Abstract
The aim of the present work was to study the effect of tobacco smoking on disease progression in rheumatoid arthritis patients and its relation to anti-cyclical citrullinated peptide (anti-CCP) antibodies. The study included 54 patients; 20 non-smokers, 9 ex-smokers, 14 mild to moderate smokers and 11 heavy smokers. Fifteen normal volunteers were also studied as controls. Disease stage was clinically and radiologically determined, rheumatoid factor (RF) and anti-CCP antibodies were measured in serum. Higher percentage of severe disease (stage III) was seen in heavy smoker patients than mild to moderate smokers (54.6% versus 35.7%) and in moderate smokers than ex-smokers (35.7% versus 33.6%). Lowest percentage of severe disease was seen in non-smokers (15%). RF and anti-CCP were significantly higher in smoker than non-smoker and in heavy than mild to moderate smoker patients (p < 0.01, p < 0.05 and p < 0.01, p < 0.001, respectively). In smoker patients, both RF and anti-CCP antibodies correlated significantly and positively with smoking index (r = 0.581, p < 0.001; r = 0.661, p < 0.001). Also, smoking index and anti-CCP correlated significantly and positively with disease stage (r = 0.424, p < 0.05; r = 0.523, p < 0.01). It appears from our results that, tobacco smoking mostly play a role in progression of rheumatoid arthritis through tissue protein citrullination. So all rheumatoid arthritis patients must quit completely to achieve a good control.
Keywords: Arthritis, Rheumatoid, Smoking, Anti-CCP
1. Introduction
Tobacco smoking constitutes a major public health problem all over the world because of its widespread consumption and difficult quitting (Renard et al., 2008). Many authors linked tobacco consumption to auto-immune disorders mainly through tissue protein citrullination which makes them escape normal immune tolerance (Costenbader and Karlson, 2006). Tobacco smoking was claimed by many research workers to be a risk factor for occurrence and progression of rheumatoid arthritis (RA) (Hutchison et al., 2001). We carried out this study to find out the relation (if any) between tissue protein citrullination, disease progression and tobacco smoking in RA patients.
2. Subjects and methods
Complying with ethical guidelines of scientific research committee at Benha University Hospitals, we selected 54 rheumatoid arthritis patients from those attending rheumatology, internal medicine and chest medicine outpatient clinics. Patients were diagnosed according to the 1987 revised criteria of American Rheumatism Association (Arnett et al., 1988).
Patients inclusion criteria: current smoker, ex-smoker and non-smoker rheumatoid arthritis patients.
Exclusion criteria: patients with diabetes mellitus, chronic renal or liver disease, thyroid disease, psoriasis or other extensive dermatosis and those on high doses of anti-inflammatory or immune-suppressive therapy where excluded to avoid the effect of these factors on blood levels of anti-CCP antibodies and rheumatoid factor (RF) (Eisenbarth and Homann, 2006).
All patients were males as no smoker females were met. Twenty patients were non-smokers and considered as group I, 9 ex-smoker patients (>5 years abstinence) considered as group II, 14 mild to moderate current smokers (smoking index < 15 pack/year) considered as group III and 11 heavy current smokers (smoking index > 20 pack/year) considered as group IV. Fifteen healthy, age matched male subjects were also selected as controls: 8 non-smokers and considered as group V, while the remaining 7 are heavy smokers and considered as group VI. The study was carried out between March (2008) and January (2009) and consent was taken from all patients and control subjects enrolled in the study.
Full history taking and clinical examination as well as general lab studies including CBC, ESR, liver and kidney functions and random blood sugar were done to all patients to confirm selection criteria. X-ray (as well as CT and/or MRI if required) examination of the affected joints was also done. Rheumatoid patients were classified according to their disease stage depending on clinical and radiological findings: stage I – mild, stage II – moderate, stage III – severe and stage IV – terminal (Steinbroker et al., 1949).
RF was measured in blood of all subjects using IgG ELISA kits from International Biological Laboratory (IBL, Germany) and patient serum samples (Swedler et al., 1997). The rheumatoid factor IgG ELISA is based on the principle of the enzyme immunoassay (EIA) where goat-IgG is bound on the surface of the microtiter strips. Diluted patient serum or ready to use standards and controls are pipetted into the wells of the microtiter plate. A binding between the rheumatoid factor of the serum and the immobilized IgG takes place. After a 1-h incubation at room temperature, the plate is rinsed with diluted wash buffer in order to remove unbound material. Then the anti-human IgG peroxidase conjugate is added and incubated for 30 min. After a further washing step, the tetramethyl-benzidine (TMB) substrate solution is pipetted inducing the development of a blue dye in the wells. The color development is terminated by the addition of a stop solution, which changes the color from blue to yellow. The resulting dye is measured spectrophotometrically at the wavelength of 450 nm. The concentration of IgG rheumatoid factor is directly proportional to the intensity of the color. Levels less than 20 U were considered negative, 20–39 U: equivocal or weakly positive, 40–59 U: positive and 60 U or more were considered strongly positive.
