Abstract
Introduction
While influenza A(H1N1)pdm09 usually causes mild illness in the majority of people, there have been reports of severe cases and deaths. As there is no documented evidence on fatal outcomes from influenza in Mongolia previously, we aimed to describe the epidemiology of fatal influenza A(H1N1)pdm09 cases to provide recommendations to assist the national influenza prevention and control strategy.
Methods
We selected influenza A(H1N1)pdm09-confirmed deaths in hospitals between 12 October 2009 and 31 January 2010 in Mongolia from the national influenza surveillance system. The mortality rate and case fatality rate (CFR) of influenza A(H1N1)pdm09-hospitalized deaths were calculated. Using country prevalence of pregnancy and chronic diseases, we calculated the relative risk of death from influenza A(H1N1)pdm09.
Results
There were 29 deaths with a mortality rate of 1.0 per 100 000 population during the study period, which was highest in children under five and the middle-aged population. Crude CFR was 2.2%. Of all fatal cases, 62% had at least one underlying condition. Most (62%) were provided antivirals, although none received these within 48 hours of symptom onset. Prevalence for pregnancy, cardiovascular and chronic liver diseases was five to 50 times higher in fatal cases compared to country prevalence.
Discussion
Mortality and crude CFR in our study was higher than in other studies. However, due to the diagnostic policy change during the epidemic, this estimate is likely to have overestimated actual case fatalities. Pregnancy, cardiovascular and chronic liver diseases were suggestive risk factors for death from influenza A(H1N1)pdm09. Strengthening hospital-based influenza surveillance is important in predicting severity of an epidemic and responding to influenza epidemics in a timely and appropriate manner.
Introduction
Influenza A(H1N1)pdm09 emerged in Mexico and the United States of America in April 2009 and spread globally, affecting many countries of the world in 2009 to 2010. Although, the majority of people with influenza A(H1N1)pdm09 experienced mild illness,1,2 there were severe cases and even deaths. The efforts devoted to understanding the severity and impact of this novel influenza virus have demonstrated a generally low case fatality rate (CFR).3–5 Pregnant women and people with underlying medical conditions are known to be at increased risk of severe and sometimes fatal illness.1
After the first case of influenza A(H1N1)pdm09 was identified on 12 October 2009 in Mongolia the epidemic peaked in November 2009, then cases gradually decreased below surveillance threshold starting the third week of 2010.6–8 There is no previously documented evidence on fatal outcomes from influenza in Mongolia. Analysing influenza fatal outcomes is important in understanding the severity and impact of influenza and guiding prevention and control strategies. Thus, we aimed to describe the epidemiological and clinical characteristics of influenza A(H1N1)pdm09 fatal cases in Mongolia.
Methods
Study design
We conducted a descriptive epidemiological study of laboratory-confirmed influenza A(H1N1)pdm09 cases reported through the national influenza surveillance system who died in hospitals between 12 October 2009 and 31 January 2010 in Mongolia. We excluded deaths reported to the surveillance system that occurred outside of hospitals due to the unavailability of case data. We selected this study period because the first confirmed A(H1N1)pdm09 case was reported on 12 October 2009 and the epidemic continued until the third week of 2010.
National influenza surveillance is conducted in Mongolia throughout the year at over 150 designated sentinel influenza-like illness (ILI) surveillance sites across the country. Category-I surveillance sites include family group practices and district hospitals in the capital city and seven other population-dense and border provinces, as well as the Mother and Child Health Center and the National Center for Communicable Diseases in the capital city. ILI cases are reported daily and nasopharyngeal samples for virological analysis are collected from the cases. The number of samples collected depends on the outbreak or epidemic.
Category-II sites include family group practices and general hospitals in seven low population-dense provinces, two border point villages and two villages with over 10 000 population, as well as two tertiary hospitals and the National Cancer Center in the capital city. ILI cases are reported weekly and samples for virological testing are only collected when there is a suspected cluster of cases. Category-III surveillance sites include family group practices and province general hospitals of seven additional provinces that report ILI cases weekly.
