Abstract
A major problem in clinical trials of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) as cancer therapy is the development of resistance to TRAIL. Therefore, agents that can overcome TRAIL resistance have great therapeutic potential. In this study, we evaluated capsazepine, a TRPV1 antagonist, for its ability to sensitize human colon cancer cells to TRAIL-induced apoptosis. Capsazepine potentiated the effect of TRAIL, as shown by its effect on intracellular esterase activity; activation of caspase-8,–9, and -3; and colony-formation assay. Capsazepine induced death receptors (DRs) DR5 and DR4, but not decoy receptors, at the transcriptional level and in a non-cell-type-specific manner. DR induction was dependent on CCAAT/enhancer-binding protein homologous protein (CHOP), as shown by (a) the induction of CHOP by capsazepine and (b) the abolition of DR- and potentiation of TRAIL-induced apoptosis by CHOP gene silencing. CHOP induction was also reactive oxygen species (ROS)-dependent, as shown by capsazepine’s ability to induce ROS and by the quenching of ROS by N-acetylcysteine or glutathione, which prevented induction of CHOP and DR5 and consequent sensitization to TRAIL. Capsazepine’s effects appeared to be mediated via JNK, as shown by capsazepine’s ability to induce JNK and by the suppression of both CHOP and DR5 activation by inhibition of JNK. Furthermore, ROS sequestration abrogated the activation of JNK. Finally, capsazepine downregulated the expression of various antiapoptotic proteins (e.g., cFLIP and survivin) and increased the expression of proapoptotic proteins (e.g., Bax and p53). Together, our results indicate that capsazepine potentiates the apoptotic effects of TRAIL through downregulation of cell survival proteins and upregulation of death receptors via the ROS–JNK–CHOP-mediated pathway.
Keywords: TRPV1 antagonist, TRAIL, Apoptosis, Death receptor, Free radicals
Introduction
Among the 18 members of the tumor necrosis factor (TNF)1 superfamily, TNF-related apoptosis-inducing ligand (TRAIL) is the only one being explored for its anticancer potential in the clinic. This is due in part to its lack of proinflammatory activity [1]. TRAIL−/− mice show an increased susceptibility to tumor initiation and metastasis [2,3], which emphasizes the critical role of TRAIL in the defense against tumors. TRAIL mediates its anticancer effects through two transmembrane agonistic receptors and three antagonistic receptors. The two transmembrane agonistic receptors are TRAIL receptor 1 (DR4) [4] and TRAIL receptor 2 (DR5) [5]. The three antagonistic receptors include the transmembrane decoy receptor (DcR) 1 and DcR2 [6,7] and the soluble receptor osteoprotegerin [8]. Engagement of DR4 or DR5 by TRAIL or agonistic antibodies can lead to activation of caspase-8 and caspase-3 and from there to apoptosis.
Unfortunately, one of the major problems with TRAIL in clinical trials is the development of resistance [9]. How tumor cells become resistant to TRAIL is open to interpretation. A wide variety of mechanisms have been proposed, including overexpression of antiapoptotic proteins such Bcl-xL [10], Bcl-2 [11], XIAP [12], survivin [13], and Mcl-1 [14]; activation of NF-κB [7], which controls the expression of several of these proteins; downregulation of DR4 [15,16] and DR5 [17]; and upregulation of decoy receptors [18–20]. Thus, it has been proposed that agents that can modulate these various mechanisms of TRAIL resistance may potentially sensitize tumors to the cytokine.
One such agent is capsazepine (Fig. 1A), a capsaicin antagonist [21] that is now widely used as a selective vanilloid type 1 receptor (TRPV1) antagonist. Capsazepine can abolish osteosarcoma-induced hyperalgesia when administered subcutaneously at doses ranging from 3 to 10 mg/kg [22], block calcium channels [23], inhibit ovariectomy-induced bone loss in vivo [24], and suppress lipopolysaccharide-induced inducible nitric oxide synthase expression in macrophages through inactivation of NF-κB [25]. However, the mechanisms underlying the anticancer effects of capsazepine are not fully understood. Whether capsazepine can sensitize tumor cells to TRAIL-induced cell death is not known. Therefore, the objective of the study reported here was to determine whether capsazepine potentiates TRAIL-induced cancer apoptosis and, if so, how. We found that capsazepine effectively enhanced TRAIL-induced apoptosis by upregulating the activation of TRAIL receptors DR4 and DR5 via the reactive oxygen species (ROS)–JNK–CHOP pathway, by downregulating the expression of cell survival proteins, and by upregulating the expression of proapoptotic proteins.
Fig. 1.
Capsazepine potentiates TRAIL-induced cell death in HCT116 cells. (A) Chemical structure of capsazepine. (B) Cells were treated with capsazepine at the indicated concentrations for 6 h and then treated with TRAIL (25 ng/ml) for 20 h. Cell death was determined by the Live/Dead cell viability assay, and five random fields were counted. (C) Cells were treated with 10 or 30 μM capsazepine for 6 h and then treated with TRAIL (25 ng/ml) for 20 h. Cell death was determined by the Live/Dead cell viability assay, and three random fields were counted. Each column represents the mean±SD (n=3; *P<0.05 and **P<0.01, compared to untreated cells; #P<0.05 and ##P<0.01, compared to TRAIL-treated cells). (D) HCT116 cells were treated with capsazepine (0, 10, and 30 μM) for 6 h and then treated with TRAIL (25 ng/ml) for 12 h. Cells (500 cells/well) were then reseeded in six-well plates and incubated. After 14 day, the cells were stained with crystal violet and counted for colony formation. Each column represents a mean±SD (n=3; *P<0.05 and **P<0.01, compared to untreated cells; #P<0.05 and ##P<0.01, compared to TRAIL-treated cells). (E) Cells were pretreated with capsazepine at the indicated concentrations for 6 h and then treated with TRAIL (25 ng/ml) for 20 h. Whole-cell extracts were prepared and analyzed by Western blotting using antibodies against caspase-9, caspase-8, caspase-3, and PARP. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading.