Tissue protein citrullination is reflected in blood as antibodies against cyclical citrullinated peptides (anti-CCP antibodies). These antibodies were measured using also an ELISA assay (DIASTAT® anti-CCP kits, Axis-Shield Diagnostics Ltd., Dundee, UK) (Niewold et al., 2007).
Sampling: serum or plasma (EDTA, lithium heparin, sodium citrate) samples; grossly haemolysed or turbid samples were not used. Samples kept at 2–8 °C if tested within 3 weeks and at −20 °C if kept for longer time.
Preparation for the assay: allow all kit components, including the microtitre strips, to warm up to 18–25 °C for 30–60 min before use. Mix reagents by gentle inversion. The reference control is not diluted but the following solutions were diluted as follows: wash buffer concentrate 1 vial in 375 mL distilled/deionised water, sample diluent concentrate 1 vial in 100 mL distilled/deionised water and positive and negative controls/samples: 10 μL in 1 mL diluted sample diluent.
Steps: pipette 100 μL reference control/calibrators in duplicate, pre-diluted (1:100) positive and negative controls, and pre-diluted (1:100) patient samples into appropriate wells. This step should not exceed 15 min for any one set of calibrators/controls/samples. Incubate 60 ± 10 min at 18–25 °C. Decant strip contents by quick inversion over a sink suitable for the disposal of biological materials, bearing in mind the potential infective hazard of the samples. Blot inverted strips well with paper towels. Wash wells three times with a minimum of 200 μL diluted wash buffer. Decant and blot after each wash step. Add 100 μL conjugate to each well. Incubate 30 ± 5 min at 18–25 °C. Repeat steps 4 and 5. Add 100 μL substrate to each well. Incubate 30 ± 5 min at 18–25 °C. Do not decant. Add 100 μL stop solution to each well, in the same order and rate as the substrate. Tap wells gently to mix. Strips then read spectrophotometrically within 24 h at 550 nm (540–565 nm).
3. Statistical analysis
Collected data were analyzed using the software KyPlot.2001, version 2-b (Kioshi, Japan) for windows. Data were presented as mean (M) ± standard error of mean (SE). Comparisons were done by parametric, two tailed t-test for unpaired values, while correlations were done by linear correlation and regression analysis.
4. Results
Clinical characteristics of all subjects included in the study are shown in Table 1. Forty-five percent of non-smoker patients were in disease stage I, 40% were in stage II and only 15% were in stage III. For ex-smokers, 22% were in stage I, 44.4% in stage II and 33.6% in stage III. In mild to moderate smoker patients, 21.4% were in stage I, 42.9% in stage II and 35.7% in stage III. More severe disease was seen in heavy smoker group as only 9% of them were in stage I, 36.4% in stage II and 54.6% in stage III. Chi square test for independence showed a positive relation between disease stage and degree of smoking (Chi square: 51.699, degree of freedom: 4, alpha probability: <0.01) (Table 2).
Table 1.
Clinical characteristics of patients and control subjects included.
| Group I | Group II | Group III | Group IV | Group V | Group VI | ||
|---|---|---|---|---|---|---|---|
| Description | NS patients | ES patients | MS patients | HS patients | NS controls | HS controls | |
| Number | 20 | 9 | 14 | 11 | 8 | 7 | |
| Age in years (M ± SD) | 37.1 ± 6.172 | 41.78 ± 5.262 | 40.71 ± 5.135 | 42 ± 4.857 | 38.13 ± 3.603 | 37.57 ± 6.55 | |
| Stage | I (%) | 45 | 22 | 21.4 | 9 | – | – |
| II (%) | 40 | 44.4 | 42.9 | 36.4 | |||
| III (%) | 15 | 33.6 | 35.7 | 54.6 | |||
| ESR: mm/h (M ± SD) | 43.43 ± 5.122 | 45.55 ± 6.453 | 49.44 ± 9.122 | 53.71 ± 7.982 | 10.53 ± 0.651 | 13.11 ± 1.234 | |
| RF: U/L (M ± SD) | 43.95 ± 6.557 | 42.78 ± 6.057 | 54.57 ± 12.92 | 68.72 ± 12.53 | 10.12 ± 3.09 | 15.71 ± 3.147 |
NS, non-smoker; ES, ex-smoker; MS, mild to moderate smokers; HS, heavy smokers.
