Abstract
The aim of this study was to determine the role of NADPH-cytochrome P450 reductase (CPR) and CPR-dependent enzymes in neural stem cell (NSC) genesis in the brain. A mouse model with globally suppressed Cpr gene expression (Cpr-low mouse) was studied for this purpose. Cpr-low and wild-type (WT) mice were compared immunohistochemically for the expression of markers of cell proliferation (Ki67), immature neurons (doublecortin, DCX), oligodendrocytes (oligodendrocyte transcription factor 2, OLIG2), and astrocytes (glial fibrillary acidic protein, GFAP) in the SVZ, and for the in vitro capability of their SVZ cells to form neurospheres and differentiate into astrocytes. We found that the abundance of SVZ cells that are positive for Ki67 or GFAP expression, but not the abundance of SVZ cells that are positive for DCX and OLIG2 expression, was significantly increased in Cpr-low mice, at various ages, compared with WT mice. Furthermore, extents of astrocyte differentiation and growth, but not neurosphere formation, from SVZ cells of the Cpr-low mice were significantly increased, compared with WT mice. These results suggest that CPR and CPR-dependent enzymes play a role in suppressing astrocytosis in the SVZ of adult mice.
Keywords: Cytochrome P450 reductase, astrocytosis, subventricular zone, neural stem cells, mice
1. Introduction
Neural stem cells (NSCs) are self-renewing and multipotent cells, capable of generating neurons, astrocytes, and oligodendrocytes. NSCs only exist in the subventricular zone (SVZ) and the subgranular zone (SGZ) of hippocampal dentate gyrus (DG) in adult mammalian brain, offering therapeutic potential for neural injury and neurodegenerative diseases [21]. NSCs and related precursor cells can be stimulated to differentiate by exogenous cues from the microenvironment or stem cell niche; the differentiation can also be regulated by many endogenous factors, including steroid hormones and cytokines [9].
Cytochrome P450 (P450) enzymes are responsible for metabolizing many foreign chemical compounds as well as endogenous substances [22]. NADPH-cytochrome P450 reductase (CPR), a 678-amino acid microsomal flavoprotein, is required for all microsomal P450 activities [16]. CPR is commonly expressed in various parts of the CNS, including at high levels in the olfactory bulb and hippocampus, two regions where neurogenesis occurs, and in both neurons and glial cells [15, 4]. A marked increase in the expression of CPR protein was observed in the brain of patients with Alzheimer disease [17], in which neurogenesis was thought to be inhibited [6]. However, the roles of CPR and CPR-dependent enzymes in the genesis or differentiation of NSCs and related precursor cells are still unknown. We assumed that suppression of CPR activity in the brain will have a significant impact on NSC genesis, possibly through changes in the homeostasis of endogenous compounds, such as sex steroid and cholesterol.
In order to investigate the role of CPR-dependent enzymes in the proliferation and differentiation of NSCs in adult mice, a unique knockdown mouse model, named Cpr-low, was used in this study. The Cpr-low mouse has 70–90% suppression in the levels of CPR expression in all organs examined, including the brain, which had 90% decrease [26]. The reason for the low CPR expression is the presence of a neo gene in its last intron, which dictates the universal nature of the down-regulation of CPR expression [23], including in the SVZ. Cpr-low mice, which have a normal life-span, showed no abnormalities in the brain when examined by histopathology [26]. However, adult Cpr-low mice exhibited lowered plasma cholesterol levels, mild hepatic lipidosis, and increased serum and tissue levels of testosterone and progesterone [26]. The Cpr-low mouse is a useful model for human patients harboring mutations that affect CPR expression. Here, we used special markers of cell proliferation and cell types for immunohistochemical analysis of cells in the SVZ of WT and Cpr-low mice. We also performed neurosphere culture assays for comparing the in vitro ability of SVZ NSCs from the two mouse strains to undergo self-renewal and differentiation [13].
2. Materials and methods
2.1 Animal models
All animal studies were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. The generation of the Cpr-low mice has been reported previously; the mice were backcrossed to a C57BL/6J background for at least 6 generations. Genotyping of the mice was conducted by PCR analysis of tail DNA for the Cpr-low allele [26]. Adult male and female mice (2, 6, and 9 months old) were used for the study, and wild-type (WT) C57BL/6J mice were use as controls.
