Abstract
Intermuscular adipose tissue (IMAT) is associated with metabolic abnormalities similar to those associated with visceral adipose tissue (VAT). Increased IMAT has been found in obese human immunodeficiency virus (HIV)-infected women. We hypothesized that IMAT, like VAT, would be similar or increased in HIV-infected persons compared with healthy controls, despite decreases in subcutaneous adipose tissue (SAT) found in HIV infection. In the second FRAM (Study of Fat Redistribution and Metabolic Change in HIV infection) exam, we studied 425 HIV-infected subjects and 211 controls (from the Coronary Artery Risk Development in Young Adults study) who had regional AT and skeletal muscle (SM) measured by magnetic resonance imaging (MRI). Multivariable linear regression identified factors associated with IMAT and its association with metabolites. Total IMAT was 51% lower in HIV-infected participants compared with controls (P = 0.003). The HIV effect was attenuated after multivariable adjustment (to −28%, P < 0.0001 in men and −3.6%, P = 0.70 in women). Higher quantities of leg SAT, upper-trunk SAT, and VAT were associated with higher IMAT in HIV-infected participants, with weaker associations in controls. Stavudine use was associated with lower IMAT and SAT, but showed little relationship with VAT. In multivariable analyses, regional IMAT was associated with insulin resistance and triglycerides (TGs). Contrary to expectation, IMAT is not increased in HIV infection; after controlling for demographics, lifestyle, VAT, SAT, and SM, HIV+ men have lower IMAT compared with controls, whereas values for women are similar. Stavudine exposure is associated with both decreased IMAT and SAT, suggesting that IMAT shares cellular origins with SAT.
Introduction
Changes in fat distribution and metabolic abnormalities have been found in human immunodeficiency virus (HIV)-infected individuals (1) in the era of combination antiretroviral (ARV) therapy. It is well established that subcutaneous adipose tissue (SAT) is decreased in HIV infection (2,3). In contrast, visceral adipose tissue (VAT) is decreased in HIV-infected men, whereas HIV-infected women may have similar VAT if they suffer from clinical peripheral lipoatrophy or increased VAT if they do not. Intermuscular adipose tissue (IMAT) is defined as the AT visible beneath the muscle fascia and between the muscle groups, as depicted in Figure 1. Little is known about the levels or distribution of IMAT in HIV infection.
Figure 1.
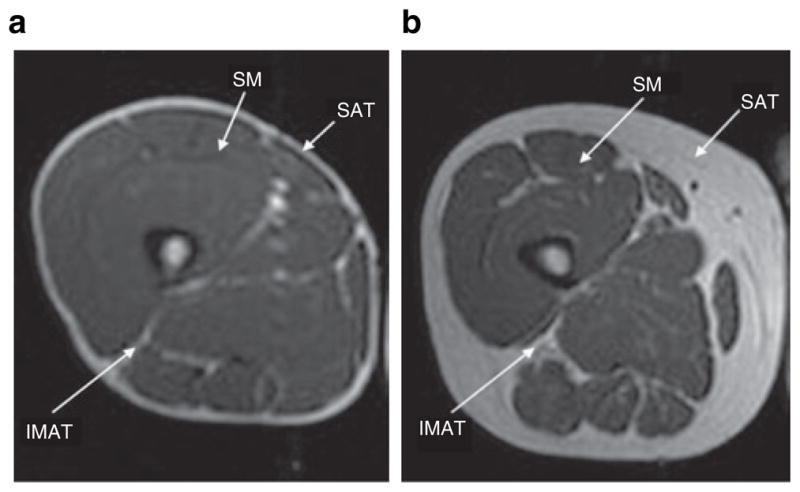
MRI slice image comparing IMAT, SAT, SM by HIV status. Axial MRI, at the level of mid-thigh. (a) An HIV-infected male, showing decreased subcutaneous and intermuscular fat. (b) An uninfected, control male, showing normal amounts of subcutaneous and intermuscular fat. Both participants have a BMI of 25. HIV, human immunodeficiency virus; IMAT, intermuscular adipose tissue; MRI, magnetic resonance imaging; SAT, subcutaneous adipose tissue; SM, skeletal muscle.
Both VAT and IMAT increase with age, whereas SAT and skeletal muscle (SM) decrease with age (4,5). IMAT is also thought to increase in sarcopenia, the loss of SM associated with aging (4,6) and with inflammatory diseases such as rheumatoid arthritis (7). However, the increase in IMAT with aging is independent of changes in weight or SAT (4). Both VAT and IMAT are associated with insulin resistance, diabetes, and triglycerides (TGs) (5,8–12). One study found IMAT to be a stronger predictor of insulin resistance than VAT (8), whereas others found that VAT was stronger than IMAT (10) or IMAT was not an independent predictor of insulin resistance after controlling for VAT (11,12). In other analyses, IMAT was an independent predictor of glucose or diabetes (5,11,12). Femoral–gluteal IMAT was associated with glucose independent of VAT, whereas the association of IMAT with TGs was attenuated by VAT (12). However, most of these studies were limited in their ability to analyze the associations of whole body, regional depots, as they did not examine upper-trunk AT, for which we have shown important associations with insulin resistance and TGs (9,13,14). It is also thought that IMAT may influence SM metabolism in a manner similar to the relationship between VAT and the liver, because of their shared vascular connection (15). These studies suggest that IMAT and VAT are biologically similar.
IMAT is preserved in subjects with certain types of severe subcutaneous lipoatrophy such as familial partial lipodystrophy (16), but decreased in other forms of lipodystrophy such as congenital generalized lipodystrophy (17). IMAT has also been reported to be elevated in obese HIV-infected women compared with obese uninfected women (18), but it is unknown whether IMAT is also elevated in normal-weight HIV-infected men and women.
