Abstract
Aloe vera L. high molecular weight fractions (AHM) containing less than 10 ppm of barbaloin and polysaccharide (MW: 1000 kDa) with glycoprotein, verectin (MW: 29 kDa), were prepared by patented hyper-dry system in combination of freeze–dry technique with microwave and far infrared radiation. AHM produced significant decrease in blood glucose level sustained for 6 weeks of the start of the study. Significant decrease in triglycerides was only observed 4 weeks after treatment and continued thereafter. No deterious effects on kidney and liver functions were apparent. Treatment of diabetic patients with AHM may relief vascular complications probably via activation of immunosystem.
Keywords: Aloe vera L., High molecular weight fractions, Hypoglycemic effect, Type 2 diabetic patients
1. Introduction
Type 2 Diabetes Mellitus (T2DM) is one of the primary threats to human health due to its increasing prevalence, chronic course and disabling complications. Synthetic hypoglycemic drugs cannot fully control glucose level as well as cause side effects prompting the patients stop taking the medication. Aloe vera is a widely distributed Liliaceae plant in tropical regions and cosmetic and medicinal products are made from the mucilaginous tissue in the centre of the A. vera called A. vera gel. The peripheral bundle of sheath cells produce intensely bitter, yellow latex, commonly termed aloe juice, or sap or aloes. Unlike aloes, A. vera gel contains no anthraquinones, which are responsible for the strong laxative effects of aloe. The pharmacological actions of A. vera was studied in vitro or in vivo (in most cases the total leaf extract was used), include anti-inflammatory, anti-arthritic, antibacterial and hypoglycemic effects (Newall et al., 1996). A. vera is a medicinal plant that is claimed to have hypoglycemic effect with fewer side effects and less expensive without toxicity (Bergfield, 2007). The hypoglycemic efficacy of aloe gel was confirmed in streptozotocin-induced diabetic rats (Bunyapraphatsara et al., 1995). However, acute and subchronic toxicity studies of aloe gel (Charles, 1981) showed no toxic effect when lyophilized aloe gel was administered orally to albino rats at doses 1, 4, 16, or 64 mg/kg body weight twice daily, and the study revealed that the LD50 is over 5 g/kg body weight. Studies in mice showed no acute toxicity in therapeutic doses; however, in high doses a decrease of CNS activity was observed (Shah et al., 1989). No changes in levels of serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), blood urea nitrogen (BUN) and creatinine were observed (Wattanasrisin, 1988). Another study showed the same result with fresh and preserved aloe gel (Jirakulchaiwong et al., 1991). All these findings led us to conduct a clinical study to investigate the efficacy of Aloe vera high molecular weight fractions (AHM) prepared by the patented hyper-dry technique in the treatment of T2DM.
2. Materials and methods
2.1. Extraction and preparation
Aloe vera gel high molecular weight fractions (AHM) were obtained from water-washed gel part of Aloe vera leaves cultivated in Okinawa, Japan. Voucher specimens of Aloe vera collected in Okinawa, were compared and determined to be Aloe vera L. plant (syn. Aloe barbadensis Miller) (Herbarium number 54-3 in Medicinal garden, Fukuyama University by Emeritus Prof. A. Yagi). AHM were processed by patented hyper-dry system in combination of freeze-dry technique with microwave and far infrared-ray radiation. AHM contain the following chemical and physical properties: MW: 1,119,500 Da by HPLC analysis; column, TSK gel GMPW, two columns in series: 10 μm, 7.8 mm × 30 cm. Eluent: 0.2 M NaNO3. Flow rate: 1.0 ml/ min. Temperature: 40 °C, detection by refractive index detector.