2.2 Immunohistochemical analysis
Age-matched Cpr-low and WT mice (3 mice/group, male and female) at the age of 2, 6, 9 months were examined by immunohistochemical (IHC) analysis for the markers described below. Mice were sacrificed by CO2 overdose. Mouse brains were embedded in OCT and frozen on dry ice, and 20-μm coronal sections (approximately at interaural 4.54–4.30 mm, bregma 0.74–0.50 mm) were prepared using a cryostat, according to a published protocol [20]. The primary and secondary antibodies were used as follows, rabbit anti-Ki67 (Thermo Scientific, 1:200) with Alexa 596-conjugated secondary antibody (Invitrogen); rabbit anti-GFAP (Dako, 1:1000) and Alexa 488-conjugated secondary antibody (Invitrogen); goat anti-DCX, (Abcam, 1:200) and Cy5-conjugated secondary antibody (Jackson Immunology Laboratory); rabbit anti-Olig2 (Dr. Charles Stile, 1:4000) and Alexa 488-conjugated secondary antibody (Invitrogen). The nuclei were stained with DAPI (Invitrogen, 1:2000). Images were taken using a 2-photon confocal microscope (Zeiss, Germany). Positive cells (identified by immunofluorescence in the nucleus (for Ki67 and Olig2) or cytoplasm (for GFAP) were counted manually (at 100 X magnification) on five slides from each brain; each slide contained both right and left SVZ. For DCX, a cytoplasmic marker for immature neurons, which are small in size, have a tendency to appear in clusters in the SVZ sections, and thus difficult to count in numbers, we used integrated areas of immunofluorescence to represent abundance of positive cells. Areas that were positive for DCX on each section were quantified using Image J software (NIH) with a threshold value of 150 (8-bit, B&W), and expressed in arbitrary units.
2.3 Neurosphere culture
NSCs from the SVZ of Cpr-low and WT mice (female, 6 month old) were dissected and plated in non-coated 6-well plates (Corning) at a density of 10,000 cells/well (5000 cells/ml, 2 ml/well) in B27 DMEM medium containing bFGF (20 ng/ml) and EGF (20 ng/ml). The pooled SVZ cells from 3–5 mice were plated in 3 wells. The numbers of neurospheres (>25 μm in diameter) were counted in each well and normalized by the total number of cells after culturing for 7 days. For neurosphere differentiation assay, neurospheres were plated in poly-L-lysine-coated 6-well plates (Corning) without the growth factors. The neurospheres, which were allowed to adhere to the plate and differentiate for 5 days, were fixed with 4% paraformaldehyde, and, following an incubation with 3% BSA, were analyzed by IHC using the antibodies to GFAP, DCX and OLIG2, as described above.
2.4 Statistical analysis
All data are expressed as means ± S.D. Statistical analysis was performed using GraphPad Prism (GraphPad, San Diego, CA). For cell type abundance in SVZ, statistical significance was assessed using two-way ANOVA (genotype and age), followed by Bonferroni Post hoc test; data from each sex were analyzed separately. Results of neurosphere culture assay were analyzed using Student’s t-test. P<0.05 was considered statistically significant.