An ILI case in the surveillance system is defined as a person with sudden onset of fever over 38 °C and cough or sore throat in the absence of other syndromic diagnoses. Data including detailed residence address, onset of illness, name of health care organization, date of presentation to health care, laboratory confirmation status and identified virus subtype are collected from each ILI case.
After the first laboratory-confirmed influenza A(H1N1)pdm09 case on 12 October 2009 in Mongolia, nasopharyngeal swabs were collected from all persons presenting to health care with an ILI. The swabs were sent to the virology laboratory of the National Center for Communicable Diseases for confirmation by real-time reverse transcription polymerase chain reaction (RT–PCR) using primers, probes and protocols supplied by the US Centers for Disease Control and Prevention.7 However, due to the rapid increase in the number of reported ILI cases within three weeks and the diagnostic capacity of the virology laboratory, the Ministry of Health changed the virologic diagnosis strategy to restrict laboratory testing to persons at risk for complications (pregnant women, young children with severe acute respiratory infection and people with chronic conditions).
Data collection and analysis
For the influenza A(H1N1)pdm09 deaths reported through the national influenza surveillance system, we retrospectively collected additional data by reviewing medical files using a pre-developed questionnaire. For each case, we collected socio-demographic data including education, employment, body weight and height, tobacco and alcohol use and clinical course of illness including signs, onset of illness, complications during the course of illness, underlying medical conditions and whether treated with antiviral medications.
The 2009 mid-term population data for age, sex and social variables including living areas, different household settings and employment were obtained from the National Statistics Office of Mongolia to calculate the population-based mortality rate of influenza A(H1N1)pdm09, defined as the number of fatal cases per 100 000 population during the study period.
As data on risk factors for non-fatal cases were not available, relative risks comparing fatal to non-fatal cases were unable to be calculated. Instead we compared the risk factors of the fatal cases to reported country prevalence data. Country prevalence data on smoking and alcohol use was obtained from the Mongolian STEPS Survey on the Prevalence of Noncommunicable Disease Risk Factors – 2009,9 and the country prevalence of pregnancy and chronic diseases were obtained from monthly morbidity and mortality reports for September 2009 through February 2010 from the Health Department of Mongolia. Body mass index (BMI) was calculated from available height and weight data as body weight in kilograms divided by the square of height in metres.
All analyses were performed using EpiInfo 3.5.2. We compared the prevalence of tobacco use, alcohol drinking and BMI between the fatal cases and population prevalence using χ2 tests. For pregnancy, cardiovascular diseases and chronic liver diseases, we calculated a prevalence risk ratio (RR) (with 95% confidence interval [CI]) by dividing the proportion of these conditions in the fatal cases to that in the general population.
As laboratory testing was restricted to high-risk persons after three weeks, the total number of cases was unknown. Therefore, the CFR was calculated by dividing hospitalized deaths into all laboratory-confirmed cases for each study month, and reported as a percentage.
Ethics clearance was not required as our study was part of an emergency response to outbreak.
Results
There were 1322 laboratory-confirmed cases including 29 confirmed fatal illnesses reported to the national influenza surveillance system between 12 October 2009 and 31 January 2010. Overall mortality rate was 1.0 per 100 000 population for this period. Crude case fatality rate (CFR) was 2.2%, ranging from 0.6% to 6.1% for the study months (Fig. 1).
Fig. 1.
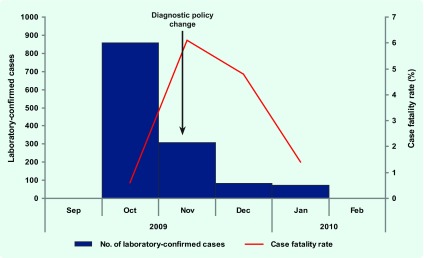
Laboratory-confirmed cases and CFR of influenza A(H1N1)pdm2009 in Mongolia, 12 October 2009– 31 January 2010
Demographic characteristics
Median age of fatal cases was 35, ranging from five months to 61 years. Population-based mortality rate was greatest in children under five (2.3 per 100 000 population), followed by persons aged 45–59 (1.7 per 100 000 population). Significant differences in mortality rates between females and males was not observed (P = 0.4) (Fig. 1).
