. Noncovalent interaction between amyloid-β-peptide (1–40) and oleuropein studied by electrospray ionization mass spectrometry. J. Am. Soc. Mass. Spectrom. 2006;17:568–575. doi: 10.1016/j.jasms.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Bianco A., Uccella N. Biophenolic components of olives. Food Res. Int. 2000;33:475–485. [Google Scholar]
- Bianco A., Naccarato G., Passacantilli P., Righi G., Scarpati M.L. Partial synthesis of oleuropein. J. Nat. Prod. 1992;55:760–766. [Google Scholar]
- Bianco A., Lo Scalzo R., Scarpati M.L. Isolation of cornoside from Olea europaea and its transformation into halleridone. Phytochemistry. 1993;32:455–457. [Google Scholar]
- Bisignano G., Tomaino A., Lo Cascio R., Crisafi G., Uccella N., Saija A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999;51:971–974. doi: 10.1211/0022357991773258. [DOI] [PubMed] [Google Scholar]
- Boccio P., DiDeo A., De Curtis A., Celli N., Iacoviello L., Rotilio D. Liquid chromatography–tandem mass spectrometry analysis of oleuropein and its metabolite hydroxytyrosol in rat plasma and urine after oral administration. J. Chromatogr. B. 2003;785:47–56. doi: 10.1016/s1570-0232(02)00853-x. [DOI] [PubMed] [Google Scholar]
- Brenes M., Garcia P., Duran M.C., Garrido A. Concentration of phenolic compounds change in storage brines of ripe olives. J. Food Sci. 1993;58:347–350. [Google Scholar]
- Capasso R., Evidente A., Scognamiglio F. A simple thin layer chromatographic method to detect the main polyphenols occurring in olive oil vegetation waters. Phytochem. Anal. 1992;3:270–275. [Google Scholar]
- Carluccio M.A., Siculella L., Ancora M.A., Massaro M., Scoditti E., Storelli C., Visioli F., Distante A., De Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003;23(4):622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- Cherif S., Rahal N., Haouala M., Hizaoui B., Dargouth F., Gueddiche M., Kallel Z., Balansard G., Boukef K. A clinical trial of a titrated Olea extract in the treatment of essential arterial hypertension. J. Pharm. Belg. 1996;51:69–71. (Article in French) [PubMed] [Google Scholar]
- Climato A., Mattei A., Osti M. Variation of polyphenol composition with harvesting period. Acta Hort. 1990;286:453–456. [Google Scholar]
- Cole M.P., Chaiswing L., Oberley T.D., Edelmann S.E., Piascik M.T., Lin S.M., Kiningham K.K., St Clair D.K. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc. Res. 2006;69:186–197. doi: 10.1016/j.cardiores.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Coni E., Benedetto R., Pasquale M., Masella R., Modesti D., Mattei R., Carlini E.A. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids. 2000;35:45–54. doi: 10.1007/s11745-000-0493-2. [DOI] [PubMed] [Google Scholar]
- Damtoft S., Franzyk H., Jensen S.R. Excelsioside, a secoiridoid glucoside from Fraxinus excelsior. Phytochemistry. 1992;31:4197–4201. [Google Scholar]
- Damtoft S., Franzyk H., Jensen S.R. Biosynthesis of secoiridoid glucosides in Oleaceae. Phytochemistry. 1993;34:1291–1299. [Google Scholar]
- Damtoft S., Franzyk H., Jensen S.R. Biosynthesis of iridoids in Syringa and Fraxinus: carbocyclic iridoid precursors. Phytochemistry. 1995;40:785–792. [Google Scholar]
- De la Puerta R., Guttierrez V.R., Hoult J.R.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999;57:445–449. doi: 10.1016/s0006-2952(98)00320-7. [DOI] [PubMed] [Google Scholar]