2.2. Sample treatment
AHM (1 g) sample was homogenized in 2 ml of 0.2 M NaNO3 in a homogenizer. Then, centrifugation of homogenate at 2000g for 1 min was done. Upper solution was introduced as 200 μl aliquots to size exclusion chromatography. Aloin content is <10 ppm by HPLC analysis (Okamura et al., 1996). Water content: 3 ± 0.5%, Colony formulating unit: <300/g, Na+: approx. 430 mg/100 g, Ca2+: approx. 2100 mg/100 g. Aloe vera high molecular weight fractions, AHM contain the following characteristics: neutral polysaccharides with MW of about 1000 kDa, containing 90% carbohydrate and 7% protein content. Glycoprotein, verectin, composed of carbohydrate and protein in a ratio of 10.7% and 82.0%, respectively, with MW of 29 kDa (Yagi et al., 1997), was obtained in a ratio of 20% by immunochemical assay in AHM.
Chemical shifts of AHM were determined in D2O with a JOEL JNM ALPHA-400 and 100 MHz for proton and carbon, respectively. The infrared spectra were obtained with a FTIR-8600PC, Shimadzu, Japan.
1H NMR (ppm, 10 mg/ml in D2O at 34.3 °C, TMS as an internal standard): 2.12 (s, CH3COO), 2.58 (br.s, –C(OH)–CH2COOH, citric acid), 2.74, 2.78 (d, –CH2CH(OH)COOH, malic acid), 3.4–4.0, (m, polysaccharides, CH(OH)CH(OH)–), 3.97 (m, –CH2CH(OH)COOH, malic acid), 4.18 (m, –CH(OH)COOH, malic acid), 5.19, 5.20 (br.s. ano-meric proton of the non-reducing carbon atom).
13C NMR (ppm, 10 mg/ml in D2O at 34.3 °C; TMS as an internal standard): 62.1 (C6), 70.9, 75.4, 77.0, 77.1, 97.1 (C1), 175.9 (CH3COO).
IR (KBr) νmax: 3381 (OH), 2922 (COOH, –NH–), 1735 (CH3COO), 1597 (–NHCO–), 1245 (–CHOH), 1033 (–CHOH).
2.3. Patients
The study protocol was approved by the committee for approval of the use of human in research of Tanta University (equivalent to the Institutional Review Board). The protocol of the study was explained to the patients and they agreed to participate by signing a written consent form. All procedures used in the protocol were in accordance to the Helsinki Declaration.
Fifteen patients (nine males and six females) with T2DM uncontrolled with their oral hypoglycemic medication (metformin 500 mg twice daily and glibenclamide 5 mg twice daily) were enrolled in the study. They were selected from the outpatient clinic of the Internal Medicine Department, Tanta University, Egypt. They were selected for the study using the following criteria:
2.4. Inclusion criteria
-
1.
Their ages ranged from 42 to 55 years old.
-
2.
High fasting blood glucose level (>200 mg/dL).
-
3.
Freely consented to participate this study.
2.5. Exclusion criteria
-
1.
Liver disease as defined by abnormal level of ALT, AST and alkaline phosphatase.
-
2.
Kidney disease as defined abnormal levels of serum BUN and creatinine.
-
3.
Patients with clinical problems causing hyperglycemia including infection, and thyroid disease.
-
4.
Patients taking medications known to modify glucose metabolism as corticosteroids.
-
5.
Morbid obesity (BMI > 40 kg/m2).
2.6. Trial term
Twelve weeks during October, 2007 and December, 2007.
2.7. Consent of patients
We obtained the consents of the patients to this treatment by document or word of mouth by free will through explaining the contents to them before preceding the enforcement of this treatment. In addition, we obtained the consent from the patient’s family if the patient was unable to judge through Hospital Committee. The research followed guidelines of the Declaration of Helsinki and Tokyo for humans.
2.8. Design
Before administering AHM, each patient’s body mass index was calculated (BMI = kg/m2), systolic and diastolic blood pressure were measured.
All the patients received two tablespoonful (0.05 g) of AHM three times daily for 12 weeks and continued taking their hypoglycemic medications. Blood samples were analyzed for fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), triglycerides (TG), cholesterol, serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT) and serum creatinine before the study. Blood samples were obtained again weekly for measurement of FBG, and every 2 weeks for TG and cholesterol analysis. The (HbA1c) values were evaluated every 4 weeks, while serum activities AST and ALT and serum creatinine levels were determined at the end of the study.
