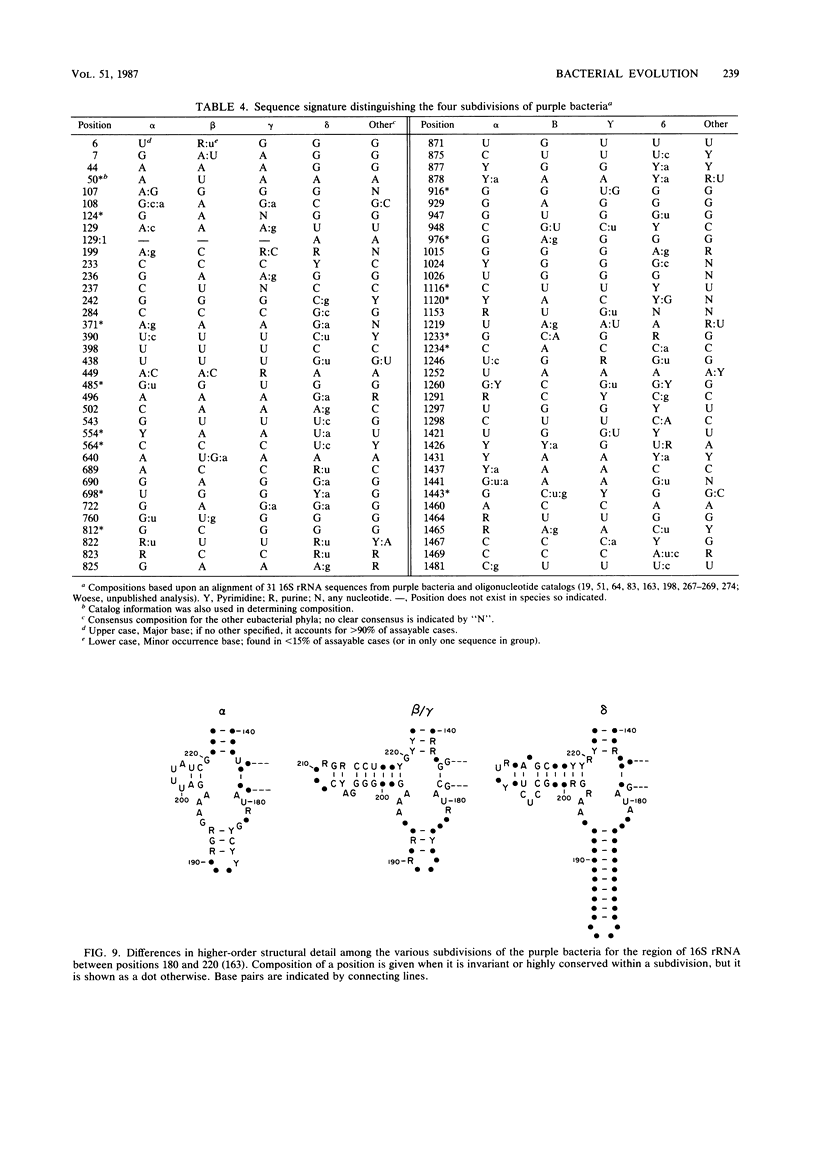
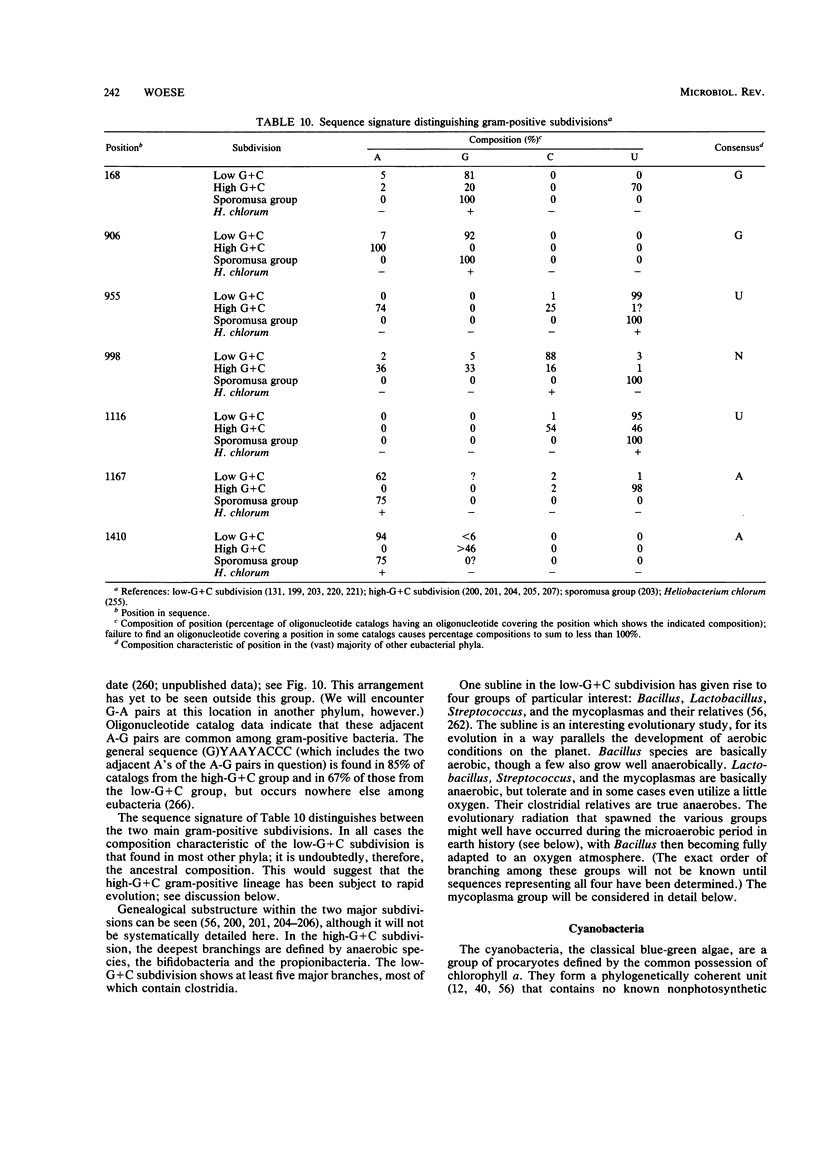
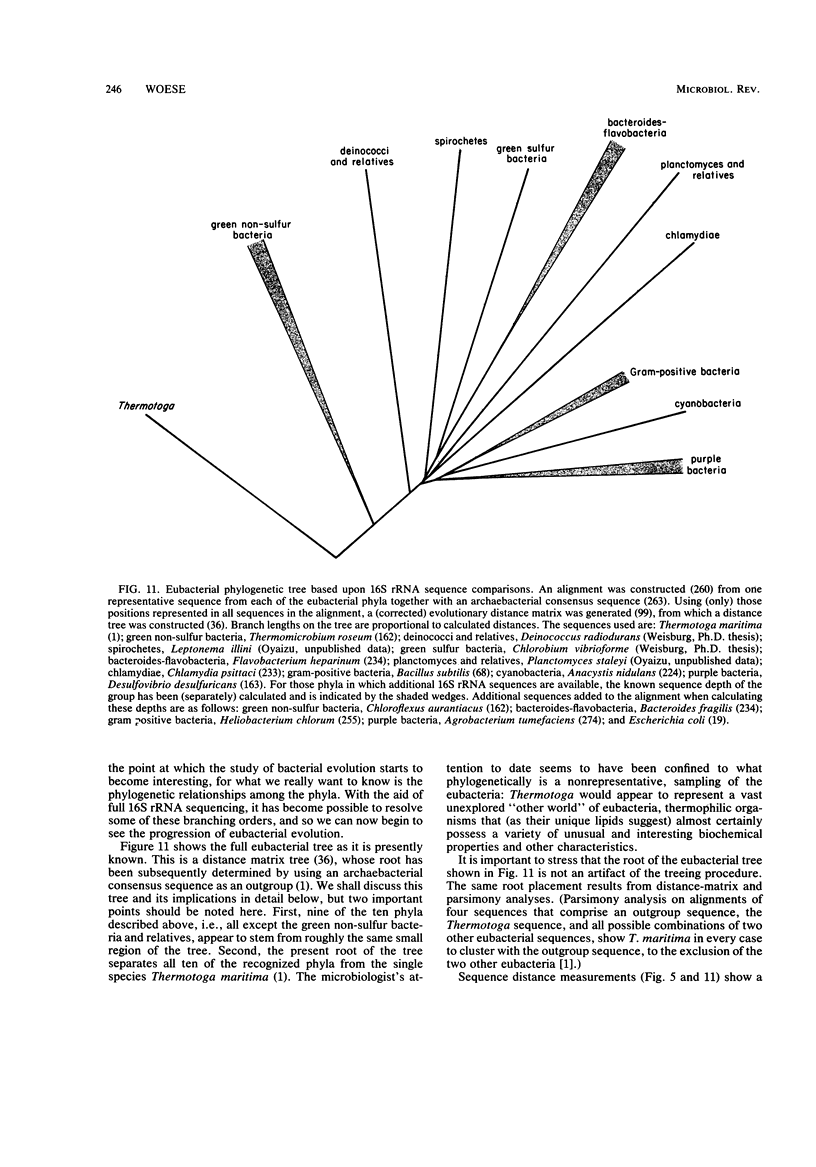
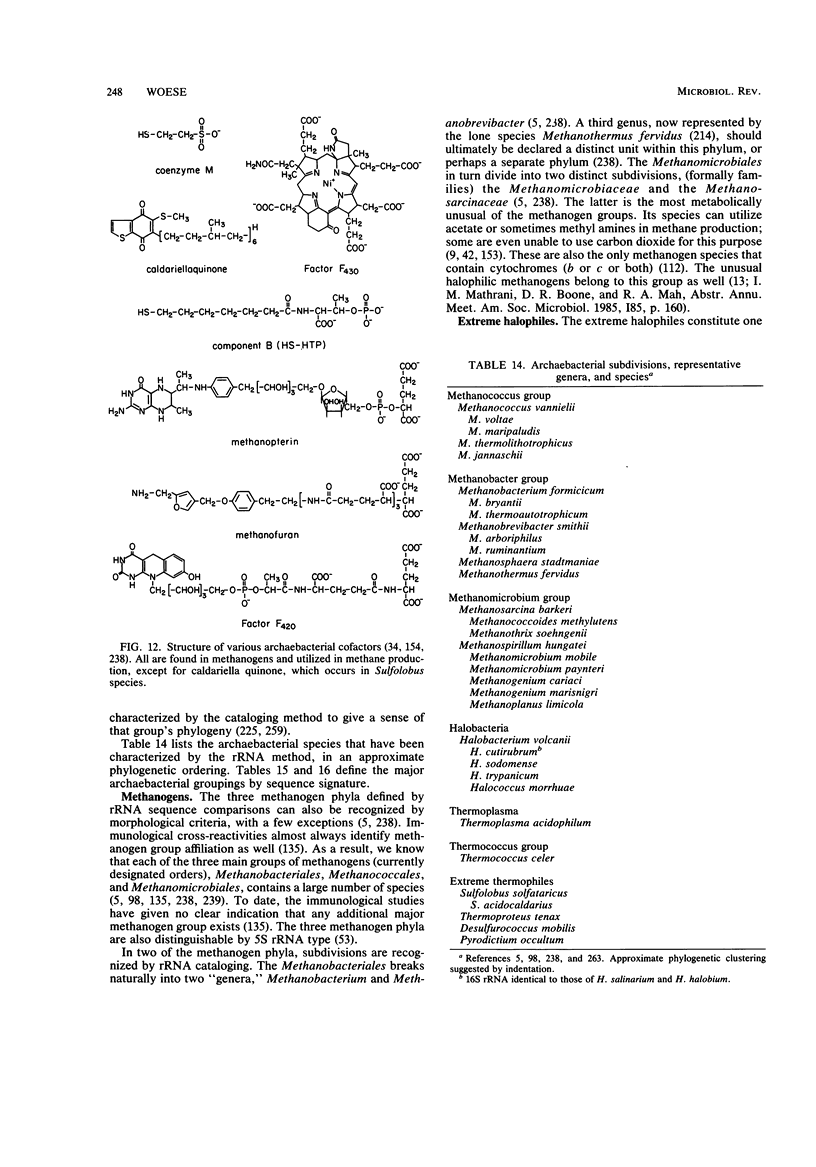
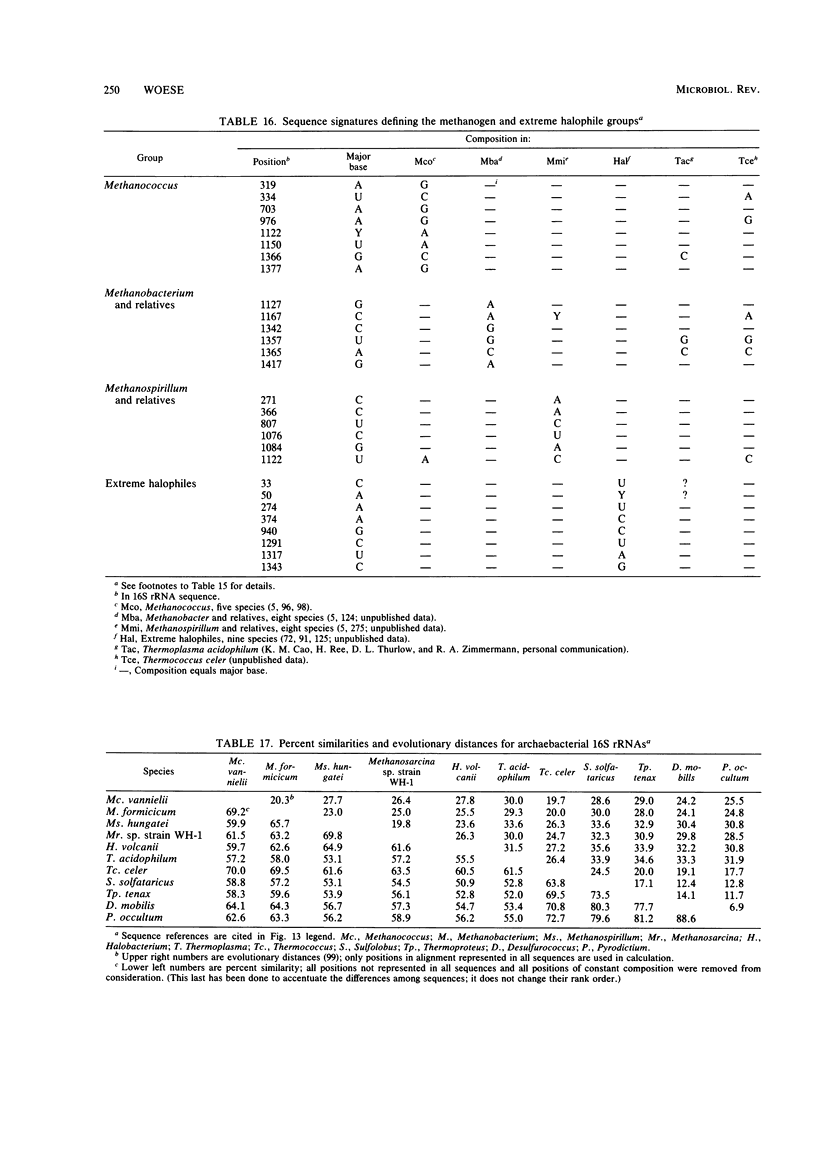
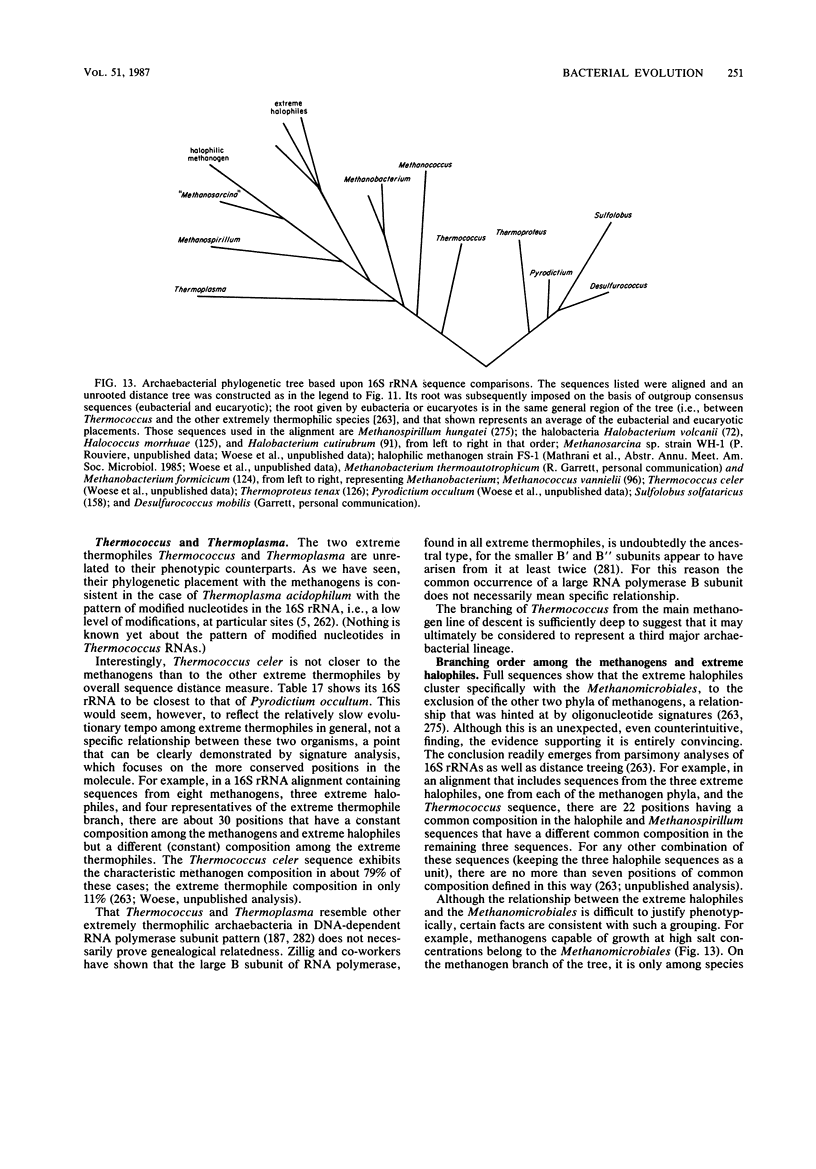
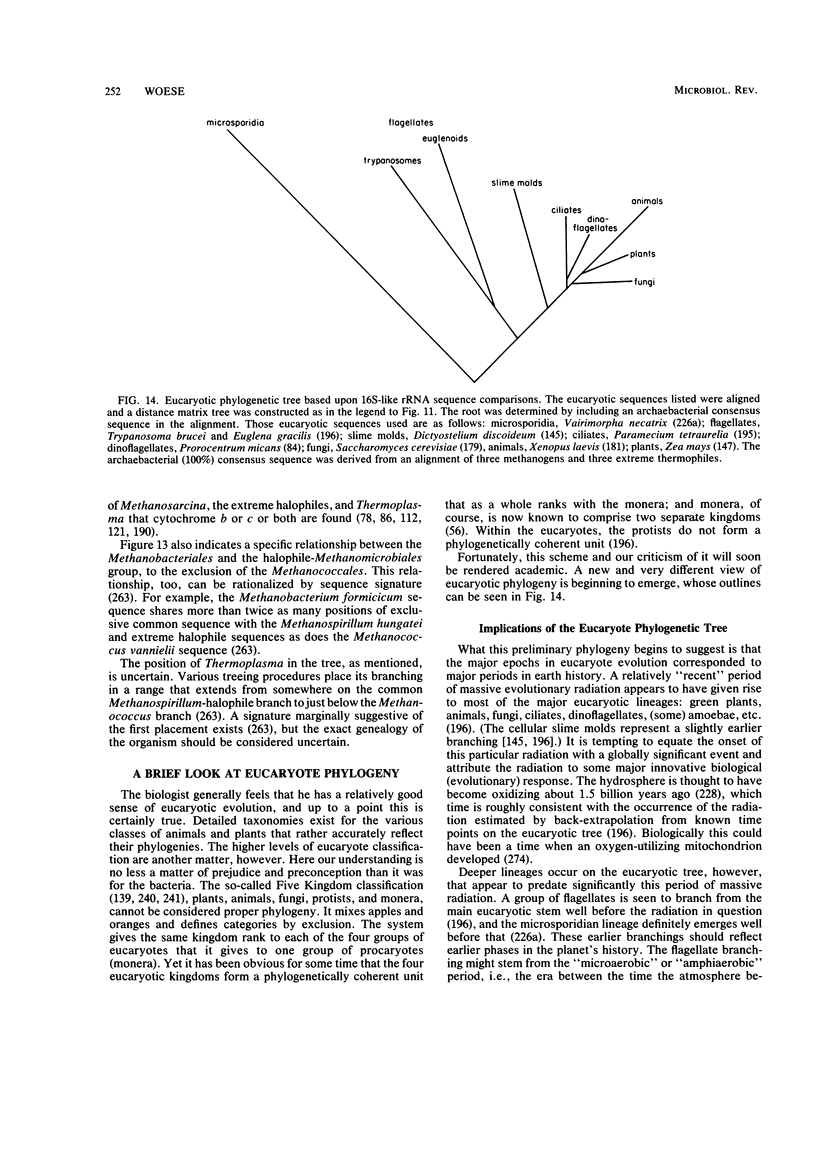
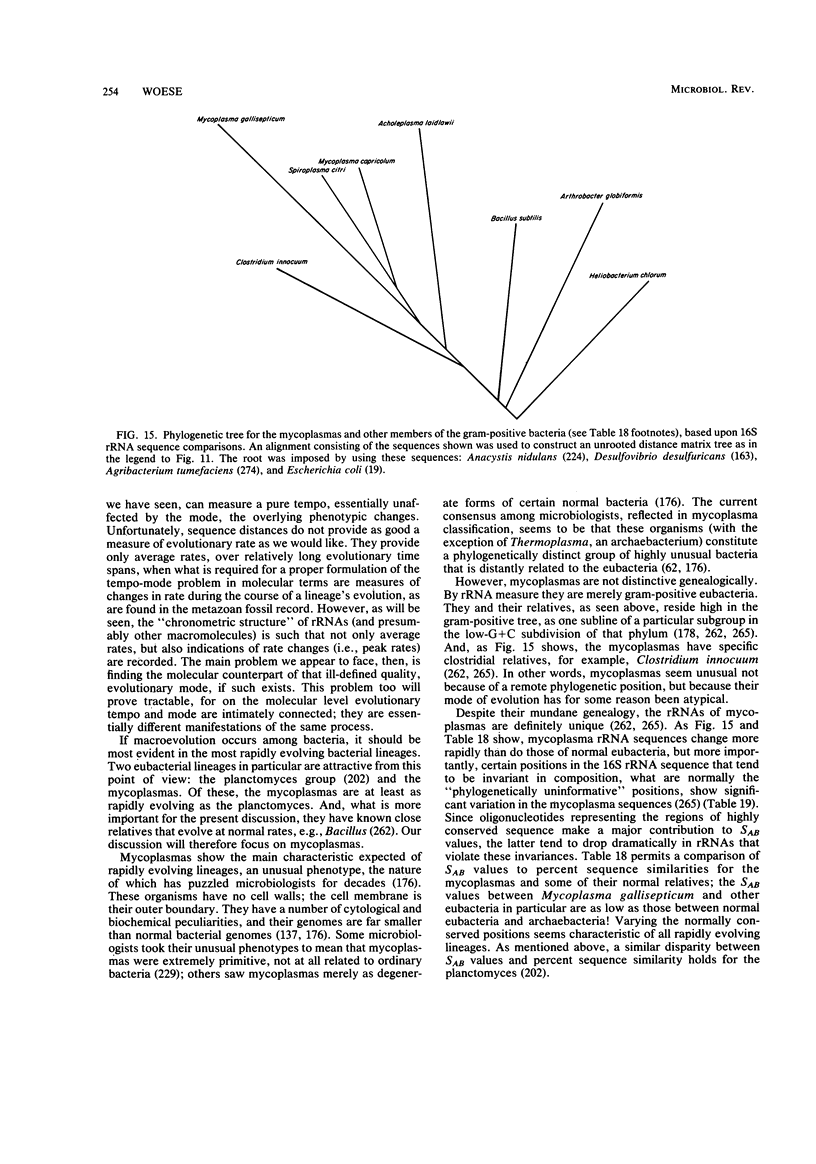
Full text
PDF



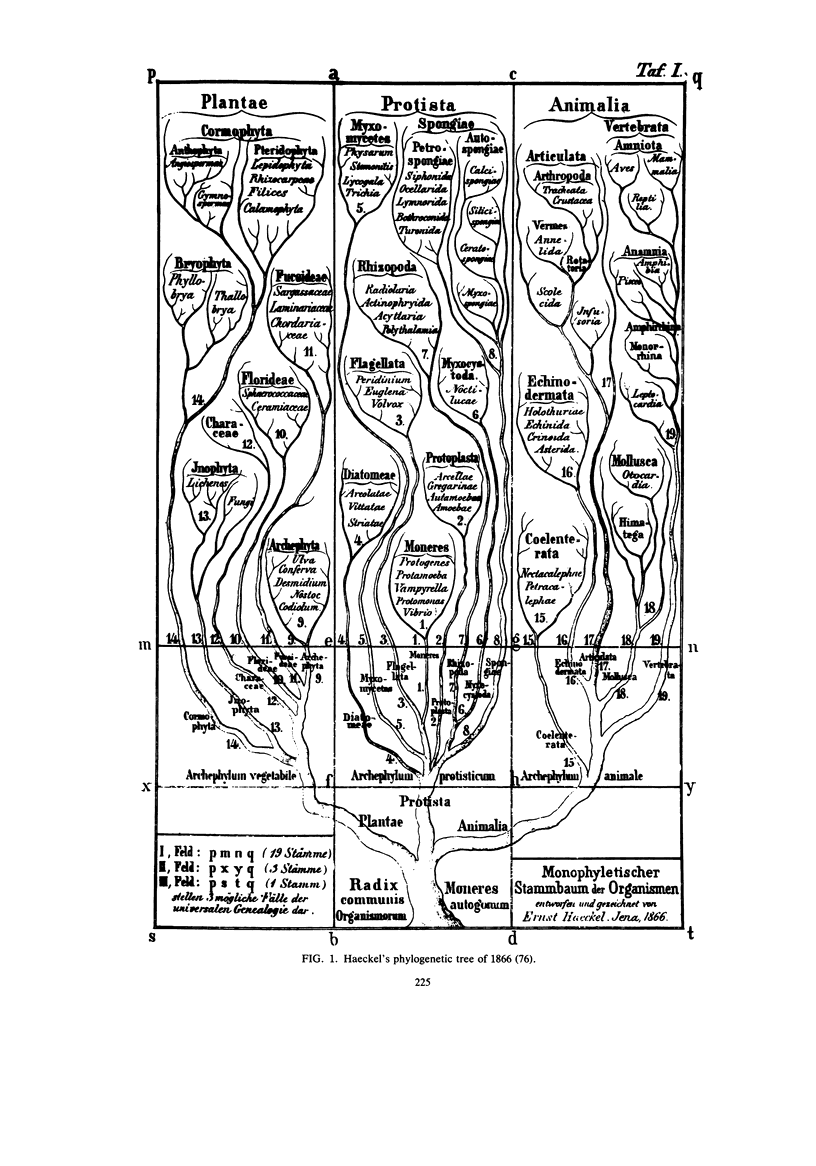



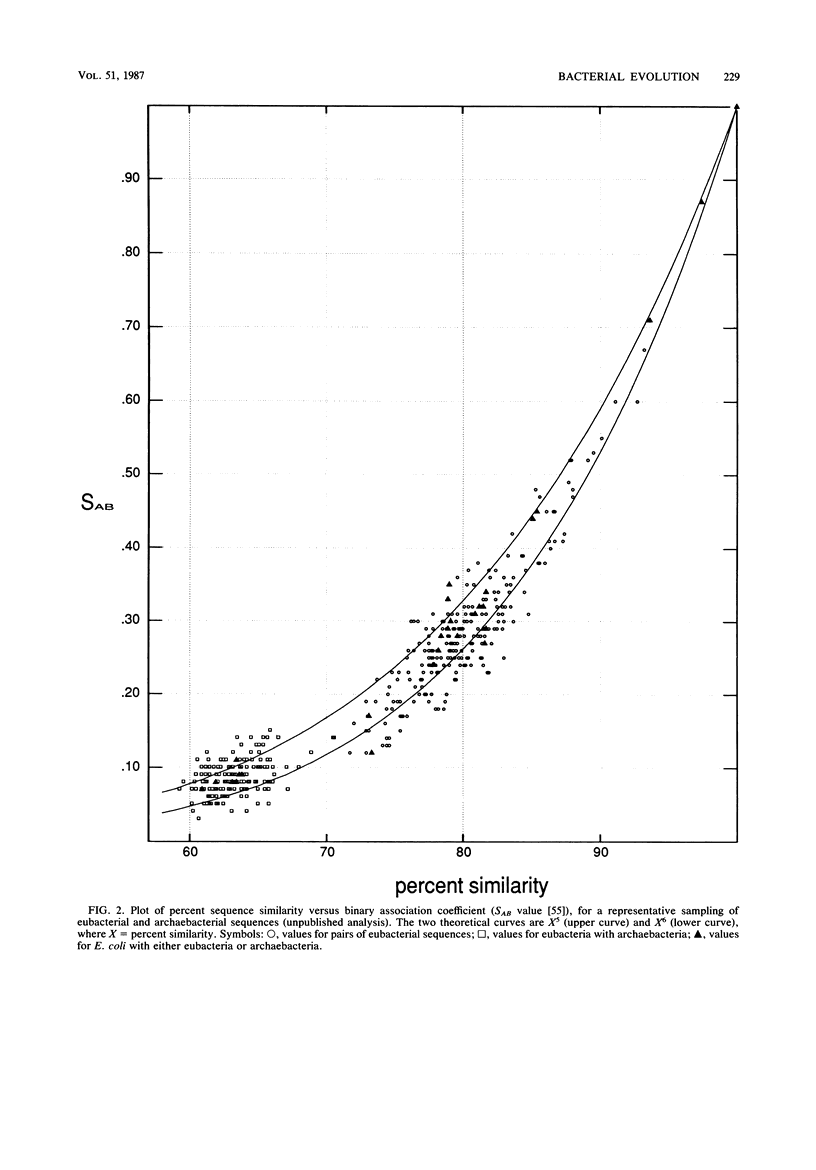
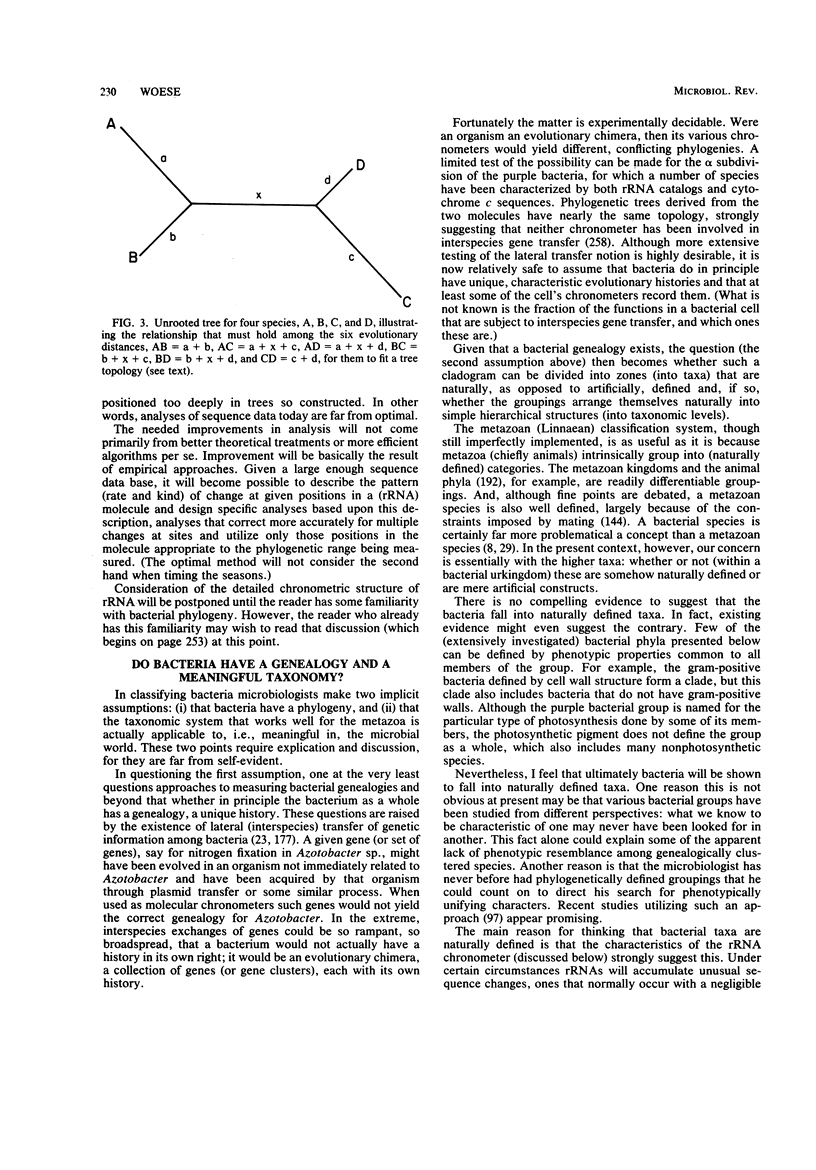
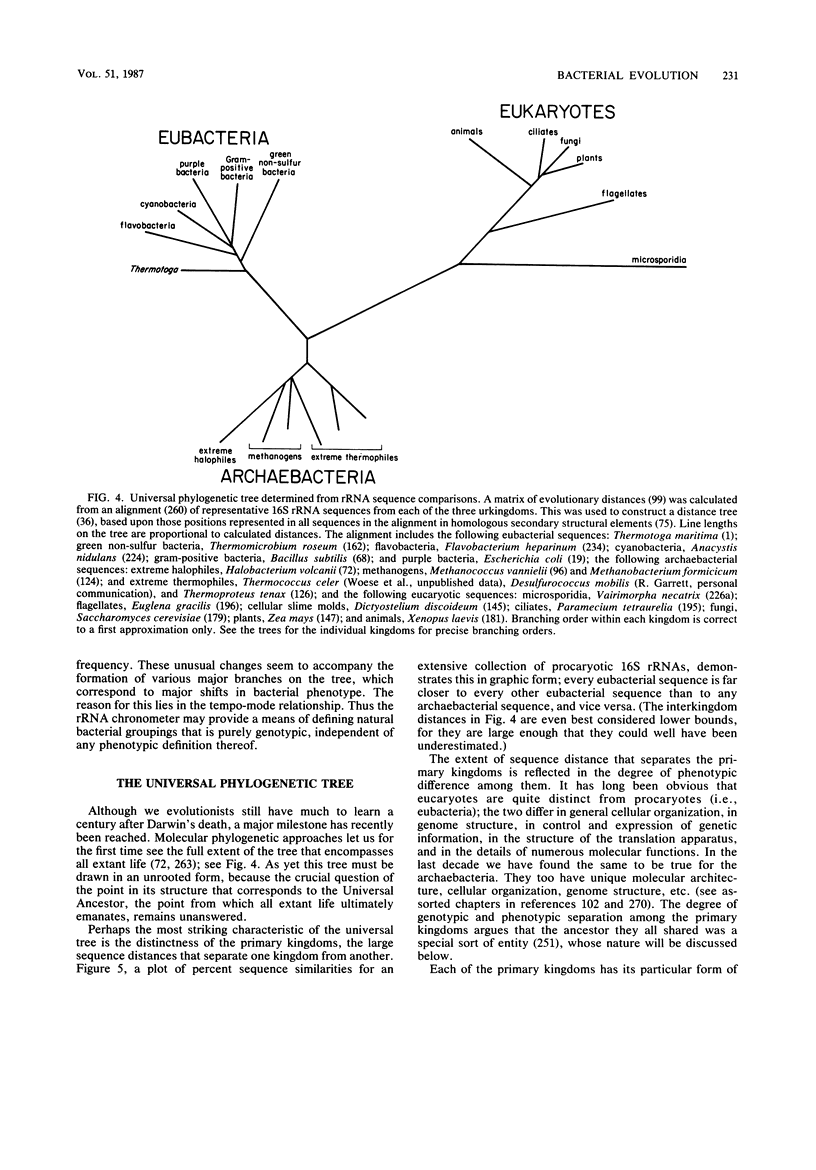

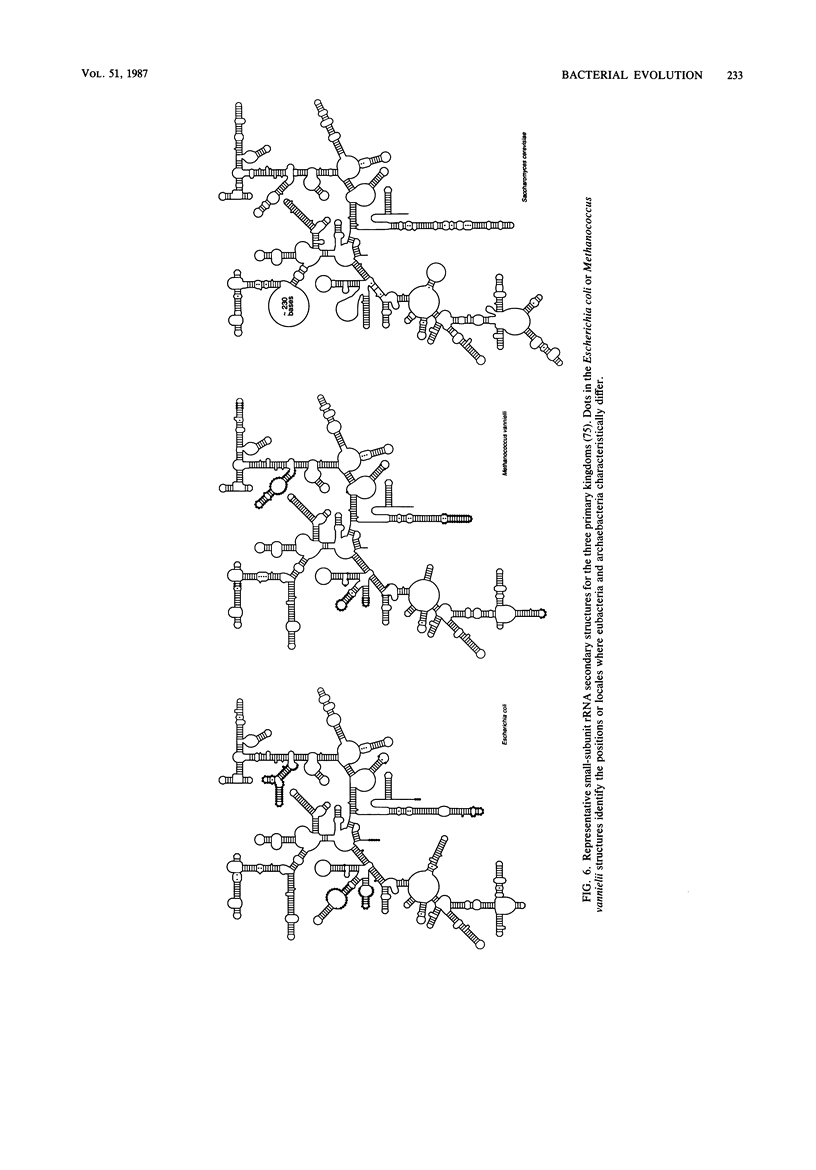
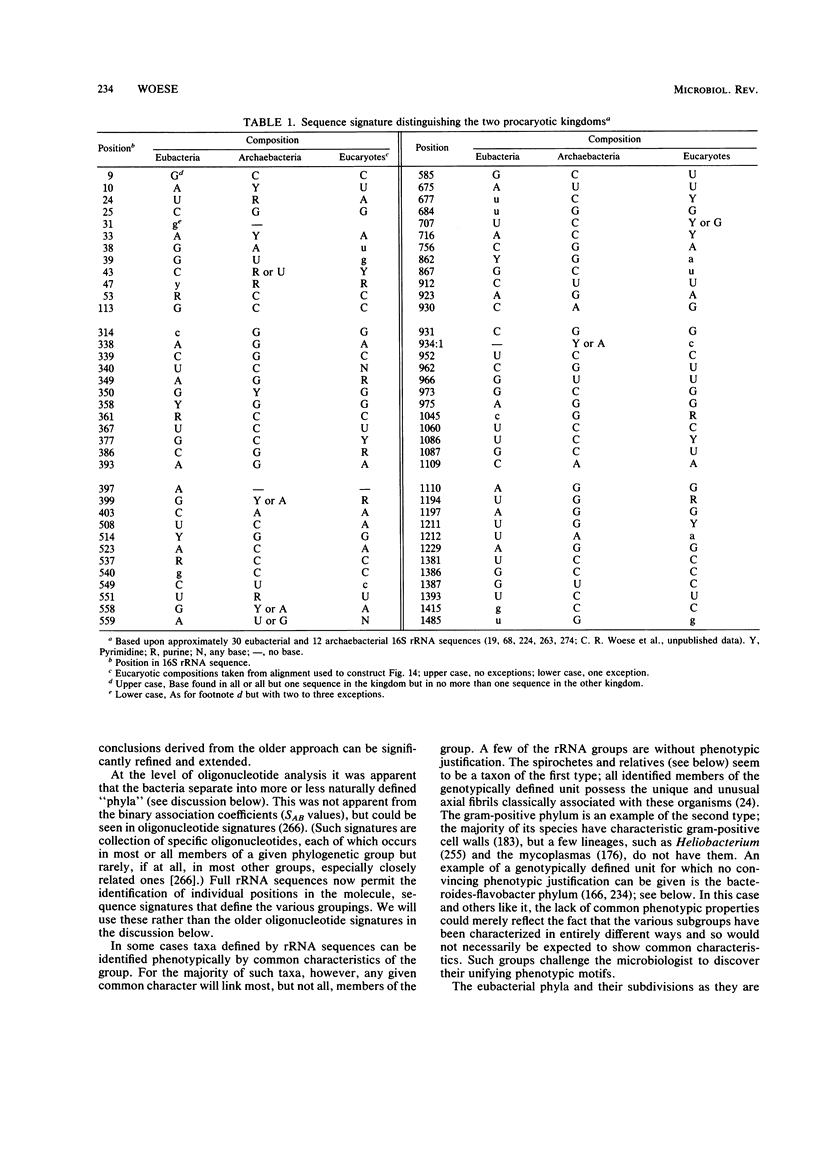
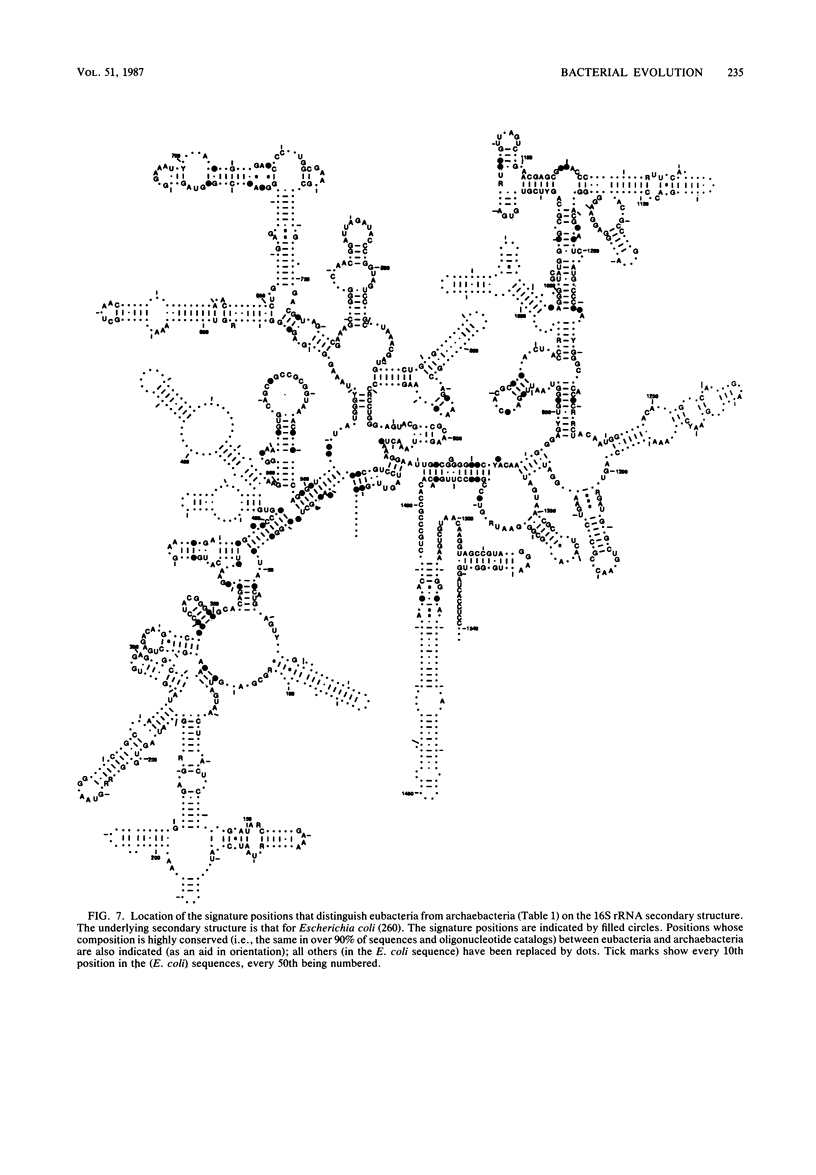

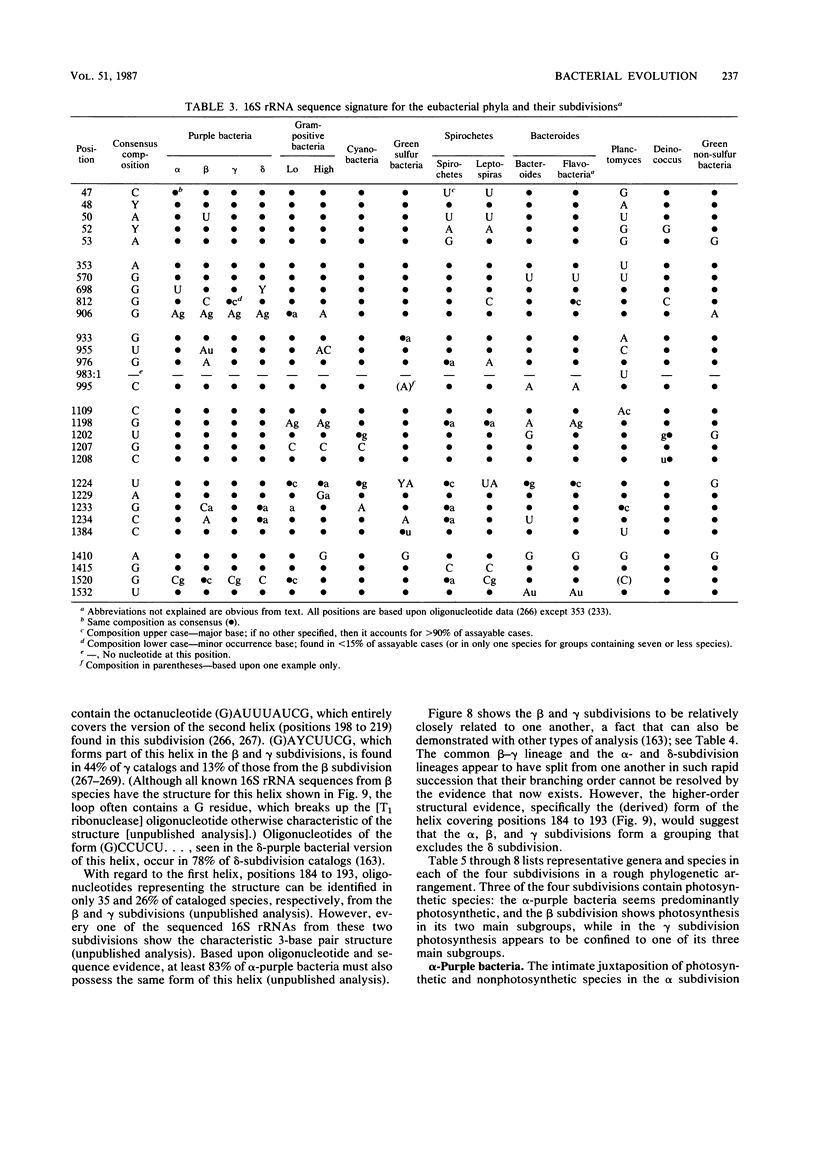

























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach-Richter L., Gupta R., Stetter K. O., Woese C. R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- Altamura S., Cammarano P., Londei P. Archaebacterial and eukaryotic ribosomal subunits can form active hybrid ribosomes. FEBS Lett. 1986 Aug 11;204(1):129–133. doi: 10.1016/0014-5793(86)81400-4. [DOI] [PubMed] [Google Scholar]
- Alvarez L. W. Experimental evidence that an asteroid impact led to the extinction of many species 65 million years ago. Proc Natl Acad Sci U S A. 1983 Jan;80(2):627–642. doi: 10.1073/pnas.80.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Daniel M., Hermoso J., Meyer T. E., Bartsch R. G., Kamen M. D. Cytochrome c2 sequence variation among the recognised species of purple nonsulphur photosynthetic bacteria. Nature. 1979 Apr 12;278(5705):659–660. doi: 10.1038/278659a0. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Magrum L. J., Fox G. E., Wolfe R. S., Woese C. R. An ancient divergence among the bacteria. J Mol Evol. 1977 Aug 5;9(4):305–311. doi: 10.1007/BF01796092. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Amano K., Hackstadt T., Perry L., Caldwell H. D. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982 Jul;151(1):420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock B. A., Stadtman T. C. Methane biosynthesis by Methanosarcina barkeri. Properties of the soluble enzyme system. Arch Biochem Biophys. 1966 Sep 26;116(1):138–152. doi: 10.1016/0003-9861(66)90022-1. [DOI] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. On the prokaryotic nature of red algal chloroplasts. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2310–2314. doi: 10.1073/pnas.72.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. Partial sequences of 16S rRNA and the phylogeny of blue-green algae and chloroplasts. Nature. 1976 Jun 24;261(5562):669–673. doi: 10.1038/261669a0. [DOI] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. Ribosomal RNA homologies and the evolution of the filamentous blue-green bacteria. J Mol Evol. 1978 Feb 21;10(4):283–291. doi: 10.1007/BF01734218. [DOI] [PubMed] [Google Scholar]