- De la Puerta R., Dominguez M.E.M., Ruiz-Guttierrez V., Flavill J.A., Hoult J.R.S. Effects of olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001;69:1213–1222. doi: 10.1016/s0024-3205(01)01218-8. [DOI] [PubMed] [Google Scholar]
- De Laurentis N., Crescenzo G., Lai O.R., Milillo M.A. Investigation on the extraction and concentration of oleuropein and flavonoids in Olea europaea L. based products. Pharm. Pharmacol. Lett. 1997;7:27–30. [Google Scholar]
- Dell’Agli M., Maschi O., Galli G.V., Fagnani R., Dal Cero E., Caruso D., Bosisio E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008;99:945–951. doi: 10.1017/S0007114507837470. [DOI] [PubMed] [Google Scholar]
- Donaire J.P., Sanchez A.J., Lopez-Gorge J., Recalde L. Metabolic changes in fruit and leaf during ripening in the olive. Phytochemistry. 1975;14:1167–1169. [Google Scholar]
- Esti M., Cinquanta L., Notte E.I., La Notte E. Phenolic compounds in different olive varieties. J. Agric. Food Chem. 1998;46:32–35. doi: 10.1021/jf970391+. [DOI] [PubMed] [Google Scholar]
- Ficarra P., Ficarra R., de Pasquale A., Monforte M.T., Calabro M.L. HPLC analysis of oleuropein and some flavonoids in leaf and bud of Olea europaea L. Farmaco. 1991;46:803–815. [PubMed] [Google Scholar]
- Gerber M. Olive oil and cancer. In: Hill M.J., Giacosa A., Caygill C.P.G., editors. Epidemiology of Diet and Cancer. Ellis Horwood; Chichester: 1994. pp. 263–275. [Google Scholar]
- German J.B., Walzem R.L. The health benefits of wine. Annu. Rev. Nutr. 2000;20:561–593. doi: 10.1146/annurev.nutr.20.1.561. [DOI] [PubMed] [Google Scholar]
- Golde T.E., Eckman C.B. Cholesterol modulation as an emerging strategy for the treatment of Alzheimer’s disease. Drug Discov. Today. 2001;6:1049–1055. doi: 10.1016/s1359-6446(01)01965-1. [DOI] [PubMed] [Google Scholar]
- Goupy P., Fleuriet A., Amiot M.J., Macheix J.J. Enzymatic browning, oleuropein content, and diphenol oxidase activity in olive cultivars (Olea europaea L.) J. Agric. Food Chem. 1991;39:92–95. [Google Scholar]
- Hamdi H.K., Castellon R. Oleuropein, a non-toxic olive iridoid, is an antitumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005;334:769–778. doi: 10.1016/j.bbrc.2005.06.161. [DOI] [PubMed] [Google Scholar]
- Heininger K. A unifying hypothesis of Alzheimer’s disease. II. Pathophysiological processes. Hum. Psychopharmacol. Clin. Exp. 1999;14:525–581. [Google Scholar]
- Hofman A., Ott A., Breteler M.M.B., Bots M.L., Slooter A.J., Harskamp V.F., van Duijn C.N., Van Broeckhoven C., Grobbee D.E. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Inouye H., Yoshida T., Tobita S., Tanaka K., Nishioka T. Absolute struktur des oleuropeins und einiger verwandter glucoside. Tetrahedron Lett. 1970;11:2459–2464. [Google Scholar]
- Jick H., Zornberg G.L., Jick S.S., Seshadri S., Drachman D.A. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Joshi G., Hardas S., Sultana R., St Clair D.K., Vore M., Butterfield D.A. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: implication for chemobrain. J. Neurosci. Res. 2007;15:497–503. doi: 10.1002/jnr.21158. [DOI] [PubMed] [Google Scholar]
- Keys A. Mediterranean diet and public health: personal reflections. Am. J. Clin. Nutr. 1995;61(6 Suppl.):1321S–1323S. doi: 10.1093/ajcn/61.6.1321S. [DOI] [PubMed] [Google Scholar]