Materials and methods
Materials
Capsazepine (Sigma–Aldrich) was dissolved in dimethyl sulfoxide (50 mM) and stored at −20 °C until needed. Soluble recombinant human TRAIL/Apo2L was purchased from PeproTech. Penicillin, streptomycin, McCoy’s 5A, Dulbecco’s modified Eagle’s medium (DMEM), and RPMI 1640 were purchased from Mediatech. Fetal bovine serum (FBS) was obtained from Atlanta Biologicals. Evodiamine was obtained from LKT Laboratories. Capsaicin, resiniferotoxin, Tris, glycine, NaCl, sodium dodecyl sulfate (SDS), bovine serum albumin, N-acetylcysteine (NAC), glutathione (GSH), and antibody against β-actin were obtained from Sigma Chemical Co. Antibody against DR5 was purchased from ProSci. Antibodies against DR4, poly(ADP-ribose) polymerase (PARP), Bcl-2, Bcl-xL, Bax, survivin, caspase-3, and caspase-8 were obtained from Santa Cruz Biotechnology. Anti-XIAP was purchased from BD Biosciences. Antibodies against DR4, DcR1, and DcR2 were kindly provided by Imgenex. Short interfering RNA (siRNA) against human CHOP (5′–AAGAACCAGCAGAGGTCACAA-3′) was purchased from Qiagen.
Cell lines
HCT116 and HT-29 (human colon adenocarcinoma), MDA-MB-231 (human breast adenocarcinoma), AsPC-1 (human pancreatic adenocarcinoma), SCC-4 (human squamous cell carcinoma), H1299 (human lung adenocarcinoma), and HeLa (human cervix adenocarcinoma) cell lines were obtained from the American Type Culture Collection. KBM-5 (human chronic myeloid leukemia) cells were gifted from Dr. Nicholas J. Donato (University of Michgan Comprehensive Cancer Center). SEG-1 (human esophageal adenocarcinoma) cells were kindly provided by Dr. Jaffer A. Ajani (The University of Texas M.D. Anderson Cancer Center). MDA-MB-231 and SEG-1 cells were cultured in DMEM with 10% FBS. HCT116 and HT-29 cells were cultured in McCoy’s 5A medium with 10% FBS. AsPC-1 and H1299 cells were cultured in RPMI 1640 with 10% FBS. KBM-5 cells were cultured in Iscove’s modified Dulbecco’s medium with 15% FBS and 100 U/ml penicillin and 100 mg/ml streptomycin.
Live/dead assay
To measure cell death, we used the Live/Dead cell viability assay (Invitrogen), which assays cell viability in terms of intracellular esterase activity and plasma membrane integrity. Briefly, cells were incubated with capsazepine for 6 h and then treated with TRAIL (25 ng/ml) for 20 h at 37 °C. Cells were stained with 5 μM ethidium homodimer and 5 μM calcein-AM (Live/Dead method) at 37 °C for 30 min. Cells were analyzed under a fluorescence microscope (Labophot-2; Nikon, Tokyo, Japan).
Clonogenic assay
Treated and untreated cells were seeded in six-well-plates, allowed to form colonies for 14 day, and then stained as described elsewhere. Plates were scanned and colonies counted using Cell-Profiler cell image analysis software.
Analysis of cell surface expression of DR4 and DR5
To analyze the cell surface expression of DR4 and DR5, cells were treated with capsazepine for 24 h, stained with phycoerythrin-conjugated mouse monoclonal anti-human DR4 or DR5 (R&D Systems) for 45 min at 4 °C according to the manufacturer’s instructions, resuspended in phosphate-buffered saline, and finally analyzed by flow cytometry with phycoerythrin-conjugated mouse IgG2B as an isotype control.
RNA analysis and reverse transcription–PCR
Total RNA was extracted from treated cells according to the manufacturer’s instructions (Invitrogen).
Measurement of reactive oxygen species
To assay intracellular ROS, HCT116 cells were preincubated with 20 μM dichlorofluorescein diacetate (DCF DA) for 15 min at 37 °C and then treated with capsazepine. After 1 h of incubation, the increase in fluorescence resulting from the oxidation of DCF DA to DCF was measured by flow cytometry. The mean fluorescence intensity at 530 nm was calculated for at least 10,000 cells at a flow rate of 250–300 cells/s.
Western blot analysis
After the above specified treatments were completed, cells were incubated on ice for 30 min in 0.5 ml of ice-cold whole-cell lysate buffer (10% NP-40, 5 M NaCl, 1 M Hepes, 0.1 M EGTA, 0.5 M EDTA, 0.1 M phenylmethylsulfonyl fluoride, 0.2 M sodium orthovanadate, 1 M sodium fluoride, aprotinin (2 μg/ml), and leupeptin (2 μg/ml)). Proteins were then fractionated by SDS–polyacrylamide gel electrophoresis, electrotransferred to nitrocellulose membranes, blotted with each antibody, and assayed for antibody binding by enhanced chemiluminescence (GE Healthcare).
Transfection with siRNA
HCT116 cells were plated in each well of six-well plates and allowed to adhere for 24 h. On the day of transfection, 12 μl Hiperfect transfection reagent (Qiagen) was added to 50 nM siRNA in a final volume of 100 μl culture medium. After 24 h of transfection, the cells were treated with capsazepine for 6 h and then exposed to TRAIL for 20 h.
Statistical analysis
Statistic results in vitro were calculated using Student’s t test and analyzed in Microsoft Excel.