2.9. Serum determinations
Fasting blood glucose was determined by glucose oxidase technique (Trinder, 1969), using kits obtained from Stanbio Laboratory, Inc., San Antonio, TX. The reaction depends on the enzymatic oxidation of glucose to gluconic acid and H2O2. The formed hydrogen peroxide reacts under the influence of peroxidase with phenol and aminophenazone to form red–violet quinoneimine dye measured colorimetrically, using UV-160A Shimadzu Spectrophotometer.
Serum AST and ALT activities were assayed according to the method of Reitman and Frankel (1957), using kits obtained from Quimica Clinica Aplicadea S.A., Amposta/Spain. The ketoacids produced by the enzyme action reacts with 2,4-dinitrophenylhydrazine producing hydrazone complex measureed colorimetrically at 505 nm.
Serum creatinine was measured according to Heinegard and Tiderstrom (1973) method, using kits obtained from Stanbio Laboratory, Inc., San Antonio, TX. Creatinine in picric acid protein free solution reacts with alkali to form reddish-brown complex measured colorimetrically at 520 nm.
Serum total cholesterol was determined using kits obtained from Stanbio Laboratory, Inc., San Antonio, TX. After enzymatic hydrolysis of the cholesterol ester, the free cholesterol was oxidized with cholesterol oxidase. The produced H2O2 reacts with phenol and aminophenazone to form red quinineimine dye measured colorimetrically at 500 nm (Allein et al., 1974).
The triglycerides were determined using kits obtained from Reactivos Spinreact, S.A. The triglycerides were enzymatically hydrolyzed to glycerol which phosphorylated by ATP and oxidized to H2O2. The intensity of the final red color is proportional to the total triglycerides concentration which measured at 505 nm (Young and Pestaner, 1975).
Glycosylated Hb (% of total Hb) was measured by chromatographic pectrophotometric method according to Bisse and Abraham (1985) method, using kits obtained from Biosystems S.A. Barcelona (Spain).
2.10. Statistical analysis
Student’s paired t-test was used to determine the difference in biochemical changes between the patients before and after the administration of AHM. *P < 0.001.
3. Results
1H NMR studies showed that AHM have polysaccharide moieties with acetyl group in contamination of malic and citric acid by comparison of chemical shifts with those of International Aloe Science Council data base. On 13C NMR study AHM is proved to contain acetyl group in polysaccharide moiety, suggesting that acetyl group was preserved by patented hyper-dry technique.
The demographic parameters of the patients are shown in Table 1. The BMI values calculated for each patient showed that all the patients were overweight. Blood pressure measures showed that none of them was hypertensive. No alterations in the patient’s blood pressure or a significant change in their body weight could be detected during the study. There were no adverse effects reported by the patients due to the intake of AHM. It was well tolerated and there were no complaint about its taste or smell. Treatment the patients with AHM for 12 weeks with their oral hypoglycemic agents resulted in a significant decrease in fasting blood glucose by 32% compared to before administration (P < 0.001) (Fig. 1).
Table 1.
Demographic parameters of the diabetic patient.
| Parameters | Mean | SD |
|---|---|---|
| Age (years) | 48 | 4 |
| Diagnosis of DM | 2 | 0.8 |
| Body weight (kg) | 74.5 | 4.4 |
| Length (m) | 1.6 | 0.3 |
| BMI (kg/m2) | 29.5 | 2.2 |
| Systolic pressure (mmHg) | 125 | 8.6 |
| Diastolic pressure (mmHg) | 84 | 5.7 |
Figure 1.
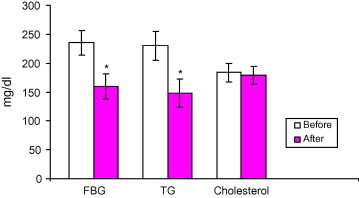
Effect of treatment with Aloe vera gel on the serum level of fasting blood glucose, triglyceride, and cholesterol (mg/dl) in type 2 diabetic patients uncontrolled with their oral hypoglycemic medications. Data are expressed as mean ± SD. ∗ Indicates significantly different from corresponding values before AHM administration for 12 weeks (p < 0.001). Before = before AHM administration. After = after AHM administration for 12 weeks.