- Keys A., Menotti A., Karvonen M., Aravanis C., Blackburn H., Buzina R., Djordjevic B.S., Dontas A.S., Fidanza F., Keys M.H. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- Khayyal M.T., el-Ghazaly M.A., Abdallah D.M., Nassar N.N., Okpanyi S.N., Kreuter M.H. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneitmittelforschung. 2002;52:797–802. doi: 10.1055/s-0031-1299970. [DOI] [PubMed] [Google Scholar]
- Kwiecien I., Michalska M., Wlodek L. The selective effect of cystathionine on doxorubicin hepatotoxicity in tumor-bearing mice. Eur. J. Pharmacol. 2006;21:39–46. doi: 10.1016/j.ejphar.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Le Tutour B., Guedon D. Antioxidant activities of Olea europaea leaves and related phenolic compounds. Phytochemistry. 1992;31:1173–1178. [Google Scholar]
- Limiroli R., Consonni R., Ottolina G., Marsilio V., Bianchi G., Zetta L. 1H and 13C NMR characterization of new oleuropein algycones. J. Chem. Soc. Perkin Trans. 1. 1995:1519–1523. [Google Scholar]
- Limiroli R., Consonni R., Ranalli A., Bianchi G., Zetta L. 1H NMR study of phenolics in the vegetation water of three cultivars of Olea europaea: similarities and differences. J. Agric. Food Chem. 1996;44:2040–2048. [Google Scholar]
- Manna C., Migliardi V., Golino P., Scognmiglio A., Galletti P., Chiariello M., Zappia V. Oleuropein prevents oxidative myocardial injury by ischemia and reperfusion. J. Nutr. Biochem. 2004;15:461–468. doi: 10.1016/j.jnutbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Markesbery W.R., Carney J.M. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann B., Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osim E.E., Mbajiorgu E.F., Mukarati G., Vaz R.F., Makufa B., Munjeri O., Musabayane C.T. Hypotensive effect of crude extract Olea africana (Oleaceae) in normo and hypertensive rats. Cent. Afr. J. Med. 1999;45:269–274. doi: 10.4314/cajm.v45i10.8498. [DOI] [PubMed] [Google Scholar]
- Panizzi L., Scarpati M.L., Oriente E.G. Structure of oleuropein bitter glycoside with hypotensive action of olive oil. Note II. Gazzetta Chimica Italiana. 1960;90:1449–1485. [Google Scholar]
- Panizzi L., Scarpati M.L., Oriente E.G. Ricerca Sci. 1958;28:994. [Google Scholar]
- Panza F., Solfrizzi V., Colacicco A.M., D’Introno A., Capurso C., Torres F., Del Parigi A., Capurso S., Capurso A. Mediterranean diet and cognitive decline. Public Health Nutr. 2004;7:959–963. doi: 10.1079/phn2004561. [DOI] [PubMed] [Google Scholar]
- Perrinjaquet-Moccetti T., Busjahn A., Schmidlin C., Schmidt A., Bradl B., Aydogan C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother. Res. 2008;22:1239–1242. doi: 10.1002/ptr.2455. [DOI] [PubMed] [Google Scholar]
- Petroni A., Blasevich M., Salami M., Papini N., Montedoro G.F., Galli C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res. 1995;78:151–160. doi: 10.1016/0049-3848(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Refolo L.M., Malester B., LaFrancois J., Bryant-Thomas T., Wang R., Tint G.S., Sambamurti K., Duff K., Pappolla M.A. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Refolo L.M., Pappolla M.A., Francois L.J., Malester B., Schmidt S.D., Thomas-Bryant T., Tint G.S., Wang R., Mercken M., Petanceska S.S., Duff K.E. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- Ryan D., Robards K., Prenzler P., Jardine D., Herlt T., Antolovich M. Liquid chromatography with electrospray ionisation mass spectrometric detection of phenolic compounds from Olea europaea. J. Chromatogr. A. 1999;855:529–537. doi: 10.1016/s0021-9673(99)00719-0. [DOI] [PubMed] [Google Scholar]