Table 2.
Disease stage according to smoking index in studied patients and Chi square test.
| Stage | Group |
||||||
|---|---|---|---|---|---|---|---|
| I (non-smokers) (%) | II (ex-smokers) (%) | III (moderate smokers) (%) | IV (heavy smokers) (%) | Chi square | Degree of freedom | Alpha probability | |
| I | 45 | 22 | 21.4 | 9 | 51.699 | 4 | <0.001⁎⁎ |
| II | 40 | 44.4 | 42.9 | 36.4 | |||
| III | 15 | 33.6 | 35.7 | 54.6 | |||
Significant.
RF was negative in smoker and non-smoker controls (14.125 ± 1.092 and 15.414 ± 1.147 U/L [M ± SE], respectively) and was positive and significantly higher in smoker than non-smoker patients (p < 0.01 and p < 0.001 for moderate and heavy smokers, respectively). Also heavy smoker patients have significantly higher RF titer than mild to moderate smokers (p < 0.05). Ex-smoker patients also showed significantly higher titer than heavy smoker controls (p < 0.001) but showed a non-significant difference from non-smoker patients (p > 0.05) (Table 3).
Table 3.
Comparison of rheumatoid factor (U/L) and anti-CCP antibodies (U/mL) between different groups included in the study.
| Group I | Group II | Group III | Group IV | Group V | Group VI | |
|---|---|---|---|---|---|---|
| RF (M ± SE) | 43.95 ± 1.446 | 42.777 ± 2.019 | 54.571 ± 3.454 | 68.72 ± 3.778 | 14.125 ± 1.092 | 15.414 ± 1.189 |
| Anti-CCP (M ± SE) | 27.55 ± 1.531 | 29.444 ± 3.082 | 35.5 ± 1.666 | 47 ± 2.268 | 4.562 ± 0.290 | 7.571 ± 0.996 |
| Comparison | RF | Anti-CCP |
|---|---|---|
| Groups V and VI | p > 0.05⁎ | p < 0.01⁎⁎ |
| Groups IV and VI | p < 0.001⁎⁎ | p < 0.001⁎⁎ |
| Groups I and III | p < 0.01⁎⁎ | p < 0.01⁎⁎ |
| Groups III and IV | p < 0.05⁎⁎ | p < 0.001⁎⁎ |
| Groups I and II | p > 0.05⁎ | p > 0.05⁎ |
| Groups II and VI | p < 0.001⁎⁎ | p < 0.001⁎⁎ |
Non-significant.
Significant.
Comparison of anti-CCP antibodies between different groups showed that, although blood levels in normal controls were very low, yet they were higher in heavy smokers (7.571 ± 0.996 versus 4.562 ± 0.290 U/mL [M ± SE]) and the difference between them was significant (p < 0.01). Blood levels in heavy smoker patients were much higher than heavy smoker controls and the difference between both was significant (p < 0.001). Also, a significant difference was seen between mild to moderate smoker and non-smoker patients (p < 0.01). In patients, heavy smokers showed much higher levels of anti-CCP antibodies than mild to moderate smokers (47 ± 2.268 versus 35.5 ± 1.666 U/mL [M ± SE]) and the difference was significant (p < 0.001). A significant difference was also seen between ex-smoker patients and heavy smoker controls (p < 0.001). No significant difference was seen between ex-smoker and non-smoker patients (p > 0.05) (Table 3).
RF and anti-CCP antibodies showed significant positive correlation to SI (r = 0.592, p < 0.001and r = 0.661, p < 0.001, respectively). SI and anti-CCP antibodies also showed a significant direct relation to disease stage (r = 0.424, p < 0.05 and r = 0.523, p < 0.01, respectively) (Table 4). Hand X-rays of three patients with different stages of RA are shown in Fig. 1.
Table 4.
Correlation between smoking index and each of RF, anti-CCP antibodies and disease stage in smoker patients.
| M ± SE | Correlation | Correlation coefficient (r) | p-value | |
|---|---|---|---|---|
| SI | 14.6 ± 2.917 | SI with RF | 0.581 | <0.001⁎⁎ |
| RF | 60.8 ± 4.114 | SI with anti-CCP | 0.661 | <0.001⁎⁎ |
| Anti-CCP | 29.36 ± 5.154 | SI with disease stage | 0.424 | <0.05⁎⁎ |
| Disease stage | 2.28 ± 0.211 | Anti-CCP with disease stage | 0.523 | <0.01⁎⁎ |
SI, smoking index; RF, rheumatoid factor.