- Boublik M., Wydro R. M., Hellmann W., Jenkins F. Structure of functional A salina -- E coli hybrid ribosome by electron microscopy. J Supramol Struct. 1979;10(4):397–404. doi: 10.1002/jss.400100403. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Cammarano P., Teichner A., Londei P., Acca M., Nicolaus B., Sanz J. L., Amils R. Insensitivity of archaebacterial ribosomes to protein synthesis inhibitors. Evolutionary implications. EMBO J. 1985 Mar;4(3):811–816. doi: 10.1002/j.1460-2075.1985.tb03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. Evolutionary significance of accessory DNA elements in bacteria. Annu Rev Microbiol. 1981;35:55–83. doi: 10.1146/annurev.mi.35.100181.000415. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Clayton B. J., Clayton R. K. Properties of photochemical reaction centers purified from Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1978 Mar 13;501(3):470–477. doi: 10.1016/0005-2728(78)90114-7. [DOI] [PubMed] [Google Scholar]
- DOOLITTLE R. F., BLOMBAECK B. AMINO-ACID SEQUENCE INVESTIGATIONS OF FIBRINOPEPTIDES FROM VARIOUS MAMMALS: EVOLUTIONARY IMPLICATIONS. Nature. 1964 Apr 11;202:147–152. doi: 10.1038/202147a0. [DOI] [PubMed] [Google Scholar]
- Daniels C. J., Gupta R., Doolittle W. F. Transcription and excision of a large intron in the tRNATrp gene of an archaebacterium, Halobacterium volcanii. J Biol Chem. 1985 Mar 10;260(5):3132–3134. [PubMed] [Google Scholar]
- De Ley J., De Smedt J. Improvements of the membrane filter method for DNA:rRNA hybridization. Antonie Van Leeuwenhoek. 1975;41(3):287–307. doi: 10.1007/BF02565064. [DOI] [PubMed] [Google Scholar]
- De Rosa M., De Rosa S., Gambacorta A., Minale L. Caldariellaquinone, a unique benzo(b)thiophen-4,7-quinone from Caldariella acidophila, an extremely thermophilic and acidophilic bacterium. J Chem Soc Perkin 1. 1977;(6):653–657. doi: 10.1039/p19770000653. [DOI] [PubMed] [Google Scholar]
- De Rosa M., Gambacorta A., Gliozzi A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol Rev. 1986 Mar;50(1):70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast 16S rRNA gene and its surrounding regions of Chlamydomonas reinhardii. Nucleic Acids Res. 1982 Dec 11;10(23):7609–7620. doi: 10.1093/nar/10.23.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easteal S., Oakeshott J. G. Estimating divergence times of Drosophila species from DNA sequence comparisons. Mol Biol Evol. 1985 Mar;2(2):87–91. doi: 10.1093/oxfordjournals.molbev.a040342. [DOI] [PubMed] [Google Scholar]
- Errede B., Kamen M. D. Comparative kinetic studies of cytochromes c in reactions with mitochondrial cytochrome c oxidase and reductase. Biochemistry. 1978 Mar 21;17(6):1015–1027. doi: 10.1021/bi00599a012. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Magrum L. J., Balch W. E., Wolfe R. S., Woese C. R. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Garrett A. J., Harrison M. J., Manire G. P. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol. 1974 Jan;80(1):315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Das J., Maniloff J. Lack of repair of ultraviolet light damage in Mycoplasma gallisepticum. J Mol Biol. 1977 Oct 25;116(2):337–344. doi: 10.1016/0022-2836(77)90221-2. [DOI] [PubMed] [Google Scholar]