- Servili M., Baldioli M., Selvaggini R., Macchioni A., Montedoro G. Phenolic compounds of olive fruit: one and two-dimensional nuclear magnetic resonance characterization of Nuzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999;47:12–18. doi: 10.1021/jf9806210. [DOI] [PubMed] [Google Scholar]
- Singh I., Mok M., Christensen A.M., Turner A.H., Hawley J.A. The effect of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008;18:127–132. doi: 10.1016/j.numecd.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Soler-Rivas C., Espin J.C., Wichers H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000;80:1013–1023. [Google Scholar]
- Solfrizzi V., Panza F., Torres F., Mastroianni F., Del Parigi A., Venezia A., Capurso A. High monounsaturated fatty acids intake protects against age-related cognitive decline. Neurology. 1999;52:1563–1569. doi: 10.1212/wnl.52.8.1563. [DOI] [PubMed] [Google Scholar]
- Somova L.I., Shode F.O., Ramnanan P., Nadar A. Antihypertensive, antiatherosclerotic, and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003;84:299–305. doi: 10.1016/s0378-8741(02)00332-x. [DOI] [PubMed] [Google Scholar]
- Stewart W.F., Kawas C., Corrada M., Metter E.J. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- Stoclet J.C., Chataigneau T., Ndiaye M., Oak M.H., Bedoui J.E., Chataigneau M., Schini-Kerth V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Tan H.W., Tuck K.L., Stupans I., Hayball P.J. Simultaneous determination of oleuropein and hydroxytyrosol in rat plasma using liquid chromatography with fluorescence detection. J. Chromatogr. B. 2003;785:187–191. doi: 10.1016/s1570-0232(02)00855-3. [DOI] [PubMed] [Google Scholar]
- Trichopoulou A., Costacou T., Bamia C., Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- Visioli F., Galli C. Oleuropein protects low density lipoprotein from oxidation. Life Sci. 1994;55:1965–1971. doi: 10.1016/0024-3205(94)00529-x. [DOI] [PubMed] [Google Scholar]
- Visioli F., Galli C. Antiatherogenic components of olive oil. Curr. Atheroscler. Rep. 2001;3:64–67. doi: 10.1007/s11883-001-0012-0. [DOI] [PubMed] [Google Scholar]
- Visioli F., Bellosta S., Galli C. Oleuropein, the bitter principles of olives, enhances nitric oxide production by mouse macrophages. Life Sci. 1998;62:541–546. doi: 10.1016/s0024-3205(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Visioli F., Caruso D., Galli C., Viappiani S., Galli G., Sala A. Olive oil rich in natural catecholic phenols decrease isoprostane excretion in humans. Biochem. Biophys. Res. Commun. 2000;278:797–799. doi: 10.1006/bbrc.2000.3879. [DOI] [PubMed] [Google Scholar]
- Visioli F., Galli C., Galli G., Caruso D. Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002;104:677–684. [Google Scholar]
- Visioli F., Bogani P., Galli C. Healthful properties of olive oil minor components. In: Boskou D., editor. Olive oil, Chemistry and Technology. AOCS Press; Champaign, IL: 2006. pp. 173–190. [Google Scholar]
- Vissers M.N., Zock P.L., Roodenburg A.J.C., Leenen R., Katan M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002;132:409–417. doi: 10.1093/jn/132.3.409. [DOI] [PubMed] [Google Scholar]
- Wang L., Geng C., Jiang L., Gong D., Liu D., Yoshimura H., Zhong L. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur. J. Nutr. 2008;47:235–243. doi: 10.1007/s00394-008-0717-8. [DOI] [PubMed] [Google Scholar]