Previous studies of IMAT in HIV infection (18,19) and in the general population (5,15,20–22) have been small or conducted in elderly or obese populations. No large, nationally representative study has been conducted to examine IMAT levels and their associations in a population of HIV-infected and control subjects. We hypothesized that IMAT would be similar or increased among HIV-infected compared to healthy controls, because of its reported metabolic similarities to VAT (8,10) and because of IMAT’s conservation in other forms of partial lipodystrophy (16). A primary aim of the second exam of the study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) was to determine levels of IMAT and factors associated with IMAT in a large, nationally representative, multiethnic cohort of HIV-infected subjects and controls. An additional aim was to compare the factors associated with IMAT to those associated with other types of AT (VAT and SAT). Furthermore, we determined the associations of all three types of AT with metabolic factors.
Methods and Procedures
This cross-sectional study includes 425 HIV-infected and 211 control participants who had IMAT measured at the second FRAM exam. FRAM was initially designed to evaluate the prevalence and correlates of changes in fat distribution, insulin resistance, and dyslipidemia in a nationally, representative sample of HIV-infected participants and a population-based group of controls in the United States. The methods and retention data have been described in detail previously (23,24).
Study population
The first FRAM examination enrolled 1,183 HIV-infected participants and 297 HIV-uninfected controls from 2000 to 2002. HIV-infected participants were initially recruited from 16 HIV/infectious disease clinics or cohorts, and controls were recruited from two centers of the Coronary Artery Risk Development in Young Adults (CARDIA) study that were part of an ancillary study, the Visceral Fat and Metabolic Rate in Young Adults study (VIM). These controls have a BMI distribution similar to the CARDIA parent study and include both whites and African Americans. The protocol was approved by institutional review boards at all sites.
We report here on the subset of 425 HIV-infected and 211 control participants who had IMAT measured at the second FRAM exam, conducted ~5 years later. Because HIV-infected participants had a higher rate of missing magnetic resonance imaging (MRI), we compared characteristics of those with and without measured IMAT (data not shown). Those without IMAT had higher BMI and lower CD4 count, but otherwise had similar demographic and clinical characteristics as those with IMAT. Because of the greater percentage of HIV-infected participants without IMAT, we adjusted analyses as described below to address the concern of selection bias.
MRI
Whole-body MRI was performed to quantify regional and total SM and AT volumes, as described in detail previously (2,3,23,25). All scans were read by the same analyst at the Obesity Research Center, St. Luke’s Roosevelt Hospital, New York. Imaging techniques and anatomical sites (based on bone landmarks) were identical between HIV-infected and control participants.
Volumes were normalized by dividing by height2 with summaries back-transformed to 1.75 m of height, as in previous analyses (2,3). We did not adjust to BMI, as BMI is influenced by the phenomenon being studied: quantity of fat. Anatomic sites considered in this analysis were: leg, lower trunk (abdomen and back), upper trunk (chest and back), arm, and VAT.
IMAT was defined as the AT visible beneath the muscle fascia and between the muscle groups, as depicted in Figure 1. The gray level intensity (threshold value) of the AT in the SAT region was first determined; this value was reduced by 20% to determine the threshold for IMAT.
Other measurements
Height and weight were measured by standardized protocols. Standardized questionnaires that were validated in a general population were used to determine demographic characteristics; medical history; risk factors for HIV; food intake; physical activity; and use of alcohol, tobacco, and illicit drugs (23,26). Total physical activity score was calculated using the validated CARDIA activity questionnaire as described elsewhere (27,28). Research associates interviewed subjects and reviewed medical charts regarding ARV medication use. A diagnosis of AIDS was made by CD4 lymphocyte count <200 or history of opportunistic infection (OI).
Hepatitis C virus-RNA testing was performed on frozen sera using Bayer Versant 3.0 branched DNA assay (Leverkusen, Germany) in the entire cohort. C-reactive protein (CRP) and fibrinogen were measured using a BNII nephelometer, as described (29). Glucose and insulin were measured in a central laboratory (Linco Research, St Louis, MO). CD4 lymphocyte count and percent, HIV-RNA level in HIV-infected participants, and other blood specimens were analyzed in a single centralized laboratory (Covance, Indianapolis, IN). Insulin resistance was calculated using the homeostatis model assessment (HOMA) from fasting glucose (mg/dl) and insulin (μU/ml) specimens as: (insulin × glucose)/405.
Statistical methods
We compared demographic and clinical characteristics of HIV-infected and control participants using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Spearman correlation coefficients were calculated to examine the associations of numerical values with IMAT. Analyses that compared characteristics of HIV-infected participants with controls excluded six HIV-infected with recent OI and 164 HIV-infected who were outside the 38–52 years age range of controls.
We used multivariable linear regression (with bootstrapped confidence intervals; 30) to evaluate the association of HIV and other factors with IMAT. IMAT and other right-skewed depots were log-transformed; results were back-transformed to produce estimated percentage differences. All analyses were adjusted using inverse probability weighting to address the impact of selection bias (31). Multiple imputation was performed for missing covariates using the Markov chain Monte Carlo method for arbitrary missing data (32). Linearity was tested for continuous measures by adding quadratic terms to the models and by examining generalized additive models (33, 34). Models were constructed in a staged fashion using HIV status, demographics (age, gender, and race), lifestyle factors, inflammatory markers (CRP and fibrinogen), and other MRI depots (SM, VAT, and SAT) as predictor variables. Models were built in a forward stepwise manner, with P = 0.05 for entry and retention. Interactions of HIV status with gender, ethnicity, and age were assessed, and stratified results were examined when interactions reached statistical significance.
Similar models were constructed for total SAT and VAT to address the question of whether IMAT and VAT are biologically similar. We also examined associations of IMAT, regional SAT, and VAT with HOMA and TGs.
Candidate lifestyle factors included physical activity (quartiled), smoking, alcohol use, adequate food intake, and illicit drug use. HIV-related candidates included AIDS diagnosis (by CD4 or OI), reported HIV duration, HIV-RNA level (log10), current and nadir CD4 count (log2), hepatitis C virus infection (by hepatitis C virus-RNA detection), days since last OI, recent OI status (last 100 days), and risk factors for HIV acquisition. In multivariable models controlling for the above factors, we also evaluated ARV exposure as previously defined (2).
Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Subjects
IMAT values were available for 636 participants whose characteristics are presented in Table 1. HIV-infected and control participants were similar in age, height, and percentage whites and African Americans, but HIV-infected participants were more often men (67% vs. 51%) by design. CRP levels were higher in HIV-infected participants, but fibrinogen levels were similar. BMI was higher in controls. Nearly all HIV-infected participants had used Highly Active Anti-Retroviral Therapy (HAART) (94% in men and 96% in women). Median (HAART) duration in those with a history of use was 5.9 years (6.2 years in men and 5.6 years in women).
Table 1.
Subject characteristics by HIV status
| Age-restricted, OI-excluded
|
All HIV+ (n = 425) | ||
|---|---|---|---|
| HIV+ (n = 255) | Control (n = 211) | ||
| Age (years) | 45.0 (42.0–49.0) | 46.0 (43.0–49.0) | 47.0 (42.0–53.0) |
| Gender | |||
| Male | 170 (67%) | 108 (51%)** | 291 (68%) |
| Female | 85 (33%) | 103 (49%) | 133 (31%) |
| Transgendered | 0 | 0 | 1 (1%) |
| Race | |||
| Whites | 125 (49%) | 121 (57%) | 205 (48%) |
| African Americans | 103 (40%) | 90 (43%) | 175 (41%) |
| Hispanics | 15 (6%) | 0 | 27 (6%) |
| Others | 12 (5%) | 0 | 18 (4%) |
| Height (cm) | 172.7 (165.9–179.8) | 172.4 (164.7–177.9) | 172.7 (166.0–179.0) |
| BMI (kg/m2) | 24.7 (22.0–27.6) | 27.7 (24.1–31.7)*** | 24.8 (22.1–27.5) |
| CRP (mg/l) | 1.5 (0.8–3.8) | 1.3 (0.6–2.8)* | 1.5 (0.8–4.0) |
| Fibrinogen (mg/dl) | 363.4 (314.6–432.7) | 365.1 (316.9–419.9) | 367.5 (312.6–450.1) |
| Current CD4 (cells/μl) | 434 (288–635) | 435 (287–633) | |
| HIV-RNA (1,000/ml) | 0.4 (0.4–1.9) | 0.4 (0.4–1.2) | |
| HAART use (ever) | 243 (95%) | 403 (95%) | |
| HAART duration in users (y) | 6.0 (4.5–7.8) | 5.9 (4.3–7.8) | |
Data are presented as median (IQR) or numbers (percent). Test of HIV in the age range of controls vs. control:
P < 0.05,
P < 0.001,
P < 0.0001.
Only subjects with IMAT measured are included. The age-restricted HIV (n = 255) are a subset of all HIV (n = 425).
AR, age restricted; HAART, Highly Active Anti-Retroviral Therapy; HIV, human immunodeficiency virus; IQR, interquartile range; OI, opportunistic infection.
IMAT levels in HIV-infected and control subjects
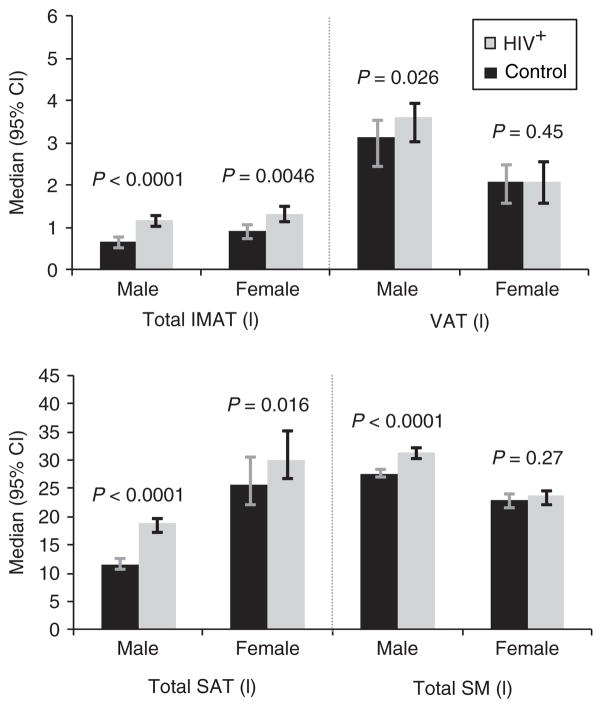
Compared with controls, total IMAT volumes were lower in HIV-infected men (median 0.66 l vs. 1.16 l, P < 0.0001) and women (0.91 l vs. 1.31 l, P = 0.0046) (Figure 2). Similar patterns were seen for limb IMAT (data not shown). Total SAT was also lower in HIV-infected men and women (Figure 2). VAT and SM levels were also lower in HIV-infected men compared with control men (P = 0.026 and P < 0.0001, respectively), but were similar in HIV-infected and control women.
Figure 2.
Adipose and muscle tissue levels (height normalized) by HIV status. Age restricted to 38–52 years. AT, adipose tissue; CI, confidence interval; HIV, human immunodeficiency virus; IMAT, intermuscular AT; SAT, subcutaneous AT; SM, skeletal muscle; VAT, visceral AT.
Overall, total IMAT levels were 51% lower in HIV-infected participants compared with controls (95% confidence interval: −69 to −21, P = 0.003). The association of HIV with IMAT showed little change after adjustment for demographics, lifestyle factors, and CRP (−50%, 95% confidence interval: −57 to −42, P < 0.0001). However, after additional adjustment for SM, VAT, upper-trunk SAT, and leg SAT, the HIV effect was attenuated, but HIV infection remained associated with 19.5% lower IMAT (95% confidence interval: −30 to −8, P = 0.0014). Analyses that did not account for those with missing IMAT showed similar results to the main analyses that adjusted for selection bias (unweighted HIV effect: −20.4%, P = 0.0004). In fully adjusted models, there was an HIV by gender interaction (P = 0.015); the association of HIV with lower IMAT remained strong in men (−28%, P < 0.0001), but was substantially attenuated in women (−3.6%, P = 0.70).