- Golding G. B. Estimates of DNA and protein sequence divergence: an examination of some assumptions. Mol Biol Evol. 1983 Dec;1(1):125–142. doi: 10.1093/oxfordjournals.molbev.a040303. [DOI] [PubMed] [Google Scholar]
- Graf L., Roux E., Stutz E., Kössel H. Nucleotide sequence of a Euglena gracilis chloroplast gene coding for the 16S rRNA: homologies to E. coli and Zea mays chloroplast 16S rRNA. Nucleic Acids Res. 1982 Oct 25;10(20):6369–6381. doi: 10.1093/nar/10.20.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Gupta R., Lanter J. M., Woese C. R. Sequence of the 16S Ribosomal RNA from Halobacterium volcanii, an Archaebacterium. Science. 1983 Aug 12;221(4611):656–659. doi: 10.1126/science.221.4611.656. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Noller H. F., Woese C. R. Higher order structure in ribosomal RNA. EMBO J. 1986 May;5(5):1111–1113. doi: 10.1002/j.1460-2075.1986.tb04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Hallberg C., Baltscheffsky H. Solubilization and separation of two b-type cytochromes from a carotenoid mutant in Halobacterium halobium. FEBS Lett. 1981 Mar 23;125(2):201–204. doi: 10.1016/0014-5793(81)80718-1. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Sickinger H. D., Oesterhelt D. Anaerobic growth of halobacteria. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3821–3825. doi: 10.1073/pnas.77.7.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Matsubara H. Halobacterium halobium ferredoxin. A homologous protein to choroplast-type ferredoxins. FEBS Lett. 1977 May 15;77(2):308–310. doi: 10.1016/0014-5793(77)80257-3. [DOI] [PubMed] [Google Scholar]
- Henderson E., Oakes M., Clark M. W., Lake J. A., Matheson A. T., Zillig W. A new ribosome structure. Science. 1984 Aug 3;225(4661):510–512. doi: 10.1126/science.6429855. [DOI] [PubMed] [Google Scholar]
- Herzog M., Maroteaux L. Dinoflagellate 17S rRNA sequence inferred from the gene sequence: Evolutionary implications. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8644–8648. doi: 10.1073/pnas.83.22.8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Schnabel R., Sentenac A., Zillig W. Archaebacteria and eukaryotes possess DNA-dependent RNA polymerases of a common type. EMBO J. 1983;2(8):1291–1294. doi: 10.1002/j.1460-2075.1983.tb01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami M., Muto A., Yamao F., Osawa S. Nucleotide sequence of the rrnB 16S ribosomal RNA gene from Mycoplasma capricolum. Mol Gen Genet. 1984;196(2):317–322. doi: 10.1007/BF00328065. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog Chem Fats Other Lipids. 1978;15(4):301–342. doi: 10.1016/0079-6832(77)90011-8. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Clark M. W., Henderson E., Fay S. P., Oakes M., Scheinman A., Thornber J. P., Mah R. A. Eubacteria, halobacteria, and the origin of photosynthesis: the photocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3716–3720. doi: 10.1073/pnas.82.11.