Significant.
Figure 1.
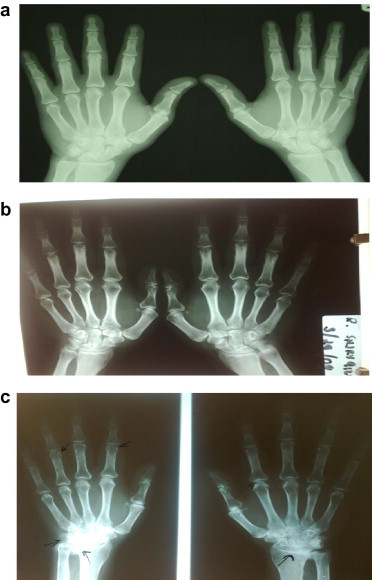
X-ray of hands of three of RA patients included in our study. (a) Stage I disease: soft tissue swelling in proximal inter-phalangeal joint. (b) Stage II disease: juxta-articular osteoporosis with erosions in the left first proximal interphalangeal joint. (c) Marked juxta-articular osteoporosis in both hands with marginal erosions in proximal interphalangeal joints and ulnar deviation.
5. Discussion
Tobacco smoking is one of the environmental factors that predispose to rheumatoid arthritis and is linked epidemiologically to more severe forms of the disease (Hutchison et al., 2001; Majka and Holers, 2006). Citrullination of tissue proteins caused by tobacco smoking was considered by some to be the main mechanism by which smoking induces and worsens auto-immune diseases (Costenbader and Karlson, 2006). We carried out this study to discover any possible effect of tobacco smoking on disease progression in rheumatoid arthritis patients as well as its relation to tissue protein citrullination as reflected by blood levels of anti-CCP antibodies.
In this study, higher percentage of advanced disease was found in heavy smoker patients than all other groups and in ex-smokers and mild to moderate smokers than non-smokers. A significant direct relation was also found between severity of smoking (SI) and disease stage in smoker patients. This means that tobacco smoking could worsen disease condition in these patients. This agrees with Saag et al. (1997), Fredreck (2000), and Mikuls et al. (2008). Saag et al. assessed the severity of rheumatoid disease by radiological evidence of bone destruction and found that patients who smoked more than 25 pack/year had the severest form of the disease and found also that subcutaneous nodules were a common feature in them. They concluded that tobacco smoking adversely influence the severity of RA in a potentially dose dependent fashion. Fredreck found a direct relation between the overall severity of rheumatoid arthritis and degree of smoking as well as RF seropositivity although he could not find a relation to the current disease activity. Mikuls and coworkers studied disease severity and seropositivity in 300 of African Americans with recent onset RA and found more severe forms with more skin nodules in current smokers than non-smokers and ex-smokers and in heavy than moderate smokers.
Surprisingly and in disagreement with our results, Finckh et al. (2007) studied 554 RA patients over a 3 years period and said that disease progression and joint damage has the same rate in both smokers and non-smokers. Moreover they claimed that heavy smokers have a lesser tendency for disease progression and concluded that tobacco smoking does not appear to accelerate joint damage in rheumatoid patients. Also Westhoff et al. (2008) showed that, although tobacco smoking decreased the efficacy of anti RA drugs and caused higher seropositivity for RF, yet it does not affect radiological progression of the disease.
In our study, RF titer was significantly higher in heavy smoker patients than mild to moderate smokers and in these patients than in patients who never smoked. Also, a significant direct relation was seen between RF and smoking severity (SI). This result is supported by the work of many authors. Stolt et al. (2003) studied 679 patients within 4 year period (1996–2000) in a defined area of Sweden and found high risk for sero-positive RA among current smokers and ex-smokers than non-smokers. Although Finckh et al. (2007) found no significant difference between smokers and non-smokers in disease progression, yet they found higher levels of RF in smoker patients. Also, Saag et al. (1997) showed a significant association between SI and titer of RF. As stated before, Mikuls et al. (2008) during their work on African American patients found high seropositivity for RF among heavy smoker patients. On the other hand, Lee et al. (2009) randomly studied RF in sera of 241 RA patients from a large rheumatology clinic aiming at evaluation of their role in the clinical situation of rheumatoid disease. They found a non-significant difference in RF titer between smokers and non-smokers, although the difference for anti-CCP antibodies was significant.