Factors associated with IMAT in HIV-infected and control subjects
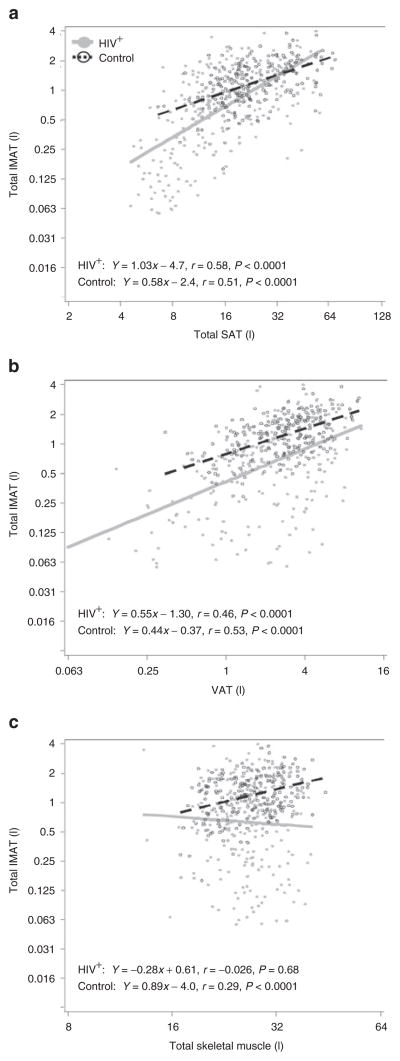
In univariate analyses, total SAT showed a positive association with IMAT in both HIV-infected (r = 0.58, P < 0.0001) and control participants (r = 0.51, P < 0.0001, Figure 3a). The difference in IMAT between HIV-infected and control participants narrowed with increasing total SAT (P = 0.0003, test for HIV × SAT interaction). Although HIV-infected participants with lower SAT had decreased IMAT relative to controls, those with higher SAT had high levels of IMAT similar to controls with high SAT (HIV vs. control: P = 0.28 at 45 l total SAT). Similarly, total fat and each regional SAT depot showed strong, positive associations with IMAT (P < 0.0001, data not shown).
Figure 3.
Association of adipose tissue and skeletal muscle with IMAT in age-restricted HIV-infected and control participants (height-normalized IMAT, SM, and SAT volumes). Age restricted to 38–52 years. Axes are log2-scaled (test for HIV × SM interaction, P = 0.003). Closed symbols and solid lines are HIV+, Open symbols and dashed lines are controls. AT, adipose tissue; HIV, human immunodeficiency virus; IMAT, intermuscular adipose tissue; SAT, subcutaneous AT; VAT, visceral AT.
VAT was also positively associated with IMAT in HIV-infected (r = 0.46, P < 0.0001) and control participants (r = 0.53, P < 0.0001, Figure 3b). However, IMAT levels were higher in controls compared to HIV regardless of VAT level.
The association of SM with IMAT more clearly differed by HIV status (P = 0.003, test for HIV × SM interaction). There was a positive correlation of IMAT with SM in controls (r = 0.29, P < 0.0001), but little correlation in HIV-infected participants (r = −0.026, P = 0.68, Figure 3c). Plots of regional SM vs. regional IMAT showed similar patterns (data not shown).
We therefore used multivariable analysis to determine which factors were independently associated with IMAT (Table 2). After adjustment for demographics, lifestyle factors, and body composition including AT, the positive association of SM with IMAT in controls weakened, whereas the negative association in HIV-infected participants strengthened.
Table 2.
Multivariate associations of body composition with total IMAT in all control and HIV-infected participants
| Body composition measure (per doubling) | Controls (n = 211)
|
HIV+ (n = 425)
|
|---|---|---|
| Adjusted R2 = 0.510
|
Adjusted R2 = 0.539
|
|
| % Estimatea (95% CI) | % Estimatea (95% CI) | |
| SMb | 21.5 (−13.5, 71.7), P = 0.27 | −46.9 (−61.0, −27.6), P < 0.0001 |
| VAT | 23.6 (11.3, 35.9), P < 0.0001 | 42.8 (30.5, 56.2), P < 0.0001 |
| Upper-trunk SAT | 15.6 (−1.7, 37.0), P = 0.071 | 31.2 (10.7, 55.5), P = 0.0018 |
| Lower-trunk SAT | −17.8 (−33.3, 2.7), P = 0.080c | 1.8 (−15.3, 22.4), P = 0.85c |
| Leg SAT | 12.5 (−2.1, 27.2), P = 0.10 | 65.3 (46.4, 86.6), P < 0.0001 |
| Arm SAT | −2.3 (−24.9, 25.0), P = 0.77c | 5.2 (−15.5, 31.0), P = 0.65c |
IMAT is height-normalized and log-transformed; results are back-transformed to calculate percent effects. Body composition measures are log2 transformed. Models also control for demographic and lifestyle factors. HIV+ model with HIV-related factors shows similar associations for MRI and other factors.
Factors did not enter into the model above, but are shown controlling for other factors in the model. Significant P values are shown in boldface.
CI, confidence interval; HIV, human immunodeficiency virus; IMAT, intermuscular adipose tissue; MRI, magnetic resonance imaging; SAT, subcutaneous adipose tissue; SM, skeletal muscle; VAT, visceral adipose tissue.
Associations with IMAT are as percent per doubling of each depot.
Test for HIV × SM interaction, P = 0.003.
VAT was associated with higher IMAT in both control and HIV-infected participants, but was stronger in HIV-infected participants (43% vs. 24%; HIV × VAT interaction: P = 0.0005, Table 2).