Results
The objective of this study was to determine whether capsazepine can sensitize human tumor cells to TRAIL-induced apoptosis and, if so, the underlying molecular mechanism responsible for this effect. Most of the experiments reported here were carried out in the human colorectal cancer cell line HCT116 but were also conducted in other cell lines to confirm applicability to other types of cancer cells.
Capsazepine potentiates TRAIL-mediated cell death
As shown by the cell viability assay, HCT116 cells were moderately sensitive to either capsazepine or TRAIL alone. However, when cells were incubated with combination of capsazepine and TRAIL, apoptosis was significantly potentiated compared with the cytokine alone (>40% vs 8%; Fig. 1B).
To correlate the effect of TRPV1 agonist or antagonist on DR expression with the effect on TRAIL-induced apoptosis, we pretreated cells with 30 μM TRPV1 antagonist (capsazepine) or agonist (capsaicin, evodiamine, or resiniferatoxin) for 6 h and then exposed them to TRAIL for 20 h. HCT116 cells so treated were moderately sensitive to either compound or TRAIL alone. Only capsazepine (Fig. 1C, upper left) and resiniferatoxin (Fig. 1C, lower right) could potentiate the cell death induced by TRAIL, although the effect of resiniferatoxin was not as impressive as that of capsazepine. The other two agonists, capsaicin and evodiamine, only minimally affected TRAIL-induced cell death (Fig. 1C, upper right and lower left).
We also examined whether capsazepine enhances the effect of TRAIL on long-term colony formation assay, which more closely mirrors the situation in vivo. Whereas capsazepine or TRAIL administered alone had little effect on the colony-forming ability of HCT116 cells, their administration in combination significantly enhanced it (Fig. 1D).
Because TRAIL is known to mediate apoptosis through the activation of caspase-8, caspase-9, and caspase-3, we examined the effect of capsazepine on the activation of these caspases and on TRAIL-induced cleavage of their substrate PARP. Whereas TRAIL administered alone had little effect on caspase activation and PARP cleavage, the addition of capsazepine greatly enhanced it (Fig. 1E). Taken together, our results indicate that capsazepine can enhance TRAIL-induced apoptosis.
TRPV1 antagonist capsazepine induces TRAIL receptor expression
We also assessed the ability of TRPV1 antagonist to modulate TRAIL receptor expression in HCT116 cells. Capsazepine upregulated DR5 and DR4 in HCT116 in a dose-dependent manner (Fig. 2A). Treatment of cells with 50 μM capsazepine optimally enhanced the upregulation of DRs without affecting cell viability.
Fig. 2.
TRPV1 antagonist capsazepine upregulates death receptor expression. (A) HCT116 cells were treated with various doses of capsazepine for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using TRAIL receptor antibodies. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (B) HCT116 cells were incubated with resiniferatoxin, capsaicin, or evodiamine at the indicated concentrations for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting using relevant antibodies. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (C) Cells were exposed to 50 μM evodiamine, capsaicin, resiniferatoxin, or capsazepine alone or in combination with each other compound for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting for DR4 and DR5 expression. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading.
Resiniferatoxin, a TRPV1 agonist, induces TRAIL receptor expression
To examine the ability of TRPV1 agonists, including capsaicin, to modulate the expression of TRAIL receptors, we exposed cells to the same concentration of capsazepine for 24 h, processed the cells to obtain whole-cell extracts, and then examined those extracts for expression of DR4 and DR5 proteins. Under these conditions, the TRPV1 agonist resiniferatoxin induced the expression of TRAIL receptors, whereas the other two TRPV1 agonists capsaicin and evodiamine did so only minimally (Fig. 2B).
Upregulation of TRAIL receptors by capsazepine is TRPV1-independent
To determine whether the upregulation of TRAIL receptors by capsazepine occurs through the TRPV1 receptor, we treated cells with TRPV1 agonist and antagonist alone or in combination and then assessed them for induction of death receptors. As shown in Fig. 2C, capsazepine (lane 2) and resiniferatoxin (lane 3) upregulated the expression of DR4 and DR5, whereas capsaicin (lane 4) and evodiamine (lane 5) did not. In addition, none of the three agonists could reverse the effect of capsazepine on induction of TRAIL receptors (Fig. 2C, lanes 6–8), suggesting that the upregulation of TRAIL receptors by capsazepine is not mediated through TRPV1 activation.
Capsazepine induces the expression of DRs but not DcR
We also examined the effect of capsazepine on decoy receptors. Capsazepine significantly increased the expression of both DR4 and DR5 (Fig. 3A) in a dose-dependent manner, but had no effect on the expression of decoy receptor DcR1 or DcR2 (Fig. 3A). Capsazepine also induced both DR4 and DR5 (Fig. 3B) in a time-dependent manner.
Fig. 3.
Capsazepine upregulates the expression of death receptors. HCT116 cells (4×105 cells/well) were treated with capsazepine (A) at the indicated concentrations and (B) for the indicated time periods. Whole-cell extracts were then prepared and analyzed by Western blotting for TRAIL receptor and CHOP expression. β-Actin was used as an internal control to show equal protein loading. (C) Capsazepine-induced gene expression of DRs was examined in HCT116 cells treated with capsazepine (50 μM) for the indicated time periods. Total RNA was extracted and examined for expression of DRs by RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to show equal RNA loading. (D) Capsazepine increased cell surface expression of DR5. Cell surface expression of DR4 and DR5 was measured by flow cytometry of HCT116 cells after capsazepine treatment for 24 h, using anti-DR4 and anti-DR5 antibodies conjugated with phycoerythrin. The filled gray peaks represent cells stained with a matched control phycoerythrin (PE)-conjugated IgG isotype antibody. The open peaks represent cells stained with PE-conjugated antibody against an individual DR. (E) Upregulation of DRs by capsazepine is not cell-type-specific. Cells (4×105 cells) were treated with 50 μM capsazepine for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting for DR4 and DR5 expression. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading.