As regards anti-CCP antibodies, our present work showed significantly higher levels of these antibodies in heavy smokers than mild to moderate smokers and in the later patients than non-smokers. Also, a highly significant direct relation was found between these antibodies and SI. This means that tobacco smoking proportionally increases these antibodies in RA patients who smoke. In other words, it increases tissue protein citrullination which could enhance auto-immunity. This agrees with many published research works. In the African American group of patients studied by Mikuls et al. (2008) significantly higher levels of anti-CCP antibodies were found in heavy smokers than moderate smokers and in mild to moderate smokers than non-smokers. Lee et al. (2009) found a highly significant difference in these antibodies between smoker and non-smoker RA patients, although RF was not significantly different between them as stated before. Michou et al. (2008) studied the relation between human leukocyte antigen (HLA) and anti-CCP antibodies in a French population with RA and found significantly higher levels of these antibodies in smokers with a direct relation to the cumulative dose of smoking.
In this study, we found a positive Chi square test as well as a significant direct relation between anti-CCP antibody levels and disease stage which implies a possible role of these antibodies in the pathology of rheumatoid disease. More over it is also possible that smoking increases the risk of RA and worsens the ongoing disease through these antibodies. This agrees with Navarro et al. (2006), van Oosterhout et al. (2008), and Turesson et al. (2005). All of these authors showed a more severe form of the disease besides a higher incidence of extra articular joint affection in association with higher levels of these antibodies.
Although not exactly known how does tobacco smoking increases the risk of RA and worsens its course, yet tissue protein citrullination is considered by some authors to be the main pathogenetic mechanism (van Gaalen et al., 2005). Kobayashi et al. (2008) proposed another mechanism by which this hazardous effect of smoke happens. These authors showed that tobacco smoking induces an inflammatory cytokine response especially of IL-1β and TNFα. This inflammatory response is mediated by hydrocarbons (the main constituent of tobacco smoke, especially 2,3,7,8-tetrachlorodibenzo-p-dioxin) and is most marked in the synovial fluid.
Genetic factors, especially genes encoding for HLA-DRB1 and protein tyrosine phosphatase, non-receptor type 22 (PTPN22) antigens, were also claimed to predispose smokers for RA and RA patients for more severe forms of the disease (Michou et al., 2008).
Finally, RA appears to results from an interaction between genetics, the immune system and the environment.
6. Conclusion
From our results we can conclude that, tobacco smoking mostly play a role in progression of rheumatoid arthritis, most likely, through tissue protein citrullination. So, for achieving optimal control of rheumatoid arthritis, all smoker patients must quit.
References
- Arnett F.C., Edworthy S.M., Bloch D.A., Mcshane D.J., Fries J.F., Cooper N.S., Healey L.A., Kaplan S.R., Liang M.H., Luthra H.S., Medsger T.A., Mitchell D.M., Neustadt D.H., Pinals R.S., Schaller J.C., Sharp J.T., Wilder R.L., Hunder G.G. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Costenbader K.H., Karlson E.W. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15(11):737–745. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- Eisenbarth, S.C., Homann, D., 2006. Type 1 Diabetes: Cellular, Molecular and Clinical Immunology. Primer: Immunology and Autoimmunity. An Online CME Topic 8/2008 (Chapter 1). <http://uchsc.edu/misc/diabetes/books/type1/type1_ch1.html>.