Model fit was better when individual SAT depots were in the model than when total SAT was used. Of the regional depots, leg SAT and upper-trunk SAT were positively associated with IMAT in both control and HIV-infected participants, but the associations were stronger in HIV-infected participants (P < 0.0001). Similar associations were seen when limb IMAT was the outcome (data not shown). When the analysis was stratified by gender, we found a stronger association of regional SAT with IMAT in men compared with women in both HIV-infected and controls, but the HIV vs. control differences remained. The estimated effect size for leg SAT was 94% in HIV-infected men (P < 0.0001), 24% in control men (P = 0.035), 28% in HIV-infected women (P = 0.0027), and 5.5% in control women (P = 0.53).
Comparison of factors associated with IMAT, Total SAT, and VAT in HIV infection
We constructed similar multivariable models in order to compare the associations of demographic and HIV-related factors with IMAT, total SAT, and VAT (Table 3). Female gender was strongly associated with higher IMAT and total SAT, but was weakly associated with lower VAT. African-American ethnicity had little association with IMAT and was modestly associated with higher total SAT, but was strongly associated with lower VAT. Increasing age was associated with higher VAT, but its association with IMAT was weaker.
Table 3.
C omparison of factors independently associated with IMAT, SAT, and VAT in all HIV+ participants (n = 425)
| Measure | Total IMAT
|
Total SAT
|
VAT
|
|---|---|---|---|
| Adjusted R2 = 0.182
|
Adjusted R2 = 0.434
|
Adjusted R2 = 0.263
|
|
| % Estimate (95% CI) | % Estimate (95% CI) | % Estimate (95% CI) | |
| Demographics | |||
| Female vs. male | 68.9 (36.3, 109.4), P < 0.0001 | 121.8 (97.2, 149.5), P < 0.0001 | −4.7 (−19.9, 13.5), P = 0.59 |
| African American vs. white | 0.9 (−18.2, 24.5), P = 0.93 | 12.1 (1.1, 24.3), P = 0.030 | −38.0 (−47.1, −27.4), P < 0.0001 |
| Other vs. white | −18.9 (−39.6, 9.0), P = 0.17 | 12.5 (−3.8, 31.5), P = 0.14 | −19.2 (−38.9, 6.9), P = 0.14 |
| Age (per decade) | 6.8 (−2.7, 17.1), P = 0.17 | −3.3 (−7.8, 1.4), P = 0.17 | 13.9 (5.4, 23.1), P = 0.0010 |
| HIV-related factors | |||
| HIV-RNA (log10) | 5.8 (−4.6, 17.4), P = 0.28 | 1.0 (−3.9, 6.3), P = 0.69 | −11.5 (−19.3, −2.9), P = 0.0098 |
| Current CD4 (log2) | −4.1 (−10.0, 2.2), P = 0.20 | −0.3 (−3.4, 2.8), P = 0.85 | −1.4 (−7.4, 4.9), P = 0.65 |
| HCV-RNA+ | −12.6 (−29.3, 8.1), P = 0.21 | −4.8 (−14.3, 5.7), P = 0.36 | −23.6 (−37.5, −6.4), P = 0.0092 |
| AIDS by CD4/OI | 2.0 (−16.8, 25.1), P = 0.85 | −9.5 (−17.7, −0.5), P = 0.040 | −13.1 (−24.4, 0.0), P = 0.050 |
| Stavudine (per year of use) | −7.1 (−10.5, −3.6), P < 0.0001 | −2.6 (−4.2, −0.9), P = 0.0030 | −1.3 (−3.8, 1.4), P = 0.34 |
| Didanosine (per year of use) | −8.9 (−13.7, −3.8), P = 0.0008 | −2.5 (−4.7, −0.2), P = 0.031 | −2.5 (−6.4, 1.6), P = 0.23 |
| Nelfinavir (per year of use) | −6.9 (−10.6, −3.0), P = 0.0006 | −2.2 (−4.6, 0.3), P = 0.082 | −4.7 (−8.4, −0.8), P = 0.018 |
| Lamivudine (per year of use) | 1.7 (−1.3, 4.9), P = 0.28 | 0.1 (−1.3, 1.6), P = 0.84 | 2.7 (0.4, 5.1), P = 0.022 |
IMAT, SAT, and VAT are height-normalized and log-transformed; results are back-transformed to calculate percent effects. Models also control for lifestyle factors. HIV-related factors with P > 0.05 did not enter into the model above, but are shown controlling for other factors in the model. Significant P values are shown in boldface.
CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IMAT, intermuscular adipose tissue; OI, opportunistic infection; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
With regard to HIV-related factors (Table 3), HIV-RNA was strongly negatively associated with VAT, but showed little association with IMAT or SAT. Hepatitis C virus-RNA was also negatively associated with VAT, with weaker negative associations for IMAT and SAT. History of AIDS was associated with lower VAT and SAT, but appeared to have little association with IMAT.
A different pattern was seen with stavudine. Duration of stavudine use was strongly associated with lower IMAT, showed significant, but somewhat less, association with lower total SAT and little association with VAT. Duration of didanosine and nelfinavir use was strongly associated with lower IMAT, with weaker associations with lower total SAT and VAT. Lamivudine use showed a positive association with VAT, with smaller associations for IMAT and SAT. Associated factors were generally similar in gender-stratified analysis. ARV duration was shorter in women, and a few ARV associations appeared to be stronger in men; however, no ARV by gender interactions reached statistical significance, and the small differences in duration of ARV did not account for the gender difference in IMAT. The largest gender difference in ARV was in the association of stavudine with SAT (men: −3.3%, P < 0.0001, women 0.4%, P = 0.86, HIV × gender P = 0.092) and IMAT (men: −8.4%, P < 0.0001, women −2.9%, P = 0.43).