Although the highest mortality rate was in rural residents (1.5 per 100 000 population) followed by urban residents (1.0 per 100 000) and provincial centres (0.3 per 100 000), a significant difference was not observed in mortality rates by geographical location (P = 0.06). When mortality rates per household types were compared, although traditional households had a rate of 1.4 per 100 000 compared to non-traditional households (0.7 per 100 000), a significant difference was not observed (P = 0.07). Of the 20 cases appropriate for the analysis of employment status (excluding children, soldiers, students and retired people), being unemployed had the highest and statistically significant (P ≤ □0.05) mortality rate (12.0 per 100 000) over the employed group (0.8 per 100 000) (Table 1).
Table 1. Demographic characteristics and mortality rates from fatal cases of influenza A(H1N1)pdm09, Mongolia, October 2009 to January 2010.
| Variables | Cases | Mortality rate per 100 000 | P-value | |
|---|---|---|---|---|
| n | % | |||
| Age group | ||||
| Below 5 years old | 6 | 20.7 | 2.3 | |
| 5–24 | 4 | 13.8 | 0.4 | |
| 25–44 | 12 | 41.4 | 1.4 | |
| 45–59 | 6 | 20.7 | 1.7 | |
| 60 years old and above | 1 | 3.4 | 0.6 | |
| Sex | 0.40 | |||
| Male | 12 | 41.4 | 0.9 | |
| Female | 17 | 58.6 | 1.2 | |
| Geographical location | 0.06 | |||
| Urban | 11 | 37.9 | 1.0 | |
| Provincial centre | 2 | 6.9 | 0.3 | |
| Rural | 16 | 55.2 | 1.5 | |
| Household type | 0.07 | |||
| Non-traditional | 10 | 34.5 | 0.7 | |
| Traditional* | 19 | 65.5 | 1.4 | |
| Employment | < 0.05 | |||
| Employed | 8 | 27.6 | 0.8 | |
| Unemployed | 12 | 41.4 | 12.0 | |
| Other (children, soldier, student, retired) | 9 | 31.0 | - | |
| Education | - | |||
| Primary & secondary school education | 12 | 41.4 | - | |
| College | 6 | 20.7 | - | |
| University | 5 | 17.2 | - | |
| Children | 6 | 20.7 | - | |
* Ger is the traditional household, which is a portable, felt-covered, wood lattice-framed dwelling, traditionally used by nomads in Mongolia. Most rural Mongolians and some parts of the population in the capital city still live in this traditional dwelling.
Clinical information
All cases (100%) presented with fever, as per the case definition, followed by cough (89.7%) and shortness of breath (65.5%), while the least common symptoms were sore throat (10.3%), diarrhoea (6.9%) and vomiting (6.9%). No cases manifested signs such as skin rash and sneezing.
All cases had medical complications, with pneumonia diagnosed in 27 (93.1%) and Acute Respiratory Distress Syndrome (ARDS) in 15 (57.1%) cases (Table 2).
Table 2. Symptoms, complications and underlying medical conditions of fatal cases of influenza A(H1N1)pdm09, Mongolia, October 2009 to January 2010 (n = 29).
| Signs and symptoms | Cases | % |
|---|---|---|
| Fever | 29 | 100.0 |
| Cough | 26 | 89.7 |
| Chest pain | 8 | 27.6 |
| Shortness of breath | 19 | 65.5 |
| General malaise | 19 | 65.5 |
| Myalgia | 7 | 24.1 |
| Headache | 5 | 17.2 |
| Sore throat | 3 | 10.3 |
| Diarrhoea | 2 | 6.9 |
| Vomiting | 2 | 6.9 |
| Runny nose | 2 | 6.9 |
| Others (nose bleeding, confusion, chills, skin rash, sneezing) | 6 | 20.6 |
| Complications | 29 | 100.0 |
| Pneumonia | 27 | 93.1 |
| ARDS | 15 | 51.7 |
| Disseminated intravascular coagulation | 2 | 6.9 |
| Liver dysfunction | 6 | 20.7 |
| Renal insufficiency | 1 | 3.4 |
| At least one underlying medical condition | 18 | 62.1 |
| Cardiovascular disease | 7 | 24.1 |
| Pregnancy | 7 | 24.1 |
| Chronic liver disease | 5 | 17.2 |
| Blood system disorder | 3 | 10.3 |
| Other conditions* | 3 | 10.3 |
| Chronic lung disease | 2 | 6.9 |
| Allergy | 1 | 3.4 |
* Post surgery, multiorgan anomaly and low birth weight with rachitis
The median interval from symptom onset to initial presentation to health care was three days (range: 0–14 days) and cases were hospitalized for a mean of five days (range: 0–20 days) after symptom onset. Median time between onset of symptom and death was 9.5 days (2–25 days). In 18 cases (62.1%) Oseltamivir (Tamiflu) was given orally, but none of the cases received antiviral medication within the recommended 48 hours of symptom onset.