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A., Henderson E., Oakes M., Clark M. W. Eocytes: a new ribosome structure indicates a kingdom with a close relationship to eukaryotes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3786–3790. doi: 10.1073/pnas.81.12.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Light energy conversion in Halobacterium halobium. Microbiol Rev. 1978 Dec;42(4):682–706. doi: 10.1128/mr.42.4.682-706.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. I. Spectrophotometric identification of the cytochromes of Halobacterium cutirubrum. Arch Biochem Biophys. 1968 Dec;128(3):716–724. doi: 10.1016/0003-9861(68)90080-5. [DOI] [PubMed] [Google Scholar]
- Larsen H. The fourth A. J. Kluyver memorial lecture delivered before the Netherlands Society for Microbiology on April 27th, 1972, at the Delft University of Technology, Delft. The halobacteria's confusion to biology. Antonie Van Leeuwenhoek. 1973;39(3):383–396. doi: 10.1007/BF02578880. [DOI] [PubMed] [Google Scholar]
- Leffers H., Garrett R. A. The nucleotide sequence of the 16S ribosomal RNA gene of the archaebacterium Halococcus morrhua. EMBO J. 1984 Jul;3(7):1613–1619. doi: 10.1002/j.1460-2075.1984.tb02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. A. Prochloron and the theory of symbiogenesis. Ann N Y Acad Sci. 1981;361:325–329. doi: 10.1111/j.1749-6632.1981.tb46528.x. [DOI] [PubMed] [Google Scholar]
- Li W. Y., Reddy R., Henning D., Epstein P., Busch H. Nucleotide sequence of 7 S RNA. Homology to Alu DNA and La 4.5 S RNA. J Biol Chem. 1982 May 10;257(9):5136–5142. [PubMed] [Google Scholar]
- Ludwig W., Seewaldt E., Kilpper-Bälz R., Schleifer K. H., Magrum L., Woese C. R., Fox G. E., Stackebrandt E. The phylogenetic position of Streptococcus and Enterococcus. J Gen Microbiol. 1985 Mar;131(3):543–551. doi: 10.1099/00221287-131-3-543. [DOI] [PubMed] [Google Scholar]
- Ludwig W., Stackebrandt E. A phylogenetic analysis of Legionella. Arch Microbiol. 1983 Aug;135(1):45–50. doi: 10.1007/BF00419481. [DOI] [PubMed] [Google Scholar]
- Luehrsen K. R., Fox G. E., Kilpatrick M. W., Walker R. T., Domdey H., Krupp G., Gross H. J. The nucleotide sequence of the 5S rRNA from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1981 Feb 25;9(4):965–970. doi: 10.1093/nar/9.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehrsen K. R., Nicholson D. E., Jr, Fox G. E. Widespread distribution of a 7S RNA in archaebacteria. Curr Microbiol. 1985;12:69–72. doi: 10.1007/BF01567394. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Carlson J., Hagen G., Rubenstein I., Oleson A. Cloning and sequencing of the ribosomal RNA genes in maize: the 17S region. DNA. 1984;3(1):31–40. doi: 10.1089/dna.1.1984.3.31. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Cusanovich M. A., Kamen M. D. Evidence against use of bacterial amino acid sequence data for construction of all-inclusive phylogenetic trees. Proc Natl Acad Sci U S A. 1986 Jan;83(2):217–220. doi: 10.1073/pnas.83.