3. Results
3.1 Abundances of Ki67-positive cells and GFAP-positive cells in the SVZ of Cpr-low and WT mice
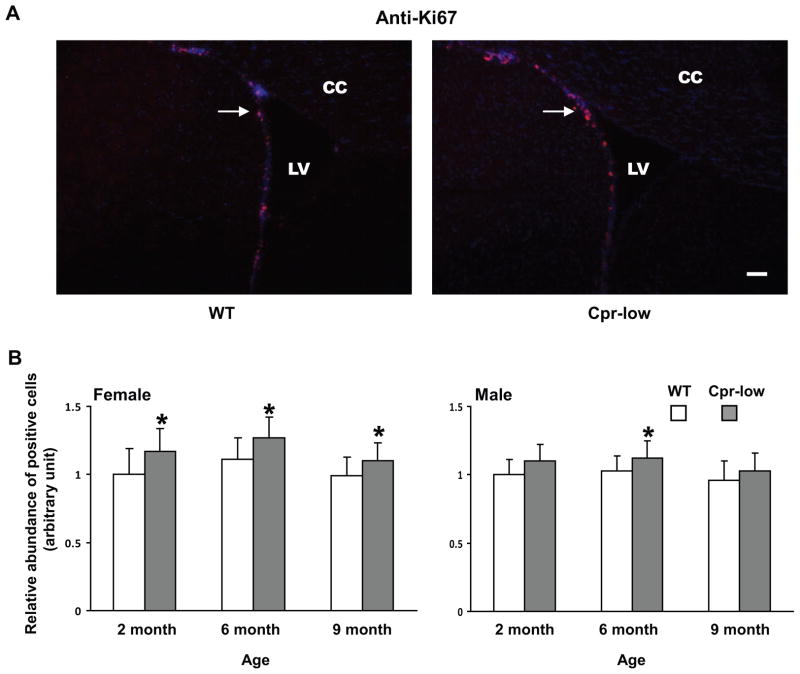
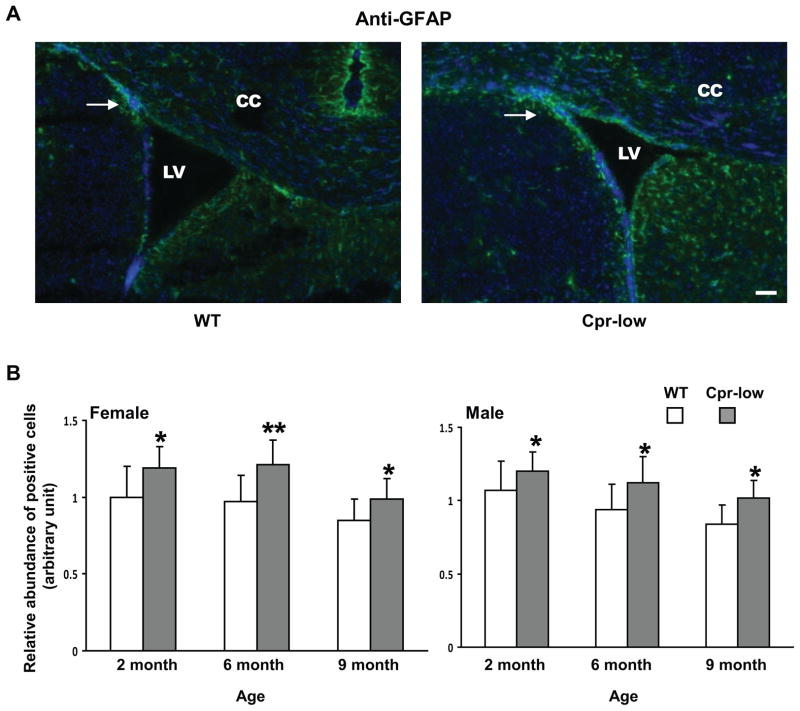
Ki67-positive cells and GFAP-positive cells were detected in the SVZ of both Cpr-low mice and WT mice (Fig. 1--1 and Fig. 1-2). The abundance of Ki67-positive cells was significantly higher in Cpr-low mice than in WT mice, for both males (at 6 months) and females (at 2, 6, and 9 months) (Fig. 1--1, A and B). Similarly, the abundance of GFAP-positive cells was significantly higher in both male and female Cpr-low mice than in WT mice, at 2, 6 and 9 months of age (Fig. 1-2, A and B).