- Finckh A., Dehler S., Costenbader K.H., Gabay C. Cigarette smoking and radiographic progression in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2007;66:1066–1071. doi: 10.1136/ard.2006.065060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredreck W. The effect of smoking on clinical, laboratory, and radiographic status in rheumatoid arthritis. Journal of Rheumatology. 2000;27:630–637. [PubMed] [Google Scholar]
- Hutchison D., Shepstone L., Moots R., Lear J.T., Lynch M.P. Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Annals of Rheumatic Diseases. 2001;60:223–227. doi: 10.1136/ard.60.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Okamoto H., Iwamoto T., Toyama Y., Tomatsul T., Yamanaka H., Momohara S. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology. 2008;47:1317–1322. doi: 10.1093/rheumatology/ken259. [DOI] [PubMed] [Google Scholar]
- Lee D.M., Phillips R., Hagan E.M., Chibnik L.B., Costenbader K.H., Schur P.H. Quantifying anti-cyclic citrullinated peptide titres: clinical utility and association with tobacco exposure in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2009;68:201–208. doi: 10.1136/ard.2007.084509. [DOI] [PubMed] [Google Scholar]
- Majka D.S., Holers V.M. Cigarette smoking and the risk of systemic lupus erythematosus and rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;65:561–563. doi: 10.1136/ard.2005.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michou L., Teixeira V.H., Pierlot C., Lasbleiz S., Bardin T., Dieudé P., Prum B., Cornélis F., Petit-Teixeira E. Associations between genetic factors, tobacco smoking and autoantibodies in familial and sporadic rheumatoid arthritis. Annals of the Rheumatic Diseases. 2008;67:466–470. doi: 10.1136/ard.2007.075622. [DOI] [PubMed] [Google Scholar]
- Mikuls T.R., Hughes L.B., Westfall A.O., Holers V.M., Parrish L., van der Heijde D., van Everdingen M., Alarcón G.S., Conn D.L., Jonas B., Callahan L.F., Smith E.A., Gilkeson G., Howard G., Moreland L.W., Bridges S.L. Cigarette smoking, disease severity and autoantibody expression in African Americans with recent-onset rheumatoid arthritis. Annals of the Rheumatic Diseases. 2008;67:1529–1534. doi: 10.1136/ard.2007.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C., Bañales J.L., Nava A., Mejia M., Carrillo G., Torres M., Reyes P., Selman M. Sera anti-cyclic citrulinated peptide antibodies differentiate interstitial lung disease related to rheumatoid arthritis from other lung diseases. Annals of the Rheumatic Diseases. 2006;65(Suppl. 2):313. [Google Scholar]
- Niewold T.B., Harrison M.J., Pajet S.A. Anti-CCP antibody as a diagnostic and prognostic tool in rheumatoid arthritis. The Quarterly Journal of Medicine. 2007;100:193–201. doi: 10.1093/qjmed/hcm015. [DOI] [PubMed] [Google Scholar]
- Renard S.I., Hepp L.M., Daughton D.M. Cigarette smoking and disease. In: Fishman A.P., Elias J.A., Fishman J.A., Grippi M.A., Senior R.M., Pack A.I., editors. Fishman’s Pulmonary Diseases and Disorders. fourth ed. McGraw-Hill, Inc.; 2008. pp. 747–760. (Chapter 43) [Google Scholar]
- Saag K.G., Cerhan J.R., Kolluri S., Ohashi K., Hunninghake G.W., Schwartz D.A. Cigarette smoking and rheumatoid arthritis severity. Annals of the Rheumatic Diseases. 1997;56:463–469. doi: 10.1136/ard.56.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbroker O., Trager C.H., Batterman R.C. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659–662. doi: 10.1001/jama.1949.02900430001001. [DOI] [PubMed] [Google Scholar]
- Stolt P., Bengtsson C., Nordmark B., Lindblad S., Lundberg I., Klareskog L., Alfredsson L. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Annals of the Rheumatic Diseases. 2003;62:835–841. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedler W., Wallman J., Froelich C.J., Teodorescu M. Routine measurement of IgM, IgG, and IgA rheumatoid factors: high sensitivity, specificity, and predictive value for rheumatoid arthritis. Journal of Rheumatology. 1997;24:1037–1044. [PubMed] [Google Scholar]
- Turesson C., Jacobsson L.T.H., Sturfelt G., Matteson E.L., Rönnelid J. Antibodies to cyclic citrulinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2005;64(Suppl. 3):618. doi: 10.1136/ard.2006.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen F., Ioan-Facsinay A., Huizinga T.W., Toes R.M. The devil in the details: the emerging role of anticitrulline autoimmunity in rheumatoid arthritis. The Journal of Immunology. 2005;175:5575–5580. doi: 10.4049/jimmunol.175.9.5575. [DOI] [PubMed] [Google Scholar]
- van Oosterhout M., Bajema I.M., Levarht N.W.N., Toes R.E.M., Huizinga T.W.J., van Laar J.M. Synovial differences between anti-CCP positive and anti-CCP negative rheumatoid arthritis. Annals of the Rheumatic Diseases. 2008;66(Suppl. 2):82. [Google Scholar]
- Westhoff G., Rau R., Zinkl A. Rheumatoid arthritis patients who smoke have a higher need for DMARDs and feel worse, but they do not have more joint damage than non-smokers of the same serological group. Rheumatology. 2008;47:849–854. doi: 10.1093/rheumatology/ken057. [DOI] [PubMed] [Google Scholar]