Association of body composition with HOMA and TGs
We next examined the association of AT depots with insulin resistance (HOMA) and TGs in models adjusting for demographics, lifestyle factors, and HIV-related factors (Table 4). As expected, VAT and upper-trunk SAT showed strong positive, independent associations with HOMA in both control and HIV-infected participants. In addition, we found that lower- trunk IMAT was independently and negatively associated with HOMA in HIV-infected participants. Before controlling for IMAT, VAT showed a positive association with HOMA in HIV-infected participants (10.2%, P = 0.046); this association strengthened after controlling for IMAT (20.7%, P < 0.0001), with little change seen in the upper-trunk SAT effect. Gender-stratified analysis revealed generally similar associations in men and women with two exceptions. Upper-trunk IMAT had a weaker association with HOMA in HIV-infected men (5.3%, P = 0.17) than in HIV-infected women (15.8%, P = 0.023). Leg SAT may be associated with lower HOMA in HIV-infected men (−19%, P = 0.034), but not in HIV-infected women (0.1%, P = 0.97).
Table 4.
Multivariable associations of body composition with HOMA and triglycerides by HIV status
| Body composition measure (per doubling)a | HOMA
|
Triglycerides
|
||
|---|---|---|---|---|
| Control, % estimate (95% CI) | HIV+, % estimate (95% CI) | Control, % estimate (95% CI) | HIV+, % estimate (95% CI) | |
| VAT | 27.2 (7.1, 51.0), P = 0.0060 | 20.7 (10.6, 31.8), P < 0.0001 | 46.9 (31.1, 64.6), P < 0.0001 | 22.0 (16.2, 28.1), P < 0.0001 |
| Upper-trunk IMAT | 4.6 (−3.8, 13.8), P = 0.30 | 6.0 (−1.5, 14.2), P = 0.12 | 17.4 (5.5, 30.6), P = 0.0033 | −3.0 (−6.7, 0.9), P = 0.13 |
| Upper-trunk SAT | 30.8 (3.7, 64.9), P = 0.023 | 49.0 (30.8, 69.8), P < 0.0001 | 21.8 (−3.3, 53.3), P = 0.094 | 6.4 (−5.4, 19.6), P = 0.30 |
| Lower-trunk IMAT | 3.7 (−8.8, 17.9), P = 0.58 | −12.7 (−17.3, −7.9), P < 0.0001 | −26.1 (−37.3, −13.0), P = 0.00028 | −2.8 (−7.1, 1.7), P = 0.22 |
| Lower-trunk SAT | 10.0 (−10.6, 35.3), P = 0.37 | −3.7 (−17.2, 12.0), P = 0.63 | 12.3 (−9.9, 39.9), P = 0.30 | 9.7 (−1.7, 22.4), P = 0.099 |
| Leg IMAT | 0.7 (−9.0, 11.4), P = 0.89 | 2.6 (−6.5, 12.5), P = 0.59 | −4.6 (−18.0, 11.0), P = 0.54 | −3.3 (−8.0, 1.7), P = 0.19 |
| Leg SAT | −1.0 (−18.2, 19.9), P = 0.92 | −11.1 (−22.0, 1.3), P = 0.078 | −25.0 (−36.9, −10.8), P = 0.0012 | −20.0 (−26.6, −12.7), P < 0.0001 |
Each model controls for demographics, lifestyle factors, HIV-related factors, VAT, SAT, and IMAT. Factors with P > 0.05 did not enter into the model above, but are shown controlling for other factors in the model. Significant P values are shown in boldface.
CI, confidence interval; HOMA, homeostatis model assessment; HIV, human immunodeficiency virus; IMAT, intermuscular adipose tissue; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Measures are height-normalized and log2-transformed; results are back-transformed to calculate percent effects.
VAT also showed a strong positive association with TG in both control and HIV-infected participants, whereas leg SAT was strongly negatively associated with TG in both groups. After adjusting for IMAT, there was little change in the associations of VAT and leg SAT with TG. In addition, multivariable analysis found strong independent associations of two IMAT depots with TGs: lower-trunk IMAT was negatively associated and upper-trunk IMAT was positively associated with TG in controls.
Discussion
We have shown that contrary to expectation, MRI-measured IMAT is decreased in HIV-infected participants compared with controls. The association of HIV infection with decreased IMAT remains statistically significant in men, even after adjusting for demographics, lifestyle factors, VAT, SAT, SM, and inflammatory markers. In women, there is little association of HIV infection with decreased IMAT after multivariable adjustment. As seen with SAT loss, IMAT loss may be related to exposure to the ARV drugs stavudine, didanosine, and nelfinavir. Given previous reports of a link between IMAT and metabolic abnormalities common to VAT (11,20,35,36), these results have several implications.
Although VAT and SAT were positively associated with IMAT in both HIV-infected and control subjects, the associations were stronger in HIV-infected subjects. Although CRP was elevated in our HIV-infected participants compared with controls, controlling for inflammatory markers did not weaken the strong negative association of HIV infection with IMAT.
Our finding of a negative association of SM with IMAT in HIV-infected participants is consistent with previous studies of animals and uninfected subjects. Animal studies have found a negative correlation between muscle area and percentage IMAT (37). A previous study in healthy young adults found that weeks of reduced physical activity led to increased IMAT; change in SM was strongly negatively correlated with change in IMAT (38). Physical inactivity is thought to block the breakdown or reuptake of TGs; the increase in IMAT may shift fuel metabolism away from lipid toward glucose utilization. A recent 5-year study in older adults found increases in IMAT regardless of weight gain; decreases in muscle torque and muscle quality were also seen, although muscle changes showed little correlation with IMAT (4). We observed a weak, unexpected positive association of IMAT with SM in uninfected subjects, which may be due to the younger age of our controls. Given these contradictory findings in different patient groups, additional study is needed to understand the relationships between IMAT and muscle.