Whether modulation of TRAIL receptors by capsazepine occurs at the transcriptional level was examined. As shown in Fig. 3C, capsazepine substantially induced the transcription of both DR4 and DR5 in a time-dependent manner.
Whether capsazepine induces cell surface expression of TRAIL receptors was also examined. As shown in Fig. 3D, capsazepine increased cell surface levels of both DR5 and DR4, although the level of DR4 expression was relatively lower.
Upregulation of DR4 and DR5 by capsazepine is not cell-type-specific
To determine whether upregulation of TRAIL receptors DR4 and DR5 by capsazepine was specific to HCT116 (colon adenocarcinoma cells), we also examined the receptor expression levels in pancreatic cancer (AsPC-1), esophageal cancer (SEG-1), head and neck cancer (SCC-4), cervical cancer (HeLa), colorectal cancer (HT-29), breast cancer (MDA-MB-231), lung cancer (H1299), and chronic myelogenous leukemia (KBM-5) cells that were exposed to capsazepine for 24 h. Capsazepine upregulated the expression of both DR5 and DR4 in most of these cell lines (Fig. 3E). Together, these findings suggest that the upregulation of DR5 and DR4 by capsazepine is not cell-type-specific.
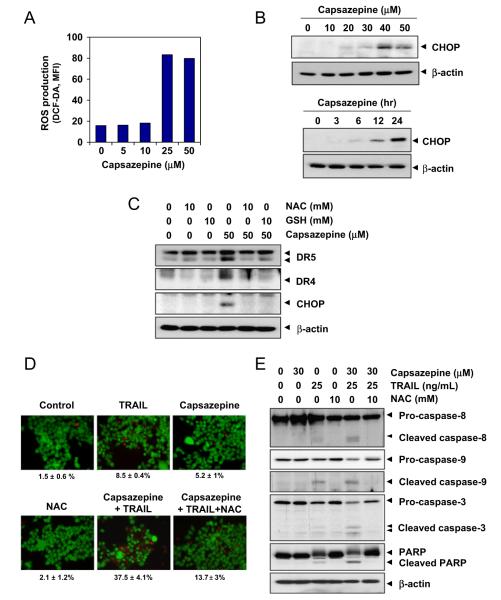
Capsazepine induces ROS generation
To determine the roles of ROS in capsazepine-induced DR expression, we assessed capsazepine’s ability to induce ROS production. As shown in Fig. 4A, the amount of ROS generated in treated cells was threefold higher than in untreated cells.
Fig. 4.
Capsazepine induces ROS generation and ROS mediate capsazepine-induced DR5 upregulation. (A) HCT116 cells (4×105 cells/well) were labeled with dichlorofluorescein diacetate, treated with the indicated doses of capsazepine for 1 h, and examined for ROS production. The ROS mean fluorescence intensity (MFI) increased with capsazepine dose. (B) HCT116 cells (4×105 cells/well) were either incubated with capsazepine at the indicated concentrations for 24 h or treated with 50 μM capsazepine for the indicated times (bottom). Whole-cell lysates were subjected to Western blotting analysis using relevant antibodies. (C) HCT116 cells were pretreated with NAC (10 mM) or glutathione (10 mM) for 1 h and then treated with 50 μM capsazepine for 24 h. Whole-cell extracts were prepared and subjected to Western blotting analysis using relevant antibodies. b (beta)-Actin was used as a loading control. (D) NAC reversed capsazepine and TRAIL-induced cell death. HCT116 cells were pretreated with NAC for 1 h and then treated with 30 μM capsazepine for 6 h. Then, the cells were treated with TRAIL (25 ng/ml) for 20 h. Cell death was assessed by using the Live/Dead cell viability assay. (E) NAC suppressed caspase activation and PARP cleavage induced by TRAIL and capsazepine. HCT116 cells were pretreated with NAC (10 mM) for 1 h and then treated with 30 μM capsazepine for 6 h. Next, the cells were treated with TRAIL (25 ng/ml) for 20 h. Whole-cell extracts were prepared and analyzed by Western blotting for caspase and PARP expression. β-Actin was used as a loading control.
Capsazepine upregulates CHOP expression
In light of numerous reports that induction of DR5 is regulated by CHOP [26,27], we assessed capsazepine’s ability to modulate the expression of CHOP. As shown by Western blot analysis, capsazepine induced CHOP expression in a dose- and time-dependent manner (Fig. 4B). Induction of CHOP occurred at 6 h and was sustained up to 24 h after capsazepine treatment (Fig. 4B, bottom).
Upregulation of DR by capsazepine requires both ROS and CHOP
We assessed the dependence of capsazepine-induced upregulation of DR4 and DR5 on ROS in cells that were pretreated with the ROS scavenger NAC or GSH for 1 h and then exposed to capsazepine for 24 h. Capsazepine significantly upregulated DR4 and DR5 expression, whereas pretreatment with NAC completely abrogated induction of DRs by capsazepine (Fig. 4C). Glutathione also blocked the effect of capsazepine on DR expression (Fig. 4C).
We also examined the effect of ROS production on capsazepine-induced CHOP expression. As revealed in Fig. 4C, pretreatment with NAC or GSH abolished the capsazepine-induced expression of CHOP, clearly indicating that ROS generation is essential for capsazepine-induced upregulation of DR4 and DR5.