The decrease in blood glucose level was significant and sustained after 6 weeks from the start of the study (Fig. 2).
Figure 2.
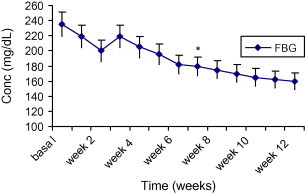
The mean changes of fasting blood glucose levels (mg/dl) in diabetic patients after administration of AHM for 12 weeks Data are expressed as mean ± SD. *Indicates significantly different from corresponding values before AHM administration for 12 weeks (p < 0.001).
The triglycerides level was decreased significantly 35% compared to before treatment with AHM (P < 0.001) (Fig. 1), the decrease was significant after 4 weeks and continues to fall throughout the study (Fig. 3).
Figure 3.
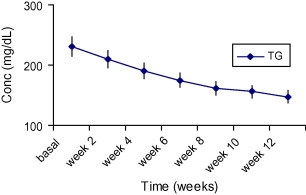
The mean changes of triglycerides levels (mg/dl) in diabetic patients after administration of AHM for 12 weeks. Data are expressed as mean ± SD.
Treatment of the patients with AHM with their oral hypoglycemic agents did not result in any change in cholesterol levels compared to before the treatment (Fig. 1).
Glycosylated Hb values calculated for each patient showed a significant reduction by 20% compared to before treatment. (P < 0.001) (Fig. 4).
Figure 4.
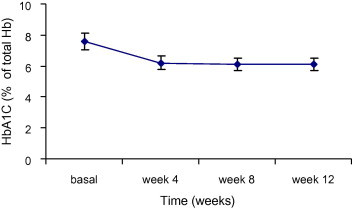
Reduction of the glycosylated hemoglobin values (% of total Hb) in diabetic patients after administration of AHM for 12 weeks. Data are expressed as mean ± SD.
Treatment of the patients with AHM did not result in any change in serum activities of AST, ALT or serum creatinine. (P < 0.001) (Figs. 5 and 6).
Figure 5.
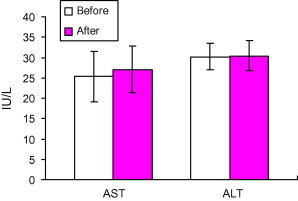
Effect of treatment with Aloe vera gel on the serum activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (IU/l) in type 2 diabetic patients uncontrolled with their oral hypoglycemic medications. Data are expressed as mean ± SD. Before = before AHM administration. After = after AHM administration for 12 weeks.
Figure 6.
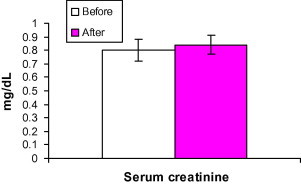
Effect of treatment with Aloe vera gel on the serum creatinine (mg/dl) in type 2 diabetic patients uncontrolled with their oral hypoglycemic medications. Data are expressed as mean ± SD. Before = before AHM administration. After = after AHM administration for 12 weeks.
4. Discussion
The present study aimed to evaluate the hypoglycemic effect of Aloe vera in diabetic patients uncontrolled with their hypoglycemic medications. Administration of AHM three times daily for 12 weeks concurrently with the oral hypoglycemic drugs resulted in significant decrease of the fasting blood glucose level. The decrease in blood glucose level was significant and sustained after 6 weeks of the start of the study. HbA1c value was reduced, a gold standard that confirms a long range level of the blood glucose, confirming the hypoglycemic effect of AHM. This results are correlated with other reports that confirm the hypoglycemic effect of aloe in experimental animals (Beppu et al., 2006; Tanaka et al., 2006; Gundidza et al., 2005).
The anti-diabetic effects of dietary administration of Aloe arborescens Miller components on multiple low-dose streptozotocin-induced diabetes in mice were investigated. The mice were fed ad libitum with basal diets supplemented with components of Aloe vera. There was no significant effect on the blood glucose level with Aloe vera leaf pulp powder. On the contrary, the whole aloe leaf significantly decreased the blood glucose level (Beppu et al., 2006).