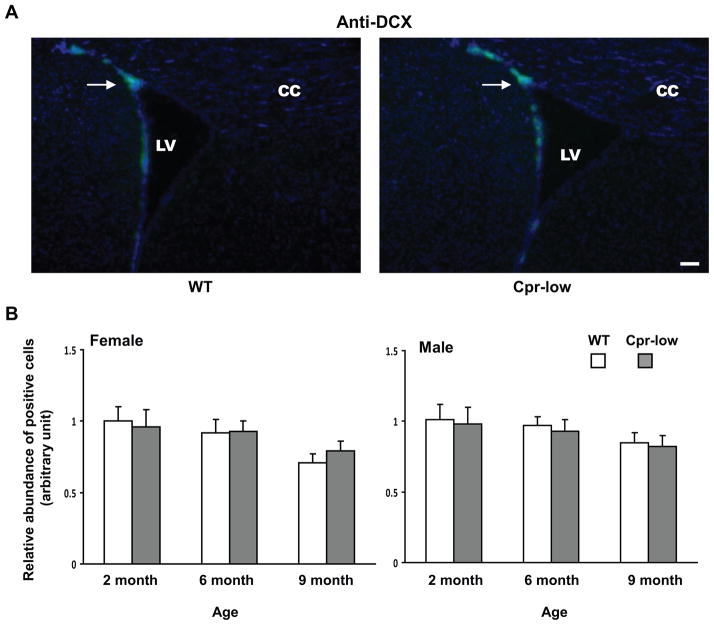
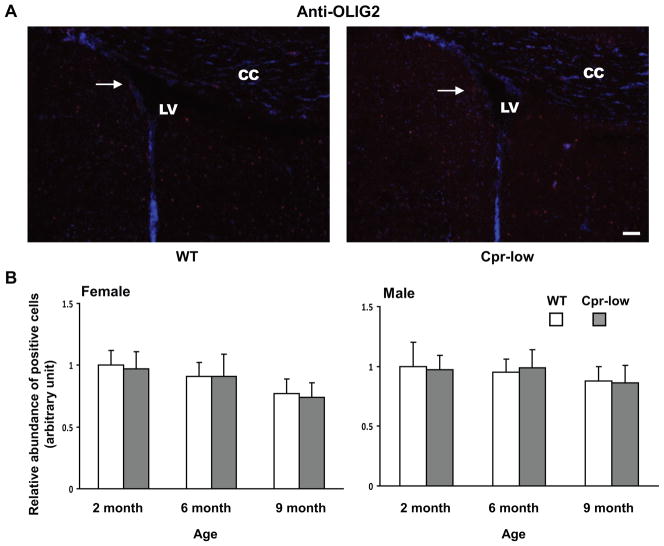
Fig. 1. Abundances of cells positive for Ki67, GFAP, DCX or OLIG2 in the SVZ of Cpr-low and WT mice at various ages.
Adult Cpr-low and WT mice (three mice per age or sex group) were studied. Coronal sections covering the SVZ region from each mouse brain were analyzed by IHC for Ki67 (Fig. 1--1, positive (proliferating) cells in red), GFAP (Fig. 1-2, positive cells (astrocytes) in green), DCX (Fig. 1-3, positive cells (immature neurons) in dark blue), OLIG2 (Fig. 1-4, positive cells (oligodendrocytes) in red). A. Representative IHC images. Images shown are for female mice at 6 months of age. All sections were counter-stained with DAPI. Examples of positive cells in the SVZ are indicated by arrows. Scale bar, 100 μm. LV, lateral ventricle; CC, corpus callosum. B. Quantitative analysis of the abundance of positive cells in each group. The values reported are means ± S.D. for three mice per group (n=3); for each mouse, the result was based on the averaged value from five separate slides, each containing both right and left SVZ. For Ki67, GFAP, and OLIG2, positive cells were counted manually, and the results are shown as relative abundance (in arbitrary units), with the mean (averaged) number of positive cells in 2-month-old male or female WT mice (which were 87, 84, and 140 for males, and 84, 82, and 137 for females, for Ki67, GFAP, and OLIG2, respectively) set as 1. For DCX, areas containing positive cells were integrated as described in Methods and the results are shown as relative abundance (in arbitrary units), with the mean value in 2-month-old male or female WT mice (36729 for females and 37731 for males) set as 1. *, P<0.05; **, P<0.01; Cpr-low vs WT mice (two-way ANOVA).