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills L. B., Stanbridge E. J., Sedwick W. D., Korn D. Purification and partial characterization of the principal deoxyribonucleic acid polymerase from Mycoplasmatales. J Bacteriol. 1977 Nov;132(2):641–649. doi: 10.1128/jb.132.2.641-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A., Goebel W. Characterization of the 7S RNA and its gene from halobacteria. Nucleic Acids Res. 1985 Oct 11;13(19):6969–6979. doi: 10.1093/nar/13.19.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll K. M., Rinehart K. L., Jr, Tanner R. S., Wolfe R. S. Structure of component B (7-mercaptoheptanoylthreonine phosphate) of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4238–4242. doi: 10.1073/pnas.83.12.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Olsen G. J., Lane D. J., Giovannoni S. J., Pace N. R., Stahl D. A. Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol. 1986;40:337–365. doi: 10.1146/annurev.mi.40.100186.002005. [DOI] [PubMed] [Google Scholar]
- Olsen G. J., Pace N. R., Nuell M., Kaine B. P., Gupta R., Woese C. R. Sequence of the 16S rRNA gene from the thermoacidophilic archaebacterium Sulfolobus solfataricus and its evolutionary implications. J Mol Evol. 1985;22(4):301–307. doi: 10.1007/BF02115685. [DOI] [PubMed] [Google Scholar]
- Oyaizu H., Debrunner-Vossbrinck B., Mandelco L., Studier J. A., Woese C. R. The green non-sulfur bacteria: a deep branching in the eubacterial line of descent. Syst Appl Microbiol. 1987;9:47–53. doi: 10.1016/s0723-2020(87)80055-3. [DOI] [PubMed] [Google Scholar]
- Pang H., Ihara M., Kuchino Y., Nishimura S., Gupta R., Woese C. R., McCloskey J. A. Structure of a modified nucleoside in archaebacterial tRNA which replaces ribosylthymine. 1-Methylpseudouridine. J Biol Chem. 1982 Apr 10;257(7):3589–3592. [PubMed] [Google Scholar]
- Pfennig N. Phototrophic green and purple bacteria: a comparative, systematic survey. Annu Rev Microbiol. 1977;31:275–290. doi: 10.1146/annurev.mi.31.100177.001423. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Thornber J. P. Isolation and spectral characterization of photochemical reaction centers from the thermophilic green bacterium Chloroflexus aurantiacus strain J-10-f1. Proc Natl Acad Sci U S A. 1983 Jan;80(1):80–84. doi: 10.1073/pnas.80.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond J. L., Langworthy T. A., Holzer G. Long-chain diols: a new class of membrane lipids from a thermophilic bacterium. Science. 1986 Mar 7;231(4742):1134–1136. doi: 10.1126/science.231.4742.1134. [DOI] [PubMed] [Google Scholar]
- Postgate J. R. Methane as a minor product of pyruvate metabolism by sulphate-reducing and other bacteria. J Gen Microbiol. 1969 Aug;57(3):293–302. doi: 10.1099/00221287-57-3-293. [DOI] [PubMed] [Google Scholar]