It has been reported that Aloe vera gel and its derived phytosterols have a long-term blood glucose level control effect and would be useful for the treatment of type 2 diabetes mellitus (Tanaka et al., 2006).
The anti-diabetic activity of Aloe excelsa on male albino rats was also studied. The study showed that the Aloe excelsa powder produced a dose-dependent reduction in blood glucose levels (Gundidza et al., 2005).
Our results are in accordance with the clinical trial on the anti-diabetic activity of Aloe vera juice by Yongchaiyudha et al. (1996), who suggests the potential use of Aloe vera as an anti-diabetic agent. Agarwal (1985) conducted a clinical trial on 5000 patients with angina pectoris. He found that adding aloe gel to the diet produced marked reduction in total cholesterol, triglycerides, fasting and post-brandial blood sugar levels in diabetic patients.
In the present study, AHM administration produced a significant decrease in the level of triglycerides, the decrease was significant after 4 weeks and continued to fall throughout the study, however, cholesterol level did not change. These result was in accordance with that obtained by Rajasekaran et al. (2006) and Lim et al. (2003).
Rajasekaran et al. (2006) studied the oral administration of Aloe vera gel extract at a dose of 300 mg/kg body weight per day to STZ-induced diabetic rats for a period of 21 days. They found that it resulted in a significant reduction in fasting blood glucose, hepatic transaminases (AST and ALT), plasma and tissue (liver) cholesterol, triglycerides, free fatty acids and phospholipids and a significant improvement in plasma insulin. In addition, the decreased plasma levels of high-density lipoprotein-cholesterol and increased plasma levels of low-density lipoprotein- and very low-density lipoprotein-cholesterol in diabetic rats were restored to near normal levels following treatment with the extract. Furthermore, they analyzed the fatty acid composition of the liver and kidney and found that the altered fatty acid composition in the liver and kidney of diabetic rats was restored following treatment with the extract. They recommended use of Aloe vera as an anti-diabetic agent.
Lim et al. (2003) studied the efficacy of dietary Aloe vera supplementation on hepatic cholesterol and oxidative status in aged rats. They found that the aloe-supplemented groups showed approximately 30% lower in the hepatic cholesterol levels. They concluded that life-long intake of aloe had superior anti-oxidative action against lipid peroxidation in vivo, as indicated by reduced levels of hepatic phosphatidylcholine hydroperoxide, and had hypocholesteremic efficacy.
In the present study, administration of AHM did not result in any change in serum AST, ALT activities, or serum creatinine, indicating its safety to liver and kidney.
Chudan et al. (2007) validated the hepatoprotective potential of Aloe barbadensis Mill against carbon tetrachloride induced hepatotoxicity. The hepatoprotective activity was evident by restoration of serum transaminases, alkaline phosphatase, bilirubin and triglycerides. It was confirmed by the restoration of lipid peroxidation, glutathione, glucose-6-phosphatase and microsomal aniline hydroxylase and amidopyrine N-demethylase towards near normal. Furthermore, histopathology of the liver tissue supported the biochemical findings.
Bolkent et al. (2004) found that the degenerative changes observed in the kidney tissue of streptozotocin-induced type 2 diabetic rats were diminished when they were given glibenclamide and aloe leaf gel and pulp extracts. Serum urea and creatinine levels were higher in diabetic rats in comparison to healthy rats. The administration of aloe gel extract and glibenclamide decreased serum urea and creatinine levels in comparison to diabetic controls. Only Aloe vera leaf gel extract showed improvement both in histological and biochemical parameters suggesting a protective effect of Aloe vera on mild damage caused by type 2 diabetes on kidney tissue.
Sulfonylurea is initially successful in treatment T2DM, but it is often associated with failure (Matthews et al., 1998). This failure also occurs during multi-drug therapy using a combination of sulfonylurea and oral anti-diabetic agents with different mechanisms poor compliance, lack of adequate diet regimen, body weight gain, deterioration of insulin sensitivity, presence of anti-islet cell and antiglutamic acid decarboxylase antibodies and decline in pancreatic β-cell function which is the strong predictor causative factor (Fukui et al., 1997).