3.2 Abundance of DCX-positive cells and OLIG2-positive cells in the SVZ of Cpr-low and WT mice
DCX-positive cells and OLIG2-positive cells were detected in the SVZ of both Cpr-low and WT mice (Fig. 1-3 and Fig. 1-4). However, the relative abundance of either DCX-positive cells or OLIG2-positive cells remained constant between Cpr-low mice and WT mice, at 2, 6, and 9 months, in both males and females (Fig. 1-3B and Fig. 1-4B).
3.3 Neurosphere formation from SVZ cells of Cpr-low and WT mice
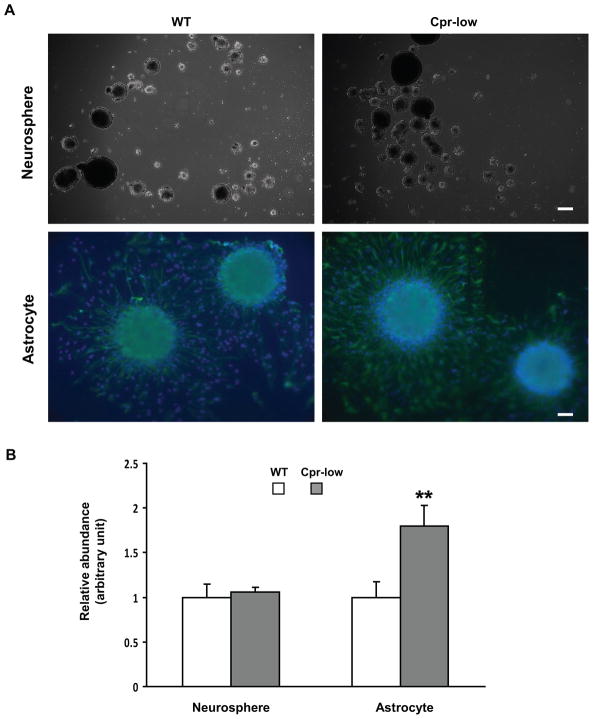
The neurosphere formation assay, which tests NSC’s in vitro capacity for proliferation, self-renewal, and multipotency, was performed for 6-month-old female WT and Cpr-low mice. Although these mice had significant differences in the abundance of Ki67-positive cells and GFAP-positive cells in vivo (Fig. 1--1 and 1-2), they did not show any significant difference in the number of neurospheres formed in vitro (Fig. 2A, upper panels, and Fig. 2B, left side).
Fig. 2. In vitro formation of neurospheres and astrocytes from SVZ cells of Cpr-low and WT mice.
SVZ cells dissected from Cpr-low and WT mice (female, 6-month old, three per group) were examined in culture for their capacity to form neurospheres and astrocytes. A. Representative images of neurospheres (upper panel; dark field) originated from SVZ cells and astrocytes (lower panel; IHC) differentiated from neurospheres are shown. The astrocytes were detected using anti-GFAP; cells were also stained with DAPI. Scale bar: 200 μm for neurosphere, 50 μm for astrocyte. B. Numbers of neurospheres and astrocytes were counted and their relative abundance was calculated by setting the averaged numbers from WT group (94 and 36, respectively) as 1. Values represent means ± S.D., n=3. **, P<0.01; Cpr-low vs WT mice (Students t-test).
3.4. Astrocyte differentiation and growth from SVZ cells of the Cpr-low and WT mice
In the neurosphere differentiation assay, the number of GFAP-positive cells (astrocytes), which were newly differentiated from SVZ cells, was significantly greater for Cpr-low mice than for WT mice (Fig. 2A, lower panels; Fig. 2B, right side). In contrast, there was no significant difference in the number of newly generated DCX-positive cells (for immature neuron) or OLIG2-positive cells (for oligodendrocyte) between the two strains (data not shown).