Although IMAT showed strong univariate correlations with both VAT and total SAT, associations of demographic and HIV-specific predictors with IMAT, VAT, and total SAT varied. Given reports in the non-HIV literature linking increased IMAT and VAT and decreased SAT with metabolic disturbances, we had expected that factors associated with IMAT would track more closely with VAT than total SAT (21); however, that pattern was not found. As previously reported in the non-HIV literature (5), we found age to be strongly associated with increased VAT, but only weakly associated with increased IMAT and decreased SAT, although our age range may be narrower than some studies. Being female was associated with more IMAT and SAT, but not VAT. Being African American was associated with less VAT, but had little association with IMAT. HIV-RNA and history of AIDS were associated with less VAT, but not IMAT.
Of interest is that exposure to stavudine leads to mitochondrial dysfunction within SAT (39). In our study, stavudine exposure was associated with lower IMAT and SAT, but showed little association with VAT. This association suggests that IMAT and SAT may share a common cellular origin. IMAT also appeared to track more closely with SAT than VAT, but we cannot rule out the possibility that IMAT has mixed cellular origins, including some IMAT that is similar to VAT or derives from the same precursors as muscle.
Previous studies had found that IMAT (18,19) is elevated in obese HIV-infected subjects compared with controls. However, such studies had small sample size. In one study, HIV-infected subjects were specifically selected for central obesity and had similar IMAT to obese controls. We have demonstrated previously in this representative cohort of HIV-infected participants that VAT is not systematically elevated relative to controls (2,3). Our HIV-infected participants with higher SAT also had high levels of IMAT similar to our controls with high SAT. However, our HIV-infected participants with low SAT had decreased IMAT compared to controls with similarly low SAT. These data also support a role of HIV-associated lipoatrophy and stavudine exposure in the decrease in IMAT seen in HIV infection.
Upper-trunk SAT and VAT were independently associated with HOMA in both HIV-infected and controls, consistent with our earlier report from the first FRAM exam (9). However, we also found that lower-trunk IMAT made an independent contribution, showing a negative association with HOMA in HIV-infected participants. Although others have previously found a positive association of central obesity with insulin resistance and TG in the non-HIV literature (11,12,40), our finding of an independent, protective role of lower-trunk IMAT in HIV infection appears to be novel.
Although increased VAT and decreased leg SAT were associated with higher TG in both HIV-infected and controls, consistent with our previous reports (13,14), we also found that increased upper-trunk IMAT and decreased lower-trunk IMAT were independently associated with higher TG in controls. By comparison, in a study of uninfected men and women, Yim et al. (12) found that femoral–gluteal IMAT was inversely associated with insulin and TG, but after controlling for VAT, the associations were no longer statistically significant. Our data are consistent with our previous conclusions that upper-body SAT had deleterious associations with TG whereas lower-body SAT was protective. As HIV lipoatrophy affects lower-body SAT more than upper-body SAT, the IMAT findings help explain the hypertriglyceridemia in HIV infection.
The primary limitation of our study was its cross-sectional design. We were unable to measure IMAT at the first FRAM exam, so changes in IMAT over time could not be studied, nor could we evaluate the causality of the associations of inflammatory markers, AT, and SM with IMAT. We were unable to assess intramyocellular lipid accumulation, as such measurements require magnetic resonance spectroscopy. Therefore, we cannot rule out the possibility of an elevation of intramyocellular fat in HIV infection, as has been reported in smaller studies of subjects with central lipohypertrophy (41,42). A previous study found increased attenuation of muscle by computed tomography in HIV infection (42). However, as attenuation in computed tomography does not differentiate between intramyocellular lipid and IMAT, results from computed tomography studies cannot be compared with our results. Strengths of this study include a large representative HIV cohort; a population-based control group of similar age, gender, and racial composition; and use of MRI for body composition analysis. This allowed study of the largest number of healthy controls to date, although none were elderly.
In conclusion, this study found that HIV infection was associated with decreased IMAT; after controlling for demographics, lifestyle factors, and body composition, HIV-infected men but not women have lower IMAT compared with controls. As seen with SAT, lower IMAT is associated with exposure to stavudine, suggesting that IMAT shares some cellular origins with SAT. Future longitudinal studies should evaluate carefully the effects of HIV infection and its therapies on intermuscular and intramyocellular AT.
Acknowledgments
R.S. contributed to the analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis; S.B.H., W.S., and M.P. contributed to the conception and design of the study, analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content and obtained funding; P.B., D.K., C.E.L., and M.G.S. contributed to the conception and design of the study, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and statistical analysis; and C.G. obtained funding, provided administrative support and supervision, and contributed to the conception and design of the study, analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis. The funder played no role in the conduct of the study, collection of the data, management of the study, analysis of data, interpretation of the data or preparation of the manuscript. A representative of the funding agent participated in planning the protocol. Sites and investigators: University Hospitals of Cleveland (Barbara Gripshover); Tufts University (Abby Shevitz (deceased) and Christine Wanke); Stanford University (Andrew Zolopa); University of Alabama at Birmingham (Michael Saag); John Hopkins University (Joseph Cofrancesco and Adrian Dobs); University of Colorado Health Sciences Center (Lisa Kosmiski and Constance Benson); University of North Carolina at Chapel Hill (David Wohl and Charles van der Horst); University of California at San Diego (Daniel Lee and W. Christopher Mathews); Washington University (E. Turner Overton and William Powderly); VA Medical Center, Atlanta (David Rimland); University of California at Los Angeles (Judith Currier); VA Medical Center, New Y ork (Michael Simberkoff); VA Medical Center, Washington DC (Cynthia Gibert); St Luke’s-Roosevelt Hospital Center (D.P.K. and Ellen Engelson); Kaiser Permanente, Oakland (Stephen Sidney); University of Alabama at Birmingham (C.E.L.); University of California at San Francisco (Morris Schambelan and Kathleen Mulligan); Indiana University (Michael Dube); FRAM 1 Data Coordinating Center: University of Alabama, Birmingham (O. Dale Williams, Heather McCreath, Charles Katholi, George Howard, Tekeda Ferguson, and Anthony Goudie). FRAM 2 Data Coordinating Center: University of Washington, Seattle (Richard A. Kronmal, PhD, Mary Louise Biggs, PhD, Joseph A.C. Delaney, PhD, and John Pearce). Image Reading Centers: St Luke’s-Roosevelt Hospital Center (S.B.H., Jack Wang, and M.P.). Tufts New England Medical Center, Boston (Daniel H. O’Leary, Joseph Polack, and Anita P. Harrington). Office of the Principal Investigator: University of California, San Francisco, Veterans Affairs Medical Center and the Northern California Institute for Research and Development: (C.G., Phyllis Tien, P.B., Dennis Osmond, M.G.S., R.S., Mae Pang, Heather Southwell). This study was supported by grants from the NIH (R01-DK57508, HL74814, and HL53359; K23 AI66943 and NIH center grants M01-RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, RR00865, and UL1 RR024131), the Albert L. and Janet A. Schultz Supporting Foundation and with resources and the use of facilities of the Veterans Affairs Medical Center, San Francisco, California. The funding agencies had no role in the collection or analysis of the data. Clinicaltrials.gov ID: NCT00331448.