Obesity increases the risk of diabetes due to insulin resistance. This may be due to the production of various factors derived from the adipocyte that act on fat, liver, or muscle to impair insulin action.
Obesity itself is also associated with hyper-insulinemia, and insulin may induce insulin resistance through down regulation of the insulin receptor. Potential candidate substances produced by fat that may cause insulin resistance include tumor necrosis factor (TNF) and other cytokines such as interleukin-6 (IL-6) and adiponectin (Hajer et al., 2008).
Aloe vera gel (AHM) act as thromboxane A2 inhibitor. It promotes vasodilatation and maintains hemostasis within the vascular endothelium as well as within the surrounding tissue (Heggers et al., 1993). In earlier study we reported that verectin, a glycoprotein, indicated anti-oxidative, anti-thromboxane A2 synthase inhibition and cyclooxygenase-2 inhibiting activities in vitro (Yagi et al., 2003), those of which may be deeply correlated with vasodilatation in DM patients. By use of polyclonal antibody to verectin generated in rabbit serum, it was demonstrated that AHM containing immunoreactive verectin in a ratio of 20% (Yagi et al., 1997, 2000a,b) inhibited serum FBG and triglyceride levels.
The present clinical study was carried out using AHM including polysaccharides (MW about 1000 kD) and verectin (MW: 29 kD), with contamination of barbaloin <10 ppm and hypoglycemic efficacy for T2DM patients by oral administration for 12 weeks was revealed without any side effects. Therefore, treatment with AHM may relief peripheral blood vessel complications in diabetic patients through activation of immunosystem. Furthermore, AHM containing about MW 1000 kD was biodegraded by intestinal microbials into oligosaccharides (Yagi et al., 1999, 2001) which inhibit absorption of glucose moiety through gut membrane (Jain et al., 2007).
AHM is a high molecular weight natural polysaccharide contains glucomannan polysaccharide with acetyl group (acemannan). The effect of mannan on triglyceride level was estimated by Boban et al. (2006) as well as by Sood et al. (2008) which is due to inhibition of the hepatic production of chylomicron.
5. Conclusion
AHM with <10 ppm of barbaloin prepared by patented hyper-dry system exhibited a significant hypoglycemic effect, it can lower not only the glucose but also the triglycerides level which are often high in diabetic patients with no hepato- and nephrotoxicity.

Acknowledgements
The authors express deep thanks to Mr. S. Yagi, Ellie Corporation, Shizuoka, Japan for providing Aloe vera high molecular weight fractions and to Mr. T. Kaku, CEO, Japan Bio Products Co. Ltd. for encouragement of the study.
References
- Agarwal O.P. Prevention of atheromatous heart disease. Angiology. 1985;36(8):485–492. doi: 10.1177/000331978503600801. [DOI] [PubMed] [Google Scholar]
- Allein C., Poon L., Chan C., Richmond W., Fu P. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- Beppu H., Shimpo K., Chihara T., Kanako T., Tamai I., Yamaji S., Ozaki S., Kuzuya H., Sonoda S. Antidiabetic effects of dietary administration of Aloe arborescens Miller components on multiple low-dose streptozotocin-induced diabetes in mice: investigation on hypoglycemic action and systemic absorption dynamics of aloe components. J. Ethnopharmacol. 2006;103(3):468–477. doi: 10.1016/j.jep.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Bergfield W.F. Final report of the cosmetic ingredient review. Int. J. Toxicol. 2007;26(Suppl. 2):1–50. doi: 10.1080/10915810701351186. (Expert Panel Chairman, 2007) [DOI] [PubMed] [Google Scholar]
- Bissé A., Abraham E.C. New less temperature sensitive microchromatographic method for the separation and quantification of glycosylated hemoglobin using a non-cyanide buffer system. J. Chromatogr. 1985;344:81–91. doi: 10.1016/s0378-4347(00)82009-5. [DOI] [PubMed] [Google Scholar]
- Boban P.T., Nambisan B., Sudhakaran P.R. Hypolipidemic effect of chemically different mucilages in rats: a comparative study. Br. J. Nutr. 2006;96:1021–1029. doi: 10.1017/bjn20061944. [DOI] [PubMed] [Google Scholar]
- Bolkent S., Akev N., Ozsoy N., Sengezer-Inceli M., Can A., Alper O., Yanadaq R. Effect of Aloe vera (L.) Burm fil. leaf gel. and pulp extracts on kidney in type II diabetic rat model. Indian J. Exp. Biol. 2004;42(2):48–52. [PubMed] [Google Scholar]
- Bunyapraphatsara N., Chasrakaew W., Pornchirasilp S., Pneungvicha P., Chokechaijaroenporn O. Antidiabetic effect of fresh and preserved aloe gel. Thai J. Phytopharm. 1995;2:1–7. [Google Scholar]
- Charles, B., 1981. Acute Oral Toxicity Study. Dawson Research Corporation, DRC 2765.