4. Discussion
In this study, we evaluated the NSCs in the SVZ of the Cpr-low mice, compared to WT mice. The NSC’s in vitro capability in neurosphere formation and differentiation was also assessed. The abundance of cells that express Ki67, a cell proliferation maker expressed in all active phases of the cell cycles [8], was increased in the SVZ, an area where NSCs and progenitor cells reside, of both male and female Cpr-low mice at various ages. Consistent with this finding, our preliminary data showed that abundance of cells expressing SOX2, an SRY box-containing protein found in multipotent neural stem cells in both embryonic and adult brain, was also increased in the SVZ of the Cpr-low mice, compared to WT mice (data not shown). Therefore, it appears that suppression of CPR expression promotes the proliferation of SVZ NSCs in vivo. Furthermore, there was a marked increase in the abundance of cells expressing GFAP, a marker for astrocytes, in the SVZ of the Cpr-low mice at various ages for both male and female, while the abundance of cells expressing DCX (a marker for immature neurons) or OLIG2 (a marker for oligodendrocytes) remained unchanged. The increase in astrocytosis in the SVZ of the Cpr-low mice was further confirmed by the results of in vitro neurosphere differentiation assay, in which a significant increase was observed in the number of astrocytes differentiated from neurospheres originated from SVZ cells of the Cpr-low mice, relative to those from WT mice.
For the first time, our study has demonstrated that global suppression of CPR/CPR-dependent activities, as occurs in the Cpr-low mice, can increase astrocytosis in the SVZ of adult mice. This novel finding supports the need for further studies on the role of CPR/P450 in brain function. In that regard, it has been reported that astrocytes can regulate surrounding neuronal cells (e.g., through the secretion of cytokines) [3]. Astrocytes are also involved in various neurodegenerative diseases [14], and in the regulation of hippocampal neurogenesis [1]. Our finding also leads to interesting questions about possible mechanistic links between CPR/P450 and astrocytosis. Given the property of the CPR/P450 enzymes as major biotransformation enzymes involved in the biosynthesis and/or degradation of a number of endogenous signaling molecules, such as sterols, retinoids, sex steroids, and eicosanoids, it is conceivable that the increased astrocytosis in the Cpr-low mice was related at least partly to alterations in the homeostasis of one or more of these molecules (as further discussed below).
One possible link between CPR and astrocytosis may be cholesterol. Although little is known about the precise role of cholesterol in astrocytosis, it seems that high cholesterol levels can increase astrocytosis in animal models [5, 27]. The suppression of CPR activity in the Cpr-low mice actually led to a decrease in serum levels of cholesterol [26], presumably as a result of decreases in the activities of lanosterol 14α-demethylase (CYP51) and squalene monooxygenase, the two CPR-dependent enzymes in cholesterol biosynthesis [12, 24]. The impact of the low CPR status on brain cholesterol level is not known. However, a decrease in circulating cholesterol level may not affect brain cholesterol level since cholesterol metabolism in the brain is somewhat different from that in the liver. CYP46A1, a CPR-dependent cholesterol 24-hydorxylase expressed predominately in the brain, is responsible for cholesterol homeostasis in the brain [10, 11]. Thus, the suppression of CPR activity in the brain of Cpr-low mice will most likely decrease the activity of CYP46A1, leading to accumulation of cholesterol in the brain. Therefore, further studies are warranted to analyze the actual cholesterol levels in the brain and the SVZ region of the Cpr-low mice, in order to define the exact role of CPR/P450-mediated cholesterol metabolism in astrocytosis in the brain.
CPR/P450 enzymes are also involved in the synthesis and metabolism of sex steroids. The suppression of CPR activity in the Cpr-low mice led to an increase in serum levels of testosterone and progesterone [26], though serum levels of estradiol remained unchanged (data not shown). Interestingly, a 3-fold increase in brain testosterone level was also observed in Cpr-low mice compared to WT mice [25]. The impact of sex steroids on astrocytosis is complex and not well understood; however, sex steroids have been reported to down-regulate reactive gliosis and astrocyte proliferation after brain injuries in rodents [2,7], which seems to argue against a possible role of sex steroids in the increased astrocytosis in the Cpr-low mice. Nonetheless, the Cpr-low mice do not show any pathological changes or injury in the brain [26]. Therefore, it remains to be determined whether the elevated testosterone and progesterone levels in the Cpr-low mice contributed to the increased astrocytosis in their SVZ.