- Qu H. L., Michot B., Bachellerie J. P. Improved methods for structure probing in large RNAs: a rapid 'heterologous' sequencing approach is coupled to the direct mapping of nuclease accessible sites. Application to the 5' terminal domain of eukaryotic 28S rRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5903–5920. doi: 10.1093/nar/11.17.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reanney D. Extrachromosomal elements as possible agents of adaptation and development. Bacteriol Rev. 1976 Sep;40(3):552–590. doi: 10.1128/br.40.3.552-590.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov P. M., Musakhanov M. M., Zakharyev V. M., Krayev A. S., Skryabin K. G., Bayev A. A. The structure of the yeast ribosomal RNA genes. I. The complete nucleotide sequence of the 18S ribosomal RNA gene from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Dec 11;8(23):5779–5794. doi: 10.1093/nar/8.23.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., VAN NIEL C. B. The concept of a bacterium. Arch Mikrobiol. 1962;42:17–35. doi: 10.1007/BF00425185. [DOI] [PubMed] [Google Scholar]
- Salim M., Maden B. E. Nucleotide sequence of Xenopus laevis 18S ribosomal RNA inferred from gene sequence. Nature. 1981 May 21;291(5812):205–208. doi: 10.1038/291205a0. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Doolittle W. F. Unusual physical organization of the Halobacterium genome. Nature. 1982 Feb 4;295(5848):384–389. doi: 10.1038/295384a0. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Stackebrandt E. Molecular systematics of prokaryotes. Annu Rev Microbiol. 1983;37:143–187. doi: 10.1146/annurev.mi.37.100183.001043. [DOI] [PubMed] [Google Scholar]
- Schnabel R., Thomm M., Gerardy-Schahn R., Zillig W., Stetter K. O., Huet J. Structural homology between different archaebacterial DNA-dependent RNA polymerases analyzed by immunological comparison of their components. EMBO J. 1983;2(5):751–755. doi: 10.1002/j.1460-2075.1983.tb01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J. Primary structure of the Paramecium tetraurelia small-subunit rRNA coding region: phylogenetic relationships within the Ciliophora. J Mol Evol. 1986;23(1):53–60. doi: 10.1007/BF02100998. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Kroppenstedt R. M., Fowler V. J. A phylogenetic analysis of the family Dermatophilaceae. J Gen Microbiol. 1983 Jun;129(6):1831–1838. doi: 10.1099/00221287-129-6-1831. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Ludwig W., Schubert W., Klink F., Schlesner H., Roggentin T., Hirsch P. Molecular genetic evidence for early evolutionary origin of budding peptidoglycan-less eubacteria. Nature. 1984 Feb 23;307(5953):735–737. doi: 10.1038/307735a0. [DOI] [PubMed] [Google Scholar]
- Stahl D. A., Luehrsen K. R., Woese C. R., Pace N. R. An unusual 5S rRNA, from Sulfolobus acidocaldarius, and its implications for a general 5S rRNA structure. Nucleic Acids Res. 1981 Nov 25;9(22):6129–6137. doi: 10.1093/nar/9.22.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Van Niel C. B. The Main Outlines of Bacterial Classification. J Bacteriol. 1941 Oct;42(4):437–466. doi: 10.1128/jb.42.4.437-466.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W. The rhodopsin-like pigments of halobacteria: light-energy and signal transducers in an archaebacterium. Trends Biochem Sci. 1985 Dec;10(12):483–486. doi: 10.1016/0968-0004(85)90210-5. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W. Walsby's square bacterium: fine structure of an orthogonal procaryote. J Bacteriol. 1981 Oct;148(1):352–360. doi: 10.1128/jb.148.1.352-360.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler-Meilicke M., Böhme C., Strobel O., Böck A., Stöffler G. Structure of Ribosomal Subunits of M. vannielii: Ribosomal Morphology as a Phylogenetic Marker. Science. 1986 Mar 14;231(4743):1306–1308. doi: 10.1126/science.231.4743.1306. [DOI] [PubMed] [Google Scholar]
- Tohdoh N., Sugiura M. The complete nucleotide sequence of 16S ribosomal RNA gene from tobacco chloroplasts. Gene. 1982 Feb;17(2):213–218. doi: 10.1016/0378-1119(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Tomioka N., Sugiura M. The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet. 1983;191(1):46–50. doi: 10.1007/BF00330888. [DOI] [PubMed] [Google Scholar]
- Tu J. K., Prangishvilli D., Huber H., Wildgruber G., Zillig W., Stetter K. O. Taxonomic relations between archaebacteria including 6 novel genera examined by cross hybridization of DNAs and 16S rRNAs. J Mol Evol. 1982;18(2):109–114. doi: 10.1007/BF01810829. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. 1987 Mar 26-Apr 1Nature. 326(6111):411–414. doi: 10.1038/326411a0. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Woese C. R. Eukaryotic ribosomes that lack a 5.8S RNA. Nature. 1986 Mar 20;320(6059):287–288. doi: 10.1038/320287a0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Morowitz H. J. Genome size and evolution. Chromosoma. 1973;40(2):121–126. doi: 10.1007/BF00321457. [DOI] [PubMed] [Google Scholar]
- Waugh D. S., Pace N. R. Catalysis by RNA. Bioessays. 1986 Feb;4(2):56–61. doi: 10.1002/bies.950040204. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Oyaizu Y., Oyaizu H., Woese C. R. Natural relationship between bacteroides and flavobacteria. J Bacteriol. 1985 Oct;164(1):230–236. doi: 10.1128/jb.164.1.230-236.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Woese C. R., Dobson M. E., Weiss E. A common origin of rickettsiae and certain plant pathogens. Science. 1985 Nov 1;230(4725):556–558. doi: 10.1126/science.3931222. [DOI] [PubMed] [Google Scholar]
- Whittaker R. H. New concepts of kingdoms or organisms. Evolutionary relations are better represented by new classifications than by the traditional two kingdoms. Science. 1969 Jan 10;163(3863):150–160. doi: 10.1126/science.163.3863.150. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Woese C. R. A proposal concerning the origin of life on the planet earth. J Mol Evol. 1979 Jul 18;13(2):95–101. doi: 10.1007/BF01732865. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Debrunner-Vossbrinck B. A., Oyaizu H., Stackebrandt E., Ludwig W. Gram-positive bacteria: possible photosynthetic ancestry. Science. 1985;229:762–765. doi: 10.1126/science.11539659. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Evolution of the genetic code. Naturwissenschaften. 1973 Oct;60(10):447–459. doi: 10.1007/BF00592854. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. The concept of cellular evolution. J Mol Evol. 1977 Sep 20;10(1):1–6. doi: 10.1007/BF01796132. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gibson J., Fox G. E. Do genealogical patterns in purple photosynthetic bacteria reflect interspecific gene transfer? Nature. 1980 Jan 10;283(5743):212–214. doi: 10.1038/283212a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gupta R., Hahn C. M., Zillig W., Tu J. The phylogenetic relationships of three sulfur dependent archaebacteria. Syst Appl Microbiol. 1984;5:97–105. doi: 10.1016/s0723-2020(84)80054-5. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Olsen G. J. Archaebacterial phylogeny: perspectives on the urkingdoms. Syst Appl Microbiol. 1986;7:161–177. doi: 10.1016/s0723-2020(86)80001-7. [DOI] [PubMed] [Google Scholar]
- Woese C. R. On the evolution of the genetic code. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1546–1552. doi: 10.1073/pnas.54.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Sogin M. L., Bonen L., Stahl D. Sequence studies on 16S ribosomal RNA from a blue-green alga. J Mol Evol. 1975 Mar 24;4(4):307–315. doi: 10.1007/BF01732533. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Ludwig W. What are mycoplasmas: the relationship of tempo and mode in bacterial evolution. J Mol Evol. 1984;21(4):305–316. doi: 10.1007/BF02115648. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Macke T. J., Fox G. E. A phylogenetic definition of the major eubacterial taxa. Syst Appl Microbiol. 1985;6:143–151. doi: 10.1016/s0723-2020(85)80047-3. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Weisburg W. G., Paster B. J., Madigan M. T., Fowler V. J., Hahn C. M., Blanz P., Gupta R., Nealson K. H. The phylogeny of purple bacteria: the alpha subdivision. Syst Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- Woese C., Sogin M., Stahl D., Lewis B. J., Bonen L. A comparison of the 16S ribosomal RNAs from mesophilic and thermophilic bacilli: some modifications in the Sanger method for RNA sequencing. J Mol Evol. 1976 Apr 9;7(3):197–213. doi: 10.1007/BF01731489. [DOI] [PubMed] [Google Scholar]
- Wright S. The shifting balance theory and macroevolution. Annu Rev Genet. 1982;16:1–19. doi: 10.1146/annurev.ge.16.120182.000245. [DOI] [PubMed] [Google Scholar]
- Yamaizumi Z., Ihara M., Kuchino Y., Gupta R., Woese C. R., Nishimura S. Archaebacterial tRNA contains 1-methylinosine at residue 57 in T psi C-loop. Nucleic Acids Symp Ser. 1982;(11):209–213. [PubMed] [Google Scholar]
- Yang D., Oyaizu Y., Oyaizu H., Olsen G. J., Woese C. R. Mitochondrial origins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Schnabel R., Stetter K. O. Archaebacteria and the origin of the eukaryotic cytoplasm. Curr Top Microbiol Immunol. 1985;114:1–18. doi: 10.1007/978-3-642-70227-3_1. [DOI] [PubMed] [Google Scholar]
- Zuckerkandl E., Pauling L. Molecules as documents of evolutionary history. J Theor Biol. 1965 Mar;8(2):357–366. doi: 10.1016/0022-5193(65)90083-4. [DOI] [PubMed] [Google Scholar]