- Chudan B.K., Saxena A.K., Shukla S., Sharma N., Gupta K.A., Suri J., Bhadauria M., Singh B. Hepatoprotective potential of Aloe barbadensis Mill against carbon tetrachloride induced hepatotoxicity. J. Ethnopharmacol. 2007;111(3):560–566. doi: 10.1016/j.jep.2007.01.008. 22. [DOI] [PubMed] [Google Scholar]
- Fukui M., Nakano K., Shigeta H., Hoshimori K., Fujii M., Kitagawa Y., Mori H., Kajiyama S., Nakamura N., Abe N., Obayashi H., Fukui I., Ohta M., Kondo M. Antibodies to glutamic acid decarboxylase in Japanese diabetic patients with secondary failure of oral hypoglycemic therapy. Diabet. Med. 1997;14:148–152. doi: 10.1002/(SICI)1096-9136(199702)14:2<148::AID-DIA317>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gundidza M., Masuku S., Humphrey G., Magwa M.L. Antidiabetic activity of Aloe excelsa. Cent. Afr. J. Med. 2005;51(11–12):115–120. [PubMed] [Google Scholar]
- Hajer G.R., Haeften T.W., Visseren F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- Heggers J.P., Pelley R.P., Robson M.C. Beneficial effects of aloe in wound healing. Phytother. Res. 1993;7:S48–S52. [Google Scholar]
- Heinegard D., Tiderstron G. Determination of serum creatinine by direct colorimetric method. Clin. Chem. Acta. 1973;43:305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- Jain A., Gupta Y., Jain S.K. Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J. Pharm. Pharm. Sci. 2007;10:86–128. [PubMed] [Google Scholar]
- Jirakulchaiwong, S., Wongkranjang, Y., Bunyapraphatsara, N., Atisuk, K., 1991. Toxicological evaluation of fresh and preserved aloe gel. Progress on terrestrial and marine natural products of medicinal and biological interest. In: Proceedings of a Symposium Held on the Occasion of the 60th Birthday of Professor N.R. Farnsworth, Chicago, Illinois al Chicago, Illinois, pp. 91–97.