It is notable that the suppression of CPR/P450 enzyme system in the Cpr-low mice led to a selective increase in the differentiation of NSCs to astrocytes, rather than to neurons or oligodendrocytes, in the SVZ of adult brain. In contrast, our previous studies had indicated that cyclin-dependent kinase inhibitors, such as p21 and p27, could suppress the proliferation of neural progenitor cells from SVZ and DG in adult brain under certain conditions [18, 19]. Therefore, it will be interesting to further identify the mechanisms that control the cell-type specific effects, particularly regarding possible interplays among differentiating precursor cells, cholesterol, sex steroids, and cyclin-dependent kinase/inhibitors.
5. Conclusions
We discovered that the abundances of proliferating cells and astrocytes were significantly increased in the SVZ of Cpr-low mice, a model with globally suppressed Cpr gene expression, while the abundance of oligodendrocytes and immature neurons remained unchanged, compared to WT mice. In neurosphere culture assays, we found a marked increase in astrocyte differentiation, but no change in neurosphere formation, in SVZ cells of Cpr-low mice. Thus, we have demonstrated for the first time that global suppression of CPR activity enhances astrocytosis in the SVZ of adult mice. Future studies are warranted to delineate the exact underlying mechanisms and possible role of CPR/P450 enzymes in brain function.
Highlights.
A mouse model with globally suppressed P450 reductase gene expression (Cpr-low).
Markers for neurogenesis were examined in SVZ of Cpr-low mice.
Abundance of cells positive for Ki67 or GFAP was increased in SVZ of Cpr-low mice.
Astrocyte differentiation and growth from SVZ cells of Cpr-low mice were increased.
Acknowledgments
We gratefully acknowledge the use of the Biochemistry, Advanced Light Microscopy, and Histopathology Core facilities of the Wadsworth Center. This work was supported in part by funds from the MOST grants 2011CB964801/2011ZX09102-10/2010DFB30270 and NNSF 81090410 (to T.C.), Neural Stem Cell Institute (to Q.S.) and NIH grants CA092596 (to X.D.), and AG026329 (to J.G.).
Abbreviations
- P450 or CYP
cytochrome P450
- CPR
NADPH-cytochrome P450 reductase
- NSC
neural stem cell
- SVZ
subventricular zone
- DG
dentate gyrus
- CNS
central nerve system
- WT
wild type
- GFAP
glial fibrillary acidic protein
- DCX
double cortin
- OLIG2
oligodendrocyte transcription factor 2
- IHC
immunohistochemistry
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci. 2012;15:1399–1406. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25:3039–3046. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth RF. Neuroinflammation in acute liver failure: mechanisms and novel therapeutic targets. Neurochem Int. 2011;59:830–836. doi: 10.1016/j.neuint.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, Snyder-Keller A, Shan Z, Zhang SZ, Wentland MP, Behr M, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Ding X, Hough LB. Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci. 2010;13:284–286. doi: 10.1038/nn.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crisby M, Rahman SM, Sylven C, Winblad B, Schultzberg M. Effects of high cholesterol diet on gliosis in apolipoprotein E knockout mice. Implications for Alzheimer’s disease and stroke. Neurosci Lett. 2004;369:87–92. doi: 10.1016/j.neulet.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1993;628:271–278. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Park RH, Kim S, Jeon YJ, Ham DS, Jung MY, Kim SS, Lee YD, Park CH, Suh-Kim H. Id proteins facilitate self-renewal and proliferation of neural stem cells. Stem Cells Dev. 2010;19:831–841. doi: 10.1089/scd.2009.0093. [DOI] [PubMed] [Google Scholar]
- 9.Kishi Y, Takahashi J, Koyanagi M, Morizane A, Okamoto Y, Horiguchi S, Tashiro K, Honjo T, Fujii S, Hashimoto N. Estrogen promotes differentiation and survival of dopaminergic neurons derived from human neural stem cells. J Neurosci Res. 2005;79:279–286. doi: 10.1002/jnr.20362. [DOI] [PubMed] [Google Scholar]