NAC reverses the effect of capsazepine on TRAIL-induced cell death
Furthermore, we examined the effect of ROS on capsazepine’s ability to enhance TRAIL-induced cell death. As shown in Fig. 4D, capsazepine’s ability to enhance TRAIL-induced cell death was markedly greater in NAC-untreated cells than in NAC-pretreated cells (38% vs 14%), indicating that ROS are required for capsazepine-induced sensitization of cancer cells to TRAIL.
Consequently, we assessed the ability of NAC pretreatment to attenuate capsazepine-induced increases in caspase-8, caspase-9, and caspase-3 activation and PARP cleavage. As shown in Fig. 4E, capsazepine potentiated TRAIL-induced caspase-8, caspase-9, and caspase-3 and PARP cleavage readily in NAC-naïve cells but markedly less so in NAC-pretreated cells (Fig. 4E), again indicating the critical role of ROS in mediating capsazepine’s effect on TRAIL-induced apoptosis.
Capsazepine upregulates DRs through JNK
Because JNK had previously been implicated in the upregulation of DRs [28–31], we assessed the ability of capsazepine to activate p38 MAPK, JNK, and ERK1/2. Capsazepine activated p38 MAPK, JNK, and ERK1/2 in a dose-dependent manner (Fig. 5A).
Fig. 5.
Capsazepine-induced DR5 upregulation requires CHOP induction through JNK activation, and NAC blocks capsazepine-induced activation of JNK signaling. (A) To determine the involvement of ERK1/2, p38 MAPK, and JNK in the upregulation of DR5, HCT116 cells were treated with 50 μM capsazepine for various time periods. Whole-cell extracts were prepared and analyzed by Western blotting for phosphorylated JNK, ERK1/2, and p38 MAPK expression. The same blots were stripped and reprobed with JNK1, ERK1/2, and p38 MAPK antibodies to verify equal protein loading. (B) HCT116 cells (4×105 cells/well) were incubated with a p38 MAPK inhibitor (SB202190), JNK inhibitor (SP600125), and ERK1/2 inhibitor (PD98059) for 1 h and then treated with 50 μM capsazepine for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting for DR5 and DR4 expression. The same blots were stripped and reprobed with antibodies for nonphosphorylated proteins to verify equal protein loading. (C) HCT116 cells were incubated with 50 μM capsazepine for the indicated time periods, and whole-cell lysates were prepared and subjected to Western blotting analysis using relevant antibodies. (D) HCT116 cells (4×105 cells/well) were pretreated with SP600125 at the indicated concentrations for 1 h and then exposed to 50 μM capsazepine for 12 h. Whole-cell extracts were prepared and subjected to Western blotting analysis using relevant antibodies. β-Actin was used as a loading control. (E) HCT116 cells (4×105 cells/well) were seeded, incubated with 10 mM NAC for 1 h, and then treated with 50 μM capsazepine for 12 h. Whole-cell extracts were prepared and subjected to Western blotting analysis using relevant antibodies. β-Actin was used as a loading control. (F) HCT116 cells (3×105 cells/well) were transfected with either CHOP siRNA or control siRNA (scrambled RNA; scRNA). Twenty-four hours after transfection, the cells were reseeded in six-well plates or chamber slides. (Left) Cells were treated with 50 μM capsazepine for 24 h, and whole-cell lysates were analyzed by Western blotting. The same blots were stripped and reprobed with anti-β-actin antibody to verify equal protein loading. (Right) Cells were exposed to 30 μM capsazepine for 6 h and then treated with 25 ng/ml TRAIL for 20 h. Cell death was assessed by using the Live/Dead cell viability assay.
We further assessed the involvement of JNK, p38 MAPK, and ERK1/2 in capsazepine-induced upregulation of DR using pharmacological inhibitors of ERK1/2 (PD98059), p38 MAPK (SB202190), and JNK (SP600125). The JNK inhibitor (SP600125) inhibited DR upregulation, whereas the ERK1/2 and p38 MAPK inhibitors did not (Fig. 5B), thereby suggesting that JNK activation is responsible for upregulation of DRs.
Our subsequent analysis of the time course of JNK activation showed that phosphorylation of c-Jun was time-dependent, that JNK was activated as little as 2 h after capsazepine treatment (Fig. 5C), and that capsazepine-induced transcription of DR4 and DR5 occurred 3 to 12 h after treatment (see Fig. 3B). Together, these time-course findings indicate that JNK activation might induce DR expression.
Inhibition of JNK blocks CHOP induction mediated by capsazepine
To determine whether JNK is involved in the induction of CHOP and DR5 upregulation, we pretreated cells with various concentrations of the specific inhibitor of JNK (SP600125) for 1 h and then exposed them to capsazepine for 12 h. Capsazepine induced phosphorylation of c-Jun, whereas SP600125 inhibited it in a dose-dependent manner (Fig. 5D). Interestingly, the inhibition of JNK activation also suppressed the induction of CHOP induced by capsazepine (Fig. 5D, third lane). Therefore, the modulation of DR5 expression correlated with activation of JNK and CHOP. Together, these results suggest that JNK mediates the induction of CHOP, thus leading to capsazepine-induced upregulation of DR5.
NAC reverses capsazepine-induced JNK activation and CHOP expression
Because the upmodulation of DRs by capsazepine occurs through JNK activation, we further examined whether ROS work upstream of JNK activation. As shown in Fig. 5E, capsazepine alone activated JNK (c-Jun phosphorylation) and the antioxidant NAC reversed such activation. In addition, NAC also inhibited the capsazepine-mediated induction of CHOP and DR expression. Collectively, these results indicate that DR5 induction is mediated through the ROS–JNK–CHOP pathway.
Capsazepine induces DR upregulation through CHOP
To determine the role of CHOP in capsazepine-induced upregulation of the death receptors, we used siRNA specific to CHOP. The upregulation of DR5 by capsazepine was effectively abolished in cells transfected with CHOP siRNA (Fig. 5F, left), but not in untransfected cells or cells transfected with control siRNA (scrambled RNA). The silencing of CHOP also significantly affected the upregulation of DR4.