- Lim B.O., Seong N.S., Choue R.W., Kim J.D., Lee H.Y., Kim S.Y., Jeon T.I., Park D.K. Efficacy of dietary Aloe vera supplementation on hepatic cholesterol and oxidative status in aged rats. J. Nutr. Sci. Vitamonol. 2003;49(4):292–296. doi: 10.3177/jnsv.49.292. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Cull C.A., Stratton I.M., Holmann R.R., Turner R.C. UKPDS 26: sulfonylurea failure in non-insulin dependent diabetic patients over 6 years. Diabet. Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Newall C.A., Anderson L.A., Phillipson J.D. Pharma-Ceutical Press; London: 1996. Herbal Medicines: A Guide for Health-care Professionals. pp. 25–27. [Google Scholar]
- Okamura N., Asai M., Hine N., Yagi A. High performance liquid chromatographic determination of phenolic compounds in aloe species. J. Chromatogr. A. 1996;746:225–231. [Google Scholar]
- Rajasekaran S., Sivagnanam K., Ravi K., Subramanian S. Beneficial effects of Aloe vera gel extract on lipid profile status in rats with streptozotocin diabetes. Clin. Exp. Pharmacol. Physiol. 2006;33(3):232–237. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of glutamic oxaloacetic and glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Shah A.H., Qureshi S., Tariq M., Ageel A.M. Toxicity studies on six plants used in the traditional Arab system of medicine. Phytother. Res. 1989;3:25–29. [Google Scholar]
- Sood N., Baker W.L., Coleman C.I. Effect of glaucoma-nnan on plasma lipid and glucose concentrations, body weight and blood pressure: Systemic review and meta analysis. Am. J. Clin. Nutr. 2008;88:1167–1170. doi: 10.1093/ajcn/88.4.1167. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Misawa E., Ito Y., Habara N., Nomaqushi K., Yamada M., Toida T., Hayasawa H., Takase M., Inaqaki M., Hiqushi R. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol. Pharm. Bull. 2006;29(7):1418–1422. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- Trinder P. Determination of blood glucose using an oxidase–peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969;22:158–161. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanasrisin, J., 1988. Effect of Aloe vera Gel on Serum Transaminase, BUN, and Creatinine Levels in Weanling Rats. MS Thesis. Faculty of Science, Mahindol University, Thailand.
- Yagi A., Egusa T., Arase M., Tanabe M., Tsuji Isolation and characterization of the glycoprotein fraction with a proliferation-promoting activity on human and hamster cells in vitro from Aloe vera gel. Planta Med. 1997;63:18–21. doi: 10.1055/s-2006-957595. [DOI] [PubMed] [Google Scholar]
- Yagi A., Egusa T., Arase M., Tanabe M., Tsuji H. Isolation and characterization of the glycoprotein fraction with a proliferation-promoting activity on human and hamster cells in vitro from Aloe vera gel. Planta Med. 1997;63:18–21. doi: 10.1055/s-2006-957595. [DOI] [PubMed] [Google Scholar]
- Yagi A., Nakamori J., Yamada T., Iwase H., Tanaka T., Kaneo Y., Qui J., Orndorff S. In vivo metabolism of aloe-mannan. Planta Med. 1999;65:417–420. doi: 10.1055/s-1999-14018. [DOI] [PubMed] [Google Scholar]
- Yagi A., Sato Y., Akasaki K., Tsuji H. Distribution of verectin in Aloe vera leaves and verectin contents in clonally regenerated plants and the commercial gel powders by immunochemical screening. Planta Med. 2000;66:180–182. doi: 10.1055/s-0029-1243127. [DOI] [PubMed] [Google Scholar]
- Yagi A., Tsunoda M., Egusa T., Akasaki K., Tsuji H. Immunochemical distribution of Avera, A. arborescens and A. chinensis gels. Planta Med. 2000;64:277–287. doi: 10.1055/s-2006-957427. [DOI] [PubMed] [Google Scholar]
- Yagi A., Hamano S., Tanaka T., Kaneo Y., Fujioka T., Mihashi K. Biodisposition of FITC-labeled aloe mannan in mice. Planta Med. 2001;67:297–300. doi: 10.1055/s-2001-14314. [DOI] [PubMed] [Google Scholar]
- Yagi A., Kabbash A., Mizuno K., Moustafa S.M., Khalifa T.I., Tsuji H. Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from Aloe vera gel. Planta Med. 2003;69:269–271. doi: 10.1055/s-2003-38481. [DOI] [PubMed] [Google Scholar]
- Yongchaiyudha S., Rungpitarangsi V., Bunyapraphatsara N., Chokechaijaroenporn O. Antidiabetic activity of Aloe vera juice l. Clinical trial in new cases of diabetes mellitus. Phytomedicine. 1996;3:241–243. doi: 10.1016/S0944-7113(96)80060-2. [DOI] [PubMed] [Google Scholar]
- Young D., Pestaner L. Enzymatic colorimetric determination of triglycerides. Clin. Chem. 1975;2:273–281. [Google Scholar]