Comparison to population prevalence
Of the 21 cases for which data for analysis of tobacco and alcohol use was available, there was no significant difference for the prevalence of smoking (23.8% compared to 27.5%, P = 0.7) or alcohol drinking (28.6% compared with 38.6%, P = 0.3) between the fatal cases of influenza A(H1N1)pdm09 and the country prevalence.
Height and weight measures were available for 11 cases, of which 45.4% had an overweight BMI and 18.2% an obese BMI. This did not significantly differ from that of the Mongolian population at 27.3%9 and 12.5%,9 respectively (P = 0.2 and P = 0.6).
Of the cases, 62.1% had at least one underlying medical condition, with the most prevalent being cardiovascular diseases (CVDs) (24.1%), pregnancy (24.1%) and chronic liver diseases (17.2%) (Table 2). The prevalence risk ratio for CVDs was 5.6 (95% CI: 2.4–13.2), for pregnancy it was 50.4 (95% CI: 21.5–118) and for chronic liver diseases it was 14.3 (95% CI: 5.5–37.5) times higher than the prevalence in the population.
Discussion
The overall mortality rate from influenzaA(H1N1)pdm09 in Mongolia between 12 October 2009 and 31 January 2010 was 1.0 per 100 000 population. This is higher than the result from other countries such as 0.7 per million population in Viet Nam10 and 0.7 per million population in Japan.11
The mortality rate for influenza A(H1N1)pdm09 by age group in our study was highest in children under five followed by persons aged 45–59. Similar findings were observed in other studies. Result from a study in Japan indicated that severe complications were common in children under five and persons over 30 years of age.11 A study in Germany observed a considerable number of severe cases of pandemic influenza among children.12 The median age of patients who died in our study was 35, which is compatible to the age of fatal cases in other countries. The median age of patients who died in Viet Nam was 29 years,10 in England it was 393 and a study in South Africa documented the median age of patients who died as 33 years.13
Of all deaths, 62% had at least one underlying medical condition, consistent with the 78% and 64% reported by Viet Nam10 and England.4 We found that pregnancy, chronic cardiovascular diseases and chronic liver diseases were the most prevalent underlying medical conditions of those who died from influenza A(H1N1)pdm09. Death in people with these conditions increased by five to 50 times compared to the prevalence of these conditions in the general population. A study in the United Kingdom observed that pregnant women were over-represented among fatal cases compared with the general population and were at increased risk of death.2,3 Rapid deterioration and death among pregnant women have also been documented in other countries including the United States of America and South Africa.13,14 More than half of those who died in our study had received antiviral medications, but none received them within the recommended 48 hours after onset of symptoms. Other studies also observed delayed antiviral use in most severe and fatal cases.4,13
Our study had several limitations. Data on hospitalized cases were not complete and were often missing information on the onset of disease and treatment aspects including specific timing and dosage of medication. Due to the diagnostic policy change to restrict virological testing to people at higher risk of complication, the denominator of laboratory-confirmed cases was underrepresented. This is reflected in the higher mortality and CFR in our study compared to the generally lower CFR observed in other studies3,10,11 and in northern hemisphere countries.12 In addition, we calculated CFR crudely using confirmed deaths as the numerator and laboratory-confirmed cases as the denominator, so this is likely to overestimate the actual CFR. Lastly, the number of deaths was very small in our analysis.