We also examined the ability of siRNA-mediated suppression of CHOP to abrogate the sensitizing effects of capsazepine on TRAIL-induced cell death. CHOP siRNA effectively diminished the effects of capsazepine on TRAIL-induced cell death (from 38 to 26%; Fig. 5F, right, middle row), whereas control siRNA had no effect (Fig. 5F, right, bottom row). These results suggest that CHOP plays a major role in upregulation of DR5 and contributes to the sensitizing effect of capsazepine on TRAIL-induced cell death.
Capsazepine downmodulates the expression of cell survival proteins
To determine whether there are mechanisms other than DR induction by which capsazepine might enhance TRAIL-induced apoptosis, we treated cells with various concentrations of capsazepine for 24 h and then examined them for expression of antiapoptotic proteins using relevant antibodies. Capsazepine downregulated the expression of cFLIP, survivin, Bcl-xL, Bcl-2, and cIAP-1 (Fig. 6A). The downregulation of cFLIP, however, was quite dramatic and dose-dependent, whereas that of Bcl-xL, Bcl-2, and cIAP-1 was much less pronounced. Overall, these results suggest that capsazepine can also sensitize tumor cells by downregulating cell survival proteins.
Fig. 6.
Capsazepine modulates expression of proteins involved in apoptosis. HCT116 cells were treated with capsazepine at the indicated concentrations for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting with the indicated antibodies to detect (A) antiapoptotic proteins and (B) proapoptotic proteins (i.e., Bax and p53). The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (C) Wild-type and p53 knockout HCT116 cells (1×106 cells/well) were treated with capsazepine at various concentrations for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting with p53 and DR5 antibodies. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (D) NAC reversed the capsazepine-induced inhibition of antiapoptotic proteins. HCT116 cells were pretreated with NAC for 1 h and then treated with 50 μM capsazepine for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading.
Capsazepine upregulates the expression of proapoptotic proteins Bax and p53
We further examined capsazepine’s ability to regulate the expression of proapoptotic proteins and found that capsazepine upregulated the expression of Bax and p53 in a dose-dependent manner (Fig. 6B). These results suggest that capsazepine can upregulate proapoptotic proteins as well.
p53 is not required for capsazepine-induced TRAIL receptor upregulation
In addition to its proapoptotic role, p53 also has been known to induce DR expression [32,33]. To determine whether p53 is needed for capsazepine-induced DR induction, we used a HCT116 variant in which p53 is deleted. Capsazepine induced DR5 as well as DR4 in both wild-type and p53-deleted cells, thus indicating that p53 plays no role in the induction of either DR by capsazepine (Fig. 6C).
Bax is not required for induction of DR by capsazepine but Is involved in potentiation of TRAIL-induced cell death
Whether the proapoptotic protein Bax is required for the expression of DR by capsazepine was examined in Bax-deleted cells. Results show that capsazepine upregulated the expression of DR5 and Bax in wild-type cells. The deletion of Bax, however, had no effect on the induction of these receptors by capsazepine (Supplementary Fig. S1A).
To determine whether Bax is needed for the effect of capsazepine on TRAIL-induced cell death, we examined PARP cleavage. Enhanced PARP cleavage was found when TRAIL was combined with capsazepine in wild-type cells. This cleavage, however, was abolished in Bax-deleted cells (Supplementary Fig. S1B), indicating the critical role of Bax protein in apoptosis but not in induction of receptors.
NAC abrogates capsazepine-induced suppression of antiapoptotic proteins
Finally, we investigated whether NAC could reverse the suppression of antiapoptotic proteins induced by capsazepine. Under our experimental conditions, pretreatment with the antioxidant NAC effectively inhibited upregulation of DR4 and DR5 and abrogated the effect of capsazepine on suppression of XIAP, survivin, and cIAP-1 (Fig. 6D), thus indicating that pretreatment of NAC can indeed reverse the effect of capsazepine on proapoptotic protein Bax but not significantly so.
Discussion
Agents that can sensitize tumor cells to TRAIL have tremendous therapeutic potential. Our present results indicate that capsazepine is one such agent and that its ability to sensitize tumor cells to TRAIL involves multiple mechanisms. First, we found that selective upregulation of agonistic TRAIL receptors DR4 and DR5 had no effect on the antagonistic decoy receptors. Second, we found that the quenching of capsazepine-induced ROS generation abolished the sensitization of cancer cells to TRAIL. Third, we found that capsazepine activated JNK, which was needed for sensitization to TRAIL. Fourth, we observed that upregulation of CHOP protein expression by capsazepine was also critical for sensitization. Fifth, we found that capsazepine downregulated cFLIP and survivin, two antiapoptotic proteins that have been linked to TRAIL resistance. Finally, we noted that capsazepine upregulated the proapoptotic p53 protein and induced expression of Bax, another mediator of apoptosis. Together, all of these mechanisms can contribute to the sensitization of tumor cells to TRAIL, and in any given tumor cell type more than one mechanism of resistance to TRAIL may be at work. Capsazepine seems to mediate its effects through multiple mechanisms.
Our present findings strongly suggest that the induction of TRAIL receptors is linked to the ROS–JNK–CHOP pathway. The role of JNK in the induction of DR5 by boswellic acid and celastrol has been reported previously [34,35]. In our present study, we found that capsazepine activated both JNK and p38 MAPK, but that only the activated JNK was involved in the induction of death receptors. In addition, we found that capsazepine could induce death receptors independent of p53, even though the proapoptotic p53 protein is known to be involved in upregulation of DR5 [36,37]. Indeed, we observed the expression of DR5 in cell lines expressing wild-type 53 (i.e., HCT116, SEG-1, and HeLa), cell lines expressing mutant p53 (i.e., MDA-MB-231, SCC-4, and KBM-5), and even p53-deleted cell lines (i.e., H1299, AsPC-1, and HT-29) (Fig. 3E). We also observed that capsazepine upregulated DR5 with similar potency in p53-knockout HCT116 cells (Fig. 6C). It should be noted, however, that capsazepine’s ability to induce death receptors was highly dependent on the expression of CHOP.