- 10.Lund EG, Guileyardo JM, Russell DW. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci U S A. 1999;96:7238–7243. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 12.Lepesheva GI, Nes WD, Zhou W, Hill GC, Waterman MR. CYP51 from Trypanosoma brucei is obtusifoliol-specific. Biochemistry. 2004;43:10789–10799. doi: 10.1021/bi048967t. [DOI] [PubMed] [Google Scholar]
- 13.Marshall GP, 2nd, Reynolds BA, Laywell ED. Using the neurosphere assay to quantify neural stem cells in vivo. Curr Pharm Biotechnol. 2007;8:141–145. doi: 10.2174/138920107780906559. [DOI] [PubMed] [Google Scholar]
- 14.Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris PJ, Hardwick JP, Emson PC. Localization of NADPH cytochrome P450 oxidoreductase in rat brain by immunohistochemistry and in situ hybridization and a comparison with the distribution of neuronal NADPH-diaphorase staining. Neuroscience. 1994;61:331–350. doi: 10.1016/0306-4522(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 16.Omura T. Structural diversity of cytochrome P450 enzyme system. J Biochem. 2010;147:297–306. doi: 10.1093/jb/mvq001. [DOI] [PubMed] [Google Scholar]
- 17.Pappolla MA, Omar RA, Chyan YJ, Ghiso J, Hsiao K, Bozner P, Perry G, Smith MA, Cruz-Sanchez F. Induction of NADPH cytochrome P450 reductase by the Alzheimer beta-protein. Amyloid as a ‘foreign body’. Journal of Neurochemistry. 2001;78:121–128. doi: 10.1046/j.1471-4159.2001.00379.x. [DOI] [PubMed] [Google Scholar]
- 18.Qiu J, Takagi Y, Harada J, Rodrigues N, Moskowitz MA, Scadden DT, Cheng T. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199:937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu J, Takagi Y, Harada J, Topalkara K, Wang Y, Sims JR, Zheng G, Huang P, Ling Y, Scadden DT, Moskowitz MA, Cheng T. p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditions. Stem Cells. 2009;27:920–927. doi: 10.1002/stem.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taupin P. Adult neural stem cells: The promise of the future. Neuropsychiatr Dis Treat. 2007;3:753–760. doi: 10.2147/ndt.s2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh JS, Miwa GT. Bioactivation of drugs: risk and drug design. Annu Rev Pharmacol Toxicol. 2011;51:145–167. doi: 10.1146/annurev-pharmtox-010510-100514. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y, Zhou X, Fang C, Li L, Kluetzman K, Yang W, Zhang QY, Ding X. Generation of a mouse model with a reversible hypomorphic cytochrome P450 reductase gene: utility for tissue-specific rescue of the reductase expression, and insights from a resultant mouse model with global suppression of P450 reductase expression in extrahepatic tissues. J Pharmacol Exp Ther. 2010;334:69–77. doi: 10.1124/jpet.110.167411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng Y, DiRusso CC, Reilly AA, Black PN, Ding X. Hepatic gene expression changes in mouse models with liver-specific deletion or global suppression of the NADPH-cytochrome P450 reductase gene. Mechanistic implications for the regulation of microsomal cytochrome P450 and the fatty liver phenotype. J Biol Chem. 2005;280:31686–31698. doi: 10.1074/jbc.M504447200. [DOI] [PubMed] [Google Scholar]
- 25.Weng Y, Xie F, Xu L, Zagorevski D, Spink DC, Ding X. Analysis of testosterone and dihydrotestosterone in mouse tissues by liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem. 2010;402:121–128. doi: 10.1016/j.ab.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu L, Gu J, Cui H, Zhang QY, Behr M, Fang C, Weng Y, Kluetzman K, Swiatek PJ, Yang W, Kaminsky L, Ding X. Transgenic mice with a hypomorphic NADPH-cytochrome P450 reductase gene: effects on development, reproduction, and microsomal cytochrome P450. J Pharmacol Exp Ther. 2005;312:35–43. doi: 10.1124/jpet.104.073353. [DOI] [PubMed] [Google Scholar]
- 27.Zatta P, Zambenedetti P, Stella MP, Licastro F. Astrocytosis, microgliosis, metallothionein-I-II and amyloid expression in high cholesterol-fed rabbits. J Alzheimers Dis. 2002;4:1–9. doi: 10.3233/jad-2002-4101. [DOI] [PubMed] [Google Scholar]