In spite of these limitations, our study demonstrated the highest mortality in younger children and middle-aged adult population, which is comparable to other findings in different settings. In addition, we found that pregnancy and chronic diseases were suggestive risk factors of death from influenza A(H1N1)pdm09 in Mongolia.
To respond to influenza epidemics quickly and appropriately, hospital-based influenza surveillance should be strengthened. Timely analysis and feedback of severe and fatal cases is important in predicting the severity of the epidemics, which is one of the shortcomings of the ILI surveillance system in Mongolia. A hospital-based influenza surveillance system that will capture possible influenza-associated hospitalizations and deaths is useful for monitoring trends and characterizing severe influenza-related diseases. Additional data on high-risk groups, outcomes and effectiveness of treatment, intervention and deaths can be collected from hospitals included in surveillance during an epidemic/pandemic period. This information can provide evidence on many issues including priority groups for vaccine and antiviral treatment, hospital bed management and estimating the severity of an epidemic.
Conflicts of interest
None declared.
Funding
None.
References
- 1.Evolution of Pandemic A (H1N1)2009, April 2009 – March 2010. Geneva: World Health Organization; 2010. http://whqlibdoc.who.int/publications/2010/9789241599924_eng.pdf accessed 13 October 2010. [Google Scholar]
- 2.Nguyen-Van-Tam JS, et al. Influenza Clinical Information Network (FLU-CIN) Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009). Thorax. 2010;65:645–51. doi: 10.1136/thx.2010.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pebody RG, et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveillance : European Communicable Disease Bulletin. 2010;15:19571. [PubMed] [Google Scholar]
- 4.Donaldson LJ, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ (Clinical Research Ed.) 2009;339(dec10 1):b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCallum L, Partridge J. Epidemiological characteristics of influenza A(H1N1) 2009 pandemic in the Western Pacific Region. Western Pacific Surveillance and Response. 2010;1(1):5–11. doi: 10.5365/wpsar.2010.1.1.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nukiwa N, et al. Evaluating influenza disease burden during the 2008–2009 and 2009–2010 influenza seasons in Mongolia. Western Pacific Surveillance and Response. 2011;2(1):16–22. doi: 10.5365/WPSAR.2010.1.1.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmaa A, et al. Epidemiologic description of pandemic influenza A(H1N1) 2009 registered in Mongolia. Mongolian Journal of Infectious Diseases. 2010;5(36) [Google Scholar]
- 8.Nyamadawa P, et al. The first wave of influenza A(H1N1) 2009 pandemics in Mongolia. Influenza and Other Respiratory Viruses. 2011;5(Suppl 1):159–94. [Google Scholar]
- 9.Mongolian STEPS survey on the prevalence of noncommunicable disease risk factors-2009. Geneva: World Health Organization and Mongolia Ministry of Health; 2010. http://www.who.int/chp/steps/2009_STEPS_Report_Mongolia.pdf accessed 15 July 2010. [Google Scholar]
- 10.Tinh PT, et al. Epidemiological and clinical characteristics of patients who died from influenza A(H1N1)pdm09 in Viet Nam. Western Pacific Surveillance and Response Journal. 2012;3(1):6–11. doi: 10.5365/wpsar.2011.2.3.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamigaki T, Oshitani H. Epidemiological characteristics and low case fatality rate of pandemic (H1N1) 2009 in Japan. PLoS Currents. 2009;1:RRN1139. doi: 10.1371/currents.RRN1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altmann M, et al. Severe cases of pandemic (H1N1) 2009 in children, Germany. Emerging Infectious Diseases. 2011;17:186–92. doi: 10.3201/eid1702.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louie JK, et al. California Pandemic (H1N1) Working Group Severe 2009 H1N1 influenza in pregnant and postpartum women in California. New England Journal of Medicine. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 14.Archer BN, et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveillance: European Communicable Disease Bulletin. 2009;14:19369. doi: 10.2807/ese.14.42.19369-en. [DOI] [PubMed] [Google Scholar]