We also found that capsazepine downregulated all of the antiapoptotic proteins tested, including cFLIP, survivin, XIAP, Bcl-xL, and Bcl-2. The effect, however, was most pronounced on cFLIP, XIAP, and survivin. Interestingly, we found that the effect of capsazepine on these proteins was mediated through ROS and that NAC reversed the downregulation. These observations of ours are important because overexpression of cFLIP, XIAP, and survivin has been shown to be tightly linked to TRAIL resistance in a variety of tumors including bladder cancer [12], breast cancer [38,39], colon cancer [40], and kidney cancer [13]. In addition, our results show that capsazepine can upregulate proapoptotic proteins p53 and Bax and thereby potentially enhance TRAIL-induced apoptosis.
Although capsazepine was discovered as a capsaicin antagonist [21,41] that binds to the vanilloid receptor TRPV1, our present evidence indicates that TRPV1 receptors are probably not involved in the upregulation of death receptors. First, the vanilloid receptor TRPV1 agonist capsaicin had minimal effect on the expression of death receptors; second, capsaicin failed to antagonize the capsazepine-induced expression of the receptors; third, evodiamine, another TRPV1 agonist, had little effect on DR expression either alone or in combination with capsazepine; fourth, the TRPV1 superagonist resiniferatoxin also induced expression of the death receptors with or without capsazepine. Together, these observations suggest that expression of DR by capsazepine most probably occurs independent of TRPV1. Interestingly, capsazepine has been shown to suppress lipopolysaccharide-induced inducible NO synthase expression through the downregulation of NF-κB activation in macrophages [25]. Whether this effect of capsazepine is mediated through the vanilloid receptor is not clear. It is possible, however, that the downregulation of NF-κB by capsazepine is involved in the downregulation of antiapoptotic proteins shown in our studies. Recently, others have shown that capsazepine could prevent ovariectomy-induced bone loss in mice at a dose as low as 1 mg/kg/day [24]. Whether this effect of capsazepine is mediated through the vanilloid receptor is also unclear. However, the evidence does suggest that capsazepine is safe and can mediate its effect at very low doses in mice. Therefore, in light of these other findings and our present findings that capsazepine can enhance the sensitivity of tumor cells to TRAIL through multiple mechanisms, future animal studies of capsazepine’s potential for sensitizing tumors to TRAIL are warranted.
Supplementary Material
Acknowledgments
We thank Jan Baker for carefully editing the manuscript. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a core grant from the National Institutes of Health (CA-106672), and a grant from the Center for Targeted Therapy of M.D. Anderson Cancer Center.
Abbreviations
- CHOP
CCAAT/enhancer-binding protein homologous protein
- cIAP
cellular inhibitor of apoptosis
- DR
death receptor
- GSH
glutathione
- JNK
c-Jun N-terminal kinases
- NAC
N-acetylcysteine
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- TRPV1
transient receptor potential cation channel subfamily V member 1
- XIAP
X-linked IAP
Footnotes
Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.freeradbiomed.2012.08.012.
References
- [1].Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- [2].Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- [3].Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R, Gliniak B. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur. J. Immunol. 2002;32:2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [4].Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- [5].Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J. Exp. Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- [8].Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- [9].Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br. J. Cancer. 2010;102:506–512. doi: 10.1038/sj.bjc.6605507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Song JJ, An JY, Kwon YT, Lee YJ. Evidence for two modes of development of acquired tumor necrosis factor-related apoptosis-inducing ligand resistance: involvement of Bcl-xL. J. Biol. Chem. 2007;282:319–328. doi: 10.1074/jbc.M608065200. [DOI] [PubMed] [Google Scholar]
- [11].Sivaprasad U, Shankar E, Basu A. Downregulation of Bid is associated with PKCepsilon-mediated TRAIL resistance. Cell Death Differ. 2007;14:851–860. doi: 10.1038/sj.cdd.4402077. [DOI] [PubMed] [Google Scholar]
- [12].Shrader M, Pino MS, Lashinger L, Bar-Eli M, Adam L, Dinney CP, McConkey DJ. Gefitinib reverses TRAIL resistance in human bladder cancer cell lines via inhibition of AKT-mediated X-linked inhibitor of apoptosis protein expression. Cancer Res. 2007;67:1430–1435. doi: 10.1158/0008-5472.CAN-06-1224. [DOI] [PubMed] [Google Scholar]
- [13].Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–923. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- [14].Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–2065. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [15].Jin Z, McDonald ER, 3rd, Dicker DT, El-Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J. Biol. Chem. 2004;279:35829–35839. doi: 10.1074/jbc.M405538200. [DOI] [PubMed] [Google Scholar]
- [16].Kurbanov BM, Fecker LF, Geilen CC, Sterry W, Eberle J. Resistance of melanoma cells to TRAIL does not result from upregulation of antiapoptotic proteins by NF-kappaB but is related to downregulation of initiator caspases and DR4. Oncogene. 2007;26:3364–3377. doi: 10.1038/sj.onc.1210134. [DOI] [PubMed] [Google Scholar]