Footnotes
Disclosure
R.S., W.S., S.B.H., C.E.L., D.P.K., M.P., P.B., M.G.S., and C.G. received funding from the supporting grants. C.G. has received prior research funding and/or honorarium from Merck, Bristol-Myers Squibb, Abbott, Serono, and Theratechnologies.
References
- 1.Grunfeld C, Kotler DP, Arnett DK, et al. Contribution of metabolic and anthropometric abnormalities to cardiovascular disease risk factors. Circulation. 2008;118:e20–e28. doi: 10.1161/CIRCULATIONAHA.107.189623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacchetti P, Cofrancesco J, Heymsfield S, et al. Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr. 2008;87:1590–1595. doi: 10.1093/ajcn/87.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song MY, Ruts E, Kim J, et al. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 7.Roubenoff R, Roubenoff RA, Cannon JG, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–2386. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunfeld C, Rimland D, Gibert CL, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–290. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 11.Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 2007;31:1400–1405. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yim JE, Heshka S, Albu JB, Heymsfield S, Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol. 2008;104:700–707. doi: 10.1152/japplphysiol.01035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currier J, Scherzer R, Bacchetti P, et al. Regional adipose tissue and lipid and lipoprotein levels in HIV-infected women. J Acquir Immune Defic Syndr. 2008;48:35–43. doi: 10.1097/QAI.0b013e318164227f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohl D, Scherzer R, Heymsfield S, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durheim MT, Slentz CA, Bateman LA, Mabe SK, Kraus WE. Relationships between exercise-induced reductions in thigh intermuscular adipose tissue, changes in lipoprotein particle size, and visceral adiposity. Am J Physiol Endocrinol Metab. 2008;295:E407–E412. doi: 10.1152/ajpendo.90397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety) J Clin Endocrinol Metab. 1999;84:170–174. doi: 10.1210/jcem.84.1.5383. [DOI] [PubMed] [Google Scholar]
- 17.Garg A, Fleckenstein JL, Peshock RM, Grundy SM. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J Clin Endocrinol Metab. 1992;75:358–361. doi: 10.1210/jcem.75.2.1639935. [DOI] [PubMed] [Google Scholar]
- 18.Albu JB, Kenya S, He Q, et al. Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am J Clin Nutr. 2007;86:100–106. doi: 10.1093/ajcn/86.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Q, Engelson ES, Ionescu G, et al. Insulin resistance, hepatic lipid and adipose tissue distribution in HIV-infected men. Antivir Ther (Lond) 2008;13:423–428. [PMC free article] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miljkovic I, Cauley JA, Petit MA, et al. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab. 2009;94:2735–2742. doi: 10.1210/jc.2008-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tien PC, Benson C, Zolopa AR, et al. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cockerham L, Scherzer R, Zolopa A, et al. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53:102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 26.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil Prev. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 29.Madden E, Lee G, Kotler DP, et al. Association of antiretroviral therapy with fibrinogen levels in HIV-infection. AIDS. 2008;22:707–715. doi: 10.1097/QAD.0b013e3282f560d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman and Hall; London: 1993. [Google Scholar]
- 31.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 32.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 33.Hastie T, Tibshirani R. Generalized Additive Models. Chapman and Hall; New York: 1990. [DOI] [PubMed] [Google Scholar]
- 34.Belsley DA, Kuh E, Welsch RE. Regression Diagnostics. Wiley; New York: 1980. [Google Scholar]
- 35.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 37.Kent KR, Davis GW, Ramsey CB, Schluter AR. Estimates of beef carcass intermuscular fat. J Anim Sci. 1991;69:4836–4844. doi: 10.2527/1991.69124836x. [DOI] [PubMed] [Google Scholar]
- 38.Manini TM, Clark BC, Nalls MA, et al. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 39.Côté HC. Possible ways nucleoside analogues can affect mitochondrial DNA content and gene expression during HIV therapy. Antivir Ther (Lond) 2005;10 (Suppl 2):M3–11. [PubMed] [Google Scholar]
- 40.Després JP. Health consequences of visceral obesity. Ann Med. 2001;33:534–541. doi: 10.3109/07853890108995963. [DOI] [PubMed] [Google Scholar]
- 41.Luzi L, Perseghin G, Tambussi G, et al. Intramyocellular lipid accumulation and reduced whole body lipid oxidation in HIV lipodystrophy. Am J Physiol Endocrinol Metab. 2003;284:E274–E280. doi: 10.1152/ajpendo.00391.2001. [DOI] [PubMed] [Google Scholar]
- 42.Torriani M, Hadigan C, Jensen ME, Grinspoon S. Psoas muscle attenuation measurement with computed tomography indicates intramuscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J Appl Physiol. 2003;95:1005–1010. doi: 10.1152/japplphysiol.00366.2003. [DOI] [PubMed] [Google Scholar]