- [17].Kang S, Park SY, Lee HJ, Yoo YH. T. R. A. I. L. upregulates decoy receptor 1 and mediates resistance to apoptosis in insulin-secreting INS-1 cells. Biochem. Biophys. Res. Commun. 2010;396:731–735. doi: 10.1016/j.bbrc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- [18].Bernard D, Quatannens B, Vandenbunder B, Abbadie C. Rel/NF-kappaB transcription factors protect against tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by up-regulating the TRAIL decoy receptor DcR1. J. Biol. Chem. 2001;276:27322–27328. doi: 10.1074/jbc.M011183200. [DOI] [PubMed] [Google Scholar]
- [19].Bouralexis S, Findlay DM, Atkins GJ, Labrinidis A, Hay S, Evdokiou A. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br. J. Cancer. 2003;89:206–214. doi: 10.1038/sj.bjc.6601021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim CH, Gupta S. Expression of TRAIL (Apo2L), DR4 (TRAIL receptor 1), DR5 (TRAIL receptor 2) and TRID (TRAIL receptor 3) genes in multidrug resistant human acute myeloid leukemia cell lines that overexpress MDR 1 (HL60/Tax) or MRP (HL60/AR) Int. J. Oncol. 2000;16:1137–1139. doi: 10.3892/ijo.16.6.1137. [DOI] [PubMed] [Google Scholar]
- [21].Urban L, Dray A. Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci. Lett. 1991;134:9–11. doi: 10.1016/0304-3940(91)90496-g. [DOI] [PubMed] [Google Scholar]
- [22].Menendez L, Juarez L, Garcia E, Garcia-Suarez O, Hidalgo A, Baamonde A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci. Lett. 2006;393:70–73. doi: 10.1016/j.neulet.2005.09.046. [DOI] [PubMed] [Google Scholar]
- [23].Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Idris AI, Landao-Bassonga E, Ralston SH. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone. 2010;46:1089–1099. doi: 10.1016/j.bone.2010.01.368. [DOI] [PubMed] [Google Scholar]
- [25].Oh GS, Pae HO, Seo WG, Kim NY, Pyun KH, Kim IK, Shin M, Chung HT. Capsazepine, a vanilloid receptor antagonist, inhibits the expression of inducible nitric oxide synthase gene in lipopolysaccharide-stimulated RAW264.7 macrophages through the inactivation of nuclear transcription factor-kappa B. Int. Immunopharmacol. 2001;1:777–784. doi: 10.1016/s1567-5769(01)00012-1. [DOI] [PubMed] [Google Scholar]
- [26].Yamaguchi H, Wang HG. C. H. O. P. is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- [27].Yoshida T, Shiraishi T, Nakata S, Horinaka M, Wakada M, Mizutani Y, Miki T, Sakai T. Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Res. 2005;65:5662–5667. doi: 10.1158/0008-5472.CAN-05-0693. [DOI] [PubMed] [Google Scholar]
- [28].Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- [29].Ohtsuka T, Zhou T, Bisindolylmaleimide V. I. I. I. enhances DR5-mediated apoptosis through the MKK4/JNK/p38 kinase and the mitochondrial pathways. J. Biol. Chem. 2002;277:29294–29303. doi: 10.1074/jbc.M203342200. [DOI] [PubMed] [Google Scholar]
- [30].Shenoy K, Wu Y, Pervaiz S. LY303511 enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via hydrogen peroxide-mediated mitogen-activated protein kinase activation and up-regulation of death receptors. Cancer Res. 2009;69:1941–1950. doi: 10.1158/0008-5472.CAN-08-1996. [DOI] [PubMed] [Google Scholar]
- [31].Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, Bailey R, Maric D, Zenklusen JC, Lee J, Fine HA. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation. Cancer Res. 2008;68:6643–6651. doi: 10.1158/0008-5472.CAN-08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tomasetti M, Andera L, Alleva R, Borghi B, Neuzil J, Procopio A. Alpha-tocopheryl succinate induces DR4 and DR5 expression by a p53-dependent route: implication for sensitisation of resistant cancer cells to TRAIL apoptosis. FEBS Lett. 2006;580:1925–1931. doi: 10.1016/j.febslet.2006.02.054. [DOI] [PubMed] [Google Scholar]
- [33].Chen JJ, Chou CW, Chang YF, Chen CC. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J. Immunol. 2008;180:8030–8039. doi: 10.4049/jimmunol.180.12.8030. [DOI] [PubMed] [Google Scholar]
- [34].Lu M, Xia L, Hua H, Jing Y. Acetyl-keto-beta-boswellic acid induces apoptosis through a death receptor 5-mediated pathway in prostate cancer cells. Cancer Res. 2008;68:1180–1186. doi: 10.1158/0008-5472.CAN-07-2978. [DOI] [PubMed] [Google Scholar]
- [35].Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J. Biol. Chem. 2010;285:11498–11507. doi: 10.1074/jbc.M109.090209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [36].Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, el-Deiry WS. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat. Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- [37].Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ;, Jr, el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998;58:1593–1598. [PubMed] [Google Scholar]
- [38].Lee TJ, Lee JT, Park JW, Kwon TK. Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIP(L) and XIAP protein levels and ERK activation. Biochem. Biophys. Res. Commun. 2006;351:1024–1030. doi: 10.1016/j.bbrc.2006.10.163. [DOI] [PubMed] [Google Scholar]
- [39].Yoshida T, Zhang Y, Rivera Rosado LA, Zhang B. Repeated treatment with subtoxic doses of TRAIL induces resistance to apoptosis through its death receptors in MDA-MB-231 breast cancer cells. Mol. Cancer Res. 2009;7:1835–1844. doi: 10.1158/1541-7786.MCR-09-0244. [DOI] [PubMed] [Google Scholar]
- [40].Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64:3006–3008. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- [41].Dickenson AH, Dray A. Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. Br. J. Pharmacol. 1991;104:1045–1049. doi: 10.1111/j.1476-5381.1991.tb12547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.